植物胚的形态建成是单细胞发育成功能性多细胞有机体的过程,是从合子的极性建立和不均等分裂开始的。极性建立是指合子中胞内物质按照一定方向不均等的分布,形成极性之后发生不均等分裂,产生远离胚孔的顶细胞以及靠近珠孔的基细胞[1]。顶细胞会分化成胚芽,进而形成根。植物胚的发育经历了合子的不均等分裂、2细胞期、8细胞期、球形胚期、心形胚期、鱼雷胚期和子叶胚等8个阶段[2]。
在植物中已发现并克隆出30 多个胚发育相关基因,表明合子的极性建立和不均等分裂由MAPK(MAP KINASE)/GRD(GROUNDED)途径和WOX(WUSCHEL RELATED-HOMEBOX)转录因子家族成员共同决定。WOX 转录因子家族含有1 个高度保守的同源异型结构域HD(homeodomain),由60~66 个氨基酸构成,可以与DNA 序列特异地结合[3-4]。前人研究表明,WOX 家族基因在胚的形成、干细胞维持、形成层分化、愈伤形成和器官发育等方面都发挥着重要的作用[5-12]。在胚胎发育的起始阶段,也就是合子第一次不对称分裂建立早期胚胎顶-基轴极性时期,WOX 转录因子家族在顶、基细胞中的差异表达是引起顶、基细胞命运分化的重要原因。此期间,WOX2 基因只在顶细胞特异表达,而WOX8/STIMPY-LIKE(STPL)基因和WOX9/STIMPY(STIP)基因在基细胞中表达,有研究表明WOX2 受到了WOX8 和WOX9 的表达调控[13-14]。Haecker 等[5]对WOX2基因无法表达的突变体所形成的胚胎进行研究,发现有一半的胚胎顶端发育出现了异常,表明WOX2 基因在胚胎顶端区域的发育中有重要作用。Nardmann等[15]在玉米中的研究表明,玉米ZmWOX2基因与拟南芥AtWOX2 基因的功能类似,都在胚胎细胞的早期发育、茎端分生组织的形态建成过程中发挥着重要作用。Li等[16]在拟南芥的研究中也发现WOX2 基因在体胚发生过程早期发挥着重要作用。这都表明WOX2基因在胚早期发育过程中发挥重要作用,并且可以作为标记基因来研究植物胚的发育。
马家柚是上饶市广丰区特有的地方良种,具有优质、丰产、耐低温和适应性强等特点,通常栽培中单果种子数为80~120 粒。前期研究通过授粉辐射酸柚花粉获得了马家柚的无核果实(专利号:ZL201610065023.X),发现第4周为游离核胚乳快速增殖阶段,第7周胚乳已经出现大量细胞化情况,而第10 周时胚部分发育到子叶胚阶段,胚乳出现解体,且通过解剖学观察发现,果实无核可能是由于胚乳退化导致胚早期发育受到抑制[17]。笔者在本研究中以此材料为基础,首先从柚中克隆了1 个CmWOX2基因,并通过实时荧光定量PCR技术分析其在正常和退化种子发育过程中的表达差异,以及在不同组织(根、茎、叶和种子)中的表达情况。通过对比分析,可以了解WOX2 基因在柚种子发育过程中的调控作用。
1 材料和方法
1.1 试验材料
试验以江西省上饶市广丰区排山镇果园中的马家柚(118°19′49.548″E,28°26′55.572″N)为材料,随机选取8年生、树势和结果量基本一致、生长健壮的马家柚作为授粉树,进行人工授粉。花粉来自1000 Gy剂量60Co-γ辐射(湖南省原子能农业应用研究所)和未辐射处理(对照)的酸柚花药。
采集对照(未辐射)和1000 Gy处理的授粉前子房以及授粉后2、4、7、10、16、22、26 周果实中种子,并将未辐射花粉处理的种子播种于含MS基本培养基的试管中,收集培养30 d(暗培养20 d,光照培养10 d,培养温度26 ℃±1 ℃)后的幼嫩植株的根、茎和叶片。所有样品材料液氮速冻,置于-80 ℃超低温冰箱储存备用。
1.2 植物总RNA的提取及cDNA合成
采用TaKaRa的植物总RNA提取试剂盒(TaKa-Ra MiniBEST Plant RNA Extration Kit)提取所有柚组织样品的RNA,并用TaKaRa 公司的Prime-ScriptTM RT 反转录试剂盒合成cDNA 第一链,保存于-20 ℃冰箱备用。
1.3 WOX2序列的克隆
通过检索和比对,获得柚WOX2基因序列(http://citrus.hzau.edu.cn/),设计特异性引物(WOX2-F:5’-GCACCAAAAACAATACGC-3’;WOX2-R:5’-GAAACAAACAGATTCGTC-3’)通过扩增获得CmWOX2基因全长序列。PCR产物、回收以及纯化后,与PMD18-T 载体(TaKaRa)连接,通过蓝白斑筛选获得阳性克隆子,进一步进行测序分析。
1.4 生物信息学分析
用DNAman 软件进行蛋白质的多序列比对以及一致性分析。系统进化树的构建采用MEGA 6软件中的Neighbor-Joining 算法进行。使用ExPASy ProtParam tool(http://www.expasy.org/prosite/)来 分析蛋白等电点、分子质量、亲水性等理化性质。使用TMHMM 2.0 工 具(http://www.cbs.dtu.dk/services/TMHMM/)进行跨膜结构域预测。使用SignalP 4.1软件(http://www.cbs.dtu.dk/services/SignalP-4.0/)预测蛋白的信号肽。
1.5 实时荧光定量PCR检测
利用Real time RT-PCR检测马家柚辐射后退化和对照种子不同发育时期,以及正常幼苗不同组织部位WOX2 基因的表达水平差异(Bio-Rad CFX 96 PCR,美国),使用的试剂来自TaKaRa 生物公司的SYBR®Premix Ex TaqTM(Tli RNaseH Plus)的试剂盒。扩增引物为WOX2-F:5’-TTCACGATGGAACCCGACAA-3’,WOX2-R:5’-TTCAGCGCTTGGTGTCCTAA-3’,利用柑橘Actin 作为内参基因(Actin-F:5’-AGAACTATGAACTGCCTGATGGC-3’;Actin-R:5’-GCTTGGAGCAAGTGCTGTGATT-3’)。每个样品设置3 次生物学重复,利用2-ΔΔCt方法进行数据分析。
1.6 数据统计分析
采用WPS 2020 对数据进行整理和绘制图表,采用SPSS 26.0 进行显著性分析,并利用Duncan’s法(p<0.05)进行均值的多重比较,利用Student’s t-test(p<0.01)进行两者之间的比较。
2 结果与分析
2.1 柚WOX2基因的克隆和生物信息学分析
以马家柚叶片总RNA 经反转录后的cDNA 为模板,用特异性引物进行PCR扩增。产物经1.0%琼脂糖凝胶电泳获得长度为1100~1200 bp 的单一条带。将该片段连接到pMD18-T 载体上转化大肠杆菌感受态细胞,获得与前面PCR扩增到的目的片段大小一致的条带(图1)。

图1 CmWOX2 扩增电泳分析
Fig.1 Gel electrophoresis of PCR amplified product of CmWOX2
M.2000 bp DNA Marker;1~7.样品序号。
M.2000 bp DNA Marker;1-7.The sample serial number.
测序结果和生物信息学分析结果表明,CmWOX2 基因(登录号:MG558352)克隆cDNA 序列长度为1177 bp,含有750 bp 的完整开放阅读框(open reading frame,ORF),编码249 个氨基酸的蛋白质;蛋白分子式为C1202H1859N345O383S14,分子质量为27.719 9 ku;含量最高的氨基酸是谷氨酸Gly(8.8%),含量最低的氨基酸是色氨酸Trp(0.8%),其中包含25 个酸性氨基酸(Asp+Glu)和23 个碱性氨基酸(Arg+Lys)。预测的等电点为6.30,不稳定指数为58.73,脂溶指数为55.26,总平均亲水性(GRAVY)为-0.770,推测其属于不稳定亲水性蛋白。通过CFSSP 在线预测CmWOX2 基因编码蛋白的二级结构(图2),结果表明,该蛋白含50.6%螺旋结构,60.2%折叠,16.1%环状结构。通过ExPASY 在线推测CmWOX2 基因编码蛋白的三维结构模型,结果(图3)表明,该蛋白大部分为螺旋结构和折叠结构,环状结构较少,预测得到的二、三级结构相似,这说明CmWOX2 基因编码蛋白的预测结果是合理可信的。
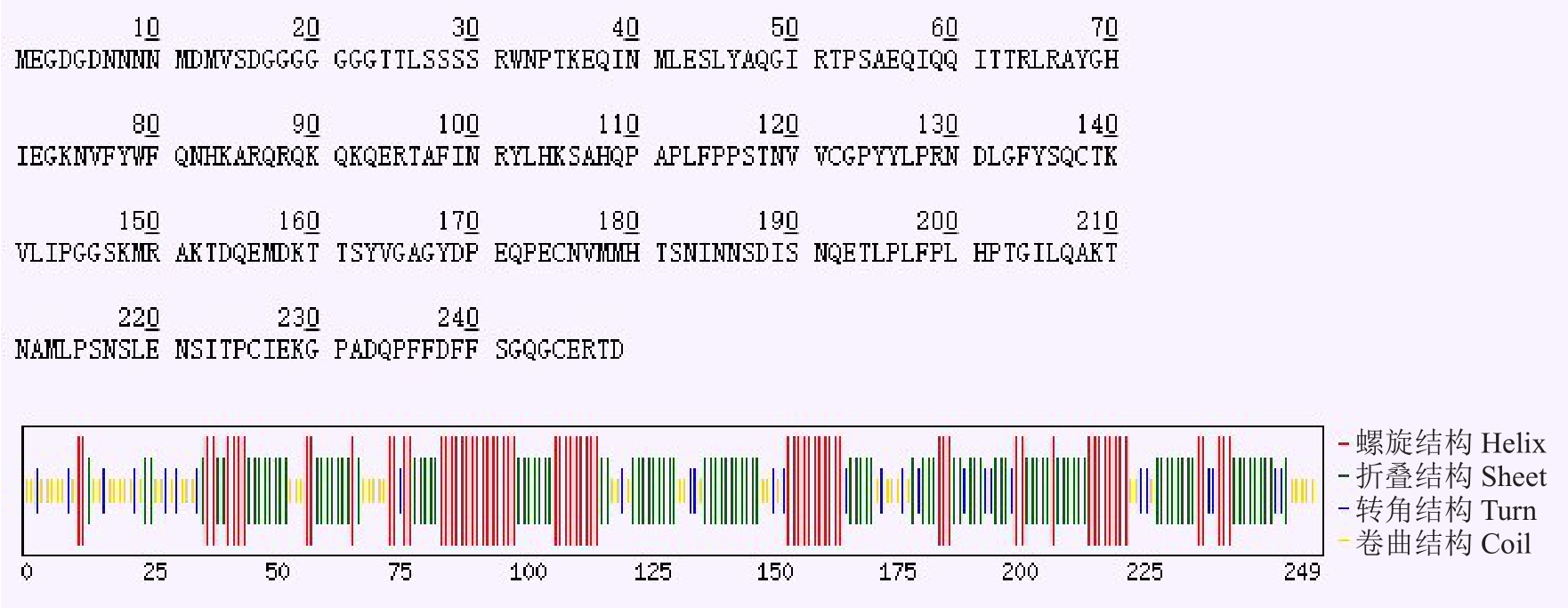
图2 CmWOX2 基因编码蛋白二级结构预测
Fig.2 Secondary structure prediction of CmWOX2 protein
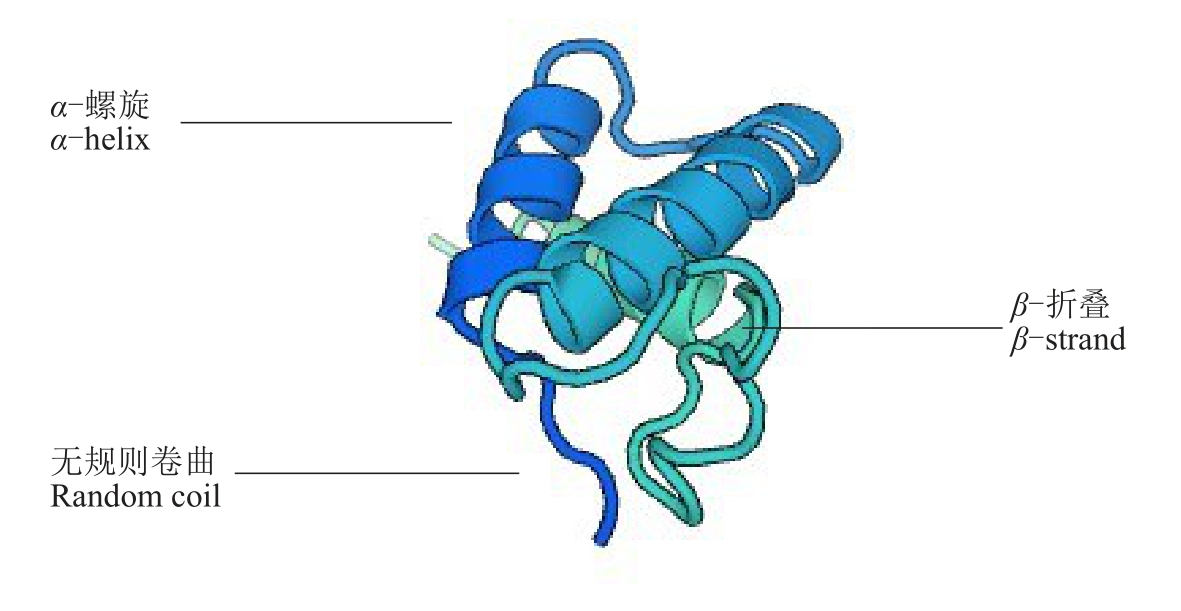
图3 CmWOX2 基因编码蛋白三维结构预测模型
Fig.3 Predicted 3D structure model of CmWOX2 protein
将柚CmWOX2基因与甜橙CsWOX2基因、葡萄VvWOX2 基因、核桃JrWOX2 基因、橡胶树HbWOX2基因、木薯MeWOX2基因、马铃薯StWOX2基因和拟南芥AtWOX2基因编码的氨基酸序列进行多序列比对分析,结果(图4)发现,8 种植物的WOX2 基因编码的氨基酸在5’homeodomain 区域高度保守,而在3’WUS-box区域CmWOX2基因、CsWOX2基因、Hb-WOX2 基因、MeWOX2 基因和AtWOX2 基因编码的氨基酸一致,但与VvWOX2基因、JrWOX2基因和St-WOX2基因编码的氨基酸存在差异。

图4 不同物种WOX2 基因编码的氨基酸序列多重比对
Fig.4 Multiple sequence alignment of WOX2 gene amino acid sequences from different species
CmWOX2.柚Citrus maxima MG 558352;CsWOX2.甜橙Citrus sinensis XP_006488662.1;VvWOX2.葡萄Vitis vinifera XP_002281161.1;Jr-WOX2.核桃Juglans regia XP_018835991.1;HbWOX2.橡胶树Hevea brasiliensis XP_021636679.1;MeWOX2.木薯Manihot esculenta XP_021611167.1;StWOX2.马铃薯Solanum tuberosum XP_006350912.1;AtWOX2.拟南芥Arabidopsis thaliana AAP37131.1。
对CmWOX2 基因编码的蛋白序列的跨膜结构域进行预测,结果表明该蛋白无跨膜区(图5)。CmWOX2 基因编码的蛋白信号肽利用SignalP 4.0 Server 在线工具进行预测,结果表明,柚CmWOX2基因编码的蛋白不含信号肽,预测其含有信号肽的概率为0.101(图6)。这表明CmWOX2 基因编码蛋白不参与细胞信号转导过程。

图5 CmWOX2 基因编码的蛋白跨膜结构域预测
Fig.5 Transmembrane domain of CmWOX2 predicted by TMHMM
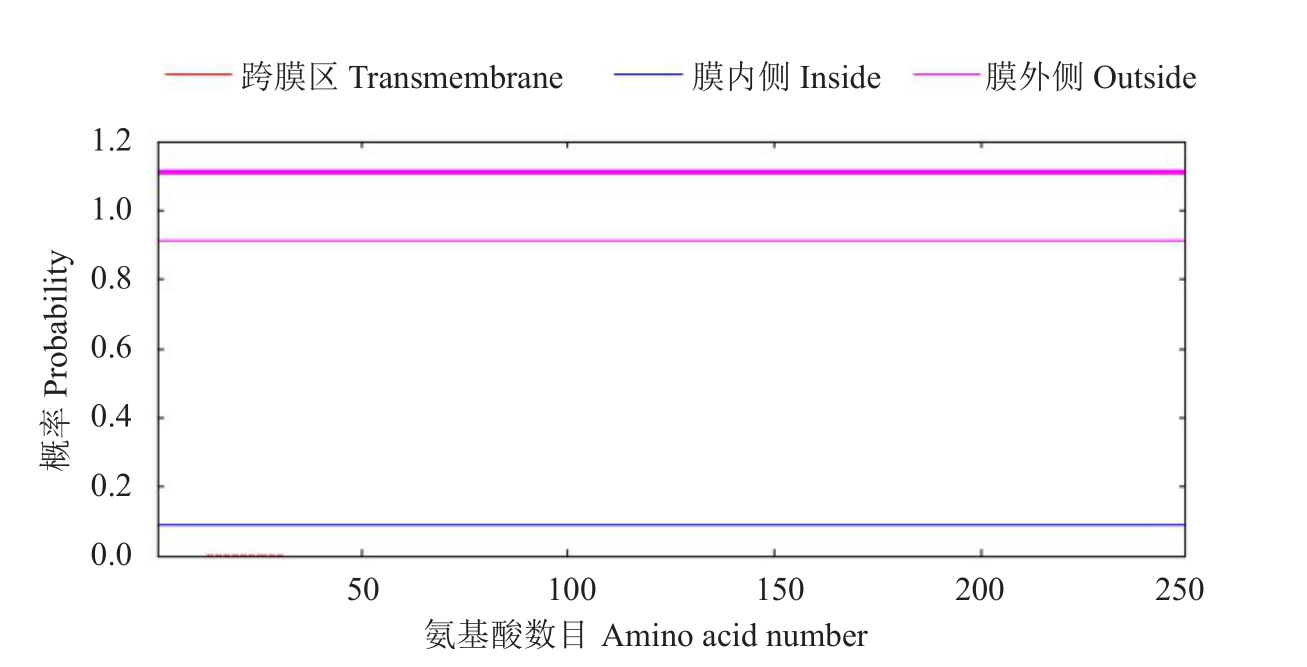
图6 柚CmWOX2 基因编码的蛋白信号肽预测
Fig.6 Prediction signal peptide of CmWOX2 by signalP
为了进一步分析CmWOX2 基因编码蛋白的进化关系,利用MEGA 5.0 对CmWOX2 基因编码的蛋白与其他20 种植物分别构建邻接法NJ(neighbor joining)系统发育树。结果(图7)表明,柚CmWOX2基因与甜橙(Citrus sinensis)CsWOX2基因编码的蛋白遗传距离最近,与荷花(Nelumwbo nucifera)NnWOX2 基因编码蛋白的遗传距离较近,与大豆(Glycine soja)GsWOX2基因编码蛋白的遗传距离较远,与醉蝶花(Tarenaya hassleriana)ThWOX2基因编码蛋白的遗传距离最远。
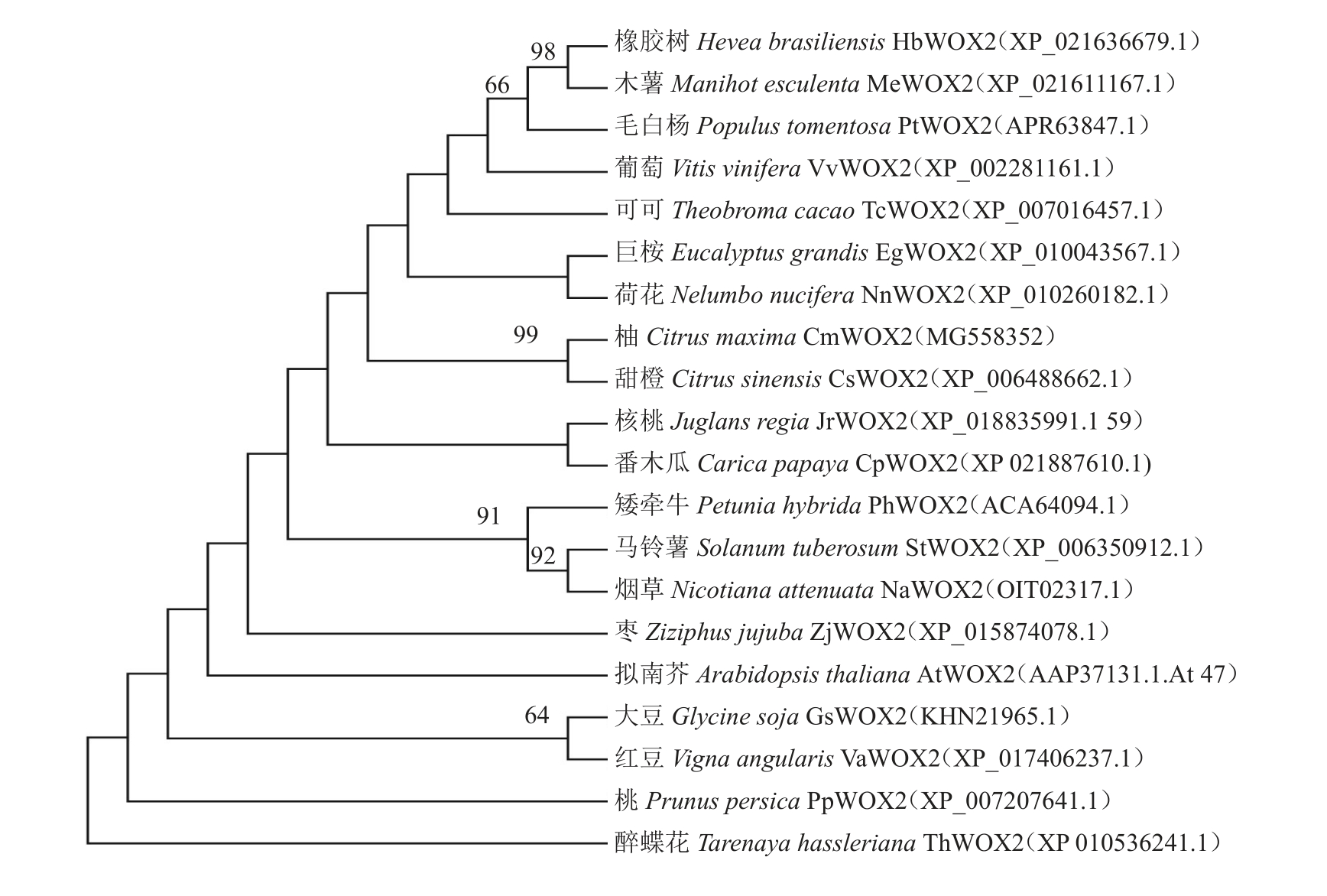
图7 CmWOX2 基因与其他植物WOX2 基因编码的蛋白系统进化关系
Fig.7 Phylogenetic relationship among CmWOX2 and WOX2 proteins of other species
2.2 CmWOX2基因的时空表达分析
马家柚中CmWOX2基因时空表达分析结果(图8)表明,该基因在种子和幼苗根、茎、叶中均有表达,在种子中的相对表达量最高,其次为根和茎,在叶中相对表达量最低(图8-A);CmWOX2 基因在未受精的子房中也出现表达,受精后在马家柚胚的发育过程中相对表达量开始增加,在10 周达到最大值,之后随种子成熟逐渐下降(图8-B);1000 Gy辐射处理花粉果实中的种子在停止发育前一直呈上升趋势,而在此期间对照种子中的CmWOX2 基因相对表达量一直都高于辐射处理,并在授粉7 周和10周时呈极显著差异。
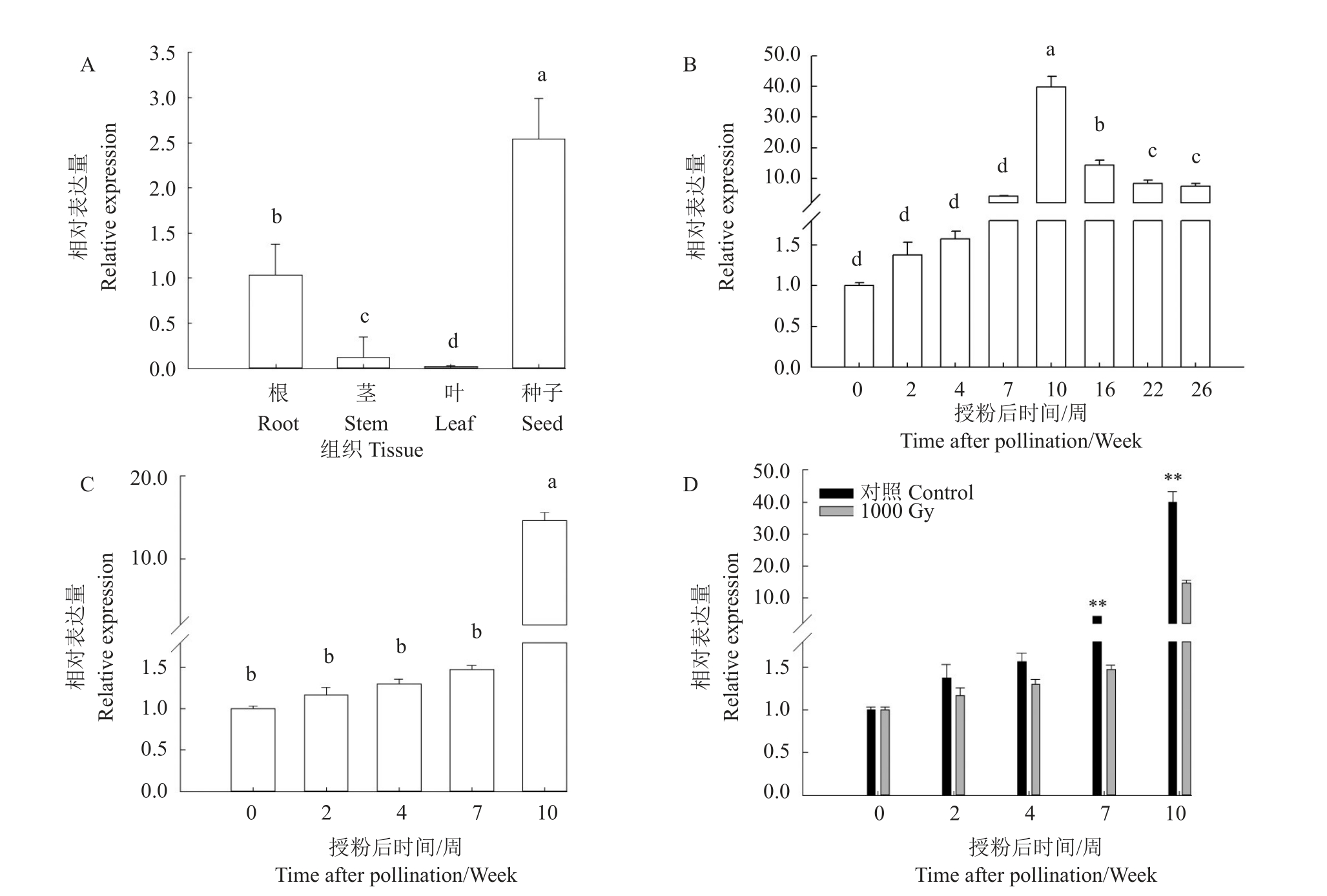
图8 CmWOX2 基因在不同组织和发育期中的相对表达量
Fig.8 Relative expression level of CmWOX2 in different tissues and developmental stages
A.幼苗时期CmWOX2在不同组织中的表达;B.在对照(CK)种子发育过程中的表达;C.在辐射(1000 Gy)种子发育过程中的表达;D.CK和1000 Gy种子发育过程中相对表达量比较。误差条表示同一遗传背景下3个生物复制的标准误差。**表示基因表达水平的统计显著变化,使用Student’t检验确定(p≤0.01)。不同小写字母表示在p<0.05差异显著。
A.Expression of CmWOX2 in different tissues at seedling period;B.Expression in CK seeds during development;C.Expression in 1000 Gy seeds during development;D.Comparison of the expression levels of CK and 1000 Gy during seed development.Error bars indicate standard errors of the three biological replicates in the same genetic background.**.Statistically significant change in gene expression levels,determined using Student’s t-test(p≤0.01).Different small letters indicate significant difference at p<0.05.
3 讨论
WOX2 基因已在多种植物中被研究,在胚胎的形成和发育过程中发挥着重要的作用。笔者从柚中克隆得到了CmWOX2基因,对其进行生物信息学分析,氨基酸序列比对结果表明,CmWOX2 基因编码的氨基酸序列与其他物种具有很高的同源率,与葡萄VvWOX2基因和马铃薯StWOX2基因编码的氨基酸序列的homeobox 区域相似性达93.65%和92.06%,并且CmWOX2基因编码的氨基酸序列具有WOX 家族现代进化支所特有的homeodomain 和WUS-box 这2 个特异结构,说明所克隆的CmWOX2基因是柚WOX家族的成员。同时发现不同物种间WOX2基因编码的氨基酸序列在5’homeodomain区域高度保守,也预示着其具有类似的功能。WOX2基因编码的氨基酸在WOX 家族中属于现代进化支,现代进化支不仅含有HD 结构域,还有一个WUS-box 结构域(一般形式是T-L-X-L-F-P-X-X,X代表任意氨基酸),这是其他进化支没有的[18]。
前人研究结果表明,WOX2 基因在胚胎的形成和发育过程中发挥着重要的作用,并且可以作为标记基因来研究植物胚胎的发育[19]。笔者在本研究中通过荧光实时定量PCR 的方法检测了马家柚中CmWOX2基因的时空表达差异,结果表明CmWOX2基因在种子中的相对表达量最高,其次是根组织,而茎和叶片中相对表达量非常低。WOX2基因在胚胎发育过程中的作用,主要表现在早期。Palovaara等[14]以挪威云杉为试验材料,研究了WOX2 基因在不同时期的表达情况,结果显示WOX2 基因在胚胎发育早期相对表达量较高,至胚胎发育成熟时其表达水平显著降低,此外Park 等[20]以黑松为试验材料也获得了相似的结果。霍胜楠[21]研究了WOX2基因在水稻胚不同发育时期的相对表达量,表明WOX2基因在幼胚中相对表达量较高,随着胚发育成熟,表达水平逐渐降低。通过前期对马家柚种子进行解剖学研究的结果可知,马家柚正常种子与其他柚类相似,在授粉后7~10 周期间,胚经历了球形胚到子叶胚的发育过程,16 周后种子成熟[17,22]。而在本研究中,与前人研究结果一致,CmWOX2 基因在合子中就开始表达,随着胚发育相对表达量逐渐升高,在球形胚到子叶胚发育关键时期达到最大值,之后随种子和胚成熟相对表达量逐渐下降。
授粉辐射花粉获得无核果实的方法已在西瓜[23]、金橘[24]以及土佐文旦柚[25]上进行了相关研究,并获得了无核、少核的果实。在前期研究中通过此方法,也已成功获得了早期胚胎退化的无核马家柚果实,使之成为了研究CmWOX2基因非常适合的材料[24]。通过表达分析发现,无核果实中的败育种子在退化前CmWOX2基因表达水平大致呈上升趋势,但明显低于正常种子,并且在授粉后7~10 周(胚发育关键时期)表现出极显著差异。以上结果都证明CmWOX2 基因在马家柚胚胎的形成发育过程中发挥着至关重要的作用,并且通过花粉辐射获得的无核果实中CmWOX2 基因相对表达量下降与胚发育异常密切相关。
[1] 王鹏凯,施季森,张艳娟,吴霜,陈金慧.植物的胚形态建成及其基因调控机制研究进展[J].南京林业大学学报(自然科学版),2013,37(5):134-138.WANG Pengkai,SHI Jisen,ZHANG Yanjuan,WU Shuang,CHEN Jinhui.The research progress of the pattern formation and gene regulation mechanism in plant embryogenesis[J].Journal of Nanjing Forestry University (Natural Sciences Edition),2013,37(5):134-138.
[2] 蒋丽,齐兴云,龚化勤,刘春明.被子植物胚胎发育的分子调控[J].植物学通报,2007,42(3):389-398.JIANG Li,QI Xingyun,GONG Huaqin,LIU Chunming.Molecular mechanisms of plant embryogenesis[J].Chinese Bulletin of Botany,2007,42(3):389-398.
[3] MUKHERJEE K,BROCCHIERI L,BÜRGLIN T R.A comprehensive classification and evolutionary analysis of plant homeobox genes[J].Molecular Biology and Evolution,2009,26(12):2775-2794.
[4] VAN DER GRAAFF E,LAUX T,RENSING S A.The WUS homeobox-containing(WOX)protein family[J].Genome Biology,2009,10(12):1-9.
[5] HAECKER A,GROSS-HARDT R,GEIGES B,SARKAR A,BREUNINGER H,HERRMANN M,LAUX T.Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana[J].Development,2004,131(3):657-668.
[6] ZHANG Z J,LAUX T.The asymmetric division of the Arabidopsis zygote:From cell polarity to an embryo axis[J].Sexual Plant Reproduction,2011,24(2):161-169.
[7] LAUX T,MAYER K F,BERGER J,JüRGENS G.The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis[J].Development,1996,122(1):87-96.
[8] ROJO E,SHARMA V K,KOVALEVA V,RAIKHEL N V,FLETCHER J C.CLV3 is localized to the extracellular space,where it activates the Arabidopsis CLAVATA stem cell signaling pathway[J].The Plant Cell,2002,14(5):969-977.
[9] OHMORI Y,TANAKA W,KOJIMA M,SAKAKIBARA H,HIRANO H Y. WUSCHEL-RELATED HOMEOBOX4 is involved in meristem maintenance and is negatively regulated by the CLE gene FCP1 in rice[J].The Plant Cell,2013,25(1):229-241.
[10] JI J B,STRABLE J,SHIMIZU R,KOENIG D,SINHA N,SCANLON M J.WOX4 promotes procambial development[J].Plant Physiology,2010,152(3):1346-1356.
[11] ZHAO Y,HU Y F,DAI M Q,HUANG L,ZHOU D X.The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice[J].The Plant Cell,2009,21(3):736-748.
[12] DEVEAUX Y,TOFFANO-NIOCHE C,CLAISSE G,THAREAU V,MORIN H,LAUFS P,MOREAU H,KREIS M,LECHARNY A.Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis[J].BMC Evolutionary Biology,2008,8:291.
[13] BREUNINGER H,RIKIRSCH E,HERMANN M,UEDA M,LAUX T.Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo[J].Developmental Cell,2008,14(6):867-876.
[14] PALOVAARA J,HALLBERG H,STASOLLA C,HAKMAN I.Comparative expression pattern analysis of WUSCHEL-related homeobox 2 (WOX2) and WOX8/9 in developing seeds and somatic embryos of the gymnosperm Picea abies[J].The New Phytologist,2010,188(1):122-135.
[15] NARDMANN J,ZIMMERMANN R,DURANTINI D,KRANZ E,WERR W.WOX gene phylogeny in Poaceae:A comparative approach addressing leaf and embryo development[J].Molecular Biology and Evolution,2007,24(11):2474-2484.
[16] LI M F,WROBEL-MAREK J,HEIDMANN I,CHEN B.Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis[J].Plant Physiology,2021,188(2):1095-1110.
[17] YANG L,LIU D C,HU W,CHUN Y,ZHANG J,LIU Y.Fruit characteristics and seed anatomy of‘Majia’pomelo pollinated with cobalt-60 gamma-ray-irradiated pollen[J].Scientia Horticulturae,2020,267:109335.
[18] KIEFFER M,STERN Y,COOK H,CLERICI E,MAULBETSCH C,DAVIES L B.Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance[J].The Plant Cell,2006,18(3):560-573.
[19] ZHOU X M,GUO Y Y,ZHAO P,SUN M X.Comparative analysis of WUSCHEL-related homeobox genes revealed their parent-of-origin and cell type-specific expression pattern during ear-ly embryogenesis in tobacco[J].Frontiers in Plant Science,2018,9:311.
[20] PARK S Y,KLIMASZEWSKA K,PARK J Y,MANSFIELD S D.Lodgepole pine:The first evidence of seed-based somatic embryogenesis and the expression of embryogenesis marker genes in shoot bud cultures of adult trees[J].Tree Physiology,2010,30(11):1469-1478.
[21] 霍胜楠.水稻胚胎发生相关基因的表达及其功能鉴定[D].泰安:山东农业大学,2008.HUO Shengnan.Isolation and characterization of rice genes involved in embryo development[D].Tai’an:Shandong Agricultural University,2008.
[22] 柴利军.沙田柚自交不亲和分子机理研究[D].武汉:华中农业大学,2012.CHAI Lijun.Molecular mechanism of Shatian pomelo self-incompatibility[D].Wuhan:Huazhong Agricultural University,2012.
[23] 苏永全,王志伟,任凯丽.二倍体西瓜无籽化生产技术[J].甘肃农业科技,2020(11):84-86.SU Yongquan,WANG Zhiwei,REN Kaili.Seedless production technology of diploid watermelon[J].Gansu Agricultural Science and Technology,2020(11):84-86.
[24] 春永强,刘勇,刘德春,郭鑫跃,洪鹏,杨莉.60Co-γ 射线辐照金柑花粉对果实生长发育及内源激素含量的影响[J].核农学报,2018,32(3):424-429.CHUN Yongqiang,LIU Yong,LIU Dechun,GUO Xinyue,HONG Peng,YANG Li.Effect of irradiating Fortunella crassifolia pollen with60Co-γ ray on fruit growth,development and endogenous hormones[J].Journal of Nuclear Agricultural Sciences,2018,32(3):424-429.
[25] OGATA T,TAKEICHI T,MATSUNAGA K,HASEGAWA K,YAMANE S,SUGIYAMA K.Seed abortion of‘Tosa-Buntan’pummelo pollinated with soft-X-irradiated pollens[J].Scientia Horticulturae,2008,116(2):180-185.