梨属于蔷薇科(Rosaceae)梨属(Pyrus)植物,具有重要的经济价值,我国梨的栽培面积和总生产量均居世界首位,2020年我国梨栽培面积和生产量分别为86.5 万hm2和1 600.0 万t,分别占世界总量的66.9% 和69.2%(https://www.fao.org/faostat/zh/#data/QCL)。在我国砂梨(Pyrus pyrifolia)主要分布在淮河及秦岭以南的长江中下游和华南高温高湿地区。
在我国梨产区,根部病害发生较普遍,常导致梨树生长衰弱、结果延迟、产量品质下降,对梨产业发展构成了严重威胁[1-2]。主要有梨根朽病[3]、梨根癌病[4]、梨白纹羽病[5]和梨根腐病[6]等,其中白纹羽病在我国砂梨主产区的老梨园和立地条件差、管理粗放的梨园较常见,染病梨树初期除表现生长衰弱外,其余与健树无明显差异。大部分根系受害后,表现树势衰弱,叶片萎凋变黄。该病危害梨苗几周内即可导致枯死,大树受害后数年内也会死亡。
白纹羽病从梨根颈处侵入,在根颈表面形成白色网纹状菌丝层,侵入皮层组织后向主根和侧根蔓延。该病主要通过病根和健根相互接触在果园中短距离传播,随着苗木和砧木的调运远距离传播[8]。该病可危害鳄梨、假人参、苹果和日本梨等多种经济林木、果树,能够降解纤维素和木质素而导致腐烂[9]。已有研究表明,白纹羽病由病原座坚壳菌(Rosellinia)所致,其无性态属于束丝菌(Dematophora)。座坚壳菌(Rosellinia)隶属子囊菌门、核菌纲、炭角菌目、炭角菌科,其主要形态学特征为菌丝层褐色、毡状;子座呈球形或近球状,表面黑色、炭质;子囊壳单个、表生,子囊孢子单胞、椭圆形;菌核在腐朽木质部上形成,黑色,近球形,直径为1 mm左右,最大者可达5 mm;老熟菌丝在分节的一端膨大,后分离形成圆形的厚垣孢子。由于该属真菌形态变化较复杂,目前在属种划分与分类鉴定等方面仍存在许多问题,部分种的特征尚不明确。德国首次报道了葡萄白纹羽病的病原菌为褐座坚壳菌(Rosellinia necatrix)[10]。日本学者[5]认为R.compacta 和R.necatrix 均能引起日本梨的白纹羽病。我国至今尚缺乏梨白纹羽病病原的系统鉴定。
笔者在本研究中对我国砂梨主产区白纹羽病的发生危害状况进行调查,并采集病根样品进行病菌分离,采用形态学及分子生物学相结合的方法对病原菌种类进行鉴定并利用柯赫式法则(Koch’s postulates)验证,以明确引起我国砂梨主产区白纹羽病的病原菌种类,旨在为该病害的有效防控提供可靠的理论依据,并为座坚壳菌的进化与分类研究提供新的有用信息。
1 材料和方法
1.1 材料
2021年3—8月,在湖北、贵州、福建、四川、山东等砂梨主产区各1~2 个白纹羽病发生严重的地区,随机选取3~5个梨园,从翠冠、翠玉、黄花、秋月和黄金等品种显现有白纹羽病典型症状的梨树上,采集根部组织作为病原鉴定样品。共采集到48 株患有白纹羽病梨树的病根,样品采集分别得到了国家梨产业技术体系武汉、贵阳、福州、成都、烟台等综合试验站的协助。
1.2 方法
1.2.1 分离及纯化 将采集的病根用自来水清洗干净并用75%酒精消毒,晾干,采用已报道的根部病害病菌分离方法[11],切取病健交界处的4~5 mm2表皮组织块放入2%NaClO 中消毒3 min,无菌水中漂洗3 次后于已灭菌滤纸片上晾干,置于PDA 平板上,25 ℃恒温培养箱中黑暗培养2 d。挑取典型菌落边缘菌丝于新的PDA 平板上,25 ℃黑暗培养2 d。进行单菌丝纯化,将纯化后的菌株转至PDA斜面4 ℃保存和25%甘油中-80 ℃保存备用。
1.2.2 形态特征观察 将分离得到的菌株25 ℃黑暗培养5 d,挑取菌落边缘菌丝块(直径5 mm),转至新的PDA 平板中央,25 ℃黑暗条件下培养,每个菌株重复3皿。每天记录菌落在培养基上的生长情况(菌落的颜色、边缘形态、菌丝的疏密程度)并测量菌落直径。在光学显微镜(Olympus BX63,Japan)下观察菌丝形态。
1.2.3 分子鉴定 将分离得到菌株的菌丝块放置在铺有已灭菌玻璃纸的PDA培养基上,25 ℃黑暗条件下培养7 d,收集菌丝。采用CTAB 法[12]提取每个分离物的基因组DNA,并通过1.2%琼脂糖凝胶电泳检测DNA 质量。使用4 对引物对ITS1/ITS4、Bt2a/Bt2b、fRPB2-5F/fRPB2-7cr和LR0R/LR7[13-16],分别对分离物rDNA-ITS 的部分区域(ITS)、β-微管蛋白(TUB)、RNA聚合酶Ⅱ的第2大亚基(RPB2)和核糖体大亚基(LSU)区域进行PCR扩增(表1)。PCR反应体系和扩增程序参考前人的方法[17-18],扩增产物由生工生物工程股份有限责任公司进行纯化测序。
表1 用于扩增ITS、TUB、RPB2 和LSU 基因序列的引物
Table 1 List of primers used for ITS,TUB,RPB2 and LSU sequence amplification

1.2.4 系统进化分析 测序序列在NCBI 数据库进行BLAST 比对后,提交序列至GenBank,并从Gen-Bank数据库中下载已报道的基因序列。采用DNAMAN 9.0软件对每个测序菌株扩增的核苷酸序列及参考菌株的相应序列进行多重比对,再通过MAFFT v.7[19]在线服务器(http://mafft.cbrc.jp/alignment/server/index)进行多重比对,必要时用MEGA 7[20]对序列进行手工矫正。将比对好的基因序列按照ITS、LSU、RPB2 和TUB 的顺序首尾相连进行拼接[21]。基因序列合并的数据集共有2773个碱基(包括比对产生的空缺),使用IQ-TREE 对合并的数据集进行遗传进化分析,采用最大似然法(Maximumlikelihood)并选用GTR+G 核苷酸替代模型构建系统进化树,1000次重复。
1.2.5 致病性测定 采用小麦粒拌土接种法[22-23],供试菌株为HB1-1-1、FJ1-2-2 和GZ1-2-3,接种材料为杜梨(Pyrus betulaefolia)生根试管苗,由中国农业大学李天忠教授团队提供。将小麦粒于水中浸泡12 h后于120 ℃高压灭菌锅中灭菌30 min,冷却后加入直径为5 mm 的菌丝块(25 ℃黑暗条件培养7 d)于25 ℃培养箱中培养14 d。将含菌的小麦粒与土壤按1:40的比例均匀混合后栽植梨苗,并以不含菌的小麦粒与土壤相同比例混合后栽植为对照。每个处理5次重复,试验3次重复。接种期间室内培养条件为温度25 ℃、12 h/12 h 光暗交替,并维持湿度为52%左右,期间观察和记录发病情况。待出现症状后,从发病根部切取组织进行病菌再分离。
2 结果与分析
2.1 梨白纹羽病的田间症状表现
田间系统调查结果显示,湖北、福建、贵州、四川、山东等砂梨主产区的翠冠、翠玉、黄花、黄金、秋月等品种均可感染梨白纹羽病。地上部症状:染病初期植株叶片变黄,树势生长衰弱,后期叶片全部萎蔫干枯凋零,枝条干枯至整株枯萎死亡(图1-A)。地下根部症状:染病植株的主根和侧根表面被白色丝网状物缠绕(图1-B、C),部分植株发病部位可见病原菌菌丝形成的白色菌索(图1-D),在植株的根茎部和主根处可见白色羽状物覆盖,为菌丝层(图1-E)。

图1 砂梨白纹羽病的田间症状表现
Fig.1 The field symptoms caused by white root rot in sand pear
A.病株枝叶枯死;B、C.病株主根和侧根表面缠绕白色菌丝体;D.白色菌索;E.白色羽状菌丝层。
A.Dryness at the leaves and branches of diseased trees;B,C.The main root and lateral root of diseased plant covered with hypha;D.White rhizomorph;E.White pinnate mycelial laye.
2.2 病原菌培养特性与形态学观察
从我国5个不同省份(湖北、贵州、福建、山东和四川)的5个梨品种(翠冠、翠玉、黄花、秋月和黄金)采集的48 份梨白纹羽病样品中,共分离纯化获得169 个分离物。根据对其菌落形态特征观察,其中128 个分离物属于座坚壳属(Rosellinia)菌株(表2)。将所获得的座坚壳属菌株于PDA 培养基上,25 ℃黑暗条件下培养,观察发现所有这些菌株的培养特性与形态特征基本一致,在此仅展示菌株HB1-1-15的相关图片。从图2可看出,菌落初期为白色,羽毛状,中央气生菌丝茂密(图2-A、B),9 d 后菌落开始有黑色素沉淀,20 d 后菌落中央为深黑色逐渐向边缘变浅(图2-C、D)。对初期和老化菌落的菌丝镜检,发现菌丝均无色透明,产生有分支,部分菌丝在隔膜处具有明显的梨形膨大(图2-E、F)。

图2 病原菌(菌株HB1-1-15)的形态特征
Fig.2 Morphological characteristics of pathogens(strain HB1-1-15)
A、B 和C、D.分别为PDA 平板上培养6 d 和20 d 的正面和背面菌落形态;E.菌丝分支;F.菌丝分隔处梨形膨大。—标尺:E、F=20 μm。
A,B and C,D.Front and back views of 6-d-old and 20-d-old PDA culture;E.Branch of mycelia;F.Pear-shaped swelling in the septa union of mycelia.—Scale bars:E,F=20 μm.
表2 从我国不同砂梨主产区分离获得的梨白纹羽病病原菌分离株
Table 2 The pathogen isolates of pear white root rot obtained from different sand pear production regions in China

2.3 多基因系统发育分析
上述结果显示本研究所分离获得的128个座坚壳属(Rosellinia)菌株的形态特征无明显差异,依据其地区和梨品种来源的不同,从中选取36个代表菌株提取基因组DNA 和ITS、TUB、RPB2 和LSU 序列PCR扩增,得到大小分别为560~575 bp、375~380 bp、929~933 bp 和937~1048 bp 的特异性片段后进行测序。将获得的基因序列在GenBank(http://www.ncbi.nlm.nih.gov)数据库中进行BLAST 比对后,选取座坚壳属(Rosellinia)不同种代表菌株的相应基因区域序列作为参考序列,以Astrocystis mirabilis HAST 94070803 作为外群,进行多基因系统发育分析,具体序列信息见表3。
表3 本研究用于系统发育分析菌株的来源及GenBank 登录号
Table 3 Source and GenBank accession numbers of strains used for phylogenetic analysis in this study

注:/.无数据;本研究获得的菌株加粗表示。
Note:/.No data;The isolates obtained in this study are expressed in bold.
多基因系统发育分析结果(图3)显示:本研究得到的36个菌株与褐座坚壳菌(R.necatrix)的CBS 325.35、CBS 267.30 和CBS 349.36 分离物聚集在同一分支,其核苷酸序列的一致率分别为ITS 99.74%、TUB 99.86%、RPB2 99.45%和LSU 100%。在此分支中,本研究得到的菌株又聚为3个亚分支,其中SC2-2-1等13个菌株与来源于荷兰黄水仙白纹羽病样品的分离株R.necatrix(CBS 325.35 和CBS 267.30)聚在亚分支II;HB2-2-4 等15 个菌株与来源于阿根廷欧锦葵白纹羽病样品的分离株R.necatrix(CBS 349.36)聚在亚分支III;HB2-2-1 等8 个菌株聚在1个亚分支I。对3 个亚分支中菌株的基因序列进行比对分析,结果显示,RPB2 序列第240 个碱基亚分支III 菌株为G,而亚分支II 菌株为A;TUB 序列第208个碱基亚分支II菌株T,亚分支III菌株为C;ITS序列的第374个碱基亚分支I菌株为A,亚分支III菌株为C。
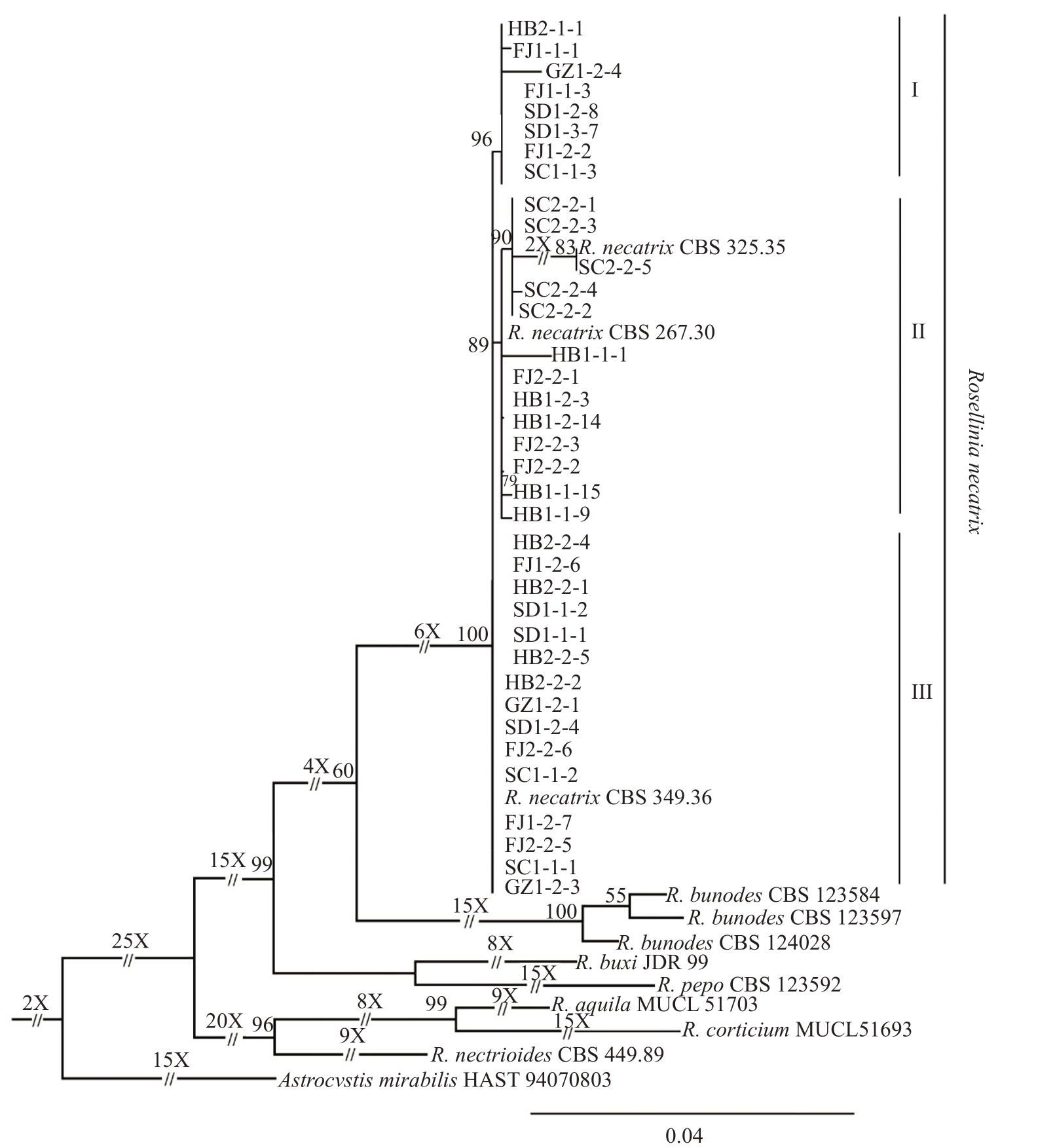
图3 本研究选取的代表菌株与参考菌株的多基因(ITS、TUB、RPB2 和LSU)序列采用最大似然法构建的系统进化树
Fig.3 Maximum-likelihood inference phylogenetic tree obtained from the combined ITS,TUB,RPB2 and LSU sequences alignments of representative strains obtained in this study and reference strains
本研究得到的36个菌株与座坚壳属6个种的代表菌株包括R.bunodes(CBS 123584、CBS 123597和CBS 124028)、R.buxi(JDR 99)、R.pepo(CBS 123592)、R.aquila(MUCL 51703)、R.corticium(MUCL 51693)和R.nectrioides(CBS 449.89)相比,其核苷酸序列的一致率分别为ITS 87.92%、TUB 90.18%、RPB2 94.34%和LSU 94.93%。座坚壳属(Rosellinia)的这7 个种的序列差异较大,分别聚集在不同分支。
上述结果表明,本研究得到的36个菌株均属褐座坚壳菌(R.necatrix),但不同来源菌株的多基因序列之间存在明显的差异。
2.4 致病性验证
本研究所分离获得的128个座坚壳属(Rosellinia)菌株中的78个菌株的品种来源为翠冠梨,致病性验证从3 个亚分支中各选取1 个品种来源均为该梨品种的菌株作为接种菌株,分别为亚分支II 中的HB1-1-15、亚分支I 中的FJ1-2-2 和亚分支III 中的GZ1-2-3。将菌株培养7 d 后的菌丝块,置于经水浸泡12 h和高压灭菌后的小麦粒中再黑暗培养14 d后作为接种源,拌土后接种于杜梨苗的根部。结果表明,接种后第3天,部分接种梨苗的地上部开始发病(图4-A);第6天,发病梨苗叶片逐渐卷曲干枯,甚至凋落,植株生长显著衰弱(图4-B);第9天,发病梨苗整株干枯死亡(图4-C),将所有枯死植株的根取出后进行观察,发现其主根和侧根均有白色丝状物覆盖,这与田间观察到的症状一致(图4-D)。15 株对照梨苗均未发病。

图4 杜梨苗接种褐座坚壳菌3 个菌株后地上部和根部的症状表现
Fig.4 Aboveground and root symptoms of P.betulifolia seedlings after inoculated with three strains of R.necatrix
A、B、C.接种后第3 天、第6 天和第9 天杜梨苗地上部症状;D.接种第9 天后杜梨苗根部症状。
A,B,C.Aboveground symptom of P.betulifolia seedlings at 3 dpi,6 dpi and 9 dpi;D.Root symptom of P.betulifolia seedlings at 9 dpi.
分别于接种后第3天、第6天和第9天调查地上部的发病株数,第9 天取出后调查地下部的发病株数。结果(表4)显示,接种后第3天,菌株HB1-1-15、FJ1-2-2 和GZ1-2-3 的发病株率分别为7%、20%和20%;接种后第6天,菌株HB1-1-15、FJ1-2-2和GZ1-2-3的发病株率分别为47%、80%和60%;接种后第9天,菌株HB1-1-15、FJ1-2-2 和GZ1-2-3 的发病株率分别为93%、100%和93%。接种后第9天,3个菌株处理植株地下部的发病株率均为100%。
表4 杜梨苗接种褐座坚壳菌3 个菌株后地上部和根部的发病株数
Table 4 Incidence for aboveground and root of P.betulifolia seedlings after inoculated with three strains of R.necatrix

对以上接种发病的梨苗随机采取病根样品进行病原菌再分离和鉴定,结果显示再分离出的病菌种类与接种菌株一致。
3 讨论
近些年来白纹羽病在我国梨产区,尤其是栽培砂梨的地区发生和危害有明显加重的趋势。本研究通过对湖北、福建、贵州、四川和山东等砂梨主产区白纹羽病的发生状况和危害特点进行系统性调查和病原菌分离纯化、形态特征观察和多基因序列分析及致病性验证,首次明确引起我国砂梨主产区白纹羽病的病原为褐座坚壳菌(R.necatrix),研究结果为我国梨产区白纹羽病的有效防控提供了理论依据。
座坚壳属(Rosellinia)隶属子囊菌门、核菌纲、炭角菌目、炭角菌科。已有研究表明,座坚壳菌在自然条件下其有性态及无性态的分生孢子非常罕见,且在实验条件下也很难诱导产生,因此该属种的形态学主要是根据其危害症状及菌丝结构特征进行鉴定[5,10,24-25]。本研究结果显示白纹羽病在染病梨的主根和侧根表面形成白色菌丝层,镜检可观察到菌丝产生分支,隔膜处呈梨形膨大,与Takemoto 等[5]和Nakamura等[24]对R.necatrix的描述一致。
近年来随着分子生物学方法的不断被应用,子囊菌的分类与种的鉴定研究取得重要进展,建立了更趋自然的分类系统[13-16,26]。采用多基因分析方法进行分子系统学研究,已成为植物病原真菌种类鉴定新的技术手段[18-20]。基于多基因包括ITS、LSU、RPB2 和TUB 等PCR 扩增和系统进化分析,相继鉴定明确了侵染林木、果树和花卉的座坚壳属(Rosellinia)的一些种[5,10,17,21]。但目前在GenBank上登陆的褐座坚壳菌(R.necatrix)基因序列很少。
本研究从我国5 个不同省份(湖北、贵州、福建、山东和四川)的5 个梨品种(翠冠、翠玉、黄花、秋月和黄金)分离得到128 个座坚壳属(Rosellinia)菌株,形态学观察结合多基因序列PCR 扩增和系统进化分析结果显示,所有分离获得的菌株培养特性与形态特征基本相同,均属褐座坚壳菌(R.necatrix),但不同来源菌株的多基因序列有异,在其系统进化树中聚在3 个不同的亚分支。从3 个亚分支中选取品种来源均为翠冠梨的3 个菌株(亚分支II中的HB1-1-15、亚分支I 中的FJ1-2-2 和亚分支III中的GZ1-2-3)接种到杜梨(Pyrus betulaefolia)生根试管苗的结果看出,这3 个菌株对杜梨的致病力存在明显差异。研究结果表明褐座坚壳菌(R.necatrix)基因序列的差异,导致了致病力的变化,而与其形态特征不相关。本研究为深入了解座坚壳菌(Rosellinia)的分子变异与致病多样性提供了新的有用信息。而有关梨白纹羽病病原褐座坚壳菌(R.necatrix)3 个亚分支菌株的致病类型及其分子机制还有待进一步研究。
梨白纹羽病为典型土传病害,针对植物土传病害的发生特点和危害方式,需要采用特殊的方法进行研究和防控[22-23]。本研究采用小麦粒拌土接种法对杜梨苗的接种结果表明,褐座坚壳菌(R.necatrix)通过侵染和危害地下部的根,导致地上部整株枯死。目前,我国栽培梨常用的砧木种类有杜梨(Pyrus betulaefolia)和豆梨(Pyrus calleryana)。因此,进一步研究褐座坚壳菌(R.necatrix)对杜梨和豆梨的侵染机制以及不同砧木对褐座坚壳菌的抵抗机制,对于梨产区白纹羽病的有效防治具有重要意义。
4 结论
我国砂梨主产区白纹羽病的病原均为褐座坚壳菌(Rosellinia necatrix),但不同来源菌株的基因序列之间存在明显差异,分别聚集在3个亚分支。
[1] 王龙,担玥玥.园林植物根部病害发生规律及防治方法[J].南方农业,2015,9(34):18-20.WANG Long,DAN Yueyue.Occurrence regularity and control methods of root diseases of garden plants[J].South China Agriculture,2015,9(34):18-20.
[2] 李改英.梨树根部主要病害的发生与防治[J].现代农村科技,2013(13):2.LI Gaiying.Occurrence and prevention of major root diseases of pear[J].Modern Rural Science Technology,2013(13):2.
[3] RAABE R D.Host list of the root rot fungus,Armillaria mellea[J].Hilgardia,1962,33(2):25-88.
[4] 杨雯,张志想,李世访.我国河南和甘肃地区梨树根癌病病原菌鉴定[J].植物保护,2020,46(6):55-59.YANG Wen,ZHANG Zhixiang,LI Shifang.Identification of pathogens of pear crown gall in Hennan and Gansu provinces of China[J].Plant Protection,2020,46(6):55-59.
[5] TAKEMOTO S,NAKAMURA H,SHIMANE S T. Rosellinia compacta,a new species similar to the white root rot fungus Rosellinia necatrix[J].Mycologia,2009,101(1):84-94.
[6] 林天元.梨根腐病病原菌鉴定及有效防控药剂筛选[D].合肥:安徽农业大学,2017.LIN Tianyuan.Pathogen identification of root rot in pear plant and fungicide screening for its efficient control[D].Hefei:Anhui Agricultural University,2017.
[7] SAWANT S S,CHOI E D,SONG J,SEO H J.Current status and future prospects of white root rot management in pear orchards:A review[J].Research in Plant Disease,2021,27(3):91-98.
[8] 代蕾,陈新雨.苗木根部病害白纹羽病和紫纹羽病的发生与防治[J].现代农村科技,2020(10):26.DAI Lei,CHEN Xinyu.Occurrence and control of seedling root diseases with white root rot and violet root rot[J].Modern Rural Science Technology,2020(10):26.
[9] 朱晓宇,杨书涛,周斌,丁慧君.白纹羽病、煤污病的诊断与防治[J].农业科技与信息,2012(9):36-37.ZHU Xiaoyu,YANG Shutao,ZHOU Bin,DING Huijun.Diagnosis and control of white root rot and sooty blotch[J].Agricultural Science Technology and Information,2012(9):36-37.
[10] PLIEGO C,CARLOS L H,RAMOS C,CAZORLA F M.Developing tools to unravel the biological secrets of Rosellinia necatrix,an emergent threat to woody crops[J].Molecular Plant Pathology,2012,13(3):226-239.
[11] LÓPEZ M,RUANO-ROSA D,LÓPEZ-HERRERA C J,MOTE E,HERMOSA R.Intraspecific diversity within avocado field isolates of Rosellinia necatrix from south-east Spain[J].European Journal of Plant Pathology,2008,121(2):201-205.
[12] PAL J,SHARMA S K,DEVI S,SHARMA R,RAJ H,KARM M,VERMA S,VEDUKOLA P R,SHARMAA.Screening,identification,and colonization of fungal root endophytes against Dematophora necatrix:A ubiquitous pathogen of fruit trees[J].Egyptian Journal of Biological Pest Control,2020,30(1):1-14.
[13] CARBONE I,KOHN L M.A method for designing primer sets for speciation studies in filamentous ascomycetes[J].Mycologia,1999,91(3):553-556.
[14] GLASS N L,DONALDSON G C.Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J].Applied Environmental Microbiology,1995,61(4):1323-1330.
[15] LIU Y J,WHELEN S,HALL B D.Phylogenetic relationships among Ascomycetes:evidence from an RNA polymerse II subunit[J].Molecular Biology &Evolution,1999,16(12):1799-1808.
[16] VILGALYS R,HESTER M.Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species[J].Journal of Bacteriology,1990,172(8):4238-4246.
[17] SU H,LI Q R,KANG J C,WEN T C,HYDE K D. Rosellinia convexa sp.nov.(Xylariales,Pezizomycotina) from China[J].Mycoscience,2016,57(3):164-170.
[18] VU D,GROENEWALD M,VRIES D,GEHRMANN T,STIELOW B,EBERHARDT U,AI-HATMI A,GROENEWALD J Z,CAEDINAL G,HOUBRAKEN J,BOEKHOUT T,CROUS P W,ROBERT V,VERKLEY G J M.Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation[J].Studies in Mycology,2019,91(1):23-36.
[19] KATOH K,STANDLEY D M.Mafft multiple sequence alignment software version 7:improvements in performance and usability[J].Molecular Biology and Evolution,2013,30(4):772-780.
[20] KUMAR S,STECHER G,TAMUA K.MEGA7:molecular evolutionary genetics analysis version 7.0 for bigger datasets[J].Molecular Biology and Evolution,2016,33(7):1870-1874.
[21] WITTSTEIN K,CORDSMEIER A,LAMBERT C,WENDT L,SIR E B,WEBER J,WURZLER N,PETRINI L E,STADLER M.Identification of Rosellinia species as producers of cyclodepsipeptide PF1022 A and resurrection of the genus Dematophora as inferred from polythetic taxonomy[J].Studies in Mycology,2020,96(1):1-16.
[22] CREEK S.Host range of Dematophora necatrix,the cause of white root rot disease in fruit trees[J].Plant Disease,1980,64(7):662-664.
[23] SZTEJNBERG A,FREEMAN S,CHET I,KATAN J.Control of Rosellinia necatrix in soil and in apple orchard by solarization and Trichoderma harzianum[J].Plant Disease,1987,71(4):365-369.
[24] NAKAMURA H,UETAKE Y,ARAKAWA M,OKABE I,MASTUMOTO N.Observations on the teleomorph of the white root rot fungus,Rosellinia necatrix,and a related fungus,Rosellinia aquila[J].Mycoscience,2000,41(5):503-507.
[25] HOOPEAN G M T,KRAUSS U.Biology and control of Rosellinia bunodes,Rosellinia necatrix and Rosellinia pepo:A review[J].Crop Protection,2006,25(2):89-107.
[26] WENDT L,SIR E B,KUHNERT E,HEITKÄMPER S,LAMBERT C,HLADKI A I,ROMERO A I,LUANGSA-ARD J J,SRIKITIKULCHAI P,PERÄOH D,STADLER M.Resurrection and emendation of the Hypoxylaceae,recognised from a multigene phylogeny of the Xylariales[J].Mycological Progress,2018,17(1-2):115-154.