改变植物株型可有效提高作物的抗逆性[1-2]、增加产量[3]以及改善品质[4]等,目前该领域研究多集中于半矮化和矮化突变体的创制和应用。Zhuang等[5]研究表明,与野生型植株相比,过表达PdC3H17 基因导致杨树(Populus)矮化,并显示出更强的光合和活性氧(reactive oxygen species,ROS)清除能力,从而增强了植株的耐旱能力。一般而言,矮化植株高度通常小于正常植株高度的1/2,而半矮化植株高度约为正常植株高度的2/3[6-7]。目前,半矮化和矮化新品种的培育已在农作物中大量开展,包括水稻(Oryza sativa)[8-10]、小麦(Triticum aestivum)[11-14]、玉米(Zea mays)[15-16]、高粱(Sorghum vulgare)[17]和大豆(Glycine max)[18]等。Zhang 等[16]利用CRISPR/Cas9技术编辑了玉米GA20ox3基因获得了半矮秆玉米植株,经外源赤霉素(gibberellins,GAs)处理可以恢复植株高度,表明GA20ox3 基因参与了GAs 的生物合成,而缺失GA20ox3 基因GAs 含量显著降低。与农作物的育种目标不同,果树由于品种化栽培的需要,嫁接砧木和接穗的种质资源创新培育往往分别开展。与接穗侧重产量和品质不同,砧木的新种质创制主要集中于株型、抗逆性以及适应性等重要性状[19-22]。目前,关于果树半矮化和矮化基因的相关研究较少,主要集中于苹果(Malus pumila)[23-24]、梨(Pyrus sorotina)[25]、桃(Amygdalus persica)[26-27]、香蕉(Musa paradisiaca)[28]和柿树(Diospyros kaki)[29]等果树中。其中,植物内源激素GAs[30]、油菜素内酯(brassinosteroids,BRs)[31]、生长素(auxin,IAA)[32]等激素被认为是导致果树矮化的重要生长调节物质,参与果树生长发育的各个阶段。Zheng 等[33]对苹果蛋白质组学分析表明,外施BRs 处理增加了内源BR、IAA 和GAs 含量,并通过多种信号蛋白改变激素信号,包括BRs、细胞分裂素(cytokinin,CTK)、脱落酸(abscisic acid,ABA)和GAs 信号,表明激素途径可能通过影响细胞生长和木质素相关蛋白加速细胞生长和木质素合成,从而共同控制苹果树的生长。
GAs 为5 大类激素之一,控制植物生长和发育的各个方面[34-37]。半矮化和矮化突变体可能有助于阐明GAs的作用方式。在一些物种中,如拟南芥[38]、水稻[39-40]和玉米[15]等描述了缺乏或改变内源GAs 会表现出矮化突变表型。GA2ox基因是降低内源生物活性GA含量并导致植株半矮化和矮化的关键基因之一[27,41]。研究表明,过量表达GA2ox 基因可导致植株矮化以及活性GAs 含量减少[42]。目前,已从香蕉[43]、梨[44]、杧果(Mangifera indica)[45]、苹果[46]、葡萄(Vitis vinifera)[47]、核桃(Juglans regia)[48]等果树中克隆获得GA2ox基因。迄今,有关薄壳山核桃GAs合成相关基因克隆的研究鲜有报道,对薄壳山核桃CiGA2ox1 基因的分离及功能研究尚未见报道。笔者在本研究中采用PCR 扩增技术从薄壳山核桃中分离克隆出CiGA2ox1基因,对其序列和表达特性进行分析,构建35S::CiGA2ox1::GFP过表达载体,通过农杆菌介导方法转化到薄壳山核桃体胚中,分析过表达CiGA2ox1 基因对薄壳山核桃组培再生植株生长的影响,为果树矮化机制研究提供理论依据。
1 材料和方法
1.1 材料
1.1.1 植物材料 本研究的植物材料源于省部共建亚热带森林培育国家重点实验室培育的薄壳山核桃(Carya illinoensis)体细胞胚(简称“体胚”)以及薄壳山核桃组培苗。体胚培养于25 ℃的暗箱中;组培苗培养于温度25 ℃、光照度1500~2000 lx、光周期16 h/8 h、相对湿度80%~90%的组织培养室中。总RNA 提取采用生长良好的薄壳山核桃体细胞胚,液氮速冻后于-80 ℃保存。烟草悬浮培养液配置:100 μL 1 mol·L-1 MgCl2、200 μL 0.5 mol·L-1 MES、15 μL,100 mmol·L-1乙酰丁香酮,ddH2O 定容至10 mL。
1.1.2 克隆菌株与试剂 大肠杆菌菌株DH5α购自于杭州有康生物技术有限公司,农杆菌菌株GV3101 购自于上海唯地生物技术有限公司。多糖多酚的总RNA 提取试剂盒购自北京天根生化科技有限公司,cDNA 反转录试剂盒、SanPrep 柱式凝胶回收试剂盒、质粒提取试剂盒、DNA Marker、各种限制性内切酶、PrimeSTAR 高保真酶、rTaq DNA 聚合酶及连接酶均购自TaKaRa 公司。过表达载体由pCAMBIA1300(简称pC1300)改造而来[49]。
1.2 方法
1.2.1 薄壳山核桃CiGA20ox 基因的克隆及过表达载体的构建 根据薄壳山核桃CiGA2ox1 基因的全长蛋白质编码区(coding sequence,CDS)序列,采用Primer 5.0 设计引物序列(表1),由上海生工生物工程有限公司合成。
表1 薄壳山核桃CiGA2ox1 基因克隆、表达及鉴定分析所用引物
Table 1 Primers used for cloning,expression and testing analysis of CiGA2ox1
注:GGATCC 和GTCGAC(下划线)分别为BamHⅠ和SalⅠ的酶切位点。
Note:GGATCC and GTCGAC(underlined)are BamHⅠand SalⅠrestriction sites,respectively.
以薄壳山核桃体胚为植物材料,提取总RNA,反转录得到的cDNA,引物 CiGA2ox1-F 和CiGA2ox1-R 用于CiGA2ox1 基因开放阅读框(open reading frame,ORF)克隆。PCR 反应体系:Prime STAR Max(2 × )酶12.5 μL,CiGA2ox1-F 和CiGA2ox1-R 引物各1.0 μL,cDNA 1.0 μL,用双蒸水补足至25 μL。反应程序:94 ℃2 min;94℃10 s;55 ℃30 s;68 ℃2 min;共32 个循环;68 ℃延伸7 min;4 ℃保存。产物回收:按照SanPrep柱式胶回收试剂盒说明书的步骤对PCR产物进行回收。
过表达载体的构建:将改造后的pC1300质粒进行BamHⅠ和SalⅠ双酶切,回收双酶切产物。利用诺唯赞One step clonging 试剂盒连接目的条带和质粒载体。将融合载体35S:CiGA2ox1::GFP 转化到大肠杆菌DH5α,PCR 检测,挑取阳性克隆,测序验证。将成功构建的载体转化农杆菌GV3101 菌株,具体步骤参照说明书,将PCR检测阳性的菌液进行保菌用于后续试验。
1.2.2 薄壳山核桃CiGA2ox1 基因的生物信息学分析 将测序验证正确的核苷酸序列和对应的氨基酸序列分别在美国国立生物技术信息中心(National Center for Biotechnology Information,NCBI)数据库中用BLASTn 和BLASTp 进行序列相似性分析;利用ORF Finder(https://www.ncbi.nlm.nih.gov/orffinder/)在线程序分析CiGA2ox1 基因的开放阅读框;应用PROSITE Scan软件预测分析蛋白保守区域;利用Expasy(http://web.expasy.org/protparam/)在线软件分析蛋白质的理化性质;TMHMM 法(http://www.cbs.dtu.dk/services/TMHMM/)分析蛋白质的跨膜区;利用Wolf psor(https://www.genscript.com/tools/wolf-psort)在线分析软件进行蛋白亚细胞定位预测;系统发育树使用MEGA 7.0 软件构建,分析CiGA2ox1 基因在进化过程中与其他物种的亲缘关系。
1.2.3 薄壳山核桃CiGA2ox1 蛋白亚细胞定位 烟草叶片的瞬时转化:将含有35S::CiGA2ox1::GFP 以及pC1300-GFP空载体的农杆菌扩大培养,8000 r·min-1离心10 min,弃上清液,收集菌液。1∶1比例悬浮液重悬,OD600为0.7~1.0。以pC1300-GFP 空载体作为阴性对照,同时以核定位蛋白Marker(由水稻ART1[50]和1 个具有RFP 信号的载体蛋白连接而来)及膜定位蛋白Marker(AtPIP2A-mCherry)[51]对烟草下表皮细胞中的细胞核和细胞膜进行准确定位。将构建的植物融合表达载体35S::CiGA2ox1::GFP,空载体pC1300-GFP 和Marker 分别转入农杆菌,注射烟草下表皮,弱光培养2 d。取标记的烟草叶片制作成装片,用蔡司LSM710 激光共聚焦显微镜观察并拍照。
1.2.4 薄壳山核桃CiGA2ox1 基因的遗传转化及表达分析 将含有35S::CiGA2ox1::GFP的农杆菌菌液在含有卡那霉素(100 mg·L-1)和利福平(100 mg·L-1)LB 液体培养基中扩大培养至OD 值为1.0 左右,6000 r·min-1离心10 min,取上清液。加入含有乙酰丁香酮(40 mg·L-1)的DKW液体培养基吹打悬浮菌液。选取生长状态良好的薄壳山核桃体胚进行遗传转化[52]。将过量表达CiGA2ox1 基因的薄壳山核桃体胚置于体式荧光显微镜(Carl Zeiss Stereo D13covery V12,Axio Cam MRc system)下,在蓝光(488 nm)激发下,观察体胚荧光激发情况。对具有绿色荧光激发的薄壳山核桃体胚进行PCR验证,引物参照表1。筛选出的阳性体胚培养至子叶胚阶段,干化处理5~7 d后进行植株再生[51]。利用实时荧光定量PCR,通过2-ΔΔCT法[53]计算CiGA2ox1 基因在薄壳山核桃阳性植株中的相对表达量,引物参照表1(QCiGA20ox1-F 及QCiGA20ox1-R)。同时分别剪取等长且生长条件较一致的野生型及阳性再生株系的顶芽,培养14 d后观察表型。
1.2.5 薄壳山核桃CiGA2ox1 转基因株系叶绿素含量测定 选取转基因株系与野生型薄壳山核桃再生株系叶片0.1g,剪碎后置于标号的试管中,向每个试管中加入10 mL 80%的丙酮溶液。放入室温、黑暗处浸提,直至试管内叶片材料全部变白。以80%的丙酮作空白对照,测定663 nm和645 nm下吸光度值。
w(叶绿素)(mg·g-1)=[(Ca+8Cb)×Vt]/FW×1000。
Ca=20.3×OD645;Cb=8.04×OD663。
其中,Ca、Cb 分别为叶绿素a、叶绿素b浓度,Vt为提取液总体积10 mL;FW为叶片鲜质量0.1 g。
1.3 数据统计与分析
利用IBM SPSS Statistics 25软件进行单因素方差分析,使用Graphpad 7.0 软件根据上述生理指标结果绘图。
2 结果与分析
2.1 薄壳山核桃CiGA2ox1基因克隆和过表达载体构建
根据设计的特异性引物,通过PCR 扩增,琼脂糖凝胶电泳获得1条1000~1500 bp之间的特异条带(图1-A),与预期结果一致。将目的条带胶回收产物与改造后的过表达载体pC1300质粒连接,转化到大肠杆菌中,挑选单克隆,对培养的菌液进行PCR鉴定。将1000~1500 bp 电泳条带(图1-B)送出测序。序列长度为1053 bp,测序比对正确的菌液保菌备用。所获过表达载体命名为35S::CiGA2ox1::GFP。
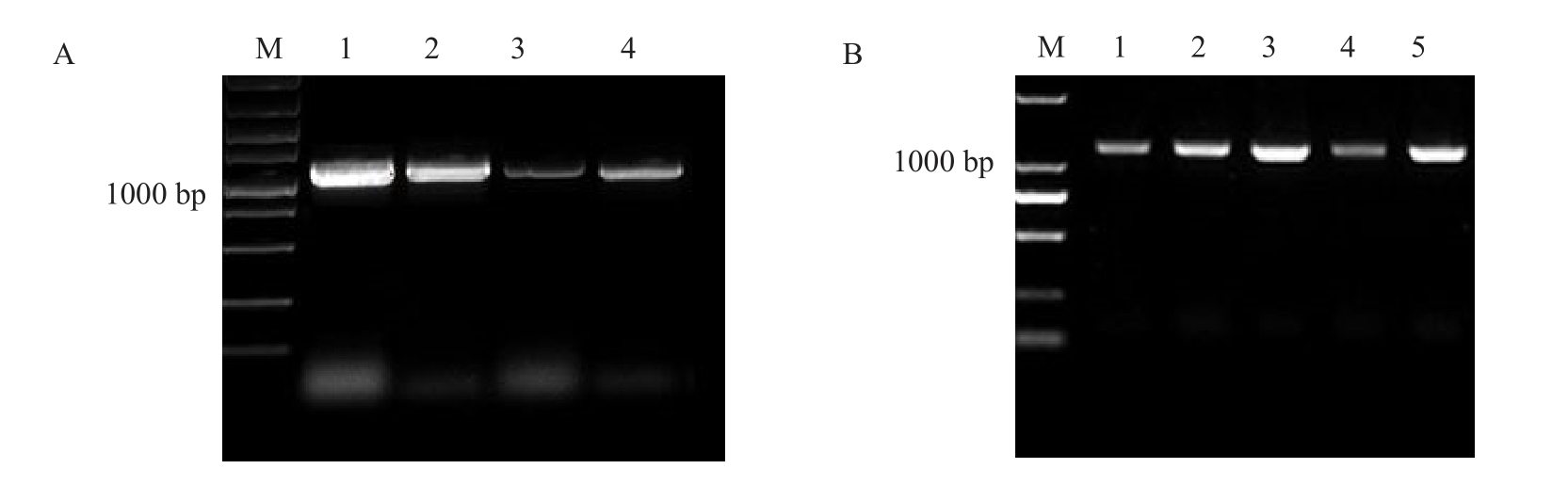
图1 薄壳山核桃CiGA2ox1 基因克隆与大肠杆菌菌液PCR 检测
Fig.1 CiGA2ox1 gene cloning and PCR detection of E.coli bacteria
A 为薄壳山核桃CiGA2ox1 基因扩增电泳图,M 为Maker DL5000,泳道1~4 为35S::CiGA2ox1::GFP 目的基因PCR 产物;B 为薄壳山核桃CiGA2ox1 基因大肠杆菌菌液PCR 检测,M 为Maker DL 2000,泳道1~5 为35S::CiGA2ox1::GFP 基因的单菌培养后的PCR 产物。
A.Amplification electrophoresis map of CiGA2ox1 gene,M.Marker DL5000,Lane 1-4 indicate PCR product of 35S::CiGA2ox1::GFP gene;B.Electrophoretogram of CiGA2ox1 Escherichia coli.M.Marker DL 2000,Lane 1-5 indicate PCR product of single colony of 35S::CiGA2ox1::GFP gene.
2.2 薄壳山核桃CiGA2ox1基因编码的蛋白氨基酸序列的理化性质分析及系统进化分析
利用ORF Finder 在线分析软件得出,薄壳山核桃CiGA2ox1 基因ORF 长度为1053 bp,编码350 个氨基酸。ExPASy 在线软件预测,薄壳山核桃CiGA2ox1 基因所编码蛋白的分子质量为39.22 ku,等电点(pI)为6.55,分子式为C1761H2750N464O516S17,总平均亲水性-0.158。Wolf psor 在线分析软件显示,薄壳山核桃CiGA2ox1 蛋白亚细胞定位50%位于细胞核,28.5%位于细胞质中。PROSITE Scan 软件预测薄壳山核桃CiGA2ox1 蛋白的保守结构域,发现该蛋白含有1 个比较保守的2OG-FeⅡ-Oxy(175~281)结构域和3 个Fe2+结合位点(205、207、262)。TMHMM 在线分析显示,CiGA2ox1 蛋白的跨膜区域值为0,该蛋白没有跨膜区域(图2)。在进化关系图中可以发现,薄壳山核桃CiGA2ox1基因编码的蛋白氨基酸序列与核桃的相似度最高,达到96.69%,同属1个小分支,亲缘关系最近(图3)。根据亲缘关系远近,可将CiGA2ox1 基因分为6 簇族。其中,薄壳山核桃CiGA2ox1 基因与核桃、苹果、沙梨、梅花、大叶栎、橡胶树、木薯、毛果杨及银白杨聚为一簇,与薄壳山核桃遗传距离较近;其次木槿、陆地棉、榴莲、可可、甜橙、野大豆和蒺藜苜蓿为第二簇;川桑、大麻、笋瓜及苦瓜聚为第三簇;马铃薯、番茄、烟草、皱叶烟草、矮牵牛和人参聚为第四簇;芝麻和夏堇为第五簇;油菜、拟南芥、鹰嘴豆及藜麦为第六簇。同一基因在同一簇族内,各物种差异性较小;而随着分支不断扩大,各物种间的亲缘关系值逐渐减弱。

图2 薄壳山核桃CiGA2ox1 蛋白跨膜区域分析
Fig.2 Prediction of CiGA2ox1 transmembrane region of hickory
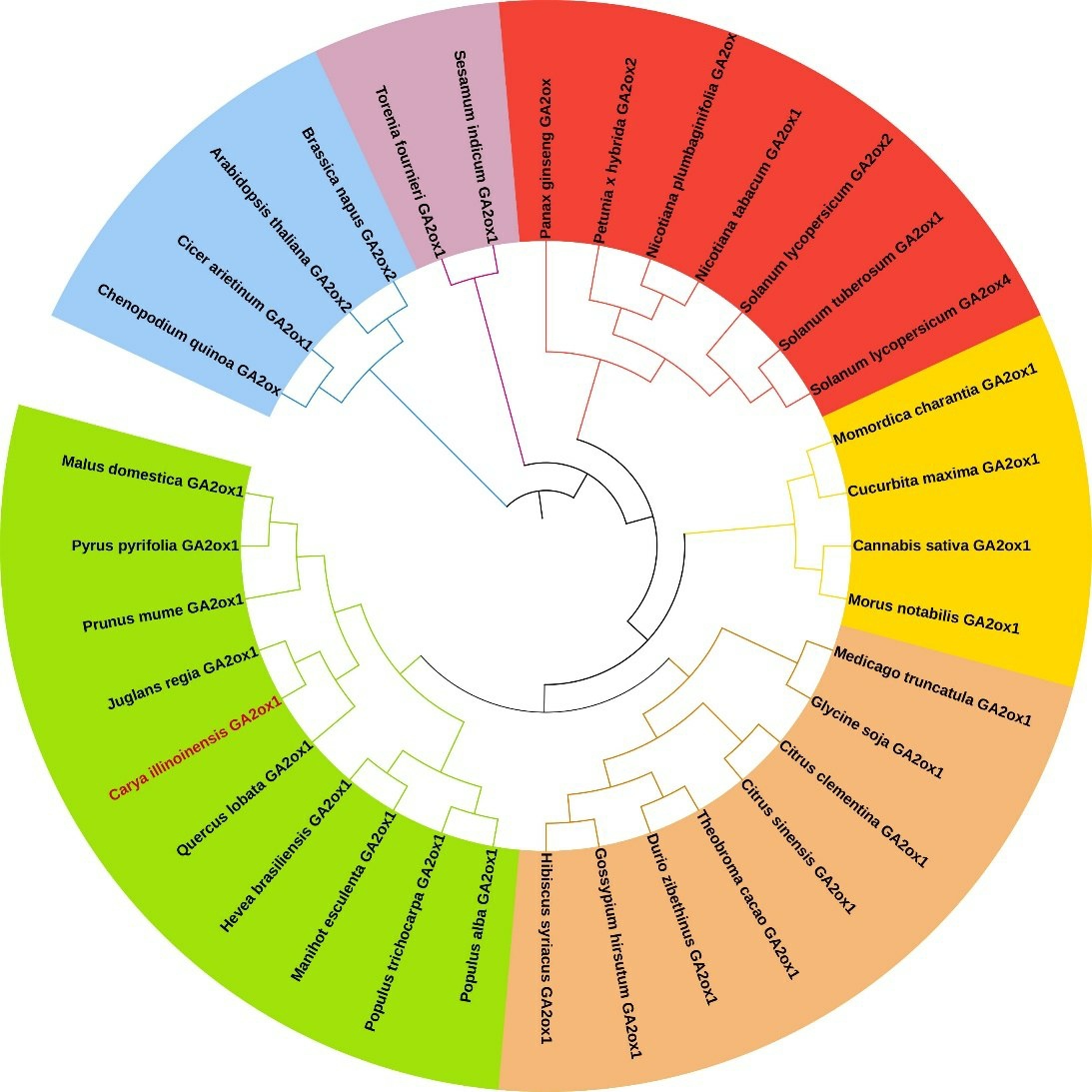
图3 不同物种GA2ox1 基因的系统发育树分析
Fig.3 Phylogenetic tree analysis of GA2ox1 in different species
藜麦Chenopodium quinoa. CqGA2ox(XM_021890681.1);鹰嘴豆Cicer arietinum.CaGA2ox1(XM_004485965.3);拟南芥Arabidopsis thaliana.AtGA2ox2(AJ132436.1);油菜Brassica napus.BnGA2ox2(XM_013797003.2);夏堇Torenia fournieri.TfGA2ox1(AB613271.1);芝麻Sesamum indicum.SiGA2ox1(XM_011084568.2);人参Panax ginseng.PgGA2ox(KT692958.1);矮牵牛Petunia x hybrida.PhGA2ox2(JQ323102.1);皱叶烟草Nicotiana plumbaginifolia.NpGA2ox(FM244693.1);烟草Nicotiana tabacum.NtGA2ox1(XM_016644757.1);番茄Solanum lycopersicum. SlGA2ox2(NM_001247409.1);马铃薯Solanum tuberosum. StGA2ox1(XM_006348309.2);番 茄Solanum lycopersicum.SlGA2ox4(NP_001234752.1);苦瓜Momordica charantia.McGA2ox1(XP_022142867.1);笋瓜Cucurbita maxima.CmGA2ox1(XM_023675454.1);大麻Cannabis sativa.CsGA2ox1(XM_030634384.1;川桑Morus notabilis.MnGA2ox1(XM_010112317.2);蒺藜苜蓿Medicago truncatula.MtGA2ox1(XM_013608940.3);野大豆Glycine soja.GsGA2ox1(XM_028332010.1);克莱门氏小柑橘Citrus clementina.CcGA2ox1(XP_006449692.1);甜橙Citrus sinensis. CsGA2ox1(XM_006477863.3);可可Theobroma cacao.TcGA2ox1(XM_018124899.1);榴莲Durio zibethinus.DzGA2ox1(XM_022877473.1);陆地棉Gossypiumhirsutum.GhGA2ox1(XM_016868828.2);木槿Hibiscus syriacus.HsGA2ox1(XM_039170793.1);银白杨Populus alba.PaGA2ox1(XM_035040541.1);毛果杨Populus trichocarpa.PtGA2ox1(XM_002300394.3);木薯Manihot esculenta.MeGA2ox1(XM_021751000.1);橡胶树Hevea brasiliensis.HbGA2ox1(XM_021813605.1);大叶栎Quercus lobata.Ql GA2ox1(XM_031106239.1);薄壳山核桃Carya illinoinensis.CiGA2ox1(XM_043095786.1);核 桃Juglans regia.JrGA2ox1(XP_018824979.1);梅花Prunus mume.PmGA2ox1(XM_008228016.1);沙梨Pyrus pyrifolia.PpGA2ox1(BAU19310.1);苹果Malus domestica.MdGA2ox1(XP_008372291.2).
2.3 薄壳山核桃CiGA2ox1基因编码蛋白的亚细胞定位
为验证CiGA2ox1基因编码蛋白的定位,将构建好的35S::CiGA2ox1::GFP 融合蛋白、pC1300-GFP空载体及2 个定位Marker(核定位蛋白Marker 及膜定位蛋白Marker)转化到烟草叶片中,在激光共聚焦显微镜观察35S::CiGA2ox1::GFP融合蛋白的定位情况。试验表明,在GFP 通道下,pC1300-GFP 空载体绿色荧光蛋白信号弥散于细胞核和细胞膜中(图4-A);在RFP 通道下,细胞核和细胞膜呈现红色荧光(图4-B);在融合通道中细胞核和细胞膜荧光信号呈黄色(图4-D)。这表明pC1300-GFP空载体、核定位蛋白Marker 及膜定位蛋白Marker 均可正常表达。而在含有35S::CiGA2ox1::GFP融合蛋白的烟草叶片中(图4-E~H)观察到绿色荧光信号、红色荧光信号和黄色荧光信号均在细胞核和细胞膜中表达。这表明CiGA2ox1 蛋白在细胞中主要定位于细胞核和细胞膜中。

图4 薄壳山核桃CiGA2ox1 基因编码蛋白在烟草叶片中的亚细胞定位
Fig.4 Fluorescent localization of CiGA2ox1 in tobacco leaf epidermal cells
A,E.GFP 通道;B,F.RFP 通道;C,G.白光通道;D,H.融合图像。A~D.pC1300 空载、膜marker 和细胞核marker;E~H. CiGA2ox1-pC1300、膜marker 和细胞核marker。比例尺50 μm。
A,E.GFP field;B,F.RFP field;C,G.Bright field;D,H.Merged field.A-D.35S-GFP indicates the empty vector,cell membrane marker and the nucleus marker;E-H.35S::CiGA2ox1::GFP indicates the fusion protein with CiGA2ox1,cell membrane marker and the nucleus marker.Bar:50 μm.
2.4 CiGA2ox1基因在薄壳山核桃中的遗传转化及阳性检测
用农杆菌侵染法将35S::CiGA2ox1::GFP过表达载体转入到薄壳山核桃体胚中,将侵染后的体胚标记为E0代,筛选培养到E3代,在体式显微镜观察转化体胚可正常生长发育和增殖,形态与野生型体胚相似。挑取15 个生长健壮的子叶胚时期的体胚进行侵染,数据统计结果表明,E1 代体胚GFP 阳性率为34.1%;E2代体胚GFP阳性获得率为32.2%;E3代体胚GFP 阳性获得率为65.9%(表2)。将转化体胚放置于体式显微镜下观察,发现转基因阳性体胚在488 nm蓝光激发下呈现绿色荧光,而野生型体胚无荧光信号(图5)。为排除荧光假阳性,进一步对荧光阳性体胚及再生植株进行PCR 验证。用外源GFP 基因(729 bp)及目的基因进行验证(引物序列见表1)。结果发现,凝胶电泳条带大小为1800 bp左右,说明转化成功(图6)。
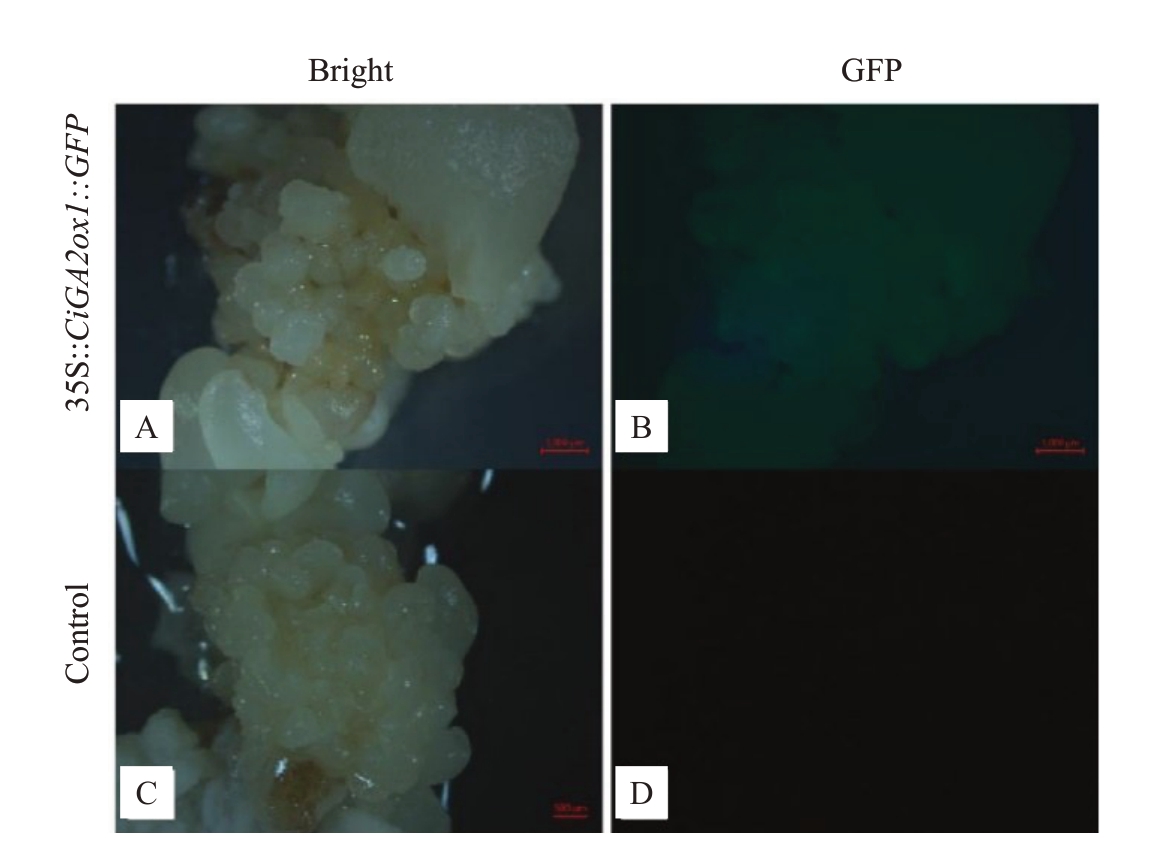
图5 薄壳山核桃35S::CiGA2ox1::GFP 转化体胚荧光表达
Fig.5 Fluorescent expression of Carya illinoinensis 35S::CiGA2ox1::GFP transformed somatic embryos
A,B.白光和蓝光激发下的转化薄壳山核桃体胚;C,D.白光和蓝光激发下的野生型薄壳山核桃体胚。比例尺:1000 μm。
A,B.Somatic embryos of transformed hickory pecans stimulated by white and blue light;C,D.Somatic embryos of wild-type hickory pecan stimulated by white and blue light.Bar:1000 μm.
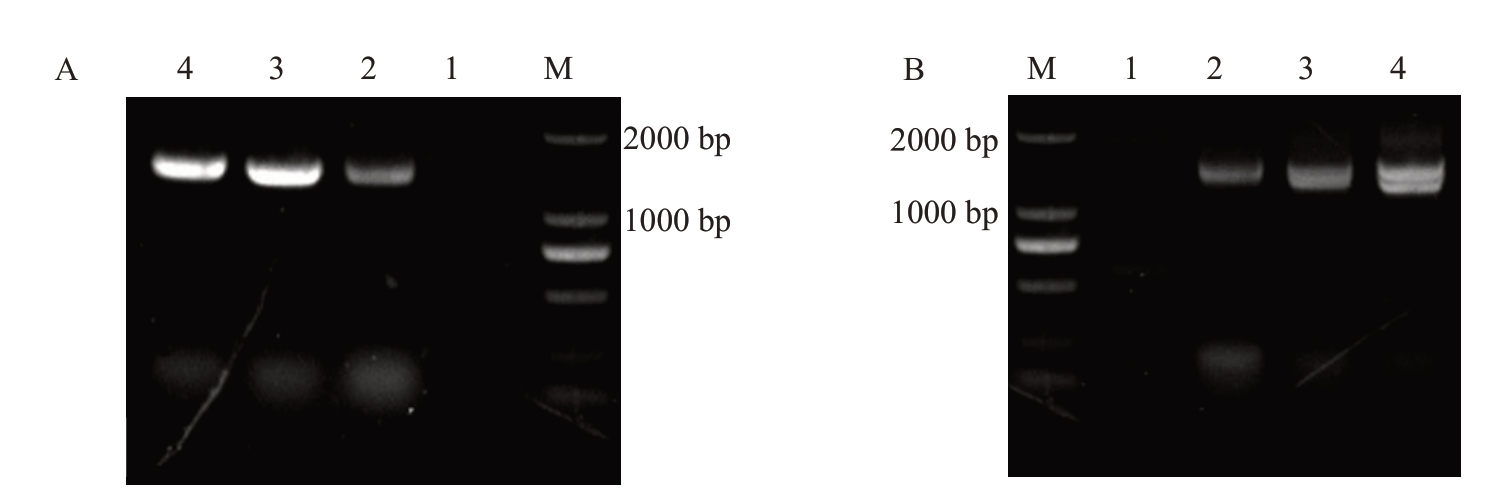
图6 薄壳山核桃体细胞胚及再生植株35S::CiGA2ox1::GFP 的PCR 检测
Fig.6 PCR assay of 35S::CiGA2ox1::GFP in overexpression Carya illinoinensis somatic embryos and regenerated plants
A:1.野生型薄壳山核桃体胚;2~4.薄壳山核桃35S::CiGA2ox1::GFP 转化体胚;B:1.野生型薄壳山核桃再生植株;2~4.薄壳山核桃35S::CiGA2ox1::GFP 转化再生植株。M.DNA marker DL2000。
A:1.Control somatic embryos;2-4.35S::CiGA2ox1::GFP somatic embryos;B:1.Control regenerated plants;2-4.35S::CiGA2ox1::GFP regenerated plants;M.DNA marker DL2000.
表2 薄壳山核桃体胚转化再生个数
Table 2 The number of transformation in somatic embryos

2.5 薄壳山核桃CiGA2ox1基因功能验证
2.5.1 薄壳山核桃CiGA2ox1 基因过表达株系株形鉴定 为了研究过表达CiGA2ox1 基因与薄壳山核桃植株高度之间的相关性,将野生型体胚和阳性体胚同时萌发。进一步对再生植株高度进行测定,CiGA2ox1基因过表达植株选取3个株系,分别命名为CiGA2ox1-OE1、CiGA2ox1-OE2、CiGA2ox1-OE3(图7)。试验结果表明,在体胚萌发后30 d 中野生型植株生长较快,而阳性再生植株生长较慢。而后高度基本保持不变。在第30天时CiGA2ox1基因过表达植株高度分别为野生型的65.1%、64.7%和59.8%,植株高度极显著减小(图8-A);第3至第4功能叶的节间长度分别为野生型的81.0%、77.7%和74.4%,节间长度极显著减小(图8-B);在叶片长度和叶片宽度指标上,叶片长度分别为野生型的72.5%、66.0%和66.1%,叶片宽度为野生型的65.1%、60.2%和55.6%,极显著减小(图8-C~D)。结果表明,薄壳山核桃35S::CiGA2ox1::GFP 再生植株与野生型植株相比,再生植株高度较矮,节间长度和叶片长度更短。

图7 阳性植株与野生型植株的表型
Fig.7 Positive plants versus wild type B

图8 薄壳山核桃35S::CiGA2ox1::GFP 再生植株表型特分析
Fig.8 Analysis of phenotypic characteristics of Carya illinoinensis 35S::CiGA2ox1::GFP regenerated plants
单因素方差分析显著性,不同大写字母表示差异极显著(p<0.01)。下同。
Significance of one-way ANOVA,different capital letters indicated extremely significant difference(p<0.01).The same below.
提取野生型及薄壳山核桃35S::CiGA2ox1::GFP再生植株的RNA进行CiGA2ox1基因相对表达量检测,实时荧光定量PCR 结果显示,薄壳山核桃35S::CiGA2ox1::GFP 再生株系CiGA2ox1 基因相对表达量均显著高于野生型植株。其中,35S::CiGA2ox1::GFP-1 株系的相对表达量为野生型的12 倍,35S::CiGA2ox1::GFP-2株系相对表达量为野生型的12.36倍,35S::CiGA2ox1::GFP-3 株系的相对表达量为野生型的13.67倍,差异均极显著(图9)。

图9 薄壳山核桃35S::CiGA2ox1::GFP 再生植株的相对表达量分析
Fig.9 Relative expression analysis of regenerated plants of Carya illinoinensis 35S::CiGA2ox1::GFP
2.5.2 薄壳山核桃CiGA2ox基因过表达株系叶绿素含量的测定 进一步研究发现,薄壳山核桃35S::CiGA2ox1::GFP 转基因再生植株叶色变深。选取3个阳性再生株系进行叶绿素含量测定(图10)。结果显示,35S::CiGA2ox1::GFP-1的叶绿素a、叶绿素b以及总叶绿素含量分别是野生型植株的1.40、1.30和1.40 倍,35S::CiGA2ox1::GFP-2 的叶绿素a、叶绿素b 以及总叶绿素含量分别是野生型植株的1.51、1.50 和1.52 倍,35S::CiGA2ox1::GFP-3 的叶绿素a、叶绿素b 以及总叶绿素含量分别是野生型植株的1.56、1.62 和1.67 倍。以上指标均显著提高。由此可以发现,过表达GA2ox1基因使得薄壳山核桃组培苗呈现更绿的叶片是因为更多叶绿素的累积。
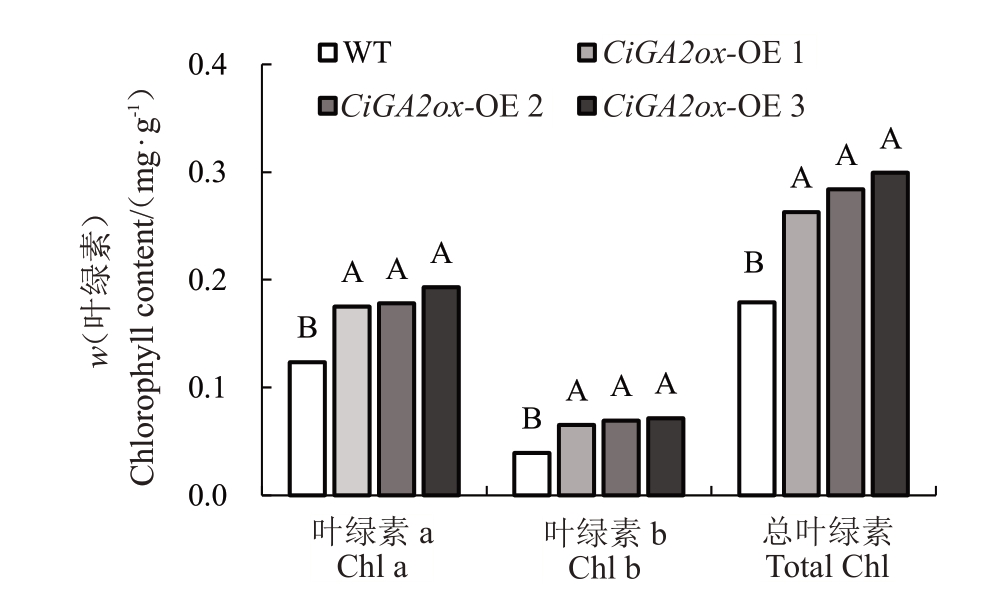
图10 薄壳山核桃35S::CiGA2ox1::GFP再生植株的叶绿素含量分析
Fig.10 Analysis of chlorophyll contents of regenerated plants in Carya illinoinensis 35S::CiGA2ox1::GFP
3 讨论
GA 生物合成代谢途径在高等植物的整个生命周期中有着极为重要的作用。GA的代谢和分解过程中涉及多种酶。这些酶通过影响生物合成与降解间的平衡,在特定发育阶段维持生物活性水平,保证植物生长发育过程的正常进行。其中,GA2ox蛋白是一种与GA 失活相关编码氧化酶,由多个基因编码[54]。通过过表达GA2ox 基因可以降低内源GA 含量,使植株矮化,延长种子的休眠时间,还可以改变植物体内叶绿素的含量[42,55]。本研究中克隆得到薄壳山核桃GA2ox 基因,经分析发现薄壳山核桃CiGA2ox1基因全长1053 bp,编码350个氨基酸。与核桃JrGA2ox 基因亲缘关系最近,同源率达到96.69%。亚细胞定位结果显示,CiGA2ox1基因编码的蛋白主要位于细胞核及细胞膜中行使功能,这同核桃JrGA2ox1 基因编码的蛋白亚细胞定位相似[48]。这表明CiGA2ox1 基因编码的蛋白可能对细胞质膜具有催化功能[54]。
半矮化和矮化树木可以高密度种植以便管理。在许多树种中,树木矮化是通过广泛使用矮化砧木实现的。砧木诱导矮化的机制已经得到了广泛的研究,但仍知之甚少[56-57]。研究表明,赤霉素代谢通路在诱导矮化砧木中起着重要作用[58]。在香波罗(Artocarpus odoratissimus)砧木上生长的面包果树(A.altilis)植株矮小,在嫁接24个月后,总植株高度减少约60%,节间长度减少约80%。这表明嫁接在香波罗砧木上面包果的矮化表型可能与内源GA含量降低有关[30]。在GA代谢中,GA2ox基因可以催化活性GA 并将其转化为非活性产物。GA2ox基因在矮化中的功能已经在一些物种中得到证实[27,59-60]。Liu等[61]报道了在柑橘中GA2ox1的表达与植物的生长呈负相关。在转基因烟草中过表达DkGA2ox1基因会导致矮秆表型和其他矮秆性状[58]。此外,GA2ox 基因转录水平升高,矮化李子杂交种的节间缩短,茎伸长减少,当用作砧木时,降低了接穗中生物活性GAs的水平[60]。笔者在本研究中通过农杆菌介导转化法获得了薄壳山核桃转基因株系,植株高度约为野生型的0.64 倍。本研究结果显示,CiGA2ox1基因过表达株系引起植株的半矮化、节间缩短和叶片长宽比降低,这与前人研究结果相符,表明薄壳山核桃CiGA2ox1 基因对植株高度具有负调节作用。
研究发现,叶绿素的降解与内源GA 含量有关[62]。以往的研究表明,内源激素含量与光合效率之间存在着一定的关系,施用烯效唑(uniconazole,UCZ)抑制了GA,而较低的GA 含量提高了少根紫萍的ABA 和玉米素核苷(trans-Zeatin-riboside,ZR)含量,从而提高了少根紫萍的叶绿素含量和净光合速率[63]。NAP转录因子通过与DELLA蛋白互作,参与GA 介导的叶绿素降解[64]。在拟南芥和水稻中过表达GA2ox基因会导致活性GAs水平降低,植株表型呈矮化和小的深绿色叶片[42,65-67]。Yan等[68]发现在拟南芥中过度表达BngGA2ox6基因会导致GA缺乏症状,同时增强总叶绿素的积累。同时,水稻半矮杆GA2ox 基因突变体叶片呈深绿色,而叶片的总叶绿素含量增加,光合作用增强[69]。本试验中,与野生型相比,CiGA2ox1基因过表达再生株系的总叶绿素含量极显著升高,这与以上研究结果相符。这表明GA 是调节叶绿素生物合成的重要信号,通过过表达GA2-氧化酶可以降低内源GA含量,进而增加植物体内叶绿素的含量[70]。
薄壳山核桃作为营养价值较高的经济作物,其种质资源的优化、生长发育的调控及品种的选育等相关的研究备受关注。由于嫁接技术的发展,薄壳山核桃半矮化/矮化栽培成为生产发展的趋势。半矮化/矮化栽培具有管理方便、适应性强等优点,为薄壳山核桃生产发展提供了更好的条件和优势。半矮化/矮化砧木种类稀少且自根苗繁殖困难,严重制约了矮化密植果树生产的进展。随着分子生物学研究发展,相关研究将越来越多转向半矮化砧木调控机制方面。植物激素对于果树的生长与发育存在着非常重要的影响。因此,通过分析探究GA 对果树半矮化的调控作用,进一步提高果树产量、品质,增强其抗逆性,可更好实现半矮化砧木的选育及应用。
4 结论
薄壳山核桃CiGA2ox1基因与核桃JrGA2ox1基因亲缘关系最近,CiGA2ox1基因编码的蛋白主要定位于细胞核及细胞膜中。通过遗传转化及功能验证初步表明,薄壳山核桃再生植株中CiGA2ox1基因相对表达量升高,可以导致薄壳山核桃植株矮化,叶绿素含量升高。为进一步开发新的半矮化薄壳山核桃突变体提供有价值的参考数据。
[1] GRUSZKA D,POCIECHA E,JURCZYK B,DZIURKA M,OLIWA J,SADURA I,JANECZKO A.Insights into metabolic reactions of semi-dwarf,barley brassinosteroid mutants to drought[J].International Journal of Molecular Sciences,2020,21(14):5096.
[2] CHEN L M,YANG H L,FANG Y S,GUO W,CHEN H,ZHANG X,DAI W,CHEN S,HAO Q,YUAN S,ZHANG C,HUANG Y,SHAN Z,YANG Z,QIU D,LIU X,TRAN L P,ZHOU X,CAO D.Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway[J].Plant Biotechnology Journal,2021,19(4):702-716.
[3] ANSARI A,WANG C L,WANG J,WANG F,LIU P,GAO Y,TANG Y,ZHAO K.Engineered dwarf male-sterile rice:A promising genetic tool for facilitating recurrent selection in rice[J].Frontiers in Plant Science,2017,8:2132.
[4] HU X M,CUI Y T,DONG G J,FENG A,WANG D,ZHAO C,ZHANG Y,HU J,ZENG D,GUO L,QIAN Q.Using CRISPRCas9 to generate semi-dwarf rice lines in elite landraces[J].Scientific Reports,2019,9(1):19096.
[5] ZHUANG Y M,WANG C P,ZHANG Y,CHEN S,WANG D,LIU Q,ZHOU G,CHAI G.Overexpression of PdC3H17 confers tolerance to drought stress depending on its CCCH domain in Populus[J].Frontiers in Plant Science,2020,10:1748.
[6] 牛良,王志强,刘淑娥,宋银花,宗学普.桃树不同生长型及其研究进展[J].果树学报,2004,21(4):354-359.NIU Liang,WANG Zhiqiang,LIU Shu’e,SONG Yinhua,ZONG Xuepu.Advances in research on growth habits of peach tree (Prunus persica)[J].Journal of Fruit Science,2004,21(4):354-359.
[7] LIANG F,XIN X Y,HU Z J,XU J,WEI G,QIAN X,YANG J,HE H,LUO X.Genetic analysis and fine mapping of a novel semidominant dwarfing gene LB4D in rice[J].Journal of Integrative Plant Biology,2011,53(4):312-323.
[8] QIN X,LIU J H,ZHAO W S,CHEN X J,GUO Z J,PENG Y L.Gibberellin 20-oxidase gene OsGA20ox3 regulates plant stature and disease development in rice[J].Molecular Plant-Microbe Interactions,2013,26(2):227-239.
[9] 涂从勇,王丰.绿色革命六十载,天下粮安系终生:半矮秆水稻之父黄耀祥院士的学术成就回顾[J].广东农业科学,2019,46(9):1-7.TU Congyong,WANG Feng.Sixty years'devotion to green revolution and a life time commitment to food security:Review on the academic achievements of Huang Yaoxiang,father of semidwarf rice breeding[J].Guangdong Agricultural Sciences,2019,46(9):1-7.
[10] JUNG Y J,KIM J H,LEE H J,KIM D H,YU J,BAE S,CHO Y,KANG K K.Generation and transcriptome profiling of Slr1-d7 and Slr1-d8 mutant lines with a new semi-dominant dwarf allele of SLR1 using the CRISPR/Cas9 system in rice[J].International Journal of Molecular Sciences,2020,21(15):5492.
[11] PENG J,RICHARDS D E,HARTLEY N M,MURPHY G P,DEVOS K M,FLINTHAM J E,BEALES J,FISH L J,WORLAND A J,PELICA F,SUDHAKAR D,CHRISTOU P,SNAPE J W,GALE M D,HARBERD N P.‘Green revolution’genes encode mutant gibberellin response modulators[J].Nature,1999,400(6741):256-261.
[12] PEARCE S,SAVILLE R,VAUGHAN S P,CHANDLER P M,WILHELM E P,SPARKS C A,AL-KAFF N,KOROLEV A,BOULTON M I,PHILLIPS A L,HEDDEN P,NICHOLSON P,THOMAS S G.Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat[J].Plant Physiology,2011,157(4):1820-1831.
[13] LI Y Y,XIAO J H,WU J J,DUAN J,LIU Y,YE X,ZHANG X,GUO X,GU Y,ZHANG L,JIA J,KONG X.A tandem segmental duplication (TSD) in green revolution gene Rht-D1b region underlies plant height variation[J].The New Phytologist,2012,196(1):282-291.
[14] WEN W,DENG Q Y,JIA H Y,WEI L,WEI J,WAN H,YANG L,CAO W,MA Z.Sequence variations of the partially dominant DELLA gene Rht-B1c in wheat and their functional impacts[J].Journal of Experimental Botany,2013,64(11):3299-3312.
[15] HU S L,WANG C L,SANCHEZ D L,LIPKA A E,LIU P,YIN Y,BLANCO M,LUBBERSTEDT T.Gibberellins promote brassinosteroids action and both increase heterosis for plant height in maize (Zea mays L.)[J].Frontiers in Plant Science,2017,8:1039.
[16] ZHANG J J,ZHANG X F,CHEN R R,YANG L,FAN K,LIU Y,WANG G,REN Z,LIU Y.Generation of transgene-free semidwarf maize plants by gene editing of Gibberellin-Oxidase20-3 using CRISPR/Cas9[J].Frontiers in Plant Science,2020,11:1048.
[17] ORDONIO R L,ITO Y,HATAKEYAMA A,OHMAE-SHINOHARA K,KASUGA S,TOKUNAGA T,MIZUNO H,KITANO H,MATSUOKA M,SAZUKA T.Gibberellin deficiency pleiotropically induces culm bending in Sorghum:An insight into Sorghum semi-dwarf breeding[J].Scientific Reports,2014,4:5287.
[18] LI Z F,GUO Y,OU L,HONG H,WANG J,LIU Z X,GUO B,ZHANG L,QIU L.Identification of the dwarf gene GmDW1 in soybean (Glycine max L.) by combining mapping-by-sequencing and linkage analysis[J].Theoretical and Applied Genetics,2018,131(5):1001-1016.
[19] TURHAN E,ERGIN S.Soluble sugars and sucrose-metabolizing enzymes related to cold acclimation of sweet cherry cultivars grafted on different rootstocks[J].The Scientific World Journal,2012,2012:979682.
[20] ZHANG H H,LI X,ZHANG S B,YIN Z,ZHU W,LI J,MENG L,ZHONG H,XU N,WU Y,SUN G Y.Rootstock alleviates salt stress in grafted mulberry seedlings:Physiological and PSII function responses[J].Frontiers in Plant Science,2018,9:1806.
[21] HAN Q Q,GUO Q X,KORPELAINEN H,NIINEMETS U,LI C.Rootstock determines the drought resistance of poplar grafting combinations[J].Tree Physiology,2019,39(11):1855-1866.
[22] HU L,LU H,LIU Q L,CHEN X,JIANG X.Overexpression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol[J].Tree Physiology,2005,25(10):1273-1281.
[23] ZHENG X D,ZHAO Y,SHAN D Q,SHI K,WANG L,LI Q,WANG N,ZHOU J,YAO J,XUE Y,FANG S,CHU J,GUO Y,KONG J.MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression[J].The New Phytologist,2018,217(3):1086-1098.
[24] GAN Z Y,WANG Y,WU T,XU X,ZHANG X,HAN Z.Md-PIN1b encodes a putative auxin efflux carrier and has different expression patterns in BC and M9 apple rootstocks[J].Plant Molecular Biology,2018,96(4/5):353-365.
[25] ZHENG X D,ZHANG H Y,XIAO Y X,WANG C,TIAN Y.Deletion in the promoter of PcPIN-L affects the polar auxin transport in dwarf pear (Pyrus communis L.)[J].Scientific Reports,2019,9:18645.
[26] CHENG J,ZHANG M M,TAN B,JIANG Y,ZHENG X,YE X,GUO Z,XIONG T,WANG W,LI J,FENG J.A single nucleotide mutation in GID1c disrupts its interaction with DELLA1 and causes a GA-insensitive dwarf phenotype in peach[J].Plant Biotechnology Journal,2019,17(9):1723-1735.
[27] CHENG J,MA J J,ZHENG X B,LÜ H,ZHANG M,TAN B,YE X,WANG W,ZHANG L,LI Z,LI J,FENG J.Functional analysis of the Gibberellin 2-oxidase gene family in peach[J].Frontiers in Plant Science,2021,12:619158.
[28] SHAO X H,WU S P,DOU T X,ZHU H,HU C,HUO H,HE W,DENG G,SHENG O,BI F,GAO H,DONG T,LI C,YANG Q,YI G.Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana[J].Plant Biotechnology Journal,2020,18(1):17-19.
[29] DONG Y H,YE X L,XIONG A S,ZHU N,JIANG L,QU S.The regulatory role of gibberellin related genes DKGA2ox1 and MIR171f_3 in persimmon dwarfism[J].Plant Science,2021,310:110958.
[30] ZHOU Y C,UNDERHILL S J R.Expression of gibberellin metabolism genes and signalling components in dwarf phenotype of breadfruit (Artocarpus altilis) plants growing on marang (Artocarpus odoratissimus)rootstocks[J].Plants,2020,9(5):634.
[31] LIU X F,ZHAO C D,GAO Y Q,XU Y,WANG S,LI C,XIE Y,CHEN P,YANG P,YUAN L,WANG X,HUANG L,MA F,FENG H,GUAN Q.A multifaceted module of BRI1 ETHYLMETHANE SULFONATE SUPRESSOR1 (BES1)-MYB88 in growth and stress tolerance of apple[J].Plant Physiology,2021,185(4):1903-1923.
[32] MA Y,XUE H,ZHANG L,ZHANG F,OU C,WANG F,ZHANG Z.Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus × domestica)[J].Scientific Reports,2016,6:26719.
[33] ZHENG L W,MA J J,ZHANG L Z,GAO C,ZHANG D,ZHAO C,HAN M.Revealing critical mechanisms of BR-mediated apple nursery tree growth using iTRAQ-based proteomic analysis[J].Journal of Proteomics,2018,173:139-154.
[34] FUENTES S,LJUNG K,SOREFAN K,ALVEY E,HARBERD N P.OSTERGAARD L.Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses[J].The Plant Cell,2012,24(10):3982-3996.
[35] BAO S J,HUA C M,SHEN L S,YU H.New insights into gibberellin signaling in regulating flowering in Arabidopsis[J].Journal of Integrative Plant Biology,2020,62(1):118-131.
[36] CASTORINA G,CONSONNI G.The role of brassinosteroids in controlling plant height in Poaceae:A genetic perspective[J].International Journal of Molecular Sciences,2020,21(4):1191.
[37] LI Q F,ZHOU Y,XIONG M,REN X Y,HAN L,WANG J D,ZHANG C Q,FAN X L,LIU Q Q.Gibberellin recovers seed germination in rice with impaired brassinosteroid signalling[J].Plant Science,2020,293:110435.
[38] FRIDBORG I,KUUSK S,MORITZ T,SUNDBERG E.The Arabidopsis dwarf mutant Shi exhibits reduced gibberellin responses conferred by overexpression of a new putative zinc finger protein[J].The Plant Cell,1999,11(6):1019-1032.
[39] HUANG J,TANG D,SHEN Y,QIN B,HONG L,YOU A,LI M,WANG X,YU H,GU M,CHENG Z.Activation of gibberellin 2-oxidase 6 decreases active gibberellin levels and creates a dominant semi-dwarf phenotype in rice (Oryza sativa L.)[J].Journal of Genetics and Genomics,2010,37(1):23-36.
[40] JI S H,GURURANI M A,LEE J W,AHN B O,CHUN S C.Isolation and characterisation of a dwarf rice mutant exhibiting defective gibberellins biosynthesis[J].Plant Biology,2014,16(2):428-439.
[41] XIE Y Y,CHEN L T.Epigenetic regulation of gibberellin metabolism and signaling[J].Plant and Cell Physiology,2020,61(11):1912-1918.
[42] HU Y X,TAO Y B,XU Z F.Overexpression of Jatropha gibberellin 2-oxidase 6 (JcGA2ox6) induces dwarfism and smaller leaves,flowers and fruits in Arabidopsis and Jatropha[J].Frontiers in Plant Science,2017,8:2103.
[43] 段雅婕,陈经烨,陈晶晶.香蕉MaGA2ox12 基因在香蕉中的克隆、亚细胞定位及表达分析[J/OL].分子植物育种:1-22[2021-11-22].http://kns.cnki.net/kcms/detail/46.1068.S.20210223.1453.025.html.DUAN Yajie,CHEN Jingye,CHEN Jingjing.Cloning,expression and subcellular localization analysis of MaGA2ox12 gene from banana[J/OL].Molecular Plant Breeding:1-22[2021-11-22].http ://kns.cnki.net/kcms/detail/46.1068.S.20210223.1453.025.html.
[44] 杨杰.梨遗传转化体系的优化与赤霉素氧化酶GA2ox8 基因的矮化功能研究[D].杨凌:西北农林科技大学,2020.YANG Jie.Optimization of genetic transformation system and dwarfing functional analysis of GA2ox8 gene and in Pyrus[D].Yangling:Northwest A&F University,2020.
[45] 张继,黄国弟,张宇,欧克纬,龙凌云,庞新华,卢业飞.杧果矮化基因GA2ox 的克隆、亚细胞定位及表达分析[J].经济林研究,2020,38(1):90-98.ZHANG Ji,HUANG Guodi,ZHANG Yu,OU Kewei,LONG Lingyun,PANG Xinhua,LU Yefei.Cloning,subcellular localization and expression analysis on dwarfing gene GA2ox in Mangifera indica[J].Non-Wood Forest Research,2020,38(1):90-98.
[46] 李飞鸿,侯应军,李雪涵,余心怡,渠慎春.苹果赤霉素氧化酶基因MdGA2ox8 的克隆及功能分析[J].中国农业科学,2018,51(22):4339-4351.LI Feihong,HOU Yingjun,LI Xuehan,YU Xinyi,QU Shenchun.Cloning and function analysis of apple gibberellin oxidase gene MdGA2ox8[J].Scientia Agricultura Sinica,2018,51(22):4339-4351.
[47] 高世敏,董阳,王武,陶建敏.葡萄赤霉素合成关键基因VvGA20ox2 的克隆、亚细胞定位和表达分析[J].江苏农业学报,2018,34(6):1331-1338.GAO Shimin,DONG Yang,WANG Wu,TAO Jianmin.Cloning,subcellular localization and expression analysis of the key gene VvGA20ox2 in gibberellin synthesis of grapevine[J].Jiangsu Journal of Agricultural Sciences,2018,34(6):1331-1338.
[48] 张佳琦,胡恒康,徐川梅,胡渊渊,黄有军,夏国华,黄坚钦,常英英,叶磊,娄和强,张启香.核桃JrGA2ox 基因的克隆、亚细胞定位及功能验证[J].林业科学,2019,55(2):50-60.ZHANG Jiaqi,HU Hengkang,XU Chuanmei,HU Yuanyuan,HUANG Youjun,XIA Guohua,HUANG Jianqin,CHANG Yingying,YE Lei,LOU Heqiang,ZHANG Qixiang.Cloning,subcellular localization and function verification of gibberellin 2-oxidase gene in walnut(Juglans regia)[J].Scientia Silvae Sinicae,2019,55(2):50-60.
[49] 魏广利,梁璧,张佳琦,胡恒康,黄有军,娄和强,张启香.山核桃赤霉素氧化酶基因CcGA3ox 的克隆和功能分析[J].果树学报,2021,38(1):13-28.WEI Guangli,LIANG Bi,ZHANG Jiaqi,HU Hengkang,HUANG Youjun,LOU Heqiang,ZHANG Qixiang.Cloning and functional analysis of CcGA3ox gene from hickory (Carya cathayensis)[J].Journal of Fruit Science,2021,38(1):13-28.
[50] YAMAGUCHI M,SASAKI T,SIVAGURU M,YAMAMOTO Y,OSAWA H,AHN S J,MATSUMOTO H.Evidence for the plasma membrane localization of Al-activated malate transporter(ALMT1)[J].Plant&Cell Physiology,2005,46(5):812-816.
[51] NELSON B K,CAI X,NEBENFÜHR A.A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants[J].The Plant Journal,2007,51(6):1126-1136.
[52] 胡恒康,江香梅,张启香,陈贝,黄坚钦.碳源对山核桃体细胞胚发生和植株再生的影响[J].浙江农林大学学报,2011,28(6):911-917.HU Hengkang,JIANG Xiangmei,ZHANG Qixiang,CHEN Bei,HUANG Jianqin.Somatic embryogenesis and plant regeneration from Carya cathayensis embryos using different carbon sources[J].Journal of Zhejiang A &F University,2011,28(6):911-917.
[53] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J].Methods,2001,25(4):402-408.
[54] THOMAS S G,PHILLIPS A L,HEDDEN P.Molecular cloning and functional expression of gibberellin 2-oxidases,multifunctional enzymes involved in gibberellin deactivation[J].Proceedings of the National Academy of Sciences of the United States of America,1999,96(8):4698-4703.
[55] ZHOU B,PENG D,LIN J Z,HUANG X,PENG W,HE R,GUO M,TANG D,ZHAO X,LIU X L.Heterologous expression of a gibberellin 2-oxidase gene from Arabidopsis thaliana enhanced the photosynthesis capacity in Brassica napus L.[J].Journal of Plant Biology,2011,54(1):23-32.
[56] ATKINSON C J,ELSE M A,TAYLOR L,DOVER C J.Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.)[J].Journal of Experimental Botany,2003,54(385):1221-1229.
[57] GREGORY P J,ATKINSON C J,BENGOUGH A G,ELSE M A,FERNANDEZ-FERNANDEZ F,HARRISON R J,SCHMIDT S.Contributions of roots and rootstocks to sustainable,intensified crop production[J].Journal of Experimental Botany,2013,64(5):1209-1222.
[58] SHEN Y Y,ZHUANG W B,TU X T,GAO Z,XIONG A,YU X,LI X,LI F,QU S.Transcriptomic analysis of interstock-induced dwarfism in sweet persimmon (Diospyros kaki Thunb.)[J].Horticulture Research,2019,6:51.
[59] BUSOV V B,MEILAN R,PEARCE D W,MA C,ROOD S B,STRAUSS S H.Activation tagging of a dominant gibberellin catabolism gene (GA 2-oxidase) from poplar that regulates tree stature[J].Plant Physiology,2003,132(3):1283-1291.
[60] EL-SHARKAWY I,EL KAYAL W,PRASATH D,FERNANDEZ H,BOUZAYEN M,SVIRCEV A M,JAYASANKAR S.Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum[J].Journal of Experimental Botany,2012,63(3):1225-1239.
[61] LIU X Y,LI J,LIU M M,YAO Q,CHEN J.Transcriptome profiling to understand the effect of Citrus rootstocks on the growth of‘Shatangju’mandarin[J].PLoS One,2017,12(1):e169897.
[62] LI J R,YU K,WEI J R,MA Q,WANG B Q,YU D.Gibberellin retards chlorophyll degradation during senescence of Paris Polyphylla[J].Biologia Plantarum,2010,54(2):395-399.
[63] LIU Y,FANG Y,HUANG M J,JIN Y,SUN J,TAO X,ZHANG G,HE K,ZHAO Y,ZHAO H.Uniconazole-induced starch accumulation in the bioenergy crop duckweed (Landoltia punctata)I:Transcriptome analysis of the effects of uniconazole on chlorophyll and endogenous hormone biosynthesis[J].Biotechnology for Biofuels,2015,8:64.
[64] LEI W,LI Y,YAO X H,QIAO K,WEI L,LIU B,ZHANG D,LIN H.NAP is involved in GA-mediated chlorophyll degradation and leaf senescence by interacting with DELLAs in Arabidopsis[J].Plant Cell Reports,2020,39(1):75-87.
[65] SCHOMBURG F M,BIZZELL C M,LEE D J,ZEEVAART J A,AMASINO R M.Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants[J].The Plant Cell,2003,15(1):151-163.
[66] LO S F,YANG S Y,CHEN K T,HSING Y I,ZEEVAART J A,CHEN L J,YU S M.A novel class of gibberellin 2-oxidases control semidwarfism,tillering,and root development in rice[J].The Plant Cell,2008,20(10):2603-2618.
[67] RIEU I,ERIKSSON S,POWERS S J,GONG F,GRIFFITHS J,WOOLLEY L,BENLLOCH R,NILSSON O,THOMAS S G,HEDDEN P,PHILLIPS A L.Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis[J].The Plant Cell,2008,20(9):2420-2436.
[68] YAN J D,LIAO X,HE R,ZHONG M,FENG P,LI X,TANG D,LIU X,ZHAO X.Ectopic expression of GA 2-oxidase 6 from rapeseed(Brassica napus L.)causes dwarfism,late flowering and enhanced chlorophyll accumulation in Arabidopsis thaliana[J].Plant Physiology Biochemistry,2017,111:10-19.
[69] LO S F,HO T H D,LIU Y L,JIANG M J,HSIEH K T,CHEN K T,YU L C,LEE M H,CHEN C Y,HUANG T P,KOJIMA M,SAKAKIBARA H,CHEN L J,YU S M.Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice[J].Plant Biotechnology Journal,2017,15(7):850-864.
[70] DIJKSTRA C,ADAMS E,BHATTACHARYA A,PAGE A F,ANTHONY P,KOURMPETLI S,POWER J B,LOWE K C,THOMAS S G,HEDDEN P,PHILLIPS A L,DAVEY M R.Over-expression of a gibberellin 2-oxidase gene from Phaseolus coccineus L.enhances gibberellin inactivation and induces dwarfism in Solanum species[J].Plant Cell Reports,2008,27(3):463-470.