薄壳山核桃[Carya illinoinensis (Wangenh.)K.Koch],是胡桃科山核桃属植物,又名长山核桃、美国山核桃。薄壳山核桃是一种重要的木本油料和珍贵的干果经济树种[1],原产于美国,20 世纪引入中国,我国引种地主要分布在湖南、江西、浙江、云南和秦淮以南、长江以北部分地区[2]。
磷(P)是一种植物必需的营养元素,在植物体内参与各种生化过程,如脂质代谢、核酸和细胞膜的生物合成等。植物以正磷酸盐的形式吸收生长发育所需的磷元素,在薄壳山核桃适生范围内,土壤有效磷普遍不足,以浙江临安为例,63%的土壤有效磷不足10 mg·kg-1,其中不足5 mg·kg-1占40%,这是由于磷(P)与铝和铁氧化物反应和土壤微生物转化,形成不能被植物吸收利用的难溶性磷和有机磷的形式[3]。生产中通过增施磷肥,提高薄壳山核桃产量。
植物为了应对缺磷环境,进化出了一系列生理和生化反应机制,典型的反应是减少光合作用和增加根/茎比(就生物量或干重而言)。在缺磷条件下,根茎比的增加主要是由于地上部分生长的减少和碳元素由茎向根的分配增加[4]。并且在拟南芥中还会通过改变根系结构,增强根中磷转运蛋白的表达,以提高磷的获取效率[5]。对于大多数植物而言,缺磷会引发根系分泌物的增加,包括酸性磷酸酶和核糖核酸酶[6]、羧酸盐和质子。这些渗出物有助于溶解固定在金属上的磷并从有机磷酸盐化合物中释放磷,从而提高植物吸收磷的效率。其他缺磷反应包括花青素在枝条中的积累(以保护叶绿体膜免受光损伤)和糖脂和硫脂的合成(以取代生物膜中的磷脂,因此可以为其他更重要的生化反应保留磷)[7]。但目前还缺乏在低磷胁迫下薄壳山核桃生长发育方面的综合研究报道。鉴于此,笔者针对低磷处理下薄壳山核桃幼苗在生理生化、细胞超微结构等层面进行较全面的研究,以期为揭示薄壳山核桃适应低磷胁迫的内在机制、提高抗逆性以及合理施磷肥以提高磷素利用效率、促进资源节约和环境保护提供参考。
1 材料和方法
1.1 试验地概况及试验材料
试验位于浙江省杭州市临安区浙江农林大学森林培育学科温室(119°43′39″E,30°15′14″N)进行。
试验材料选自浙江农林大学潘母岗实践教学基地,材料为半年生、长势一致、健康的薄壳山核桃实生苗,实生苗的种子采自浙江省杭州市的长乐林场钟山25号同一株成年树,并在10月下旬播种。
1.2 试验处理
采用水培法进行试验,40 L 的培养箱作为试验容器,用黑色泡沫板作遮光处理,霍格兰营养液(表1)每7 d 更换1 次,用通气泵保持24 h 通气。处理前用1/2 霍格兰营养液对试验材料缓苗30 d(2021年5—6月)。设置3 个磷浓度梯度:正常供磷(1 mmol·L-1 KH2PO4,CK)、低磷处理(0.5 mmol·L-1 KH2PO4,0.5 mmol·L-1 KCl,LP)、无磷处理(0 mmol·L-1 KH2PO4,1 mmol·L-1 KCl,NP),每个处理3 次重复,每个重复9 株幼苗。通过KH2PO4改变不同磷浓度梯度的P 含量,处理中不足的K+用KCl 代替,同时为排除Cl-的影响,在缓苗时加入CaCl2,其他各种元素浓度保持一致。连续处理90 d 后收获,即2021年9月收获测定指标数据。
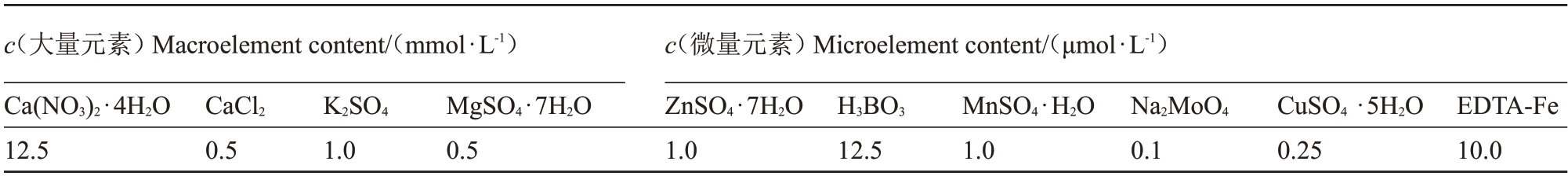
表1 霍格兰营养液配方
Table 1 Hoagland nutrient solution formula
1.3 试验方法与测定指标
1.3.1 生长指标测定 采用米尺测量幼苗地上部分长度(cm);采用游标卡尺测量地径(mm);采用万深LA-S系列植物图像分析仪扫描获得幼苗根系图片,然后用万深LA-S 系列植物图像分析仪系统对幼苗根系的根长、根径、表面积、体积等数据进行分析。
1.3.2 生理生化指标的测定(1)叶片光合参数测定:在缺磷胁迫处理90 d 后的上午9:00,从每个梯度中随机选取9 株植物,每株植物选择一片中上部的成熟叶片(顶端向下数第3~4 片叶),选择天气晴朗的上午用Li-6800 便携式光合测定系统(Li-Cor,Inc,USA)测定薄壳山核桃叶片的光合参数,测定过程中进入叶室的气体流速控制在500 μmol·s-1,相对湿度(RH)控制在60%左右,参比室CO2浓度控制在400 μmol·L-1左右,叶室温度为30 ℃左右,光合有效辐射(PAR)设定为800 μmol·m-2·s-1左右,测定得到净光合速率(Pn,μmol·m-2·s-1)、气孔导度(Gs,mol·m-2·s-1)、胞间CO2浓度(Ci,μmol·mol-1)、蒸腾速率(Tr,mol·m-2·s-1)等指标。
(2)叶片叶绿素荧光参数测定:在缺磷胁迫处理90 d 后天气晴朗的上午9:00 左右,采用Pm2500 便携式调制叶绿素慢速荧光仪对薄壳山核桃幼苗叶片进行活体测定。叶片经30 min 暗适应后测得初始荧光(F’o)和最大荧光(Fm),在光适应状态下测得实际荧光产量(F’o)、稳态荧光产量(F’s)和最大荧光产量(F’m)等荧光参数。根据所测定参数按照公式分别计算原初光能转换效率(Fv/Fo)、光系统Ⅱ的最大光能转化效率(Fv/Fm)、实际光化学效率(ΦPS Ⅱ)、光化学淬灭系数(qP)、非光化学猝灭系数(qN):

测量时,尽量使叶片均匀夹于叶夹内,并保持探头方向、角度一致,以保证均无遮阴且叶片受光方向相同。
(3)处理90 d 后叶片的叶绿素含量采用95%乙醇直接浸取法测定[8];用钼锑抗比色法测定根系磷含量;蛋白定量(TP)采用总蛋白测定试剂盒测定(序号:A045-2-2);超氧化物歧化酶(Superoxide dismutase,SOD)活性采用总超氧化物歧化酶测试盒(羟胺法)进行测定(序号:A001-1-2);过氧化物酶(Peroxidase,POD)活性采用过氧化物酶(POD)测定试剂盒(比色法)进行测定(序号:A084-3-1);丙二醛(malondialdehyde,MDA)含量采用丙二醛测定试剂盒进行测定(序号:A003-1-2);酸性磷酸酶(acid phosphatase,ACP)活性采用酸性磷酸酶测定试剂盒(分光光度法)说明进行测定(序号:A060-1-1)。
(4)超微结构观察:选取幼嫩的叶片剪成1 mm×4 mm 后放到含有2.5%的戊二醛溶液的离心管中,4 ℃固定过夜,进行双固定,然后倒掉固定液,用0.1 mol·L-1、pH7.0 的磷酸缓冲液漂洗样品;然后用一系列梯度浓度的乙醇和纯丙酮进行脱水,最后进行包埋、切片、染色和观察[9]。透射电镜中观察拍照,每个样品选取10个视野。
1.4 数据处理
采用Microsoft Excel 统计、整理试验数据;采用SPSS 25 软件对数据进行显著性(p<0.05)和相关性分析;采用GraphPad Prism 9 软件绘制柱形图等;采用Python 中的Pandas、Numpy、Seaborn 库对数据进行绘图和相关性分析。
2 结果与分析
2.1 薄壳山核桃生长指标
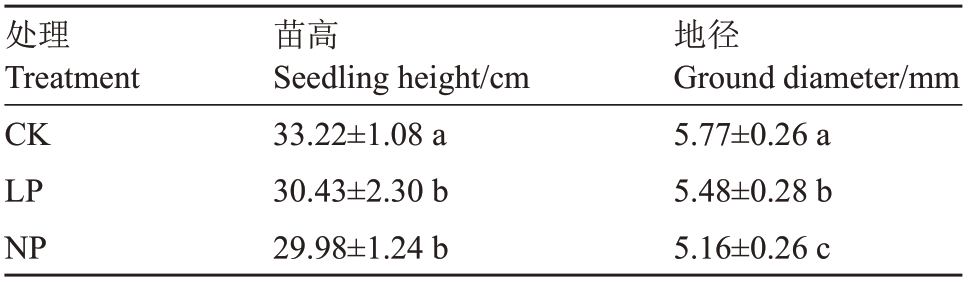
由表2和图1可知,低磷胁迫对薄壳山核桃幼苗的苗高和地径的影响差异显著(p<0.05)。LP和NP的薄壳山核桃植株的地径较CK 分别下降了5.03%、10.57%,LP和NP的薄壳山核桃植株的苗高较CK分别下降了8.40%、9.75%,LP和NP的苗高差异不显著。

图1 低磷胁迫对薄壳山核桃生长的影响
Fig.1 Effects of low phosphorus stress on the growth of pecans
表2 低磷处理对薄壳山核桃苗高、地径的影响
Table 2 Effects of low phosphorus stress on seeding height and ground diameter of pecans
注:不同小写字母表示差异显著p<0.05。下同。
Note:Different samall letters meam signicant difference at p<0.05.The same below.
2.2 低磷胁迫对薄壳山核桃幼苗根系形态的影响
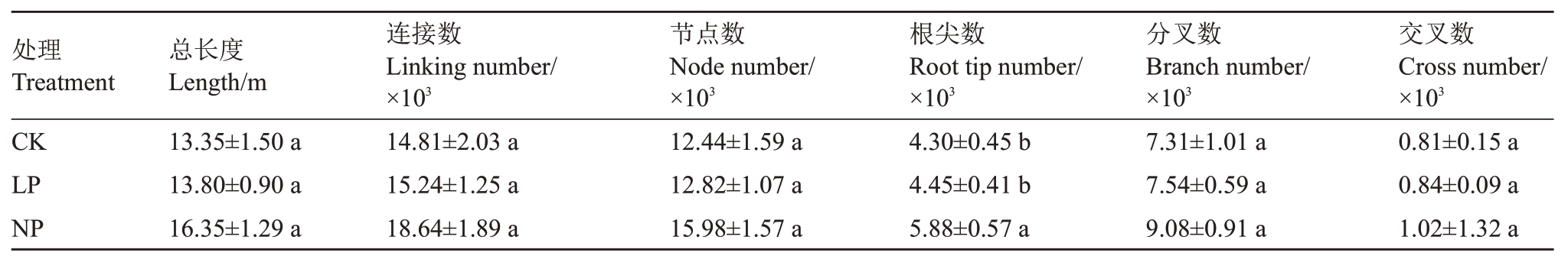
由表3可知,根系总长度、连接数、节点数、根尖数等指标都呈上升趋势,LP和NP的根尖数较CK分别上升了3.49%和36.74%,LP 较CK 差异不显著,NP 较CK 差异显著(p<0.05),表明在无磷条件下,薄壳山核桃的根会增加根的数量来吸收更多的磷以维持生命活动所需要的磷元素[10]。
表3 低磷处理对薄壳山核桃根系形态的影响
Table 3 Effects of low phosphorus stress on root morphology of pecans
2.3 低磷胁迫对薄壳山核桃幼苗根P含量的影响
由图2 可知,NP与CK的根系磷含量差异显著(p<0.05),LP和NP的根系磷含量较CK分别降低10.57%、59.21%,表明缺磷胁迫会减少薄壳山核桃磷的吸收。
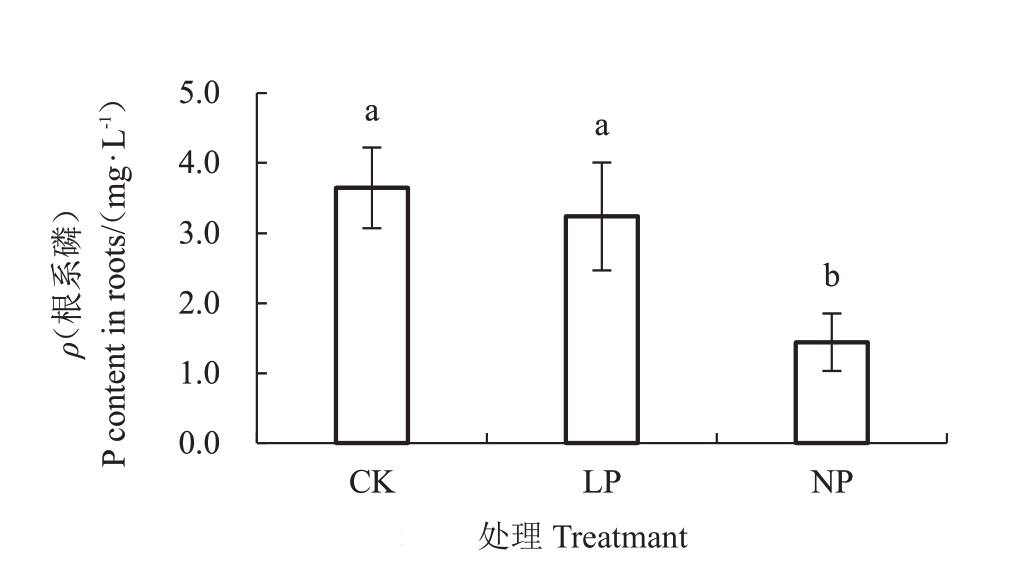
图2 低磷胁迫对薄壳山核桃幼苗根P 含量的影响
Fig.2 Effects of low phosphorus stress on P content in roots of pecans
2.4 低磷胁迫对薄壳山核桃叶片光合生理的影响
2.4.1 低磷胁迫对薄壳山核桃叶片光合作用的影响 由图3 可知,NP 与CK 的气孔导度(Gs)和蒸腾速率(Tr)差异显著(p<0.05),LP 和NP 的气孔导度较CK分别上升了15.21%、77.42%,LP和NP的蒸腾速率较CK分别上升了34.05%、73.62%。表明无磷胁迫下,薄壳山核桃幼苗为维持缺磷条件下的代谢平衡加快了蒸腾速率,气孔导度也随之增加。

图3 低磷胁迫对薄壳山核桃光合作用的影响
Fig.3 Effects of low phosphorus stress on photosynthesis of pecans
2.4.2 低磷胁迫对薄壳山核桃叶绿素含量的影响由图4 可知,NP 与CK 之间叶绿素含量差异显著(p<0.05),LP 和NP 叶绿素a 含量较CK 分别降低7.72%、30.78%,叶绿素b 含量较CK 分别降低6.50%、28.71%,总叶绿素含量较CK 分别降低9.37%、29.55%,胡萝卜素含量较CK 分别降低4.62%、29.53%。表明无磷的胁迫效应抑制了色素的合成,导致叶绿素和胡萝卜素的含量降低[11]。
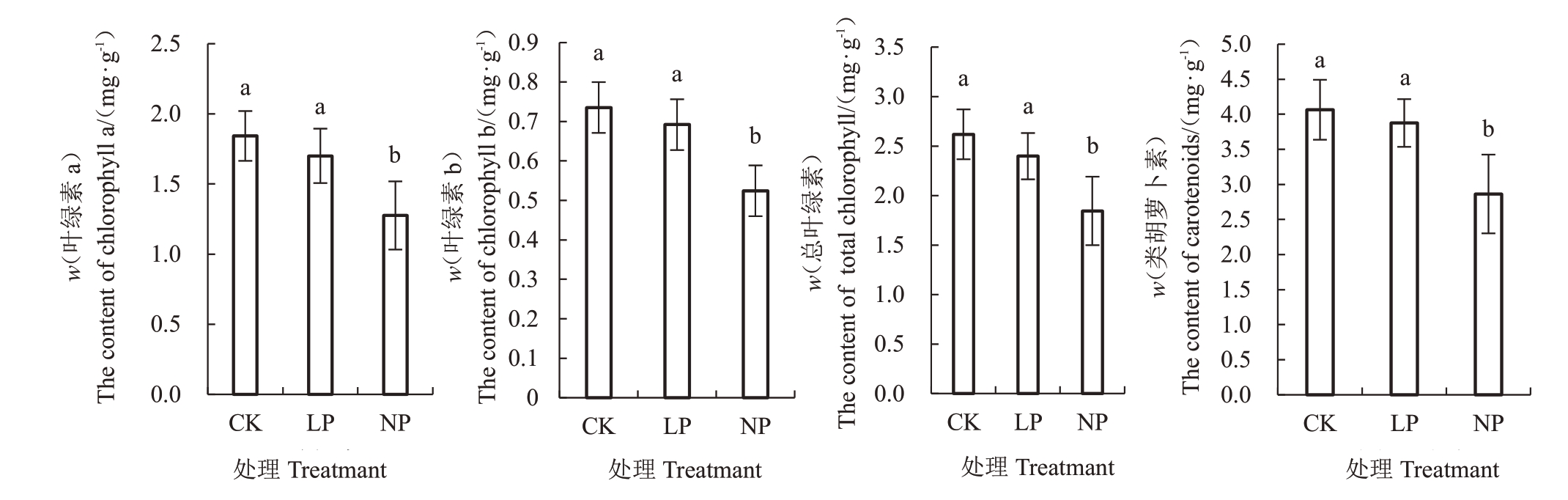
图4 低磷胁迫对薄壳山核桃叶片叶绿素含量的影响
Fig.4 Effects of low phosphorus stress on chlorophyllin content in pecans’s leaves
2.4.3 低磷胁迫对薄壳山核桃叶绿素荧光参数的影响 由表4 可知,LP 和NP 光系统Ⅱ的原初光合转化效率(Fv/Fo)较CK 分别下降了3.98%、7.67%,LP 和NP 光系统Ⅱ的最大光能转化效率(Fv/Fm)较CK 分别下降了1.28%、2.56% 。LP 和NP 光系统Ⅱ的实际光化学效率(ΦPS Ⅱ)较CK 分别上升了10.42%、12.50%,LP 和NP 的光化学淬灭(qP)较CK 分别上升了8.33%、13.89%,LP 和NP 的电子传递效率(ETR)较CK 分别上升了9.78%、12.35%。
表4 低磷胁迫对薄壳山核桃叶绿素荧光参数的影响
Table 4 Effects of low phosphorus stress on chlorophyll fluorescence parameters of pecans

2.5 低磷胁迫对薄壳山核桃幼苗抗氧化酶活性以及蛋白含量的影响
由图5 可知,LP 和NP 的蛋白含量较CK 分别显著下降了51.48%、69.21%(p<0.05)。LP 和NP的SOD 活性较CK 分别显著上升了83.28%、195.03%(p<0.05),LP 和NP 的POD 活性较CK 分别显著上升了86.63%、196.40%(p<0.05)。NP 与CK 间的MDA 含量差异显著,LP 和NP 的MDA 含量较CK 分别上升了4.93%、83.82%。LP 和NP 的ACP 活性较CK 分别显著上升了26.57%、65.18%(p<0.05)。
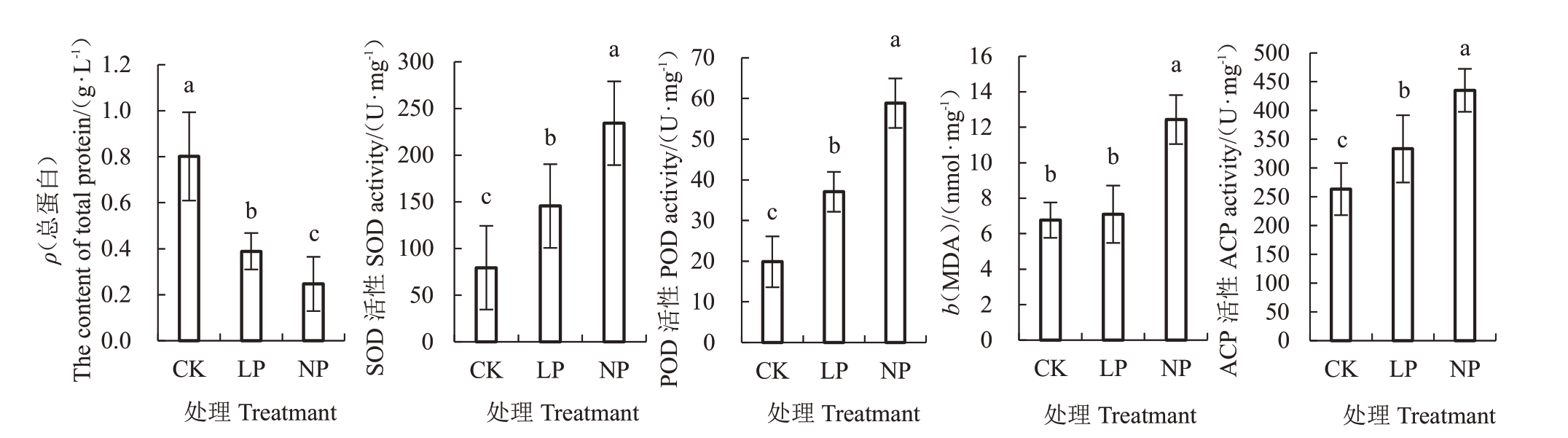
图5 低磷处理对薄壳山核桃抗氧化酶活性和蛋白含量的影响
Fig.5 Effects of low phosphorus treatment on enzyme activity and protein content of pecans
2.6 低磷胁迫对薄壳山核桃幼苗叶片叶肉细胞叶绿体超微结构的影响
由图6 可知,CK处理下薄壳山核桃幼苗叶肉细胞的叶绿体多为梭状,轮廓清晰光滑,紧贴细胞膜排布,类囊体有序堆叠,各叶绿体含扁长形、近椭圆状淀粉粒1~2个,还可见少量嗜锇颗粒分布。LP处理下的薄壳山核桃幼苗叶肉细胞的叶绿体和CK相差不大,都为梭状且紧贴细胞膜,近椭圆状淀粉粒1~2个。但NP 处理下薄壳山核桃幼苗叶肉细胞的叶绿体表现更膨大的状态,有些叶绿体已肿成半球状,向中部挤压液泡;类囊体垛叠仍然规则有序,淀粉粒数量变化小,但体积明显增大,挤占叶绿体中央;嗜锇颗粒基本不可见。

图6 薄壳山核桃叶肉细胞叶绿体超微结构
Fig.6 Chloroplast ultrastructure of mesophyll cells of pecans
A.CK 处理(标尺1 μm);B.LP 处理(标尺1 μm);C.NP 处理(标尺1 μm);D.CK 处理(标尺500 nm);E.LP 处理(标尺500 nm);F.NP 处理(标尺500 nm);CH.叶绿体;CW.细胞壁;M.线粒体;N.细胞核;OG.嗜锇颗粒;S.淀粉粒;V.液泡。
A.CK(scale 1 μm);B.LP(scale: 1 μm);C.NP(scale 1 μm);D.CK (scale: 500 nm);E: LP(scale: 500 nm);F.NP(scale: 500 nm);CH.Chloroplast;CW.Cell wall;M.Mitochondria;N.Nucleus;OG.Osmophilic granules;S.Starch granule;V.Vacuole.
2.7 低磷胁迫对薄壳山核桃幼苗各指标的相关性分析
由图7 可知,叶绿素a、叶绿素b、总叶绿素和类胡萝卜素之间的相关系数都大于0.98,蒸腾速率(Tr)与气孔导度(Gs)、胞间二氧化碳浓度(Ci)的相关系数分别为1、0.8,与净光合速率变化的相关系数为0.52,光系统II的相对电子传递效率(ETR)和光化学淬灭系数(qP)相对与净光合速率(Pn)的相关系数为0.23和0.22,表明在缺磷胁迫下,除Tr、Gs、Ci外,还存在其他影响光合作用的因素,如光系统II 的相对电子传递效率(ETR)[12-13]和光化学淬灭系数(qP)等一些叶绿素荧光参数。

图7 低磷胁迫对薄壳山核桃各指标相关性分析
Fig.7 Correlation analysis of various indexes of pecans under low phosphorus stress
2.8 低磷处理(LP)与无磷处理(NP)对薄壳山核桃幼苗影响程度的对比
由图8 可知,在低磷处理(LP)下,薄壳山核桃幼苗根系的磷含量、蛋白含量、地径相比CK 下降程度不大,无磷处理(NP)下,薄壳山核桃幼苗根系的磷含量、蛋白含量、地径相比CK 显著下降50%以上。

图8 低磷与无磷处理下胁迫程度比较
Fig.8 Comparison of stress degree between low P and none P treatments
3 讨论
薄壳山核桃幼苗可通过提高超氧化物歧化酶(SOD)、过氧化物酶(POD)的活性来清除超氧阴离子自由基和过氧化氢等物质,保护细胞免受伤害[14-15],特别是在无磷处理(NP)下,SOD、POD 的酶活性都显著上升,这与丁冬[16]研究的结果相一致,表明薄壳山核桃受到低磷胁迫时,酶活性调节能力强,植株的抗氧化能力也越强。SOD 和POD 对薄壳山核桃幼苗在缺磷条件下的氧化与抗氧化平衡起着关键的作用,有趣的是MDA 作为衡量植株脂质过氧化程度和反映细胞损伤程度的指标[17],无磷处理(NP)下虽然SOD、POD酶活性的上升在一定程度上增强了薄壳山核桃幼苗的抗氧化能力,但是MDA含量还是显著上升,表明无磷处理(NP)下薄壳山核桃幼苗还是受到了一定程度的脂质过氧化,SOD与POD未能及时清除无磷处理(NP)下的脂质过氧化,幼苗体内活性氧累积与清除的平衡仍旧遭到了破坏[18],而低磷处理(LP)下,MDA 的含量相对于CK只上升了4.36%,说明低磷处理下SOD与POD酶活性的增强可以及时清理过氧化的脂质,使幼苗适应低磷处理下的生长环境。
在缺磷胁迫下,薄壳山核桃幼苗分泌的酸性磷酸酶活性也显著增加,这与前人[19-22]在草莓、黄瓜、玉米、油菜等物种上的研究结果一致。研究普遍认为,这是植物在应对胁迫环境条件时,被诱导产生的响应机制[23],通过合成酸性磷酸酶分解环境中的有机难溶磷,在缺磷环境下,提高了幼苗对磷的利用率,从而促进有效磷的吸收。同时植物还会通过促进根的生长来增加根与营养液的接触面积,从而促进植物从外界吸收更多的磷[24-25]。本试验对薄壳山核桃缺磷胁迫下的根系形态进行了分析,无磷处理(NP)下的根尖数较正常磷(CK)显著增加,这也进一步说明薄壳山核桃幼苗耐低磷能力较强。但目前对于无磷处理(NP)下根尖数增加的作用机制尚不清楚,有待于通过转录组测序、基因芯片等分子生物学方法更加全面地分析磷胁迫下基因表达和代谢调控的作用机制。
叶绿体对外界环境敏感,在逆境胁迫下,叶绿体的超微结构都会产生一定程度的响应[26-27]。本试验中观察到,无磷处理(NP)下薄壳山核桃幼苗叶片叶绿体变成球状,基粒垛叠间距增大;叶绿体内淀粉粒体积明显膨大,填充于中部基粒片层之间。这是因为淀粉只能在叶绿体内降解,当供磷不足时,P与TP(磷酸丙糖)之间交换受到阻碍,导致叶绿体内的TP不能外运,进而转化为淀粉[28],残留在叶绿体内逐渐积聚。
在无磷处理下(NP)的胞间CO2浓度、蒸腾速率和叶绿素含量较正常磷(CK)差异显著,而低磷处理下(LP)的胞间CO2浓度、净光合速率等光合参数与叶绿素含量差异均不显著。胞间CO2浓度(Ci)反映的是其与气孔导度和叶肉细胞的光合活性两者之间的关系[29]。在无磷胁迫下,蒸腾速率和气孔导度显著上升,胞间CO2浓度没有显著差异,推断无磷胁迫下薄壳山核桃幼苗蒸腾速率的加快导致消耗了更多的能量并释放了更多的CO2,使胞间CO2浓度无显著差异。而缺磷胁迫下呼吸作用的增强,有机物快速地消耗,薄壳山核桃幼苗为快速补充消耗的有机物质而增强了光合作用,使得3 个处理下的净光合速率无较大的变化,表明薄壳山核桃幼苗的抗低磷能力较强[30]。为此加强探究磷胁迫下呼吸速率和叶绿体内有机物的合成速率的变化对磷胁迫下薄壳山核桃的抗低磷机制提供了研究方向。3个处理下叶片叶绿素的荧光参数以及净光合速率都不显著,可能是根中组装成了具有光合作用的膜结构[31],猜测是根中通过进行膜脂重塑,增加了膜上双半乳糖二甘油二酯(Digalactosyldiacylglycerol,DGDG)的含量,来进行光合作用。因为DGDG对于光合作用至关重要,而且正常情况下是只存在于叶绿体类囊体薄膜中,但在缺磷条件下就可在细胞膜或者质体膜中观察到[32],但目前还缺试验证据,有待于通过转录组测序等手段从分子层面解释膜脂重塑的分子机制。
4 结论
本研究结果表明,在低磷胁迫处理90 d后,薄壳山核桃的抗逆性较强,可以较长时间耐低磷胁迫,但是低磷胁迫下的时间也不宜过长,0.5 mmol·L-1的磷浓度还是存在胁迫的,因此在薄壳山核桃生产中,不需要经常施用磷肥,并且每次施用磷肥不要过多,综合生态环境和薄壳山核桃的生长状态,笔者推荐最适磷浓度为0.5~1 mmol·L-1之间,过多的施肥不但植物没有长得更好,还会导致严重的环境问题。综上,在对薄壳山核桃施用磷肥时,应遵循少量少施的原则。
[1] 贾晓东,刘永芝,翟敏,罗会婷,许梦洋,郭忠仁.薄壳山核桃山地嫁接与栽培技术[J].北方园艺,2018(3):195-197.JIA Xiaodong,LIU Yongzhi,ZHAI Min,LUO Huiting,XU Mengyang,GUO Zhongren.Mountain grafting and cultivation techniques of pecan tree[J].Northern Horticulture,2018(3):195-197.
[2] 张日清,李江,吕芳德,栗彬.我国引种美国山核桃历程及资源现状研究[J].经济林研究,2003,21(4):107-109.ZHANG Riqing,LI Jiang,LÜ Fangde,LI Bin.Historical survey of the introduced pecan tree in China:Distribution and resource estimate[J].Non-wood Forest Research,2003,21(4):107-109.
[3] JEZ J M,LEE S G,SHERP A M.The next green movement:Plant biology for the environment and sustainability[J].Science,2016,353(6305):1241-1244.
[4] HERMANS C,HAMMOND J P,WHITE P J,VERBRUGGEN N.How do plants respond to nutrient shortage by biomass allocation[J].Trends in Plant Science,2006,11(12):610-617.
[5] LóPEZ-BUCIO J,CRUZ-RAMíREZ A,HERRERA-ESTRELLA L.The role of nutrient availability in regulating root architecture[J].Current Opinion in Plant Biology,2003,6(3):280-287.
[6] WANG L S,LI Z,QIAN W Q,GUO W L,GAO X,HUANG L L,WANG H,ZHU H F,WU J W,WANG D W,LIU D.The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation[J].Plant Physiology,2011,157(3):1283-99.
[7] KOBAYASHI K,AWAI K,NAKAMURA M,NAGATANI A,MASUDA T,OHTA H.Type-B monogalactosyldiacylglycerol synthases are involved in phosphate starvation-induced lipid remodeling,and are crucial for low-phosphate adaptation[J].The Plant Journal for Cell and Molecular Biology,2009,57(2):322-331.
[8] 潘静,韩蕾.葡萄叶片叶绿素含量测定方法比较[J].西北园艺(综合),2017(6):58-60.PAN Jing,HAN Lei.Grape leaf chlorophyll content determination method[J].Northwest Horticulture,2017(6):58-60.
[9] XIE L,SONG X J,LIAO Z F,WU B,YANG J,ZHANG H M,HONG J.Endoplasmic reticulum remodeling induced by Wheat yellow mosaic virus infection studied by transmission electron microscopy[J].Micron,2019,120:80-90.
[10] LóPEZ-BUCIO J,CRUZ-RAMı′REZ A,HERRERA-ESTRELLA L.The role of nutrient availability in regulating root architecture[J].Current Opinion in Plant Biology,2003,6(3):280-287.
[11] 郑金凤,李成璞,董少鸣,白志英,李存东,毕常锐.低磷胁迫对小麦代换系叶绿素和类胡萝卜素含量的影响及染色体效应[J].华北农学报,2010,25(5):161-165.ZHENG Jinfeng,LI Chengpu,DONG Shaoming,BAI Zhiying,LI Cundong,BI Changrui.The effect of phosphorus deficiency stress on chlorophyll content and corticoid content and chromosome of wheat substitution lines[J].Acta Agriculturae Boreali-Sinica,2010,25(5):161-165.
[12] GENTY B,BRIANTAIS J M,BAKER N R.The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence[J].BBA-General Subjects,1989,990(1):87-92.
[13] HANELT D.9-Photosynthesis assessed by chlorophyll fluorescence[M].Netherlands:Elsevier,2018.
[14] 王文佳,李爽,马泽众,马玉玲,刘丽君,董守坤.水分胁迫对春大豆叶片保护酶活性及相对电导率的影响[J].中国农学通报,2019,35(11):14-18.WANG Wenjia,LI Shuang,MA Zezhong,MA Yuling,LIU Lijun,DONG Shoukun.Water stress affects protective enzyme activity and relative conductivity of spring soybean[J].Chinese Agricultural Science Bulletin,2019,35(11):14-18.
[15] 何洁,张显,张宁,杨小振,刘晓辉,郑俊鶱.低磷胁迫对不同西瓜品种苗期性状的影响[J].中国瓜菜,2014,27(S1):52-55.HE Jie,ZHANG Xian,ZHANG Ning,YANG Xiaozhen,LIU Xiaohui,ZHEN Junxian.Effects of low phosphorus stress on seedling traits of different watermelon varieties[J].China Cucurbits and Vegetables,2014,27(S1):52-55.
[16] 丁冬.低磷胁迫下玉米幼苗根系生理及膜脂代谢分子调控研究[D].大庆:黑龙江八一农垦大学,2020.DING Dong.Molecular regulation of physiology and membrane lipid metabolism in maize roots under low phosphorus stress[D].Daqing:Heilongjiang Bayi Agricultural University,2020.
[17] 王莹,刘晶,纪善博,杨国亭,关旸.锌胁迫对长药景天光合特性和保护酶活性的影响[J].生态学报,2016,36(22):7422-7427.WANG Ying,LIU Jing,JI Shanbo,YANG Guoting,GUAN Yang.Effects on photosynthetic characteristics and protective enzyme activity of hylotelephium spectabile to zinc stress[J].Acta Ecologica Sinica,2016,36(22):7422-7427.
[18] 张仁和,郑友军,马国胜,张兴华,路海东,史俊通,薛吉全.干旱胁迫对玉米苗期叶片光合作用和保护酶的影响[J].生态学报,2011,31(5):1303-1311.ZHANG Renhe,ZHENG Youjun,MA Guosheng,ZHANG Xinghua,LU Haidong,SHI Juntong,XUE Jiquan.Effects of drought stress on photosynthetic traits and protective enzyme activity in maize seeding[J].Acta Ecologica Sinica,2011,31(5):1303-1311.
[19] 吴妤,李卜,李晓彤,刘璐,李奕松.低磷胁迫对草莓幼苗根系生长和酸性磷酸酶活性的影响[J].中国农学通报,2013,29(34):197-200.WU Yu,LI Bo,LI Xiaotong,LIU Lu,LI Yisong.Effects of low phosphorus stress on root growth and acid phosphatase activity of strawberry seedlings[J].Chinese Agricultural Science Bulletin,2013,29(34):197-200.
[20] 庞欣,张福锁,李春俭.部分根系供磷对黄瓜根系和幼苗生长及根系酸性磷酸酶活性影响[J].植物生理学报,2000,26(2):153-158.PANG Xin,ZHANG Fusuo,LI Chunjian.Effects of the part of P-supply roots on cucumber seedling growth P concentration in shoot and root and secreted acid phosphatase activity by root[J].Plant Physiology Journal,2000,26(2):153-158.
[21] GANIE A H,AHMAD A,PANDEY R,AREF I A,YOUSUF P Y,AHMAD S,IQBAL M.Metabolite profiling of low-P tolerant and low-P sensitive maize genotypes under phosphorus starvation and restoration conditions[J].PloS One,2015,10(6):e0129520.
[22] 张海伟,黄宇,叶祥盛,徐芳森.低磷胁迫下甘蓝型油菜酸性磷酸酶对磷效率的贡献分析[J].中国科学:生命科学,2010,40(5):418-427.ZHANG Haiwei,HUANG Yu,YE Xiangsheng,XU Fangsen.Analysis of the contribution of acid phosphatase to P efficiency in Brassica napus under low phosphorus condition[J].Scientia Sinica(Vitae),2010,40(5):418-427.
[23] 陈永亮,李修岭,周晓燕.低磷胁迫对落叶松幼苗生长及根系酸性磷酸酶活性的影响[J].北京林业大学学报,2006,28(6):46-50.CHEN Yongliang,LI Xiuling,ZHOU Xiaoyan.Effects of phosphate deficiency on the growth and acid phosphatase activity of Larix gmelini seedlings[J].Journal of Beijing Forestry University,2006,28(6):46-50.
[24] DEVAIAH B N,KARTHIKEYAN A S,RAGHOTHAMA K G.WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis[J].Plant Physiology,2007,143(4):1789-801.
[25] DEVAIAH B N,MADHUVANTHI R,KARTHIKEYAN A S,RAGHOTHAMAK G.Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis[J].Molecular Plant,2009,2(1):43-58.
[26] 李江肃,王志刚.以胞间CO2为指标的光合速率探讨[J].生物学教学,2020,45(5):68-71.LI Jiangsu,WANG Zhigang.Study on photosynthetic rate using intercellular CO2 as index[J].Biology Teaching,2020,45(5):68-71.
[27] 王茹.杜仲光合生理和蒸腾特性的研究[D].合肥:安徽农业大学,2012.WANG Ru.Photosynthetic and transpiration characteristics of Eucommia ulmoides[D].Hefei:Anhui Agricultural University,2012.
[28] 万里强,石永红,李向林,何峰,贾亚雄.高温干旱胁迫下3 个多年生黑麦草品种叶绿体和线粒体超微结构的变化[J].草业学报,2009,18(1):25-31.WANG Liqiang,SHI Yonghong,LI Xianglin,HE Feng,JIA Yaxiong.Alterations in leaf cellular ultra-structure of three vari-eties of Lolium perenne subjected to high temperature and soil drought stress[J].Acta Prataculturae Sinica,2009,18(1):25-31.
[29] 尤扬,王贤荣,张晓云.低温对大花丹桂叶肉细胞超微结构的影响[J].甘肃农业大学学报,2018,53(4):100-106.YOU Yang,WANG Xianrong,ZHANG Xiaoyun.The effects of low temperature stress on mesophyll cells ultrastructure in Osmanthus fragrans Dahua Dangui[J].Journal of Gansu Agricultural University,2018,53(4):100-106.
[30] 李绍长.低磷胁迫对植物光合和呼吸作用的影响[J].石河子大学学报(自然科学版),2003,7(2):157-160.LI Shaochang.Effects of low phosphate stress on plant photosynthesis and respiration[J].Journal of Shihezi University(Natural Science),2003,7(2):157-160.
[31] KOBAYASHI K,BABA S,OBAYASHI T,SATO M,TOYOOKA K,KERÄNEN M,ARO E,FUKAKI H,OHTA H,SUGIMOTO K,MASUDA T.Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis[J].The Plant Cell,2012,24(3):1081-1095.
[32] JOUHET J,MARéCHAL E,BALDAN B,BLIGNY R,JOYARD J,BLOCK M.Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria[J].The Journal of Cell Biology,2004,167(5):863-874.