葡萄(Vitis Vinifera L.)是一种重要的经济果树。葡萄的着色过程影响最终色泽品质,是消费者选择果品的重要依据,与经济效益直接相关。特别是在南方夏季昼夜温差小的环境下,有色葡萄上色难已成为产业发展的瓶颈问题,因此亟需通过遗传育种进行色泽改良。芽变是一种体细胞突变,可产生与母树不同的性状,是果树育种的重要手段。在众多芽变中,颜色变化是最广泛和最常见的变异[1]。目前已通过芽变选育出许多呈色多样的葡萄新品种[2]。
花青苷(Anthocyanin)是一类广泛存在于植物体内的重要次生代谢物质,属黄酮类化合物,是决定葡萄果实色泽的主要物质,使葡萄呈现出红色、紫红色、紫蓝色至蓝黑色等颜色[3]。同时,研究表明花青苷具有抑制肿瘤细胞生长与转移、改善血糖平衡等作用,可用于预防心脑血管疾病[4-5],具有较高的营养功能和药理功效。
花青苷由花色素和糖基结合而成,具有C6-C3-C6碳骨架结构,由一系列结构基因编码的酶催化合成,同时结构基因的表达受MYB、bHLH、和WD40 这3类转录因子协同调控[6-7]。葡萄的花青苷主要存在于有色品种的果皮细胞液泡中,也有少量红肉品种的果肉中富含花青苷[8]。葡萄花青苷主要包括单糖苷或双糖苷化的花青素(Cyanidin, Cy)、甲基花青素(Peonidin,Pn)、花翠素(Delphinidin,Dp)、甲基花翠素(Petunidin, Pt)、二甲基花翠素(Malvidin, Mv)及其酰化修饰衍生物[3]。花青苷种类和含量受遗传背景、环境条件和栽培手段的影响,其中不同品种的差异主要由遗传因素决定[9-10]。
南太湖特早是由浙江大学主持选育的夏黑芽变新品种[11],于2019 年获得农业农村部非主要农作物品种登记证书[GPD 葡萄(2019)330044]。与母树比较,其转色期早、成熟期早,果实色泽深、香气浓,是颇具推广价值的鲜食葡萄品种,也是进行葡萄色泽相关研究的优良材料。作者课题组前期对南太湖特早的果实生长发育规律和糖酸品质有所研究[12]。Leng 等[13]通过转录组分析,得到与南太湖特早和夏黑的TSS、总花青苷含量差异相关的2 个关键基因。然而,关于南太湖特早具体花青苷组分以及果实发育过程花青苷积累规律的研究未有报道。
笔者在本研究中以南太湖特早为试材,用UPLC-Triple-TOF 5600+飞行时间液质联用仪鉴定成熟果实果皮花青苷种类,以HPLC 技术测定果实发育过程中果皮花青苷的含量变化,并与母本夏黑进行对比,为进一步研究葡萄花青苷形成和调控机制提供依据,为葡萄的色泽遗传育种提供理论基础。
1 材料和方法
1.1 试验材料
试验地位于浙江大学华家池实验教学基地内。供试材料为3 年生南太湖特早和夏黑葡萄,砧木5BB,避雨栽培。采用根域限制模式、T字形整形架式和短梢修剪,南北行向,株行距2.2 m×8.0 m。进行2 次激素处理,分别在花后3 d 和12 d,药剂为50 mg·kg-1 GA3、5 mg·kg-1 CPPU。通过花穗和果穗修剪整理果形,每穗质量500 g、666.7 m2产量1200 kg左右。
1.2 试验方法
1.2.1 取样与基础品质指标测定 2个供试品种的萌芽、开花期基本一致。于2019 年5 月29 日,即花后24 d开始,每7 d取样1次,直至成熟(可溶性固形物含量达17%)。每品种分别采集3串生长良好、大小一致、无病虫害的果穗,低温环境下运回实验室,剪粒并随即取中部果90粒,分为3个生物学重复,测定单果质量、纵横径、可溶性固形物含量(Total soluble solids content,TSS)等基础指标。取样后用液氮速冻,研磨成粉末,-80 ℃冰箱保存。
1.2.2 花青苷的提取 参考Leng 等[13]的方法并加以调整。在避光和低温的环境下,称取0.5 g果皮冻干粉末,加入3 mL含有1%甲酸的甲醇溶液,充分振荡提取,4 ℃,10 000 r·min-1离心10 min,重复提取3次,合并上清液。使用真空浓缩仪30 ℃浓缩至干,测定前以1 mL 1%甲酸的甲醇溶液复溶,过0.22 μm有机膜。
1.2.3 花青苷的测定条件 采用HPLC高效液相色谱(Waters Crop,美国)测定。色谱柱采用X-bridge C18柱(4.6 mm×150 mm,5 μm)(Agilent,美国);柱温25 ℃;进样量10 μL;流速1.0 mL·min-1;扫描波长为400~600 nm。流动相:(A)1%甲酸的水溶液,(B)1%甲酸的水溶液和乙腈(V∶V=1∶1)。洗脱程序为:0~3 min,75%~70% A;3~13 min,70%~62% A;13~25 min,62%~50%A;25~36 min,50%~27.5%A;36~41 min,27.5%~75%A;41~48 min,维持75%A。
1.2.4 花青苷的定性与定量 取成熟期的样品,采用UPLC-Triple-TOF 5600+飞行时间液质联用仪鉴定花青苷组分。正负离子扫描模式;扫描范围:100~1500 m·z-1;雾化气(GS1):55 psi;雾化气(GS2):55 psi;气帘气(CUR):35 psi;离子源温度(TEM):600 ℃(正)550 ℃(负);离子源电压(IS):5500 V(正)~4500 V(负);一级扫描:去簇电压(DP):100 V;聚焦电压(CE):10 V;二级扫描:使用TOF MS~Product Ion~IDA 模式采集质谱数据,CID 能量为(40±20)eV,进样前,用CDS泵做质量轴校正,使质量轴误差小于2 mg·kg-1。根据保留时间和质谱信息,比对中国农业大学葡萄与葡萄酒中花青苷指纹谱库鉴别花青苷具体组分。
定量:以花青素-3-葡萄糖苷(Cyanidin-3-O-glucoside)为标准物质进行外标定量,计算花青苷组分的 相 对 含 量 。 标 准 曲 线 公 式 为 :y=2×10-8x+0.003 8,相关系数R²=0.998 3,其中y为花青苷质量浓度(mg·mL-1),x为峰面积。
1.3 数据分析
采用Excel 2019 软件进行数据整理,采用IBM SPSS 26软件进行方差分析。
2 结果与分析
2.1 成熟过程果实大小、可溶性固形物含量变化及转色进程
如图1 所示,成熟过程中,2 个品种果实的纵横径变化趋势基本一致,但南太湖特早第1 次膨大期结束早于夏黑。各时期可溶性固形物含量(w,后同)均略高于夏黑,以17%为成熟标准,南太湖特早果实于花后73 d成熟,较夏黑提早7 d。
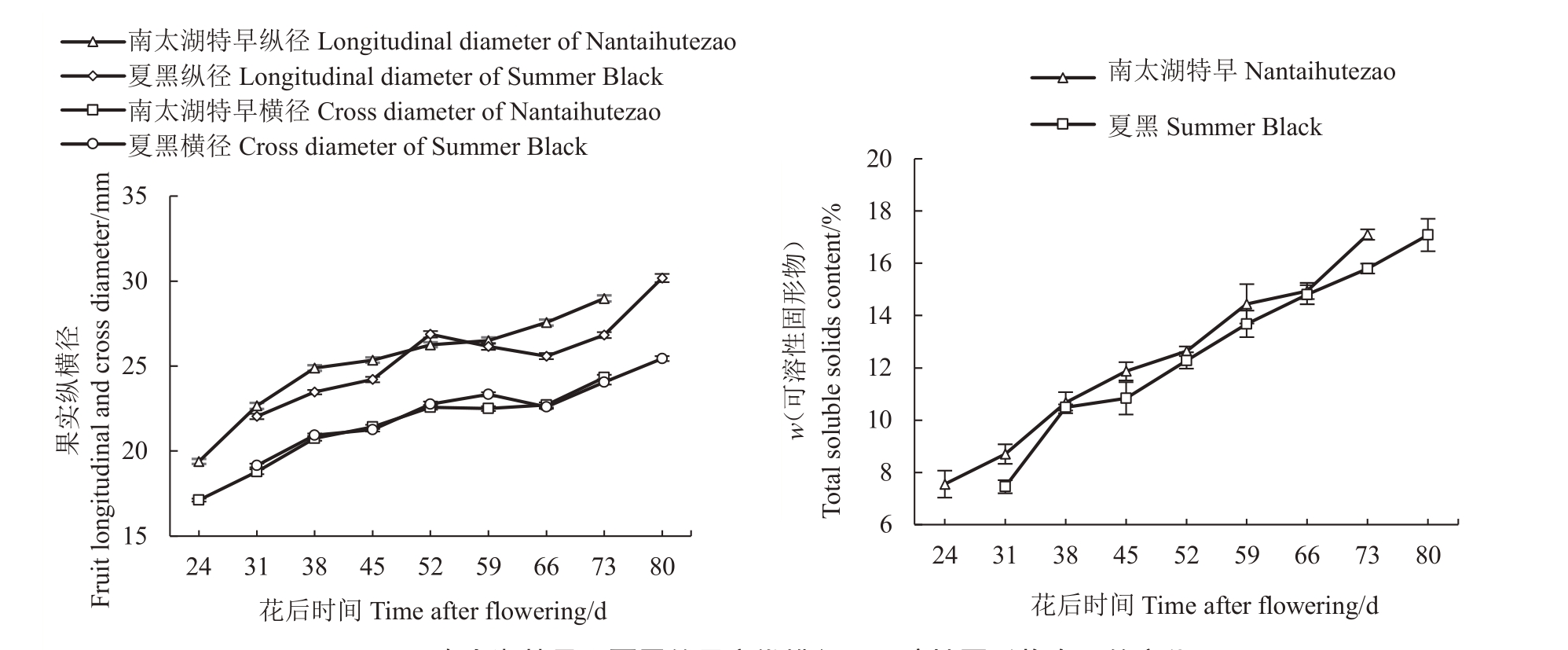
图1 南太湖特早和夏黑的果实纵横径及可溶性固形物含量的变化
Fig.1 Changes in the longitudinal and cross diameter and total soluble solids content of the Nantaihutezao and Summer Black during fruit development
如图2 所示,南太湖特早果穗在花后31 d 局部开始转色,初期上色快,至花后38 d基本全部呈紫红色,73 d果实成熟时呈紫黑色。相比之下,夏黑在花后45 d 才有较明显转色,前期上色较慢,45~66 d 呈偏红色,接近成熟时着紫红色。

图2 南太湖特早(A)和夏黑(B)果实色泽变化
Fig.2 Changes in the skin color of Nantaihutezao(A)and Summer Black(B)
2.2 成熟果实的果皮花青苷种类及含量
由表1可知,2个品种的成熟果实果皮中共检测出15种不同种类的花青苷物质。按照单体分,包括3种花翠素类(Dp)、3种甲基花翠素类(Pt)、6种二甲基花翠素类(Mv)、3 种甲基花青素类(Pn),花青素类(Cy)未检出。按照糖苷数量分,包括8 种单糖苷花青苷,7种双糖苷花青苷。按照酰基化修饰分,包括9种香豆酰化修饰、1种乙酰化修饰以及5种未修饰花青苷。其中11种物质为两品种共有,甲基花青素-3,5-O-双葡萄糖苷(Pn-DG)和二甲基花翠素-3-O-顺式香豆酰葡萄糖苷-5-O-葡萄糖苷(c-Mv-DGCo)只在南太湖特早中检出,二甲基花翠素-3,5-O-双葡萄糖苷(Mv-DG)和二甲基花翠素-3-O-乙酰葡萄糖苷(Mv-G-Ac)只在夏黑中检出。
表1 南太湖特早和夏黑成熟果实的果皮花青苷组分
Table 1 Anthocyanin components in the peel of the ripe Nantaihutezao and Summer Black fruit
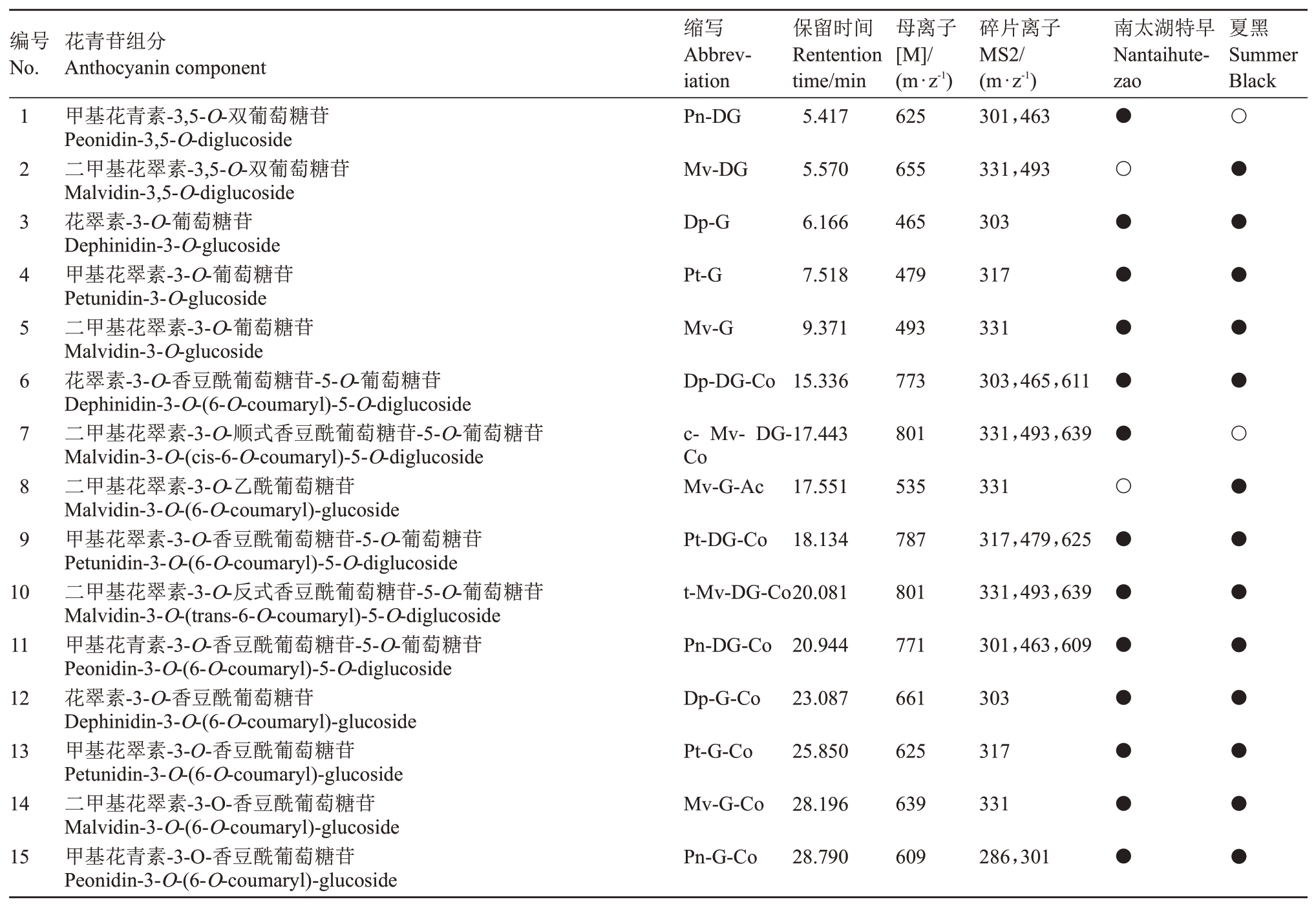
注:“●”表示检测到该物质,“○”表示未检测到该物质。
Note:“●”means the component was detected;“○”means the component was undected.
?
根据不同单体类型、葡萄糖基数量以及修饰类型,比较成熟期南太湖特早和夏黑果实的果皮花青苷含量发现(图3),南太湖特早果皮各类花青苷含量均显著高于夏黑,总含量约为夏黑的1.5倍。二甲基花翠素类(Mv)均为二者最主要的花青苷,分别占总含量的44.9%和50.6%,其次为花翠素类(Dp)和甲基花翠素类(Pt),甲基花青素类(Pn)含量最低;单葡萄糖苷为二者最主要的糖基化类型。2个品种中酰基化、甲基化修饰的花青苷含量均显著高于未酰基化和未甲基化的花青苷。
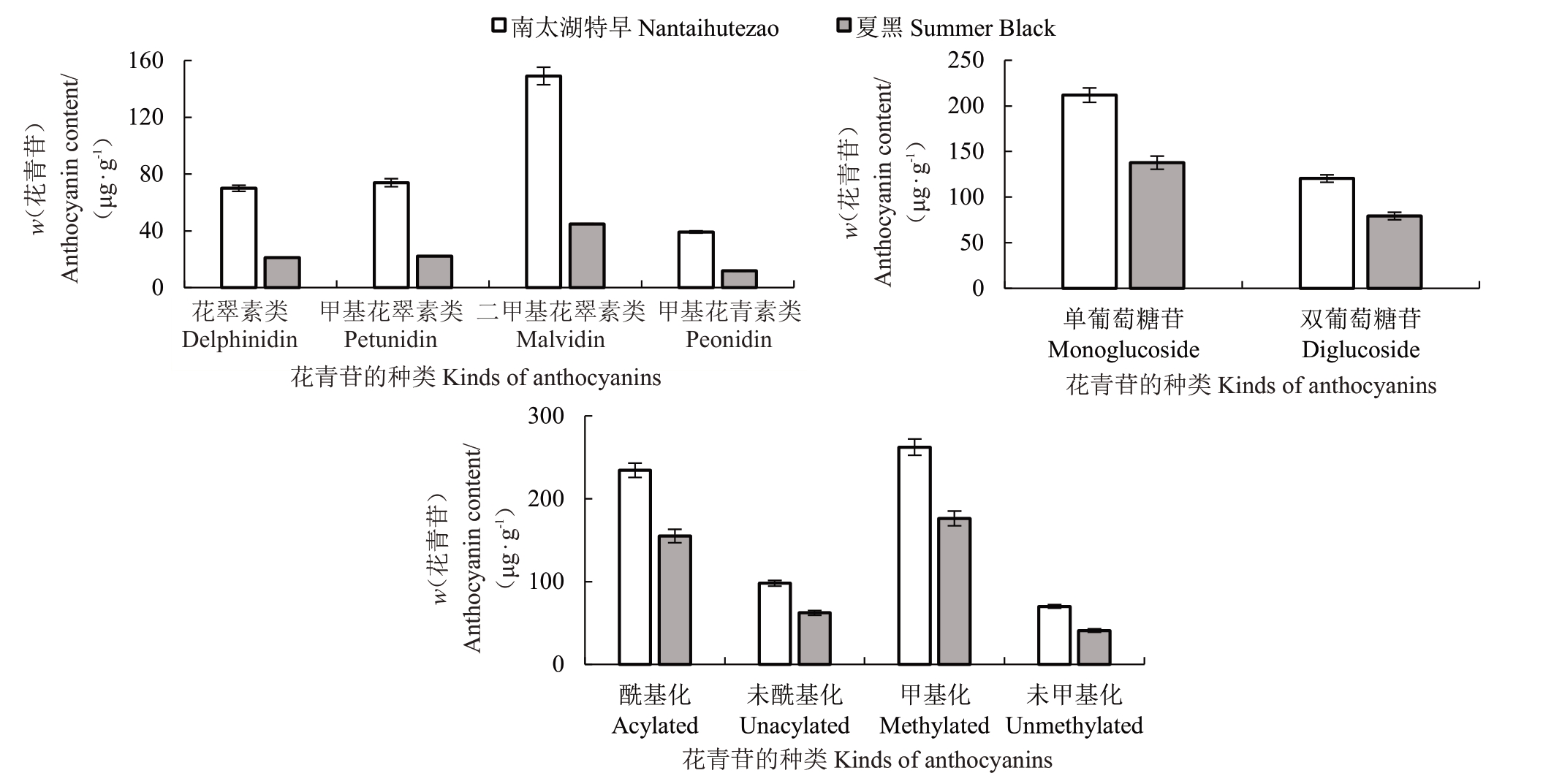
图3 南太湖特早和夏黑成熟果实果皮的各类花青苷含量
Fig.3 The content of anthocyanins in the peel of ripe Nantaihutezao and Summer Black fruit
2.3 不同发育时期果皮花青苷积累规律差异
由图4 可知,南太湖特早的果皮花青苷在花后31 d开始积累,比夏黑提早7 d。通过比较两个品种的各类花青苷积累规律发现,夏黑果皮中4 类花青苷的含量在花后38~52 d 较快积累,除甲基花青素类(Pn)基本不再增加外,另3 类在花后52 d 至成熟继续缓慢积累。相比之下,南太湖特早果皮花翠素类花青苷(Dp)、甲基花翠素类(Pt)、二甲基花翠素类(Mv)的快速积累期持续时间更长,均为花后31~52 d,并在花后52 d 达到与成熟时期相当的含量;甲基花青素类(Pn)物质在花后31~38 d迅速且大量积累,至38 d 时含量为成熟时期的73.2%,随后增速明显放缓,至成熟时达到最高。此外,南太湖特早的果皮花青苷含量在任何时期都显著高于夏黑。
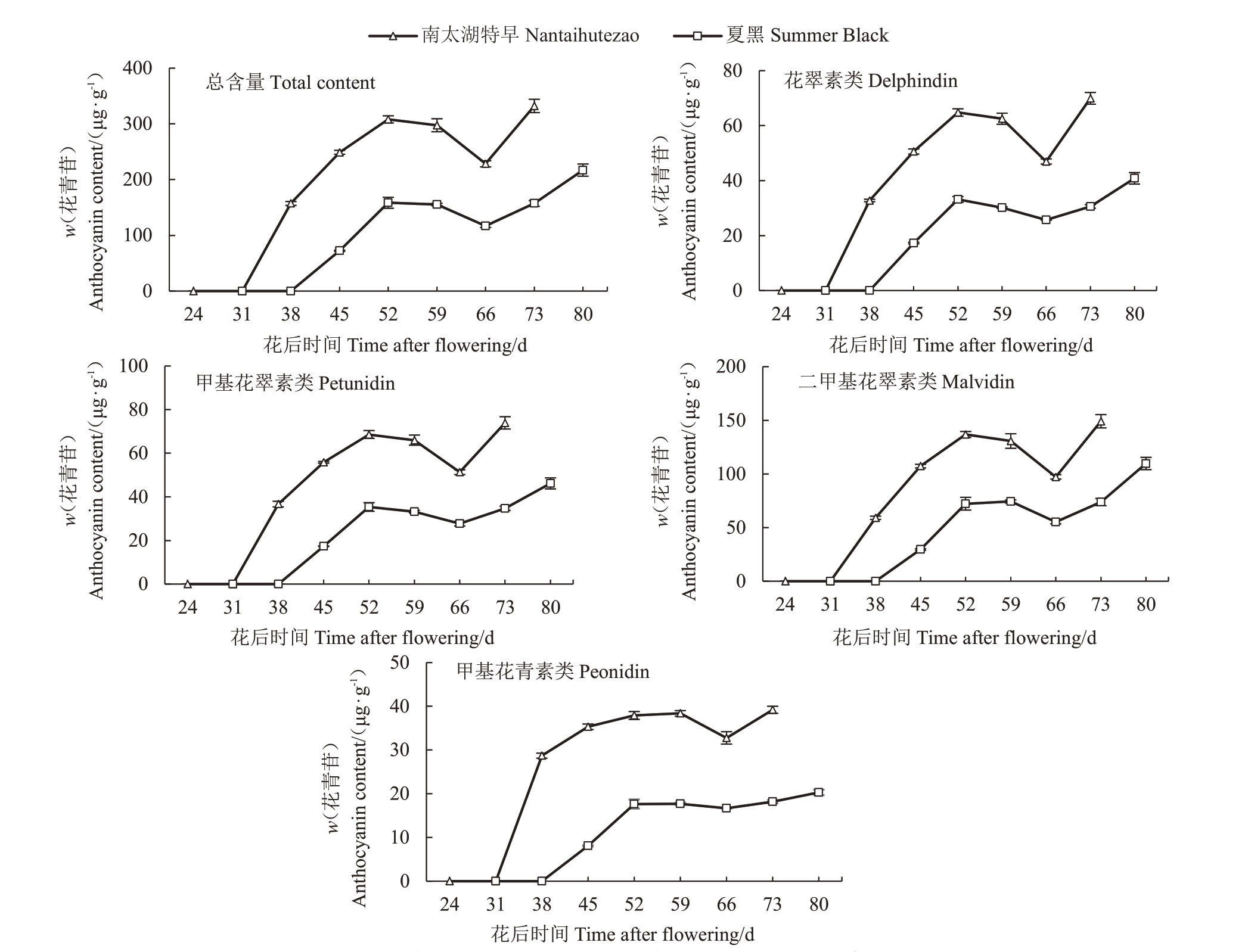
图4 南太湖特早和夏黑发育过程果皮花青苷的积累规律
Fig.4 The accumulation pattern of anthocyanin in the peel of Nantaihutezao and Summer Black during fruit development
2个品种果皮花青苷具体组分的含量变化如表2所示,南太湖特早的11种花青苷在转色初期(花后38 d)起即可全部检测到,积累规律基本均为先迅速积累,随后增速放缓,花后66 d含量有所下降,至成熟前出现第2 次快速积累,成熟期含量最高。相比之下,夏黑的果皮花青苷含量变化规律可分为2类。第1类在转色初期(花后45 d)就能检测到,38~52 d 较快积累,随后速度减缓,包括编号3、4、5、10、12、13、14、15 物质;第2 类转色初期未检测到,52 d开始检出,花后45~52 d间迅速积累,包括编号2、6、8、9、11物质,除仅在夏黑中检出的物质8外,其余均为双葡萄糖苷类花青苷。
表2 南太湖特早和夏黑果实发育过程中花青苷组分的含量变化
Table 2 Changes in the content of anthocyanin components in the peel of Nantaihutezao and Summer Black during fruit development w(/μg·g-1)
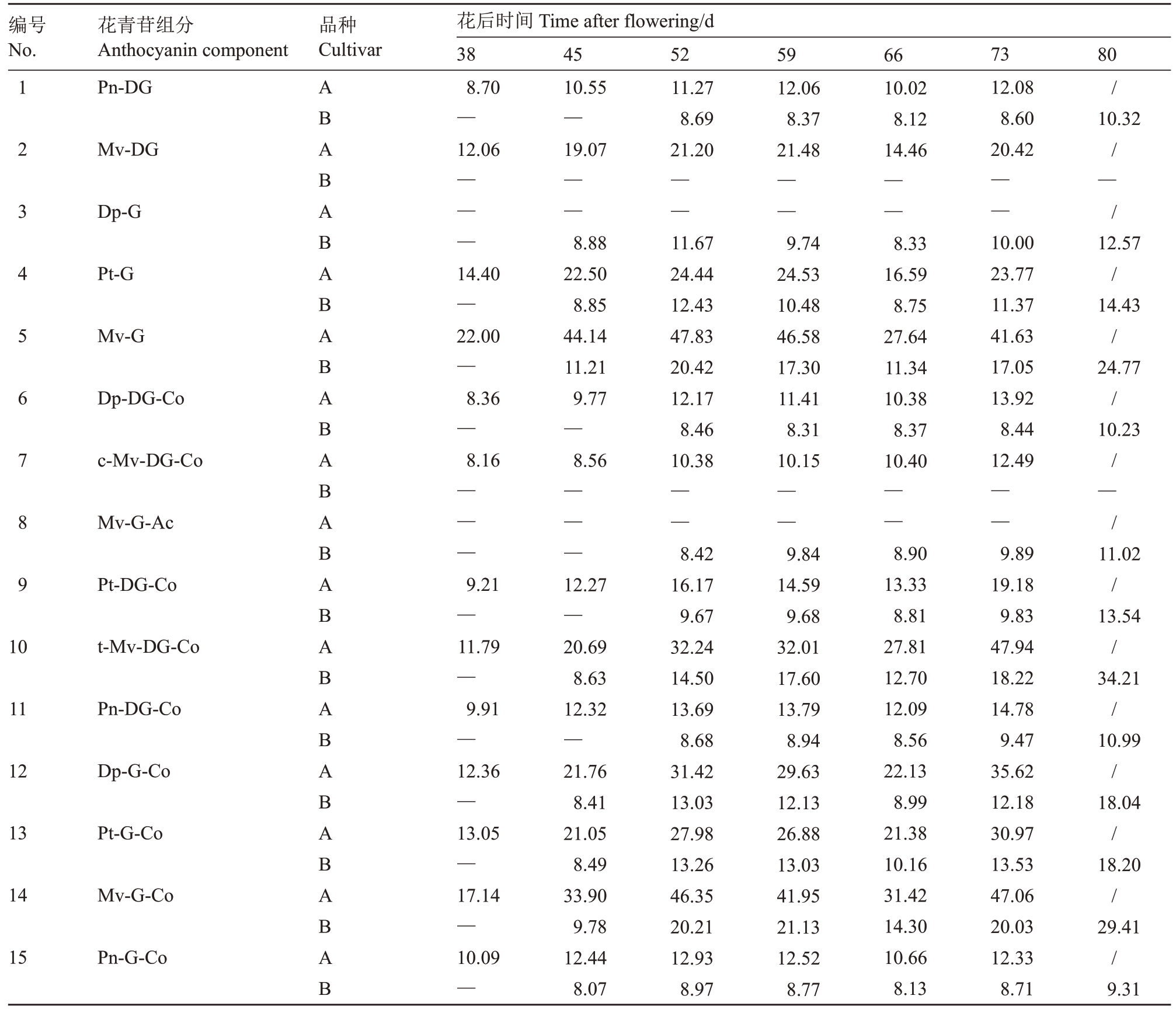
注:A 为南太湖特早;B 为夏黑。“—”表示该物质未检出;“/”表示未测定该时期。
Note:A.Nantaihutezao;B.Summer Black.“—”means the component was undected;“/”means the stage was not determined.
?
3 讨 论
受遗传背景影响,葡萄花青苷种类和基团修饰存在较大差异[9,14-15],因此花青苷组成可作为区分品种的重要依据。Zhang 等[16]发现夏黑及其红肉芽变在一些花青素类物质(Cy)的有无上存在区别,本研究中也发现类似现象。南太湖特早与其母本夏黑在成熟期时果皮花青苷组成基本一致,但Pn-DG、Mv-DG-Co 仅在南太湖特早中检测到,而Mv-DG、Mv-G-Ac仅在夏黑中检测到,表明芽变导致果皮花青苷组成发生变异。鉴于葡萄花青苷合成涉及多个编码关键酶的结构基因,同时其表达量受上游调节基因控制[17-19],推测芽变可能改变了相关基因序列或表达模式。
葡萄果实的颜色由花青苷在果皮和果肉中积累而形成。牛生洋等[20]的研究发现,小白玫瑰的芽变品种小红玫瑰果皮花青苷显著积累,使果实着色。本研究通过比较不同时期的花青苷含量,发现2 个品种在萌芽、初花和盛花期基本一致的情况下,花青苷积累规律存在明显差异,导致着色进程不同。南太湖特早于花后31 d 开始转色,比夏黑提早7 d,与二者花青苷开始合成的时期一致,表明芽变导致花青苷合成提早启动。此外,南太湖特早转色进程快于夏黑,且花后52 d的呈色与成熟期接近,与其主要花青苷类型在花后31~52 d 快速积累、至52 d 含量基本达到峰值相对应。推测这可能与南太湖特早糖类物质积累多有关,为花青苷合成提供了充足底物。相比之下,由于夏黑花青苷快速积累期较短、积累慢、含量少,导致转色缓慢。从具体组分来看,这可能与Pn-DG、Dp-DG-Co、Mv-G-Ac、Pt-DG-Co、Pn-DG-Co延迟出现有关。
葡萄受花青苷的种类和比例的影响呈现不同的颜色。花青素类花青苷衍生物(包括花青素类、甲基花青素类)含量越高,果实红色至紫红色色调越强,花翠素类花青苷衍生物(包括花翠素类、甲基花翠素类、二甲基花翠素类)含量越高,果实的蓝紫色调越强[21-22]。本试验中,南太湖特早果皮甲基花青素类(Pn)在花后31~38 d 迅速且大量积累,但花翠素类(Dp,Pt,Mv)的快速积累期自花后31 d持续至52 d,含量持续升高,解释了转色初期呈红色调,之后颜色迅速加深,到73 d 成熟时呈现紫黑色。同时也表明花翠素类,尤其是含量最高的二甲基花翠素类衍生物,是影响最终果实呈色的关键物质。
近年来,多项研究揭示了芽变导致葡萄花青苷含量改变的分子机制。与Benitaka 相比,早熟芽变品种Early-ripening Benitaka中UFGT基因的表达水平更高[23];VvMYBA1的SNP突变是引起该品种另一芽变Brazil 色泽更深的关键原因[24]。Leng 等[13]通过转录组分析发现4-coumarate-CoA ligase(VIT_02s0109g00250)和 copper amine oxidase(VIT_17s0000g09100)可能是导致南太湖特早和夏黑总花青苷积累差异的关键因子。基于此,本试验结果暗示了芽变可改变关键基因在不同发育时期的表达水平,需要进一步探究。
值得注意的是,葡萄着色过程还受光照条件的影响[25]。由于近年来浙江地区光照强度减弱、雨水多,夏黑果实上色慢、呈色浅的问题较为突出。然而在此条件下,南太湖特早果实转色进程快、成熟期色泽深,是该芽变品种的重要生物学性状,说明芽变可能增强了葡萄对光信号的响应[26],使花青苷合成通路被激活,值得进一步研究。
4 结 论
色泽是葡萄的重要外观品质,花青苷是色泽呈现的物质基础。南太湖特早与其母本夏黑相比,在成熟果实果皮花青苷组成上基本一致,但各存在2种物质单独检出的情况。2个品种均以二甲基花翠素类花青苷衍生物为主,但南太湖特早的果皮花青苷总含量为夏黑的1.5倍,且发育过程的积累规律存在较大差异。果皮花青苷合成启动提早,花翠素类(花翠素、甲基花翠素、二甲基花翠素类衍生物)物质快速积累期长、合成速度快、含量高,使南太湖特早果实转色早、上色快、呈色深,成熟时呈深紫色至紫黑色。
[1] WALKER A R,LEE E,ROBINSON S P. Two new grape cultivars,bud sports of Cabernet Sauvignon bearing pale-coloured berries,are the result of deletion of two regulatory genes of the berry colour locus[J]. Plant Molecular Biology,2006,62(4/5):623-635.
[2] 王浩,刘崇怀,樊秀彩,张颖,孙磊,姜建福,郭大龙.葡萄芽变机制研究进展及应用[J].果树学报,2022,39(3):474-482.WANG Hao,LIU Chonghuai,FAN Xiucai,ZHANG Ying,SUN Lei,JIANG Jianfu,GUO Dalong. Research progress of grape bud mutant mechanism[J].Journal of Fruit Science,2022,39(3):474-482.
[3] FLAMINI R,MATTIVI F,ROSSO M,ARAPITSAS P,BAVARESCO L. Advanced knowledge of three important classes of grape phenolics:anthocyanins,stilbenes and flavonols[J]. International Journal of Molecular Sciences,2013,14(10):19651-19669.
[4] XU M,BOWER K A,WANG S Y,FRANK J A,CHEN G,DING M,WANG S O,SHI X L,KE Z J,LUO J. Cyanidin-3-glucoside inhibits ethanol- induced invasion of breast cancer cells overexpressing ErbB2[J]. Molecular Cancer,2010,9(1):285.
[5] KHOO H E,AZLAN A,TANG S T,LIM S M.Anthocyanidins and anthocyanins:colored pigments as food,pharmaceutical ingredients,and the potential health benefits[J]. Food & Nutrition Research,2017,61(1):90-21.
[6] 刘晓芬,李方,殷学仁,徐昌杰,陈昆松.花青苷生物合成转录调控研究进展[J].园艺学报,2013,40(11):2295-2306.LIU Xiaofen,LI Fang,YIN Xueren,XU Changjie,CHEN Kunsong.Recent advances in the transcriptional regulation of anthocyanin biosynthesis[J].Acta Horticulturae Sinica,2013,40(11):2295-2306.
[7] 曹琳娇,李晓杰,焦棒棒,梁毅,马长生.蔬菜花青苷生物合成及转录调控的研究进展[J].中国瓜菜,2019,32(12):1-7.CAO Linjiao,LI Xiaojie,JIAO Bangbang,LIANG Yi,MA Changsheng.Advances in biosynthesis and transcriptional regulation of anthocyanin in vegetables[J].China Cucurbits and Vegetables,2019,32(12):1-7.
[8] 李惠清,杨航宇,陈为凯,王宇,高晓彤,何非,段长青,王军.不同品种染色葡萄转色期花青苷积累的差异性研究[J].中国酿造,2018,37(9):140-147.LI Huiqing,YANG Hangyu,CHEN Weikai,WANG Yu,GAO Xiaotong,HE Fei,DUAN Changqing,WANG Jun. Difference of anthocyanins accumulation in different varieties of teinturier grapes at veraison[J].China Brewing,2018,37(9):140-147.
[9] 邢婷婷,杨航宇,王雯染,杨晓慧,王军.14 个欧亚种红色酿酒葡萄品种(品系)的花青苷组成和含量分析[J]. 果树学报,2018,35(2):147-157.XING Tingting,YANG Hangyu,WANG Wenran,YANG Xiaohui,WANG Jun. The compositions and contents of anthocyanins of 14 red wine grape varieties or clones (Vitis vinifera)[J].Journal of Fruit Science,2018,35(2):147-157.
[10] 冀晓昊,王海波,张克坤,王孝娣,史祥宾,王宝亮,郑晓翠,王志强,刘凤之.不同颜色果袋对葡萄花青苷合成的调控[J].中国农业科学,2016,49(22):4460-4468.JI Xiaohao,WANG Haibo,ZHANG Kekun,WANG Xiaodi,SHI Xiangbin,WANG Baoliang,ZHENG Xiaocui,WANG Zhiqiang,LIU Fengzhi.The grape anthocyanin biosynthesis regulation by different color fruit bags[J].Scientia Agricultura Sinica,2016,49(22):4460-4468.
[11] 庞钰洁,李海燕,竺啸恒,高福明,殷益明,贾惠娟.“三本提”葡萄芽变“11-06-25”的遗传鉴定[J].浙江大学学报(农业与生命科学版),2017,43(1):73-80.PANG Yujie,LI Haiyan,ZHU Xiaoheng,GAO Fuming,YIN Yiming,JIA Huijuan. Genetic identification of bud sport strain“11-06-25”from“Sanbenti”grape[J]. Journal of Zhejiang University(Agriculture and Life Sciences),2017,43(1):73-80.
[12] 竺啸恒. 葡萄芽变‘11-06-25’的一串鉴定和农艺性状比较[D].杭州:浙江大学,2018.ZHU Xiaoheng.Genetic identification and agronomic traits comparison of grape bud sport strain‘11-06-25’[D]. Hangzhou:Zhejiang University,2018.
[13] LENG F,YE Y L,ZHU X H,ZHANG Y,ZHANG Z Y,SHI J Y,SHEN N,JIA H J,WANG L. Comparative transcriptomic analysis between‘Summer Black’and its bud sport‘Nantaihutezao’during developmental stages[J]. Planta,2021,253(1):12-23.
[14] 刘笑宏,郭淑华,牛彦杰,杜远鹏,翟衡.8 个葡萄品种的花色苷组分及含量分析[J].果树学报,2017,34(4):444-453.LIU Xiaohong,GUO Shuhua,NIU Yanjie,DU Yuanpeng,ZHAI Heng. Analysis of anthocyanin compositions and contents in eight grape cultivars[J]. Journal of Fruit Science,2017,34(4):444-453.
[15] DIMITROVSKA M,BOCEVSKA M,DIMITROVSKI D,MURKOVIC M.Anthocyanin composition of Vranec,Cabernet Sauvignon,Merlot and Pinot Noir grapes as indicator of their varietal differentiation[J]. European Food Research and Technology,2011,232(4):591-600.
[16] ZHANG K K,LIU Z J,GUAN L,ZHENG T,JIU S T,ZHU X D,JIA H F,FANG J G.Chages of anthocyanin components biosynthesis in Summer Black grape berries after the red flesh mutation occurred[J]. Journal of Agricultural and Food Chemistry,2018,66(35):9209-9218.
[17] TANAKA Y,OHMIYA A. Seeing is believing:engineering anthocyanin and carotenoid biosynthetic pathways[J]. Current Opinion in Biotechnology,2008,19(2):190-197.
[18] KOBAYASHI S,GOTO-YAMAMOTO N,HIROCHIKA H.Retrotransposon-induced mutations in grape skin color[J]. Science,2004,304(5673):982.
[19] KOBAYASHI S. Regulation of anthocyanin biosynthesis in grapes[J]. Journal of the Japanese Society for Horticultural Science,2009,78(4):387-393.
[20] 牛生洋,姜建福,樊秀彩,张颖,刘崇怀,王华. 葡萄‘小白玫瑰’及其突变品种‘小红玫瑰’果皮颜色变异机理分析[J].园艺学报,2017,44(2):245-254.NIU Shengyang,JIANG Jianfu,FAN Xiucai,ZHANG Ying,LIU Chonghuai,WANG Hua. Analysis on molecular basis of the color mutation in‘Muscat Rouge’and‘Muscat Blanc’grapes[J].Acta Horticulturae Sinica,2017,44(2):245-254.
[21] MATSUYAMA S,TANZAWA F,KOBAYASHI H,SUZUKI S,TAKATA R,SAITO H. Leaf removal accelerated accumulation of delphinidin-based anthocyanins in‘Muscat Bailey A’[Vitis×labruscana (Bailey) and Vitis vinifera (Muscat Hamburg)] grape skin[J]. Journal of the Japanese Society for Horticultural Science,2014,83(1):17-22.
[22] BUENO J M,SÁEZ- PLAZA P,RAMOS- ESCUDERO F,JIMÉNEZ A M,FETT R,ASUERO A G.Analysis and antioxidant capacity of anthocyanin pigments. Part II:chemical structure,color,and intake of anthocyanins[J]. Critical Reviews in Analytical Chemistry,2012,42(2):126-151.
[23] ZHAO Y,ZHAO X,ZHAO S,HAN N.A novel bud sport from the‘Benitaka’table grape cultivar (Vitis vinifera L.) improves sugar and anthocyanin accumulation at the berry ripening stage[J].South African Journal of Botany,2015,97:111-116.
[24] XU Y S,JIANG N,ZHANG Y G,WANG M,REN J P,TAO J M. A SNP in the promoter region of the VvmybA1 gene is responsible for differences in grape berry color between two related bud sports of grape[J].Plant Growth Regulation,2017,82(3):457-465.
[25] JAAKOLA L. New insights into the regulation of anthocyanin biosynthesis in fruits[J]. Trends in Plant Science,2013,18(9):477-483.
[26] 洪艳,武宇薇,宋想,李梦灵,戴思兰.光照调控园艺作物花青素苷生物合成的分子机制[J].园艺学报,2021,48(10):1983-2000.HONG Yan,WU Yuwei,SONG Xiang,LI Mengling,DAI Silan.Molecular mechanism of light-induced anthocyanin biosynthesis in horticultural crops[J]. Acta Horticulturae Sinica,2021,48(10):1983-2000.