我国是世界苹果最大的生产及贸易中心,苹果栽培面积最大,总产量最高[1]。我国桃树栽培面积约80 万hm2,也是主要栽培果树之一[2]。生产中常见桃园迹地改建苹果园的现象[3]。但是前茬土壤中所含成分错综复杂,对后茬植株的影响无法确定。桃连作障碍问题和苹果连作障碍问题已有较多报道[4-6]。研究表明,前茬土壤由于长期种植单一果树对后茬产生抑制作用[4],桃园迹地在改建苹果园时,桃园土壤对苹果植株生长、果实产量和品质等方面的影响少有报道。因此,探究桃园迹地建园对后茬苹果树体的影响,对桃园改建苹果园具有指导意义。笔者通过研究桃园迹地建园对富士苹果植株生长、光合荧光及果实品质的影响,旨在为桃园改建苹果园的认识及深入研究提供有效理论依据和参考。
1 材料和方法
1.1 试验材料与处理
本研究田间试验于2014—2021年在烟台市蓬莱区南王街道贯里村进行,室内试验在山东农业大学作物生物学国家重点实验室进行。选定有代表性的6~8年生桃园换植苹果幼树设为处理园(T),该园土壤类型为棕壤,有效磷含量(w,后同)为39.7 mg·kg-1,速效钾含量为104.7 mg·kg-1,碱解氮含量为72.7 mg·kg-1,土壤pH为6.2,有机质含量为9.03 g·kg-1;对照园(CK)为桃园邻地(小麦迹地)栽植苹果幼树,该园土壤类型为棕壤,有效磷含量为39.0 mg·kg-1,速效钾含量为109.3 mg·kg-1,碱解氮含量为76.66 mg·kg-1,土壤pH 为6.5,有机质含量为8.97 g·kg-1,栽植苹果品种均为烟富3,砧木为八棱海棠。处理园于2014年秋刨除桃树,2015年春季对照园和处理园按统一标准进行苹果幼树栽植,面积均为666.7 m2,株行距为3 m×5 m,并配以10%的嘎拉苹果树作授粉树。按生产常规统一田间管理。
1.2 指标测定
植株生物量测定:2021年4月20日调查枝条总数及开花枝条总数,计算花枝率。2021年10月18日各园区选择东、西、南、北、中五个方位,每方位选取3株树进行相关指标测定。采用卷尺测定株高、干周和冠幅;每株选取外围新梢5个,用直尺测定每个枝条节间长度和新梢长度;统计每株树外围1年生枝中长枝所占比例,计算成枝率;测量并统计短枝的数量,计算短枝率;选取未经修剪的2年生枝20个,统计萌发的芽数占总芽数的百分比,计算萌芽率。
叶片生理指标:采用CIRAS-3 便携式光合仪(PP System,英国)于8 月14 日上午8:00—11:00 测定净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)、蒸腾速率(Tr)等光合参数。采用便携脉冲调制式荧光仪(FMS-2,Hansatech,英国)测定叶绿素荧光参数,并获得相关参数。各处理选取不同方向、不同类型新梢中部健壮无病虫害叶片各10片测定,取平均值。选取树冠外围生长正常的发育枝、无果短枝、有果短枝,各在中部采集叶片100枚,采用网格法测量叶面积;感量为0.01 g 的YP1002N 型电子天平称取百叶质量,然后换算成单叶质量;参照赵世杰等[7]的方法测定叶绿素含量。按照Kazemi等[8]的方法提取叶片超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)和抗坏血酸过氧化物酶(APX)。SOD活性用氯化硝基四氮唑蓝(NBT)光化还原法[9]测定,POD活性按Omran[10]的方法测定,CAT活性用紫外分光光度法[11]测定,APX活性按照Wang等[9]的方法测定;采用硫代巴比妥酸法[7]测定丙二醛(MDA)含量;采用Bai 等[12]的方法利用紫外分光光度计测定H2O2含量;采用羟胺反应法测定超氧阴离子产生速率[13];采用Irigoyen 等[14]的方法测定叶片可溶性糖含量,采用酸性茚三酮显色法测定脯氨酸含量[7]。
果实产量及品质:2017年至2021年在各园区选择东、西、南、北、中五个方位进行样品采集,每方位选取3 株苹果植株,每株摘取10 个苹果果实统计每666.7 m2产量。2021年10月18日,每方位选取3株苹果植株,每株选取10个苹果果实,测定果实硬度、果形指数、光洁度指数、可溶性固形物含量、可溶性糖含量、可滴定酸含量、糖酸比。使用YP1002N型电子天平称取单果质量;使用GY-1硬度计测定果实硬度,使用游标卡尺测定果实纵、横径,计算果形指数;使用CI-410色差计测定果面色泽,分级标准如表1所示;使用PAL-1型数显手持糖度计测定可溶性固形物含量;采用蒽酮比色法[15]测定可溶性糖含量;采用NaOH滴定法[16]测定可滴定酸含量;由以上可溶性糖和可滴定酸的含量值计算糖酸比。
表1 果实着色和光洁度分级标准
Table 1 Grading standard for fruit coloring and finish

光洁度指数/%=[∑(各级果数×各级代表值)/(总果数×最高级值)]×100。
果面着色指数/%=[∑(各级果数×各级代表值)/(总果数×最高级值)]×100。
土壤养分测定:于2021年10月18日,每处理选取5个方位,每方位选取3 个不同苹果植株,距植株主干30 cm处清除土壤表面杂物,取0~30 cm深度土壤样品。参照鲍士旦[17]的方法测定土壤基础理化性质。
1.3 数据处理
采用SPSS 18 统计分析软件进行t检验:p<0.05,表示有显著性差异;p<0.01,表示有极显著性差异。
2 结果与分析
2.1 桃园迹地建园对苹果植株生长特性的影响
由表2可知,桃园迹地建园(T)和对照园中苹果植株株高和干周均无显著性差异,株高分别为230.7、260.7 cm,干周分别为29.0、36.0 cm。与对照园相比,桃园迹地建园条件下苹果植株冠幅和新梢长度则分别显著性降低13.1%、19.8%,苹果植株叶面积和单叶质量显著减少17.6%、7.2%,而叶绿素(a+b)含量两者之间无显著性差异。这说明桃园迹地建园不利于苹果植株的生长,对其有一定抑制作用。
表2 桃园迹地建园对苹果树体生长特性的影响
Table 2 Effects of peach orchard soil on the growth of apple trees
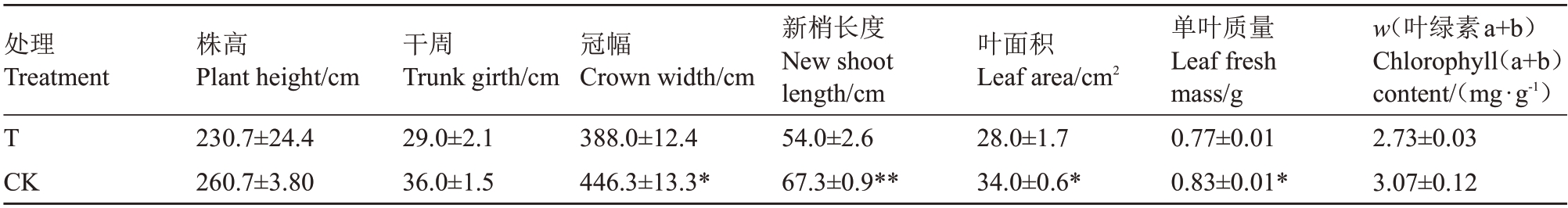
注:*p <0.05,差异显著;**p <0.01,差异极显著。下同。
Note:*indicate statistical significance(p <0.05),**indicate statistical significance(p <0.01).The same below.
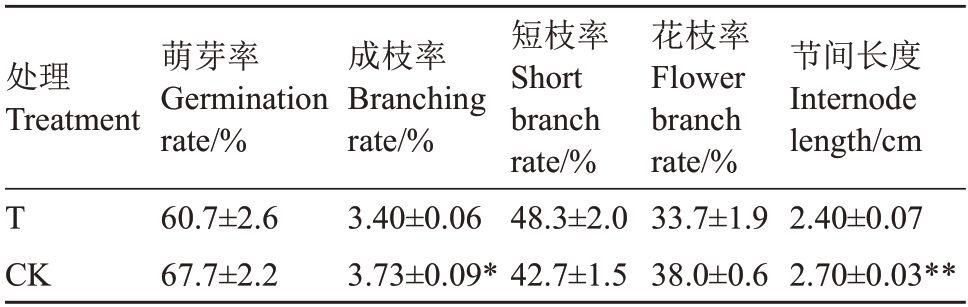
由表3可知,两处理间苹果植株枝条萌芽率、短枝率、花枝率均无显著性差异,萌芽率均在60%以上,短枝率均在40%以上。与对照园相比,桃园迹地建园使苹果植株成枝率和新梢节间长度分别显著降低8.8%、11.1%,表明桃园迹地建园导致苹果植株新梢节间长度缩短,成枝率下降。
表3 桃园迹地建园对苹果树体枝条生长的影响
Table 3 Effects of peach orchard soil on the branch growth of apple trees
2.2 桃园迹地建园对苹果植株光合特性的影响
与对照园相比,桃园迹地建园使苹果植株叶片净光合速率显著性下降12.1%,蒸腾速率、气孔导度和胞间CO2浓度两者间均无显著性差异(表4)。
表4 桃园迹地建园对苹果植株叶片光合相关参数的影响
Table 4 Effects of peach orchard soil on Pn,Gs,Ci and Tr in leaves of apple trees

Fv’/Fm’表示开放的PS Ⅱ反应中心的激发能捕获效率。ETR表示电子传递效率[18]。ΦPS Ⅱ反映光下植物叶片用于电子传递的能量占所捕获光能的比例,反映了被用于光化学途径激发能占进入PS Ⅱ总激发能的比例,是植物光合能力的一个重要指标,可以作为植物光合电子传递速率快慢的相对指标。由表5可知,与对照园相比,桃园迹地建园使苹果植株叶片Fv’/Fm’显著降低20.3%,ETR显著降低35.5%,ΦPS Ⅱ显著降低30.0%,叶片PS Ⅱ反应中心的光化学活性降低,电子传递链受到损害,激发能捕获效率降低,实际光化学效率减弱。
表5 桃园迹地建园对苹果植株叶片荧光淬灭参数的影响
Table 5 Effects of peach orchard soil on fluorescence quenching parameters in leaves of apple trees

光化学淬灭系数(qP)反映了PS Ⅱ反应中心的开放程度,1-qP 越大,对PS Ⅱ激发压越大。与对照园相比,桃园迹地建园条件下苹果植株叶片1-qP显著增加60%,说明桃园迹地建园导致苹果植株叶片PS Ⅱ反应中心关闭程度增加,受体侧接受电子能力减弱,从而加剧了对PS Ⅱ的激发压力。
NPQ为非光化学猝灭系数,反映光系统对过剩光能耗散能力。通常非光化学淬灭系数的增长可能是叶片为免受光破坏的保护机制。与对照园相比,桃园迹地建园条件下苹果植株叶片NPQ 显著增加41.3%,PS Ⅱ激发能分配方式发生改变,苹果植株叶片通过提高对过剩光能的耗散能力来适应桃园迹地土壤带来的逆境。
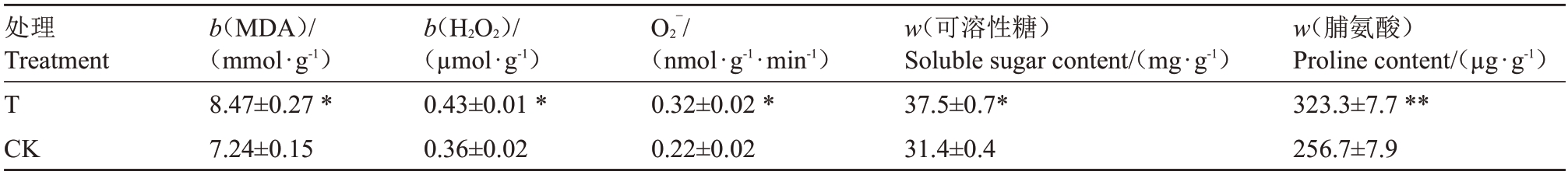
由表6 可知,桃园迹地建园导致苹果植株叶片中MDA、H2O2、可溶性糖、脯氨酸含量及O2¯活性分别比对照园显著增加17.0%、19.4%、19.4%、25.9%、45.5%。这表明桃园迹地土壤对苹果植株造成胁迫,有效提升了苹果植株叶片内的活性氧含量,膜脂过氧化程度加剧。同时,可溶性糖含量和脯氨酸含量增加。
表6 桃园迹地建园对苹果植株叶片膜透性、H2O2含量、超氧阴离子活性、可溶性糖和脯氨酸含量的影响
Table 6 Effects of peach orchard soil on MDA,H2O2,O2¯,soluble sugars and Pro in leaves of apple trees
由表7 可知,桃园迹地建园导致苹果植株叶片中超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)和抗坏血酸过氧化物酶(APX)活性分别比对照园显著降低8.8%、21.5%、18.3%、22.8%。表明与对照相比,桃园迹地建园条件下,苹果植株叶片内抗氧化酶活性降低,其对逆境抗性减弱。
表7 桃园迹地建园对苹果植株叶片抗氧化酶活性影响
Table 7 Effects of peach orchard soil on antioxidative system in leaves of apple trees

由图1可知,与对照园相比,2017年至2021年,桃园迹地建园条件下每666.7 m2苹果植株产量分别显著减少32.3%、28.6%、23.2%、18.1%、15.7%,2017年减少幅度最大,为32.3%,随年限增加,减少幅度呈现下降趋势。
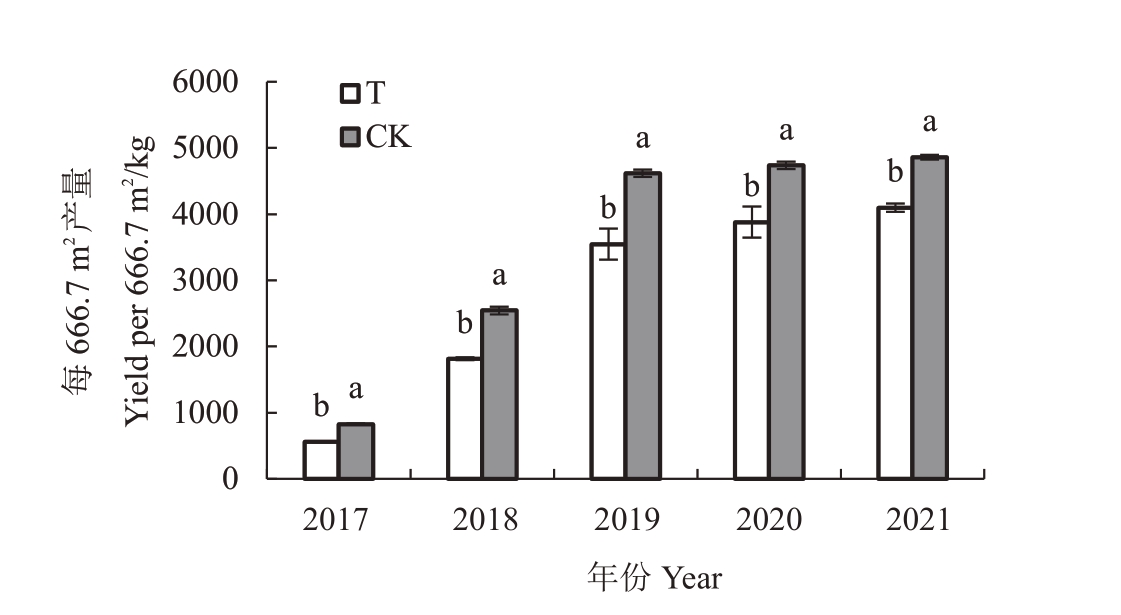
图1 桃园迹地建园对苹果产量的影响
Fig.1 Effect of peach orchard soil on yield of apples
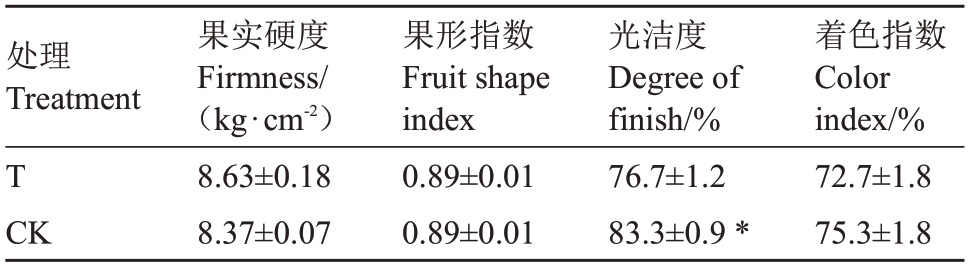
由表8 可知,与对照园相比,桃园迹地建园条件下苹果果实光洁度显著降低7.9%。对照园和桃园迹地建园条件下,苹果果实硬度分别为8.37、8.63 kg·cm-2,果形指数均为0.89,着色指数分别为75.3%、72.7%,两者间此3 项指标均无显著性差异。
表8 桃园迹地建园对苹果果实硬度、果形指数及外观品质的影响
Table 8 Effects of peach orchard soil on firmness,fruit shape index and appearance quality in apples
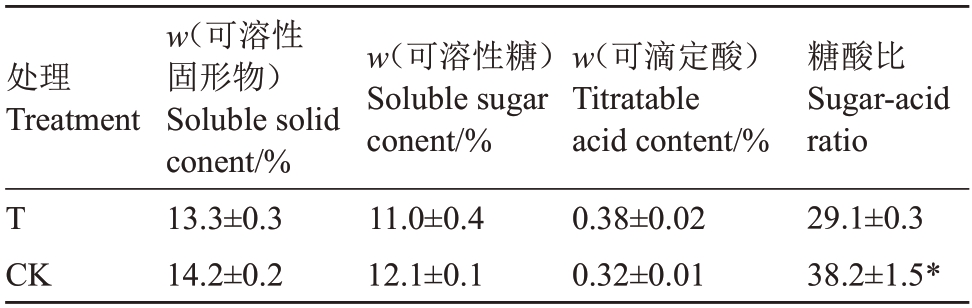
由表9可知,对照园和桃园迹地建园条件下,苹果果实可溶性固形物含量分别为14.2%、13.3%,可溶性糖含量分别为12.1%、11.0%,可滴定酸含量分别为0.32%、0.38%,两者间此3 项指标均无显著性差异。而与对照园相比,桃园迹地建园条件下苹果果实糖酸比表现为显著性下降,下降幅度达23.8%。
表9 桃园迹地建园对苹果果实糖、酸含量和糖酸比的影响
Table 9 Effects of peach orchard soil on soluble solid,soluble sugar,titratable acid content and sugar-acid tatio in apples
由表10 可知,对照园和桃园迹地建园条件下,土壤中速效钾、速效磷、全氮、有机质含量及pH值均无显著性差异。
表10 不同处理间土壤养分含量及pH 值
Table 10 Soil nutrient content and pH value in different treatments

3 讨论
生产中常见桃园迹地改建苹果园的现象。老龄苹果园改建新苹果园,往往引起苹果连作障碍,即苹果植株生长缓慢,果实产量和品质下降甚至树木死亡的现象[4-6]。而桃园迹地建园是否对苹果植株生长及果实产量、品质产生影响少有报道。前人研究认为,前茬桃园土壤中遗留积累苦杏仁苷、苯甲酸等酚酸类物质[19]。大量研究表明,酚酸类化感物质达到一定浓度可抑制作物的生长[20-22]。植物生长受到抑制时,植株内部会发生一系列生理生化变化,这些变化主要表现在酶的活性上[23]。SOD、POD、CAT、APX 等抗氧化酶活性的高低在一定程度上反映了逆境胁迫对植株的伤害大小。本研究发现,与对照园相比,桃园迹地建园条件下苹果植株叶片中保护性酶活性均不同程度显著降低,苹果植株生长发育受到抑制,苹果植株冠幅及当年新梢长度显著降低,新梢节间长度缩短,叶面积及单叶质量减少,苹果果实产量显著降低,果面光洁度及果肉糖酸比值均显著减少,表明桃园迹地建园不利于苹果植株的生长发育。
光合速率下降是植物在逆境条件下生长受抑制的重要原因之一。研究表明,化感物质通过抑制PS Ⅱ中QA和QB之间的电子传递,进而影响光合作用[24]。与对照园相比,桃园迹地建园条件下苹果植株叶片净光合速率下降,这与前人研究一致[3]。笔者深入研究发现,桃园迹地建园条件下苹果植株叶片Fv’/Fm’、ETR 及ΦPS Ⅱ显著降低,植物光合电子传递速率下降,电子传递链受损,激发能捕获效率降低,实际光化学效率减弱,植株叶片PS Ⅱ反应中心的光化学活性降低,进而光合速率下降,同时,桃园迹地建园条件下,苹果植株叶片PS Ⅱ反应中心关闭程度增加,导致受体侧接受电子的能力减弱,加剧了对PS Ⅱ的激发压力,植株叶片PS Ⅱ激发能分配方式发生改变,通过提高对过剩光能的耗散能力来适应桃园土壤带来的胁迫环境。
光是植物光合作用的启动因子和能量来源,当叶片吸收的光能不能及时转化成化学能时,容易导致活性氧的产生,对光系统造成氧化破坏,抑制光合作用。与对照园相比,桃园迹地建园条件下,前茬桃园土壤中有害微生物[3]及前茬桃树遗留积累的苦杏仁苷、苯甲酸[19]导致土壤环境不断恶化,引起苹果植株叶片内H2O2、MDA 含量及O2¯活性显著增加。活性氧和MDA过量积累,渗透物质含量上升,膜脂过氧化程度加重[9]。过量的活性氧会攻击蛋白质、核酸、脂类等生物大分子引起氧化损伤。脯氨酸和可溶性糖是逆境胁迫下植物体内保护性物质,是其适应胁迫环境的基本特征之一,逆境条件下脯氨酸的积累可能有适应意义,也可能是细胞结构受损的一种伤害反应[8]。本研究结果表明,与对照园相比,桃园迹地建园条件下苹果植株叶片内抗氧化酶活性降低,活性氧含量增加,抗氧化酶系统与活性氧之间动态平衡被打破[25],加剧了脂膜过氧化程度,同时,叶片内可溶性糖和游离脯氨酸含量增加。
4 结论
桃园迹地建园导致苹果植株叶片内抗氧化酶系统与活性氧之间动态平衡被打破,PS Ⅱ反应中心电子传递链受损,实际光化学效率减弱,净光合速率下降,苹果植株生长受到抑制,叶面积减少,枝条节间长度缩短,果实产量、糖酸比值和光洁度降低。
[1] 陈学森,韩明玉,苏桂林,刘凤之,过国南,姜远茂,毛志泉,彭福田,束怀瑞.当今世界苹果产业发展趋势及我国苹果产业优质高效发展意见[J].果树学报,2010,27(4):598-604.CHEN Xuesen,HAN Mingyu,SU Guilin,LIU Fengzhi,GUO Guonan,JIANG Yuanmao,MAO Zhiquan,PENG Futian,SHU Huairui.Discussion on today’s world apple industry trends and the suggestions on sustainable and efficient development of apple industry in China[J].Journal of Fruit Science,2010,27(4):598-604.
[2] 张亚飞,彭福田,肖元松,罗静静,杜安齐.钾肥袋控缓释对桃产量、品质及土壤氯离子含量的影响[J].中国农业科学,2020,53(19):4035-4044.ZHANG Yafei,PENG Futian,XIAO Yuansong,LUO Jingjing,DU Anqi.Effects of potassium fertilizers being bag-controlled released on fruit yield and quality of peach trees and soil chloride content[J].Scientia Agricultura Sinica,2020,53(19):4035-4044.
[3] 刘宇松,段亚楠,陈学森,沈向,尹承苗,毛志泉.老龄桃园土壤抑制平邑甜茶幼苗的生长[J].园艺学报,2017,44(10):1969-1977.LIU Yusong,DUAN Yanan,CHEN Xuesen,SHEN Xiang,YIN Chengmiao,MAO Zhiquan.Aged peach orchard soil inhibit the growth of Malus hupehensis seedlings[J].Acta Horticulturae Sinica,2017,44(10):1969-1977.
[4] SHENG Y F,WANG H Y,WANG M,LI H H,LI X,PAN F B,CHEN X S,SHEN X,YIN C M,MAO Z Q.Effects of soil texture on the growth of young apple trees and soil microbial community structure under replanted conditions[J].Horticultural Plant Journal,2020,6(3):123-131.
[5] 尹承苗,王玫,王嘉艳,陈学森,沈向,张民,毛志泉.苹果连作障碍研究进展[J].园艺学报,2017,44(11):2215-2230.YIN Chengmiao,WANG Mei,WANG Jiayan,CHEN Xuesen,SHEN Xiang,ZHANG Min,MAO Zhiquan.The research advance on apple replant disease[J].Acta Horticulturae Sinica,2017,44(11):2215-2230.
[6] LIU E T,WANG G S,LI Y Y,SHEN X,CHEN X S,SONG F H,WU S J,CHEN Q,MAO Z Q.Replanting affects the tree growth and fruit quality of Gala apple[J].Journal of Integrative Agriculture,2014,13(8):1699-1706.
[7] 赵世杰,史国安,董新纯.植物生理学试验指导[M].北京:中国农业科学技术出版社,2002.ZHAO Shijie,SHI Guoan,DONG Xinchun.Techniques of plant physiological experimental[M].Beijing:Chinese Agricultural Science and Technology Press,2002.
[8] KAZEMI N,KHAVARI-NEJAD R A,FAHIMI H,SAADATMAND S,NEJAD-SATTARI T.Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L.under nickel stress[J].Scientia Horticulturae,2010,126(3):402-407.
[9] WANG Y F,PAN F B,WANG G S,ZHANG G D,WANG Y L,CHEN X S,MAO Z Q.Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd.seedlings under replant conditions[J].Scientia Horticulturae,2014,175:9-15.
[10] OMRAN R G.Peroxide levels and the activities of catalase,peroxidase,and indoleacetic acid oxidase during and after chilling cucumber seedlings[J].Plant Physiology,1980,65(2):407-408.
[11] SINGH B,SHARMA S,SINGH B.Antioxidant enzymes in cabbage:Variability and inheritance of superoxide dismutase,peroxidase and catalase[J].Scientia Horticulturae,2010,124:9-13.
[12] BAI R,MA F,LIANG D,ZHAO X.Phthalic acid induces oxidative stress and alters the activity of some antioxidant enzymes in roots of Malus prunifolia[J].Journal of Chemical Ecology,2009,35(4):488-494.
[13] 王爱国,罗广华.植物的超氧物自由基与羟胺反应的定量关系[J].植物生理学通讯,1990(6):55-57.WANG Aiguo,LUO Guanghua.Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants[J].Plant Physiology Communications,1990(6):55-57.
[14] IRIGOYEN J,EINERICH D,SÁNCHEZ-DÍAZ M.Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa(Medicago sativa)plants[J].Physiologia Plantarum,1992,84(1):55-60.
[15] LIU H,FU Y M,HU D W,YU J,LIU H.Effect of green,yellow and purple radiation on biomass,photosynthesis,morphology and soluble sugar content of leafy lettuce via spectral wavebands“knock out”[J].Scientia Horticulturae,2018,236(16):10-17.
[16] WANG X X,FU X L,CHEN M,HUAN L,LIU W H,QI Y H,GAO Y G,XIAO W,CHEN X D,LI L,GAO D S.Ultraviolet B irradiation influences the fruit quality and sucrose metabolism of peach (Prunus persica L.)[J].Environmental and Experimental Botany,2018,153:286-301.
[17] 鲍士旦.土壤农化分析[M].3 版.北京:中国农业出版社,2000:33-105.BAO Shidan.Soil chemical analysis[M].3rd ed.Beijing:China Agriculture Press,2000:33-105.
[18] 徐新翔,侯昕,王芬,贾志航,葛顺峰,姜远茂.低钾胁迫对苹果砧木M9T337 幼苗光合荧光特性及13C 吸收分配的影响[J].园艺学报,2020,47(3):529-540.XU Xinxiang,HOU Xin,WANG Fen,JIA Zhihang,GE Shunfeng,JIANG Yuanmao.Effects of low potassium stress on photosynthetic fluorescence characteristics and13C absorption and distribution of M9T337 seedlings[J].Acta Horticulturae Sinica,2020,47(3):529-540.
[19] CHEN R,JIANG W T,LIU Y S,WANG Y F,FAN H,CHEN X S,SHEN X,YIN C M,MAO Z Q.Amygdalin and benzoic acid on the influences of the soil environment and growth of Malus hupehensis Rehd.seedlings[J].ACS Omega,2021,6(19):12522-12529.
[20] 王艳芳,潘凤兵,展星,王功帅,张国栋,胡艳丽,陈学森,毛志泉.连作苹果土壤酚酸对平邑甜茶幼苗的影响[J].生态学报,2015,35(19):6566-6573.WANG Yanfang,PAN Fengbing,ZHAN Xing,WANG Gongshuai,ZHANG Guodong,HU Yanli,CHEN Xuesen,MAO Zhiquan.Effects of five kinds of phenolic acid on the function of mitochondria and antioxidant systems in roots of Malus hupehensis Rehd.seedlings[J].Acta Ecologica Sinica,2015,35(19):6566-6573.
[21] 姜伟涛,尹承苗,段亚楠,相立,王玫,陈学森,沈向,张民,毛志泉.根皮苷和串珠镰孢菌加重苹果连作土壤环境及其对平邑甜茶生长的抑制[J].园艺学报,2018,45(1):21-29.JIANG Weitao,YIN Chengmiao,DUAN Yanan,XIANG Li,WANG Mei,CHEN Xuesen,SHEN Xiang,ZHANG Min,MAO Zhiquan.Phloridzin and fusarium moniliforme aggravated thereplanted soil environment and inhibited the growth of Malus hupehensis seedlings[J].Acta Horticulturae Sinica,2018,45(1):21-29.
[22] 尹承苗,胡艳丽,王功帅,张先富,周慧,沈向,陈学森,毛志泉.苹果连作土壤中主要酚酸类物质对平邑甜茶幼苗根系的影响[J].中国农业科学,2016,49(5):961-969.YIN Chengmiao,HU Yanli,WANG Gongshuai,ZHANG Xianfu,ZHOU Hui,SHEN Xiang,CHEN Xuesen,MAO Zhiquan.Effect of main phenolic acids of the apple replanted soil on the roots of Malus hupehensis Rehd.seedlings[J].Scientia Agricultura Sinica,2016,49(5):961-969.
[23] 李林懋,门兴元,叶保华,于毅,张安盛,李丽莉,周仙红,庄乾营.不同生长时期冬枣受绿盲蝽危害后应激防御酶活性的变化[J].中国农业科学,2014,47(1):191-198.LI Linmao,MEN Xingyuan,YE Baohua,YU Yi,ZHANG Ansheng,LI Lili,ZHOU Xianhong,ZHUANG Qianying.Defense enzyme activity of winter jujube at different stages induced by the damage of Apolygus lucorum[J].Scientia Agricultura Sinica,2014,47(1):191-198.
[24] NIMBAL C I,PEDERSEN J F,YERKES C N,WESTON L A,WELLER S C.Phytotoxicity and distribution of sorgoleone in grain Sorghum germplasm[J].Journal of Agricultural and Food Chemistry,1996,44(5):1343-1347.
[25] 王艳芳,潘凤兵,付风云,相立,张先富,陈学森,沈向,毛志泉.甲壳素对连作平邑甜茶生长、光合及抗氧化酶的影响[J].园艺学报,2015,42(1):10-18.WANG Yanfang,PAN Fengbing,FU Fengyun,XIANG Li,ZHANG Xianfu,CHEN Xuesen,SHEN Xiang,MAO Zhiquan.Effects of chitin on growth,photosynthesis and antioxidative system of Malus hupehensis seedlings under replant condition[J].Acta Horticulturae Sinica,2015,42(1):10-18.