草莓(Fragaria×ananassa Duch.)属于多年生草本果树,具有独特的风味和极高的营养、经济价值,其栽培面积和产量位于世界小浆果首位[1]。改善草莓果实品质是目前草莓生产面临的突出问题。草莓果实品质形成受植物激素、矿质营养和生态环境等因素的调节[2-4]。花色素苷含量在植物色泽形成过程中发挥重要作用,是果蔬气味和颜色的重要组成部分,因此是评价果实品质的重要指标[5-6]。
花色素苷参与植物的发育和防御,能够影响种子品质、植物产品的涩味,提高植物产品的农艺、工业和营养价值[7]。此外,花色素苷还具有清除自由基、抗氧化和抑菌等食品保健功能,对人类健康也具有重要意义[8]。花色素苷属于类黄酮化合物[7],许多植物中的合成途径已经明确[9],催化酶及其编码基因已经得到广泛鉴定,包括查尔酮合成酶(CHS)、查尔酮异构酶(CHI)、黄烷酮-3-羟化酶(F3H)、二氢类黄酮还原酶(DFR)、花色素苷合成酶(ANS)、类黄酮3,5-糖基转移酶(UFGT)[10-11]。光和温度等环境因素通过影响花色素苷合成相关基因的表达起作用,目前已有报道FvMYB10、FaRIF 和FvTCP9 等转录因子参与调控草莓果实花色素苷的生物合成[12-14]。
研究表明,植物碱性螺旋-环-螺旋(basic helixloop-helix,bHLH)家族的部分成员在植物花色素苷合成过程也发挥着重要的调控作用[15]。bHLH 转录因子作为植物第二大类转录因子,不仅在植物生长发育和生理代谢途径中具有重要作用[16],还参与调节类黄酮与花青素的合成,植物形态建成以及胁迫响应等过程[17]。bHLH 家族成员包含两个保守结构域:碱性区域和螺旋-环-螺旋(helix-loop-helix,HLH)区域[18],既可以作为转录激活子又可以作为转录抑制子起作用。目前已经在草莓[19]、杜梨(Pyrus betulaefolia)[20]、苹果(Malus domestica)[21]、葡萄(Vitis vinifera)[22]和黑果枸杞(Lyciun ruthenicum)[23]等植物中鉴定出bHLH转录因子。本研究在转录组分析的基础上,发现了一个bHLH 超家族转录因子Fab-HLH148,并对FabHLH148进行了克隆、生物信息学分析、表达模式检测以及功能的初步分析,为提高草莓果实品质提供理论基础。
1 材料和方法
1.1 试验材料与试剂
植物材料是北京农学院温室种植的红颜草莓。栽培条件为温度17~26 ℃、相对湿度为60%~80%、光照14 h/黑暗10 h。根据已有研究将草莓果实分为7 个生长阶段,分别是小绿(small green,SG)、大绿(large green,LG)、褪绿(de-green,DG)、白果(white,Wt)、始红(initial red,IR)、片红(partial red,PR)和全红(full red,FR),分别在开花后9、13、16、19、21、22和25 d后拍照。所用烟草为北京农学院科技综合楼植物培养室种植的本氏烟草。
RNA提取试剂盒购自北京华越洋公司,反转录试剂盒购自YEASEN公司,凝胶回收试剂盒和质粒提取试剂盒购自Axygen 公司,核酸内切酶购自NEB公司,无缝克隆以及Phanta高保真酶购自诺唯赞公司,植物花色素苷提取试剂盒购自索莱宝公司,所用感受态购自上海唯地生物有限公司,所用引物由上海生工生物有限公司合成,测序由睿博兴科公司完成。
1.2 FabHLH148基因生物信息学分析
草莓FabHLH148 全长CDS 序列分析采用NCBI Blast程序比对;利用NCBI Conserved Domains进行蛋白质的保守结构域预测;利用在线软件ExPASy分析蛋白理化性质;利用DNA star 软件进行蛋白质二级结构分析;利用在线软件SWISS-MODEL 进行蛋白质三级结构预测。利用MEGA 5.1构建系统进化树。
1.3 草莓总RNA提取与cDNA第1条连的合成
草莓总RNA 的提取参照北京华越洋公司的植物总RNA 提取试剂盒说明书进行,利用ND5000 超微量分光光度计测定及调整RNA浓度,反转录实验参照YEASEN 公司Hifair® Ⅲ1st Strand cDNA Synthesis SuperMix for qPCR(gDNA digester plus)试剂盒说明书进行。
1.4 FabHLH148基因CDS全长的克隆
使用SnapGene 软件,根据NCBI 中FvbHLH148(GeneBank 登录号:XM_004295010.2)基因的CDS区设计基因克隆引物(表1)。以红颜草莓全红果实的cDNA 为模板进行PCR 扩增,PCR 体系参照诺唯赞公司2×Phanta®Mix Master Mix 说明书,程序为:95 ℃,3 min;95 ℃,15 s;65 ℃,15 s;72 ℃,1 min;循环34次;72 ℃,5 min。PCR产物经琼脂糖凝胶检测正确后,使用Axygen 凝胶回收试剂盒进行纯化回收。纯化后的目的基因片段连接pEASY®-Blunt Simple 克隆载体,转化Trans1 T1 感受态细胞,进行阳性克隆筛选并测序。
表1 本研究中所用引物
Table 1 The specific primers of this study

1.5 FabHLH148过表达载体构建
采用同源重组的方法,设计同源重组的引物(表1)。以测序成功的pEASY®-Blunt Simple-Fab-HLH148 质粒为模板,克隆带有pSuper1300:GFP 过表达载体同源臂的基因片段,并将pSuper1300:GFP载体用SalⅠ-HF、SpeⅠ-HF 内切酶酶切,经琼脂糖凝胶回收线性化的载体片段。通过诺唯赞ClonExpress®ⅡOne Step Cloning Kit进行连接,转化DH5α大肠杆菌感受态细胞,筛选阳性克隆进行测序。
1.6 FabHLH148 RNAi载体构建
通过Gateway的方法构建FabHLH148 RNAi载体,设计入门载体引物(表1)。克隆FabHLH148 基因32~383 bp 为目的序列,通过琼脂糖凝胶电泳验证PCR 结果,胶回收目的片段经BP 反应构入pDONR221 载体,反应体系参照赛默飞Gateway®BP ClonaseTMⅡEnzyme Mix 说明书,反应程序为25 ℃过夜,反应结束后加入1 μL Proteinase K,37 ℃反应10 min。将BP 反应产物转化大肠杆菌Trans1-T1 感受态细胞,筛选阳性克隆并进行测序。提取入门载体pDONR221- FabHLH148352质粒通过LR 反应构入pK7GWIWG(Ⅱ)RR,得到RNAi载体,反应体系参照赛默飞Gateway®LR ClonaseTMⅡEnzyme Mix 说明书进行。筛选阳性克隆,提取FabHLH148 RNAi 载体质粒并转化GV3101 农杆菌感受态细胞。
1.7 亚细胞定位
将 Super1300:FabHLH148-GFP质粒转化GV3101 农杆菌感受态。筛选扩繁阳性克隆,制备农杆菌侵染液,侵染5~6 周长势良好的烟草叶片。48 h 后剪取注射部位的烟草叶片,避光浸泡在稀释好的DNA 荧光染料DAPI 染液(1 μg·mL-1,现配现用)中30~60 min,用激光共聚焦显微镜观察Fab-HLH148亚细胞定位。试验3次重复。
1.8 FabHLH148瞬时侵染草莓果实
扩繁阳性FabHLH148 OE、转化空载体的农杆菌(CK)和RNAi农杆菌,制备农杆菌侵染液,挑取长势相近的褪绿时期果实每种农杆菌各注射10个,注射后做好标记并在注射0、3、5 和7 d 时拍照记录表型,注射7 d后取样,去掉瘦果只切取注射部分液氮速冻后保存在-80 ℃冰箱。试验3次重复。
1.9 草莓果实花色素苷含量测定
取-80℃保存的FabHLH148 OE、CK以及RNAi组果实,液氮研磨成粉末后称取0.1 g,用于花色素苷的提取,试验步骤以及花色素苷含量的测定参照索莱宝植物花色苷含量检测试剂盒(索莱宝,北京,中国)说明书进行。试验3次重复。
1.10 FabHLH148荧光定量分析
RT-qPCR 检测基因相对表达量,反应体系参照Trans-Start® Top Green qPCR Super Mix 试剂盒(全式金,中国)说明书,PCR 反应体系为10 μL,反应程序为94 ℃,30 s;94 ℃,5 s;60 ℃,15 s;72 ℃,10 s;40 个循环。草莓FabHLH148 基因和内参基因的荧光定量的引物见表1。
2 结果与分析
2.1 FabHLH148生物信息学分析
根据实验室前期获得的八倍体草莓果实转录组测序结果[24],筛选得到一个从始红(IR)到片红(PR)时期表达显著升高的基因(图1),该基因与二倍体草 莓 FvbHLH148(GenBank登录号 :XM_004295010.2)相似度最高,因此将其命名为Fab-HLH148。FabHLH148 基因CDS 全长684 bp,共编码228 个氨基酸。对氨基酸序列分析,发现其在第153~202 位氨基酸处包含一个保守的bHLH 结构域(图2),表明其属于bHLH超家族。氨基酸理化性质分析结果表明:FabHLH148预测编码的蛋白质分子式为C1080H1809N353O312S5,分子质量为24.89 ku,理论等电点(pI)为11.56,为碱性蛋白,含有负电荷氨基酸残基(Asp+Glu)共10 个,正电荷氨基酸残基(Arg+Lys)共42 个,不稳定系数为54.49,亲水性平均值为-0.636,由此推断该蛋白为不稳定的疏水性蛋白。FabHLH148 二级结构预测结果(图3)显示,其含有的α-螺旋、β-折叠、无规则卷曲、柔性区域等基本上分布均匀,其三级结构预测如图4 所示。从NCBI数据库获得不同种植物的FabHLH148同源蛋白序列进行比对,构建系统进化树(图5),其中二倍体草莓(Fragaria vesca subsp.vesca)FvbHLH148蛋白(GenBank 登录号:XP_004295058.1)与八倍体草莓FabHLH148 的相似度最高,达99.56%,其次是与月季(Rosa chinensis)RcbHLH147蛋白(GenBank登录号:XP_024197449.1),序列相似度达79.25%。
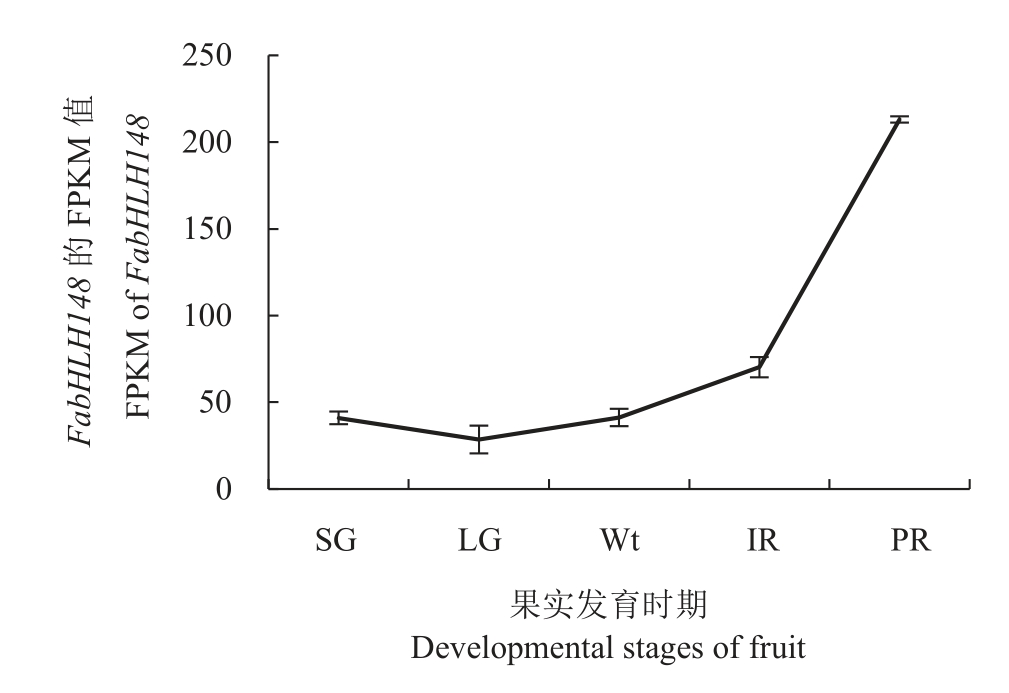
图1 FabHLH148 基因在草莓果实不同发育时期的FPKM 值
Fig.1 FPKM of FabHLH148 in different development periods of strawberry fruit
SG.小绿果时期;LG.大绿果时期;Wt.白果时期;IR.始红果时期;PR.片红果时期。下同。
SG.Small green fruit;LG.Large green fruit;Wt.White fruit;IR.Initial red fruit;PR.Partial red fruit.The same below.
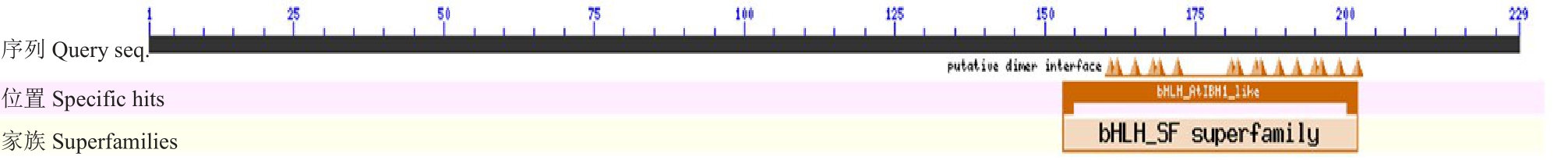
图2 FabHLH148 的结构域分析
Fig.2 Analysis of conserved domain of FabHLH148
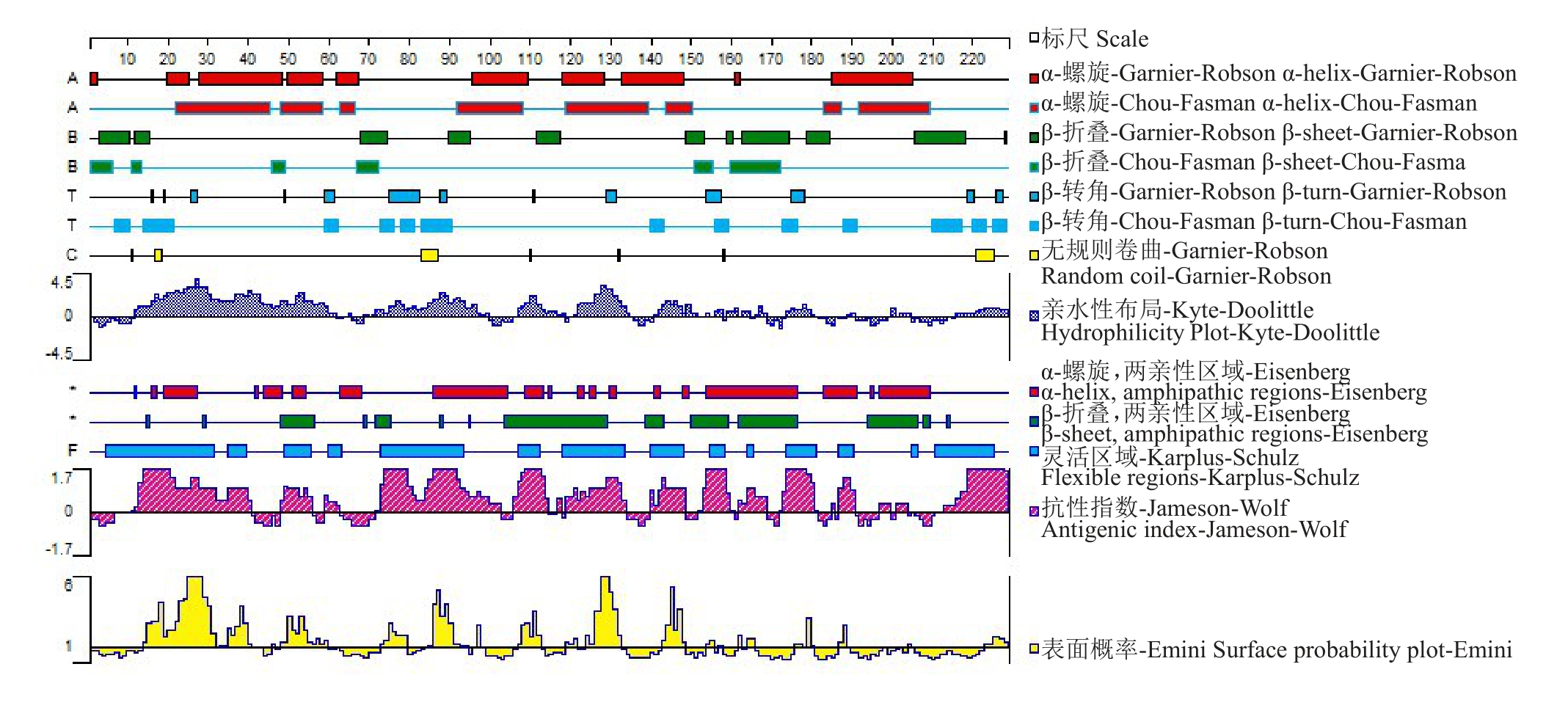
图3 FabHLH148 蛋白质二级结构分析
Fig.3 Analysis of protein secondary structure of FabHLH148
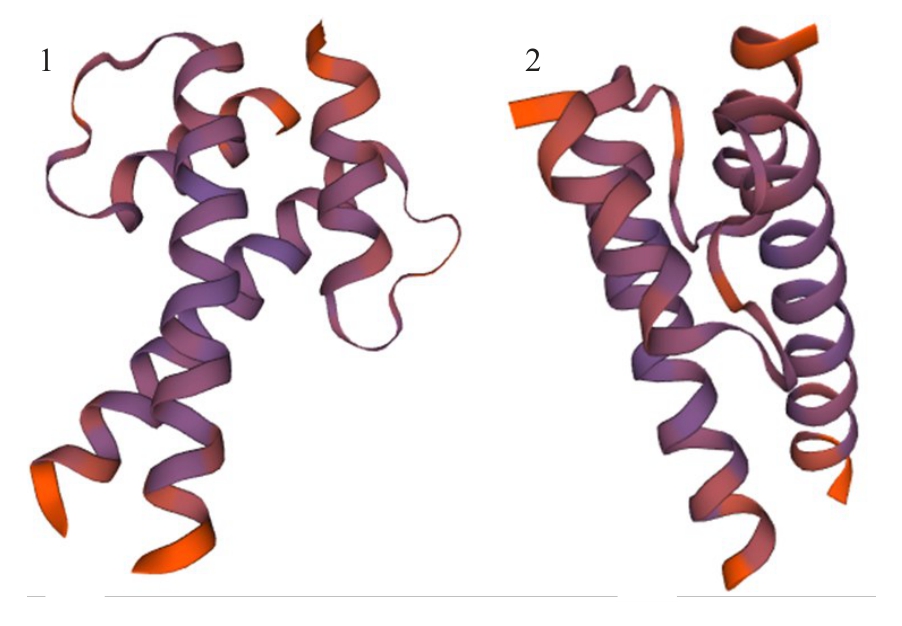
图4 SWISS-MODEL 预测FabHLH148 蛋白质三级结构
Fig.4 The tertiary structure of FabHLH148 protein predicted with SWISS-MODEL
1~2.FabHLH148 蛋白质三级结构不同视图。
1-2.Different views of the tertiary structure of FabHLH148 protein.
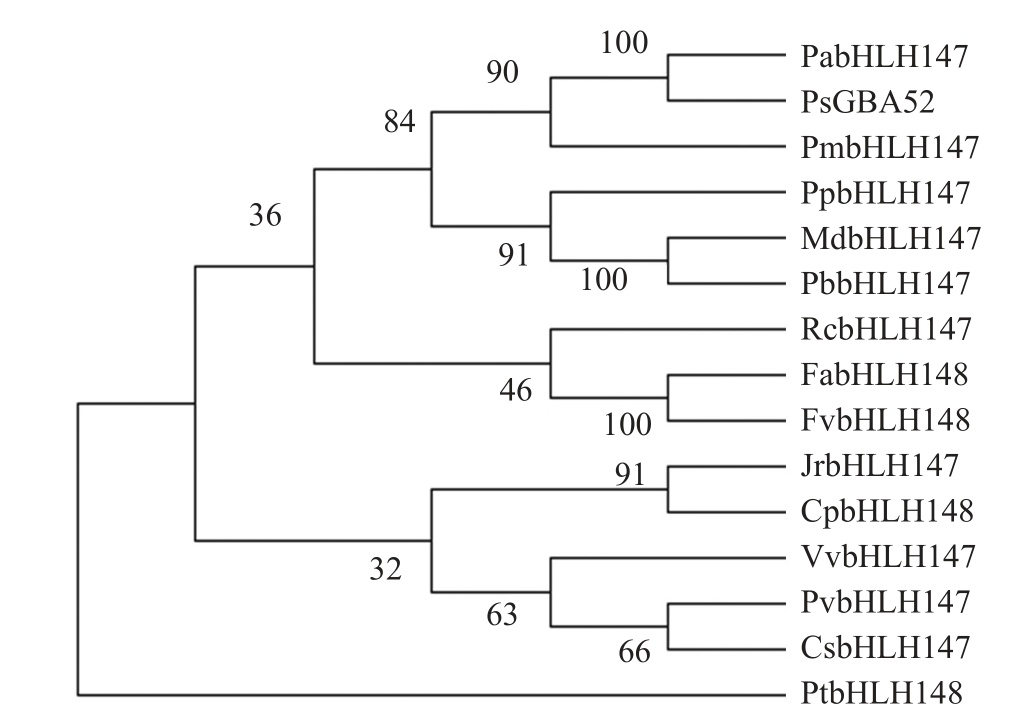
图5 FabHLH148 同源蛋白的系统进化树分析
Fig.5 Phylogenetic tree analysis of FabHLH148 homologous proteins
Pa.樱桃;Ps.山杏;Pm.梅花;Pp.桃;Md.苹果;Pb.杜梨;Rc.月季;Fa.草莓;Fv.森林草莓;Jr.核桃;Cp.番木瓜;Vv.葡萄;Pv.阿月浑子;Cs.黄瓜;Pt.毛果杨。
Pa. Prunus avium;Ps. Prunus sibirica;Pm. Prunus mume;Pp.Prunus persica;Md. Malus domestica;Pb. Pyrus bretschneideri;Rc.Rosa chinensis;Fa. Fragaria×ananassa;Fv. Fragaria vesca subsp.vesca;Jr.Juglans regia;Cp.Carica papaya;Vv.Vitis vinifera;Pv.Pistacia vera;Cs.Cucumis sativus;Pt.Populus trichocarpa.
2.2 FabHLH148亚细胞定位
为观察FabHLH148的亚细胞定位,进行了农杆菌介导的烟草叶肉细胞瞬时转化实验。实验结果表明FabHLH148-GFP 蛋白在细胞核和细胞质中有定位,且在细胞核中与DNA荧光染料DAPI共定位(图6)。细胞核定位为FabHLH148 行使转录因子功能提供了空间基础。

图6 FabHLH148 亚细胞定位
Fig.6 Subcellular localization of FabHLH148
GFP.绿色荧光蛋白通道;DAPI.DNA 荧光染料通道;BF.明场;Merge.合并图片。Bars=20 μm。GFP.Signal(green);DAPI.Signal(blue);BF.Bright field;Merge.Overlay signals.Bars:20 μm.
2.3 FabHLH148的表达模式
为检测FabHLH148在草莓中的表达模式,通过RT-qPCR 检测其在草莓不同组织部位的表达(图7-A),发现其在草莓不同组织部位均有表达,在全红果实中的相对表达量最高,在瘦果中的相对表达量最低。其次,还检测了FabHLH148在草莓果实不同发育时期的表达水平,结果显示该基因从褪绿到片红时期的表达量缓慢升高,片红到全红时期表达量迅速升高(图7-B),这为FabHLH148 在草莓果实成熟中发挥功能提供了基础。

图7 FabHLH148 在草莓不同组织部位(A)及发育时期(B)的相对表达量
Fig.7 Relative expression level of FabHLH148 in different tissues and developmental stages of strawberry
A.FabHLH148 在草莓不同器官的相对表达量;B.FabHLH148 在草莓果实不同发育时期的相对表达量。
A.Relative expression level of FabHLH148 in different organs of strawberry;B.Relative expression level of FabHLH148 in different developmental stages of strawberry fruit.
2.4 FabHLH148促进草莓果实着色
为进一步明确FabHLH148 在草莓果实成熟中的功能,分别构建FabHLH148 OE 和RNAi 载体,转化农杆菌,通过农杆菌介导瞬时侵染草莓果实,记录表型(图8-A),检测FabHLH148表达水平(图8-B),并测定不同转基因果实的花色素苷含量(图8-C)。结果表明,FabHLH148过量表达能够促进草莓果实着色以及花色素苷的积累;通过RNAi 降低Fab-HLH148表达则抑制草莓果实着色以及花色素苷的积累。以上结果初步说明FabHLH148 在草莓果实着色过程中起重要作用。

图8 FabHLH148 正调控草莓果实成熟
Fig.8 FabHLH148 positively regulated strawberry fruit ripening
A.包含FabHLH148 OE、CK 和FabHLH148 RNAi 农杆菌注射草莓进行表型观察。B.RT-qPCR 分析FabHLH148 OE、CK 和FabHLH148 RNAi 组果实中FabHLH148 基因的相对表达量,采用算法为2-△△ct。C.FabHLH148 OE、CK 和FabHLH148 RNAi 组草莓果实的花色素苷含量。不同小写字母表示在p<0.05 差异显著。
A.RT-qPCR was used to analyze the relative expression levels of FabHLH148 in FabHLH148 OE,CK and FabHLH148 RNAi group of strawberry fruits,and the algorithm was2-△△ct.B.FabHLH148 OE,CK and FabHLH148 RNAi Agrobacterium were injected into de-green strawberry fruit for phenotypic observation.C.Anthocyanin content of FabHLH148 OE,CK and FabHLH148 RNAi group of strawberry fruit.Different small letters indicate significant difference at p<0.05.
3 讨论
已有研究表明bHLH转录因子参与花色素苷调控。Cui 等[25]研究表明PyMYB10、PybHLH 和Py-WD40 转录因子形成三元复合体MBW(MYBbHLH-WD40)来调控云南红梨中花色素苷的合成与积累。来自bHLH 超家族Ⅲ亚家族的TT8(Transparent testa 8)、GL3(Glabra 3)和EGL3(Enhancer of Glabra 3)能够与TTG1(Transparent testaglabra1,WD40 蛋白家族)和MYB(myeloblastosis protein,MYB 蛋白家族)形成MBW 复合物,调控拟南芥和番茄中花色素苷的合成[24-28]。在苹果中发现两种bHLH转录因子MdbHLH3[29]和MdbHLH33[30]能够调控花色素苷的积累:Xie 等[31]从苹果中分离得到MdbHLH3,其通过N 端的两个区域(1~23 和186~288位氨基酸)与MdMYB1互作,激活下游花色素苷合成相关基因MdDFR 和MdUFGT 的表达,从而促进低温条件下苹果花色素苷的积累;而MdbHLH33通过与MdMYB16 互作形成异源二聚体来减弱Md-MYB16 对花色素苷的抑制作用[32],从而促进花色素苷的积累。NtbHLH1 能够作为调节因子与NtMYB6互作从而促进中国水仙中花色素苷的积累[33]。以上研究表明bHLH 家族转录因子主要与MYB 超家族互作来调控植物花色素苷的合成与积累。范祺祺[34]通过对彩色马铃薯块茎花色素苷合成代谢的转录组学研究,筛选出193 个注释在bHLH 类转录因子中的差异基因。王沛捷等[35]从紫色马铃薯bHLH转录因子家族中鉴定出5 个参与花色素苷合成的基因。吴楠[36]在前期卵叶牡丹叶片转录组数据的基础上,筛选出与叶片花色苷合成相关的bHLH 转录因子PqbHLH1,并获得异源表达PqbHLH1 的拟南芥株系,与野生型拟南芥相比,其主根颜色呈红褐色,积累有少量的花色素苷,此外,过表达株系中AtCHS、AtCHI、AtF3H、AtUFGT、AtF3’H 和AtFLS 等基因的表达水平较野生型均显著增加。Zhao等[37]从红颜草莓以及其突变体小白、白雪公主中筛选出来自不同组织和果实不同发育时期的113个同源bHLH基因,RT-qPCR 结果显示,7 个选定的FabHLH 基因(Fab-HLH17、FabHLH25、FabHLH27、FabHLH29、Fab-HLH40、FabHLH80 和FabHLH98)响应果实花色素苷生物合成和激素信号传导;蛋白质互作预测揭示了4种bHLHs(FabHLH25、FabHLH29、FabHLH80和FabHLH98)参与果实花色素苷生物合成和激素信号转导。以上研究表明bHLH转录因子家族在植物花色素苷合成与积累过程中具有重要的调控作用。但关于bHLH 转录因子家族转录因子Fab-HLH148少有研究。
笔者在本试验中成功从红颜草莓克隆出Fab-HLH148 基因,蛋白质保守结构域分析发现Fab-HLH148 具有一个bHLH 超家族结构域。其预测编码的氨基酸序列同二倍体森林草莓(Fragaria vesca subsp.vesca)、月季(Rose chinensis)具有较高的同源性,尤其是蔷薇科植物同源性较高,其编码的蛋白功能可能类似。亚细胞定位分析显示FabHLH148 主要在细胞核中表达。通过RT-qPCR 检测Fab-HLH148 的时空表达,其在草莓的不同组织部位均有表达,在果实中较高表达,且在全红时期表达量达到峰值,暗示其在草莓果实着色过程中起重要作用。瞬时侵染草莓果实验结果表明过表达Fab-HLH148能够促进草莓果实着色以及花色素苷的积累,通过RNAi 降低FabHLH148 表达水平则结果相反。以上结果表明FabHLH148 参与草莓果实颜色发育。探究草莓中FabHLH148 转录因子的表达模式及功能,为bHLH 超家族在草莓生长发育以及形态建成等相关研究奠定基础,为提高草莓果实品质相关研究提供参考。
4 结论
从八倍体红颜草莓中克隆转录因子Fab-HLH148,并提供一系列证据表明FabHLH148 能够促进红颜草莓果实成熟过程中花色素苷的积累,其调控的分子机制有待进一步研究。
[1] WANG F,ZHANG F,CHEN M M,LIU Z H,FU J F,MA Y.Comparative transcriptomics reveals differential gene expression related to Colletotrichum gloeosporioides resistance in the octoploid strawberry[J].Frontiers in Plant Science,2017,7:9-14.
[2] IATROU M,PAPADOPOULOS A.Influence of nitrogen nutrition on yield and growth of an everbearing strawberry cultivar(cv.Evie Ⅱ)[J].Journal of Plant Nutrition,2016,39(1):1499-1505.
[3] KIM J,LEE J G,HONG Y,LEE E J.Analysis of eight phytohormone concentrations,expression levels of ABA biosynthesis genes,and ripening-related transcription factors during fruit development in strawberry[J].Journal of Plant Physiology,2019,239:52-60.
[4] BAI Q,SHEN Y Y,HUANG Y.Advanced in mineral nutrition transport and signal transduction in Rosaceae fruit quality and postharvest storage[J].Frontiers in Plant Science,2021,12:620018.
[5] 张琼,王红清,冷平.草莓果实发育过程中花色苷和黄酮醇类物质的形成机制[J].园艺学报,2003,35(12):1735-1741.ZHANG Qiong,WANG Hongqing,LENG Ping.Formation mechanism of anthocyanins and flavonoids during strawberry fruit development[J].Acta Horticulture Sinica,2003,35(12):1735-1741.
[6] HONDA T,SAITO N.Recent progress in the chemistry of polyacylated anthocyanins as flower color pigments[J].HeteroCycles,2010,33(13):633-692.
[7] 李兴国,于泽源.花青苷的研究进展[J].北方园艺,2003(4):6-8.LI Xingguo,YU Zeyuan.Research progress of anthocyanin[J].Northern Horticulture,2003(4):6-8.
[8] 张学明,齐晓光,陈玉波,姚环宇,郑亚杰.草莓类黄酮化合物的研究进展[J].北方园艺,2020(1):128-133.ZHANG Xueming,QI Xiaoguang,CHEN Yubo,YAO Huanyu,ZHENG Yajie.Research advances of flavonoids in strawberry[J].Northern Horticulture,2020(1):128-133.
[9] 周明,沈勇根,朱丽琴,艾啸威,曾璟,杨洪烨.植物黄酮化合物生物合成积累及调控的研究进展[J].食品研究与开发,2016,37(18):216-221.ZHOU Ming,SHEN Yonggen,ZHU Liqin,AI Xiaowei,ZENG Jing,YANG Hongye.Research progress on biosynthesis,accunulation and regulation of flavonoids in plants[J].Food Research and Development,2016,37(18):216-221.
[10] RAHUL K,ASHIMA K,SHARAMA A K.Role of plant hormones and their interplay in development and ripening of fleshy fruits[J].Journal of Experimental Botany,2013,65(16): 4561-4575.
[11] TANAKA Y,SASAKI N,OHMIYA A.Biosynthesis of plant pigments:Anthocyanins,betalains and carotenoids[J].The Plant Journal,2008,54(4):733-749.
[12] MAO W W,HAN Y,CHEN Y,SUN M,FENG Q,LI L,LIU L,ZHANG K,WEI L,HAN Z,LI B.Low temperature inhibits anthocyanin accumulation in strawberry fruit by activating Fv-MAPK3-induced phosphorylation of FvMYB10 and degradation of chalcone synthase 1[J].The Plant Cell,2022,34(4):1226-1249.
[13] MARTÍN-PIZARRO C,VALLARINO J G,OSORIO S,MECO V,URRUTIA M,PILLET J,CASAÑAL A,MERCHANTE C,AMAYA I,WILLMITZER L,FERNIE A R,GIOVANNONI J J,BOTELLA M A,VALPUESTA V,POSÉ D.The NAC transcription factor FaRIF controls fruit ripening in strawberry[J].The Plant Cell,2021,33(5):1574-1593.
[14] XIE Y G,MA Y Y,BI P P,WEI W,LIU J,HU Y,GOU Y J,ZHU D,WEN Y Q,FENG J Y.Transcription factor FvTCP9 promotes strawberry fruit ripening by regulating the biosynthesis of abscisic acid and anthocyanins[J].Plant Physiology and Biochemistry,2020,146:374-383.
[15] 刘化禹.蓝果忍冬花色苷合成bHLH 转录因子筛选及LcTT8功能验证[D].哈尔滨:东北农业大学,2019.LIU Huayu.Screening of bHLH transcription factors for anthocyanin biosynthesis of blue honeysukle and functional verification of LcTT8[D].Harbin:Northeast Agricultural University,2019.
[16] 王力伟,赵沛义,王朝,房永雨,刘红葵,郭慧琴,王蕴华,何江峰.梭梭HabHLH74 转录因子基因的克隆及在大肠杆菌中的表达[J].畜牧与饲料科学,2020,41(5):13-18.WANG Liwei,ZHAO Peiyi,WANG Zhao,FANG Yongyu,LIU Hongkui,GUO Huiqin,WANG Yunhua,HE Jiangfeng.Gene cloning of transcription factor HabHLH74 from Haloxylon ammodendron and expression in Escherichia coli[J].Animal Husbandry and Feed Science,2020,41(5):13-18.
[17] 杨鹏程,周波,李玉花.植物花青素合成相关的bHLH 转录因子[J].植物生理学报,2012,48(8):747-758.YANG Pengcheng,ZHOU Bo,LI Yuhua.Plant anthocyanin synthesis-related bHLH transcription factor[J].Plant Physiology Journal,2012,48(8):747-758.
[18] 丁冬,李嘉欣,魏玉磊.拟南芥和玉米中膜结合bHLH 转录因子的鉴定与分析[J].植物生理学报,2020,56(4):700-710.DING Dong,LI Jiaxin,WEI Yulei.Identification and analysis of bHLH transcription factors binding to membrane in Arabidopsis and maize[J].Plant Physiology Journal,2020,56(4):700-710.
[19] TISZA V,KOVACS L,BALOGH A.Characterization of FaSPT,a SPATULA gene encoding a bHLH transcriptional factor from the non-climacteric strawberry fruit[J].Plant Physiology and Biochemistry,2010,48(10):822-826.
[20] 李晓刚,李慧,杨青松,蔺经,常有宏.杜梨bHLH 转录因子家族两成员的序列特征及对非生物胁迫的转录响应[J].江苏农业科学,2017,45(22):40-45.LI Xiaogang,LI Hui,YANG Qingsong,LIN Jing,CHANG Youhong.Sequence characterization and transcription response to abiotic stress of two members of bHLH transcription factor family in Pyrus betulaefolia Bunge[J].Jiangsu Agricultural Sciences,2017,45(22):40-45.
[21] 杨金华.苹果bHLH 转录因子家族的鉴定及表达分析[D].杨凌:西北农林科技大学,2017.YANG Jinhua.Identification and expression analysis of apple basic helix-loop-helix transcription factor family[D].Yangling:Northwest A&F University,2017.
[22] 陈凯利.葡萄风信子MYB 和bHLH 转录因子对花青苷合成的调控研究[D].杨凌:西北农林科技大学,2017.CHEN Kaili.The regulation of anthocyanin biosynthesis by MYB and bHLH transcription factors in grape hyacinth [D].Yangling:Northwest A&F University,2017.
[23] 包雪梅,胡娜,宗渊,王洪伦.转录组学挖掘黑果枸杞中花青素生物合成代谢关键bHLH 调控基因[J].分子植物育种,2021,9(16):1-20.BAO Xuemei,HU Na,ZONG Yuan,WANG Honglun.Transcriptomics mining the key bHLH regulatory genes for anthocyanin biosynthesis of Lyciun ruthenicum Murray[J].Molecular Plant Breeding,2021,9(16):1-20.
[24] GUO J,WANG S,YU X,DONG R,LI Y,MEI X,SHEN Y Y.Polyamines regulate strawberry fruit ripening by abscisic acid,auxin,and ethylene[J].Plant Physiology,2018,177(1):339-351.
[25] CUI D,ZHAO S,XU H.The interaction of MYB,bHLH and WD40 transcription factors in red pear (Pyrus betulaefolia)peel[J].Plant Molecular Biology,2021,106(4):407-417.
[26] FWLLER A,MACHEMER K,BRAUN E L,GROTEWOLD E.Evolutionary and comparative analysis of MYB and bHLH plant transcription factors[J].The Plant Journal,2011,66(1):94-116.
[27] MIGUEL N G,BLAIR P B,KERSTIN D,ALMUTH H,JENNY A,JAN S,MUKHTAR S M,MALIN E.Identification of norwayspruce MYB-bHLH-WDR transcription factor complex members linked to regulation of the flavonoid pathway[J].Frontiers in Plant Science,2017,8:1-10.
[28] SALVATIERRA A,PIMENTEL P,MOYA-LEON M A,HERRERA R.Increased accumulation of anthocyanins in Fragaria chiloensis fruits by transient suppression of FcMYB1 gene[J].Phytochemistry,2013,90:25-36.
[29] RUMI T W,OTA K,HAYASHI N,YAMADA K,SANO R,WADA T.Expression and protein localization analyses of Arabidopsis GLABRA3 (GL3) in tomato (Solanum lycopersicum) root epidermis[J].Plant Biotechnology,2017,34(2):215-227.
[30] ZHOU L L,SHI M Z,XIE D Y.Regulation of anthocyanin biosynthesis by nitrogen in TTG1-GL3/TT8-PAP1-programmedred cells of Arabidopsis thaliana[J].Planta,2012,236(3): 825-837.
[31] XIE X B,LI S,ZHANG R F,ZHAO L,CHEN Y C,ZHAO Q,YAO Y X,YOU C X,ZHANG X S,HAO Y J.The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit coloration in response to low temperature in apples[J].Plant,Cell&Environment,2012,35(11):1884-1897.
[32] 许海峰.bHLH33 与MYB 抑制子参与苹果花青苷生物合成的分子机理[D].泰安:山东农业大学,2020.XU Haifeng.The molecular underlying anthocyanin biosynthesis in apple using bHLH33 and MYB repressors[D].Tai’an:Shandong Agricultural University,2020.
[33] FAN Y X,PENG J Y,WU J C,ZHOU P;HE R J,ALLAN A C,ZENG L H.NtbHLH1,a JAF13-like bHLH,interacts with Nt-MYB6 to enhance proanthocyanidin accumulation in Chinese narcissus[J].BMC Plant Biology,2021,21:275-275.
[34] 范祺祺.彩色马铃薯块茎花色苷合成代谢的转录组学研究[D].长沙:湖南农业大学,2019.FAN Qiqi.Transcriptomics study on anthocyanin anabolism of colored potato tubers[D].Changsha:Hunan Agricultural University,2019.
[35] 王沛捷,马艳红,武小娟,范菠菠,赵勤娜.紫色马铃薯bHLH转录因子家族的鉴定及表达分析[J].基因组学与应用生物学,2021,7(2):21-45.WANG Peijie,MA Yanhong,WU Xiaojuan,FAN Bobo,ZHAO Qinna.Identification and expression analysis of bHLH transcription factor family genes in purple potato[J].Genomics and Applied Biology,2021,7(2):21-45.
[36] 吴楠.卵叶牡丹叶片花色苷合成相关bHLH 筛选、克隆与功能分析[D].杨凌:西北农林科技大学,2021.WU Nan.Screening,cloning and functional analysis of bHLH related to anthocyanin synthesis of leaf in Pesonia qiui[D].Yangling:Northwest Agriculture and Forestry Technology University,2021.
[37] ZHAO F L,LI G,HU P P,ZHAO X,LI L J,WEI W,FENG J Y,ZHOU H C.Identification of basic/helix-loop-helix transcription factors reveals candidate genes involved in anthocyanin biosynthesis from the strawberry white-flesh mutant[J].Scientific Reports,2018,8(1):2721.