新中国成立七十多年来,我国柑橘产业因低温冻害造成的损失高达上百亿[1-2]。培育柑橘抗寒新品种、了解柑橘抗寒机制成为领域内的热点研究方向[3]。柑橘由于多胚和有性世代周期长,常规育种和分子标记辅助育种培育抗寒品种难度较大[4]。柑橘的商用栽培品种多数通过芽变选种而来,低温冻害后筛选获得柑橘耐寒芽变材料,对培育柑橘耐寒新品种十分珍贵。2018 年12 月至2019 年1 月我国湘北地区遭受了大范围的低温冰冻和辐射霜冻,石门县秀平镇和夹山镇最低温度达到-8.6 ℃和-9.6 ℃,导致柑橘大面积遭受3 级至4 级冻害。冻后恢复救灾时,课题组从石门城郊一个冻害严重的纽荷尔脐橙果园发现1 根疑似耐寒突变体的芽变枝条,其上着生的数条一年生春梢叶片仍为绿色,同一株树上其他枝条及同一果园其他树均枝叶枯死,该材料2019年春嫁接繁殖保存。
植物抗寒性研究通常有田间直接鉴定和实验室间接鉴定[5-6]。实验室间接鉴定主要根据植物叶片结构差异和低温胁迫下细胞膜受损程度、渗透调节物质、过氧化物酶系统等指标判断其抗寒性差异[7-9]。笔者对疑似耐寒突变体的纽荷尔脐橙芽变材料进行DNA遗传鉴定以及叶片和果实性状的鉴定,测定叶片组织结构以及低温胁迫下电解质渗透率、脯氨酸含量、超氧化物歧化酶活性等耐寒相关指标,初步评价该材料是否发生了遗传性状变化,其耐寒能力是否较对照增强,为材料的下一步研究和利用提供必要依据。
1 材料和方法
1.1 试验材料与处理
2019 年春将纽荷尔脐橙疑似耐寒芽变(突变体,图1)的枝条以及对照品种纽荷尔脐橙(野生型)枝条高接于3 年生盆栽冰糖橙树上(基砧为枳),2020 年春从高接树上取突变体材料及对照枝条嫁接于1年生枳容器苗上扩繁群体。盆栽繁殖材料均放置在露地,常规水肥管理和病虫害防治。高接的变异和对照材料于2020年和2021年少量开花结果,进行叶片性状与果实性状观测;枳砧扩繁材料未开花结果,取嫩叶提取DNA 进行SSR 分子标记,取成熟春梢叶片制作石蜡切片观察叶片组织结构,12月取成熟春梢叶片用去离子水洗净晾干后置于人工气候箱(型号LEDR-400)-6 ℃处理0、2、4、6、8、10 h用于电解质渗出率、脯氨酸含量和超氧化物歧化酶活性的测定。

图1 纽荷尔脐橙疑似耐寒突变体
Fig.1 Newhall navel orange putatively cold resistant mutant
1.2 观测指标与方法
采用改良CTAB[10]法提取DNA,SSR分子标记参照李益等[11]的方法;叶片和果实性状观测参考柑橘DUS 测试指南[12],果实品质分析参照GB/T 8210—2011[13];石蜡切片观测叶片解剖结构参照帅焕丽等[14]的方法,将染色步骤改为0.5%甲苯胺蓝染色液稀释100倍浸泡3 min;电解质渗出率的测定参考徐新娟等[15]的方法;脯氨酸含量和超氧化物歧化酶活性的测定参照试剂盒方法(脯氨酸试剂盒、超氧化物歧化酶试剂盒为苏州科铭生物技术有限公司产品)。
2 结果与分析
2.1 纽荷尔脐橙突变体遗传变异性鉴定
纽荷尔脐橙突变体树势中等,树姿开张,刺少,春梢叶片披针形,叶缘波状缘,叶端急尖且有缺刻,其翼叶较为窄,呈楔形,与野生型无明显区别(图2)。突变体和野生型的春梢长度、春梢叶片长度、叶片宽度、翼叶长度、翼叶宽度、叶柄长度等均无显著性差异(表1)。
表1 纽荷尔脐橙突变体和野生型春梢长度和叶片性状观测
Table 1 Observation of spring shoot length and spring-flush leaves of Newhall navel orange mutant and wild type
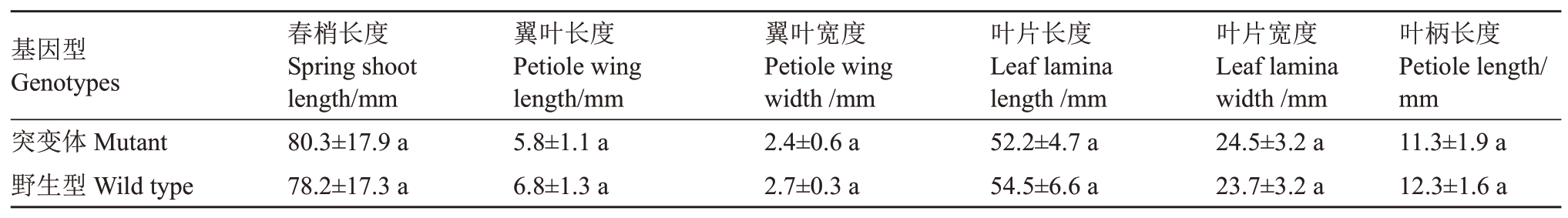
注:表中同一指标不同小写字母表示基因型之间显著性差异为p <0.05 差异水平。
Note:For each index,different lowercase letters show significant differences among genotypes at p <0.05.
?
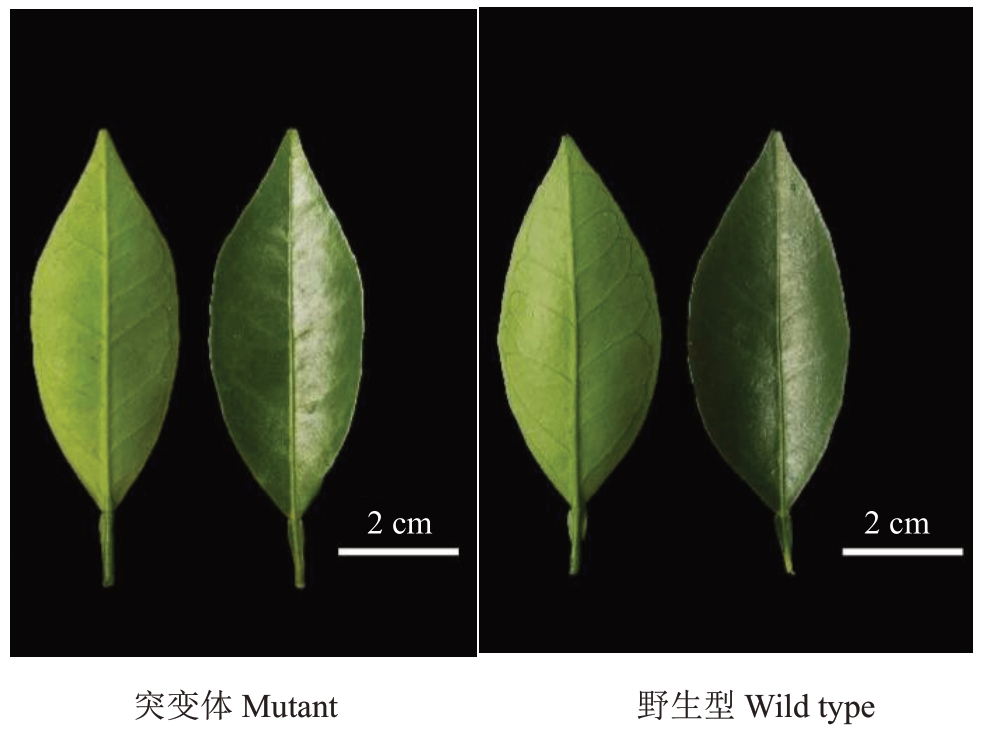
图2 纽荷尔脐橙突变体和野生型叶片比较
Fig.2 Comparison of spring-flush leaves between Newhall navel orange mutant and wild type
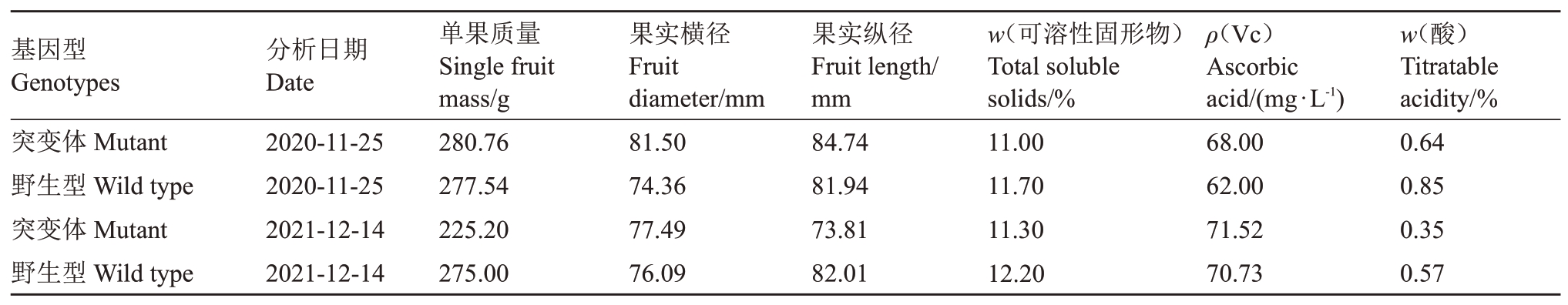
纽荷尔脐橙突变体果实椭圆形,果顶浑圆,基部钝圆,闭脐,果面橙红色,平滑光亮,油胞较稀疏,微凸,与野生型果实性状无异(图3)。连续两年的果实品质比较发现,突变体果实含酸量较野生型偏低(表2)。
表2 纽荷尔脐橙突变体和野生型果实品质比较
Table 2 Comparison of fruit quality of Newhall navel orange between mutant and wild type
?
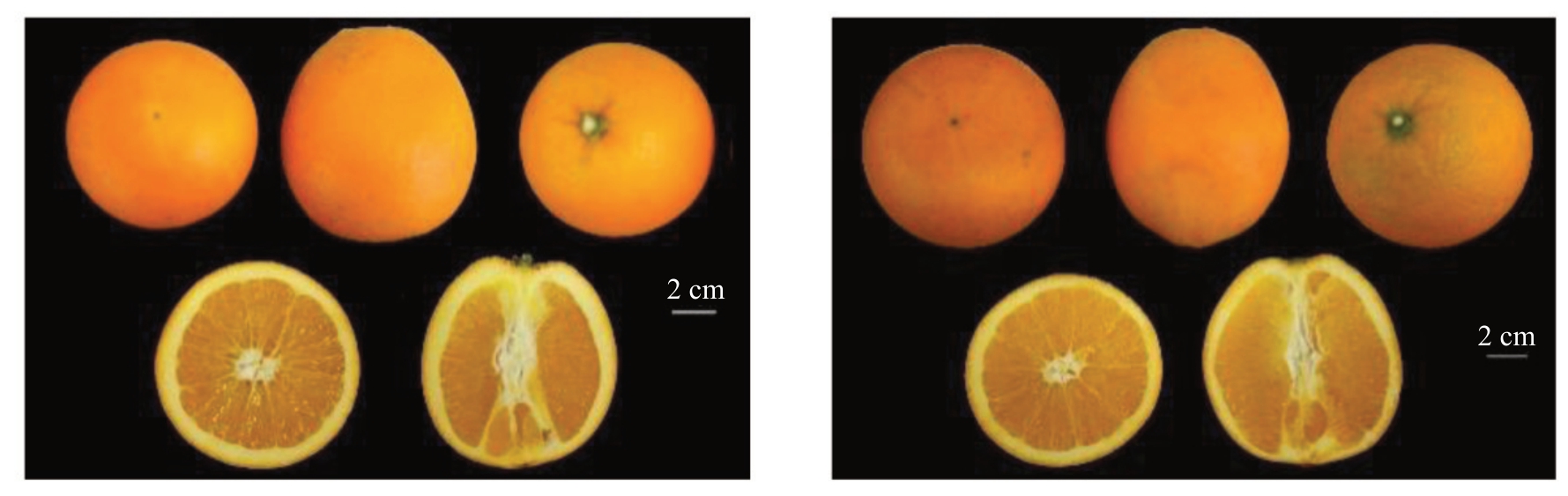
图3 纽荷尔脐橙突变体和野生型果实比较
Fig.3 Comparison of fruit orange between Newhall navel orange mutant and wild type
采用李益等[11]筛选的140 对区分橙类的SSR 引物以纽荷尔脐橙突变体和野生型叶片DNA 为模板进行PCR扩增,产物经SDS-PAGE胶分离,1对引物扩增出差异条带(图4),引物P68(F: GCTGTTTAGGGAAGCAGTCA; R: TATCCACCATCACGGCTGT)扩增的差异带位于100~250 bp。
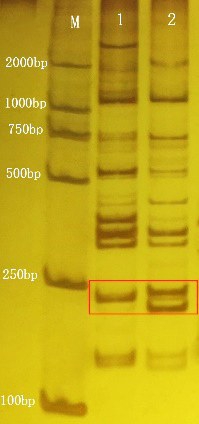
图4 纽荷尔突变体和野生型SSR 分子标记PAGE 凝胶
Fig.4 SSR molecular marker band pattern of Newhall navel orange mutant and wild type on PAGE gel
M.DNA ladder;1.野生型;2.突变体。
M.DNA ladder;Lane 1.Wild type;Lane 2.Mutant.
2.2 纽荷尔突变体抗寒性初步评价
石蜡切片观察发现,纽荷尔脐橙突变体和野生型叶片栅栏组织均为2~3层,多数为2层(图5)。在同一显微镜下以相同放大倍数通过电脑软件测量各组织厚度(表3),表明突变体叶片、栅栏组织、海绵组织等厚度、细胞结构紧密度,栅栏组织与海绵组织的厚度的比值均显著或极显著大于野生型。
表3 纽荷尔脐橙突变体和野生型叶片结构比较
Table 3 Comparison of leaf structure between Newhall navel orange mutant and wild type
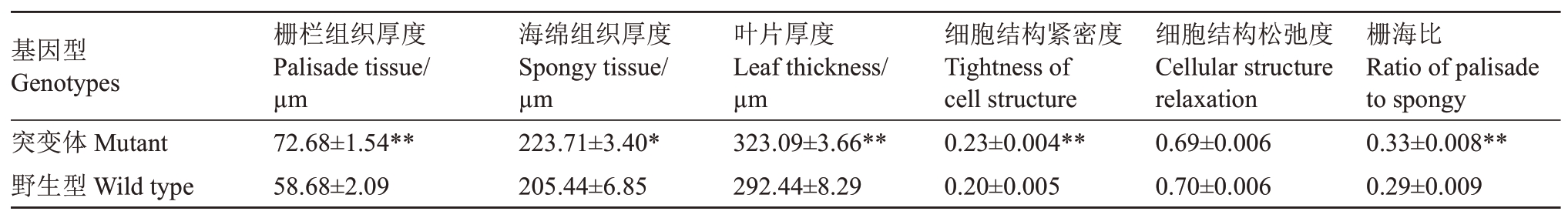
注:细胞结构紧密度为栅栏组织与叶片厚度的比值,细胞结构松弛度为海绵组织与叶片厚度的比值;*表示差异显著(p <0.05),**表示差异极显著(p <0.01)。
Note:Tightness of cell structure is the ratio of palisade tissue to leaf thickness,and Cellular structure relaxation is the ratio of sponge tissue to leaf thickness.*means the significant difference at p <0.05,**means the extremely significant difference at p <0.01.
?
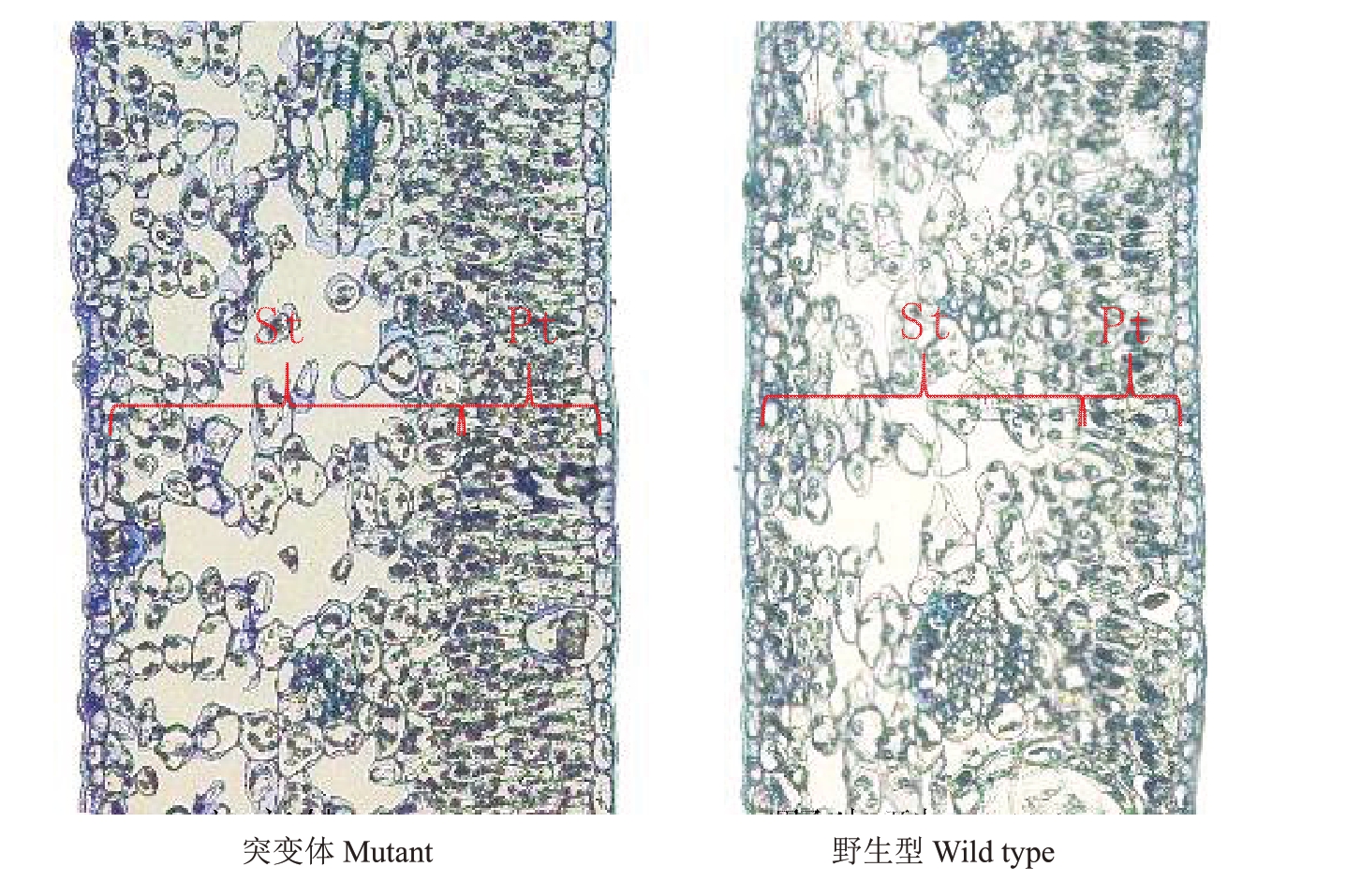
图5 纽荷尔突变体和野生型叶片横切剖面图
Fig.5 Cross-section of Newhall navel orange mutant and wild type paraffin section
Pt.栅栏组织;St.海绵组织。
Pt.Palisade tissue;St.Spongy tissue.
图6表明,纽荷尔脐橙突变体和野生型叶片电解质渗出率随着-6 ℃处理时间的增加均呈“S”形变化,突变体叶片的电解质渗出率在处理过程中始终低于野生型,0~4 h 两者的差异不大,处理6 h 突变体叶片电解质渗出率显著低于野生型,突变体叶片-6 ℃处理8 h 的电解质渗出率相当于野生型叶片-6 ℃处理6 h的水平,即大于50%。
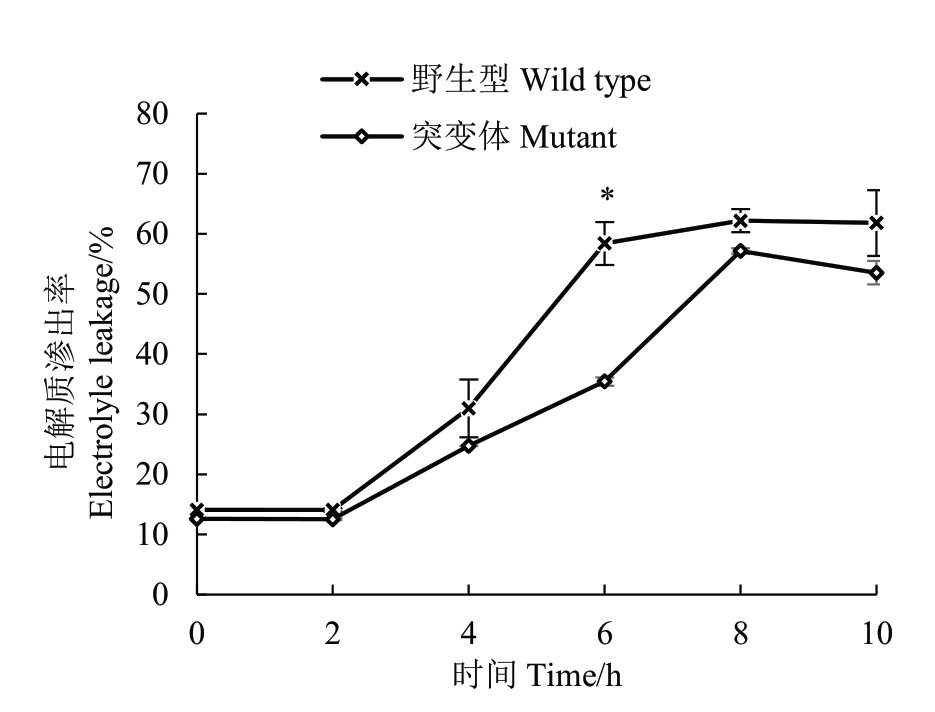
图6 纽荷尔脐橙突变体和野生型叶片-6 ℃不同时间处理下电解质渗出率的变化
Fig.6 Electrolyte changes of Newhall mutant and wild type leaves treated at-6 ℃
纽荷尔脐橙突变体叶片中脯氨酸含量在-6 ℃处理0~10 h时均极显著高于野生型,未低温处理(0 h)时突变体是野生型的1.4倍,低温处理后脯氨酸含量的差异增大,差异最大时前者是后者的2.3倍(图7)。
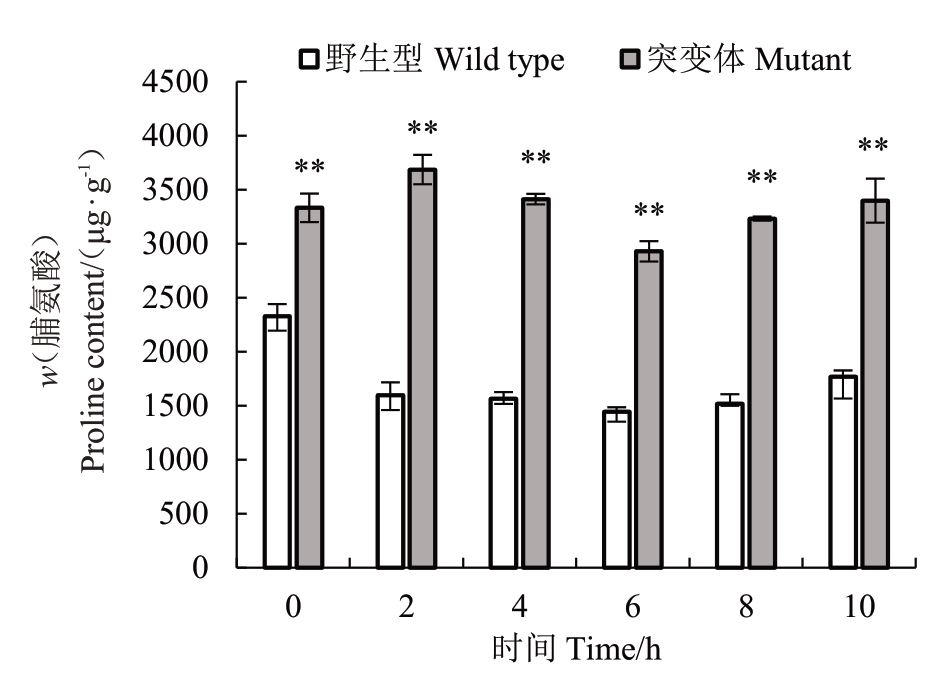
图7 纽荷尔脐橙突变体和野生型叶片--6 ℃不同时间处理下脯氨酸含量的变化
Fig.7 Proline content in leaves of Newhall mutant and wild type treated at-6 ℃
图8中,-6 ℃处理下纽荷尔脐橙突变体叶片中超氧化物歧化酶活性未低温处理(0 h)显著高于野生型,低温处理2~10 h 过程中仍显著或极显著高于野生型,两者的超氧化物歧化酶活性变化趋势基本相同,均在-6 ℃处理0~6 h酶活性呈上升趋势,之后呈下降趋势。
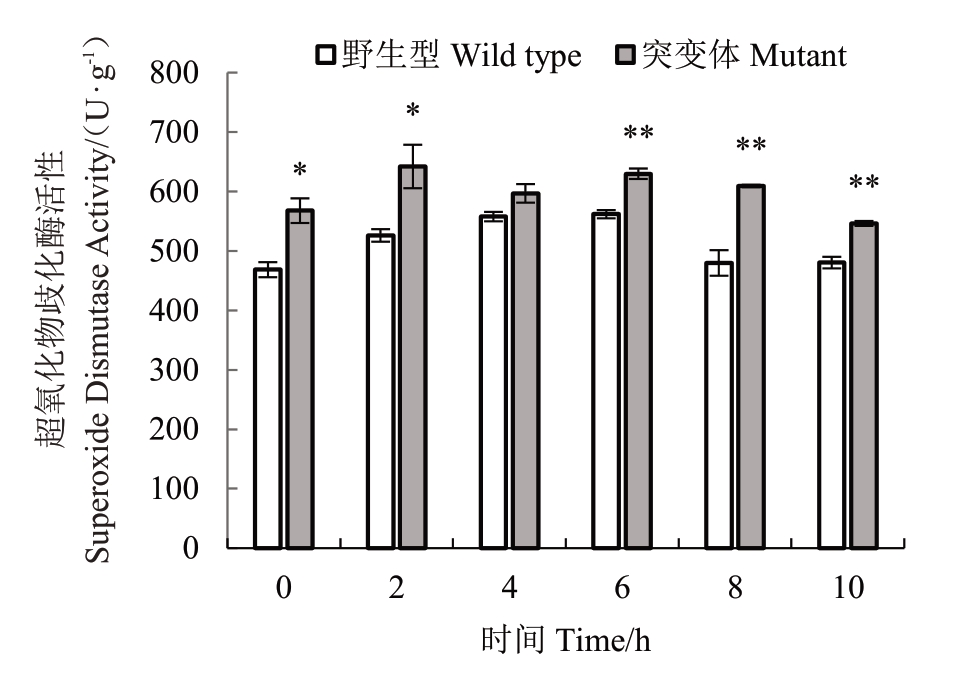
图8 纽荷尔脐橙突变体和野生型叶片-6 ℃不同时间处理超氧化物歧化酶活性的变化
Fig.8 Changes of superoxide dismutase activity in leaves of Newhall mutant and wild type treated at-6 ℃
3 讨 论
纽荷尔脐橙突变体春梢及叶片、果实外观性状等与野生型没有明显差异,果实内在品质由于高接树结果量少,品质数据达不到生物统计分析要求,突变体与野生型的果实内质是否存在明显差异尚不能确定,但2 a重复观测表明纽荷尔突变体果实含酸量有低于野生型的趋势。采用SSR分子标记技术鉴别柑橘芽变材料的遗传变异已有报道,李娜等[16]和周嘉等[17]分别用SSR分子标记技术鉴定了普通冰糖橙芽变品种和南丰蜜橘芽变品种的遗传变异,笔者在本研究中也用该技术证明了纽荷尔脐橙突变体与野生型在DNA分子水平存在差异,是发生了遗传改变的突变体。
叶片的组织结构与其抗寒性密切相关,尤其是组织结构紧密度、栅栏组织与海绵组织的比值受环境条件影响较小,是反映抗寒性的常用指标[18]。吴林等[19]对18种越橘进行抗寒性比较,发现叶片组织结构紧密度、栅海比与抗寒性强弱呈正相关,田间表现抗寒性强的品种其叶片组织结构紧密度大、栅海比值也大,本研究中纽荷尔脐橙突变体叶片组织结构紧密度和栅海比值极显著高于野生型;马翠兰等[20]对耐寒性强弱不同的8个柚类品种研究结果也表明,抗寒性强的品种其叶片电解质渗出率低,本试验中突变体叶片的电解质渗出率未低温处理以及-6 ℃处理2~10 h均低于野生型,-6 ℃处理8 h的电解质渗出率相当于野生型叶片-6 ℃处理6 h的水平;有人对6个石榴品种抗寒性研究发现,叶片中脯氨酸含量高则其抗寒性强[21],柑橘属植物在遭受低温胁迫时脯氨酸含量增加,抗寒性强的品种,脯氨酸含量高[22-23],笔者研究发现,纽荷尔脐橙突变体叶片中脯氨酸含量极显著高于野生型,-6 ℃低温处理使突变体与野生型叶片脯氨酸含量差异增大;超氧化物歧化酶活性的变化与植物的胁迫耐受力有关[24],超氧化物酶活性下降,表示植物细胞酶系统遭受破坏,对细胞内自由基清除能力降低,也有研究报道抗寒性强的品种超氧化物歧化酶活性高,抗寒性弱的品种超氧化物歧化酶活性始终保持在较低水平[25-26]。笔者在本研究中发现突变体超氧化物歧化酶活性在未低温处理(0 h)时已显著高于野生型,低温处理2 h 至10 h 过程中仍显著或极显著高于野生型。因而,综合叶片的组织结构、电解质渗出率、脯氨酸含量、超氧化物歧化酶活性等指标可初步推断,纽荷尔脐橙疑似耐寒突变体的抗寒性较野生型有所增强。
4 结 论
纽荷尔脐橙疑似耐寒突变体春梢叶片性状、果实性状等与野生型没有差异,果实含酸量连续2 a低于野生型,SSR分子标记显示DNA水平发生了遗传变异,叶片的组织结构、电解质渗出率、脯氨酸含量、超氧化物歧化酶活性等抗寒性相关指标显示突变体的抗寒性较野生型强。该突变体可能是果实含酸量较低、耐寒性增强、DNA 水平发生了遗传改变的纽荷尔脐橙芽变。
[1] 彭良志.对2008 年柑桔冻害的思考和冻害果园管理建议[J].中国果业信息,2008,25(3):1-3.PENG Liangzhi. Thoughts on citrus freezing injury in 2008 and suggestions on management of freezing injury orchards[J]. China Fruit News,2008,25(3):1-3.
[2] 邓家锐,丁德宽,敖义俊,蒋景龙,余小丽,郑其峰.对北缘柑橘产区冻害的分析与思考[J].果农之友,2020(9):1-4.DENG Jiarui,DING Dekuan,AO Yijun,JIANG Jinglong,YU Xiaoli,ZHENG Qifeng.Analysis and thinking on freezing damage of citrus production areas in the northern margin[J]. Fruit Growers’Friend,2020(9):1-4.
[3] 马文涛,樊卫国.贵州野生柑橘的抗寒性测定和综合评价[J].西北植物学报,2014,34(10):2063-2069.MA Wentao,FAN Weiguo. Determination and comprehensive evaluation on cold-tolerance of wild citrus from guizhou[J].Acta Botanica Boreali-Occidentalia Sinica,2014,34(10):2063-2069.
[4] PARMAR N,SINGH K H,SHARMA D,SINGH L,KUMAR P,NANJUNDAN J,KHAN Y J,CHAUHAN D K,THAKUR A K.Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops:a comprehensive review[J/OL]. 3 Biotech,2017,7:239. DOI:10.1007/s13205-017-0870-y.
[5] STEPONKUS P L. The effects of low temperatures on biological systems[J].Cryobiology,1989,26(1):100-102.
[6] 何利刚,王志静,宋放,吴黎明,蒋迎春,彭家清,黄咏明,刘涛.抗寒柑橘新品种‘龟井2501’[J].园艺学报,2021,48(S2):2813-2814.HE Ligang,WANG Zhijing,SONG Fang,WU Liming,JIANG Yingchun,PENG Jiaqing,HUANG Yongming,LIU Tao.A novel citrus cultivar‘Guijing 2501’with cold tolerance[J].Acta Horticulturae Sinica,2021,48(S2):2813-2814.
[7] 罗鑫辉,刘明月,黄科,刘玉兵,彭淼,王军伟.不同品种番茄幼苗低温适应性评价及光合特性分析[J].中国瓜菜,2021,34(8):49-55.LUO Xinhui,LIU Mingyue,HUANG Ke,LIU Yubing,PENG Miao,WANG Junwei.Evaluation of low temperature adaptability and analysis of photosynthetic characteristics in different tomato varieties[J]. China Cucurbits and Vegetables,2021,34(8):49-55.
[8] 李冰,张敬敬,高秀瑞,史宇凡,潘秀清,武彦荣.低温胁迫下不同基因型西瓜抗寒性综合评价[J].中国瓜菜,2019,32(4):16-19.LI Bing,ZHANG Jingjing,GAO Xiurui,SHI Yufan,PAN Xiuqing,WU Yanrong. Comprehensive evaluation of cold resistance of different watermelon under low temperature stress[J].China Cucurbits and Vegetables,2019,32(4):16-19.
[9] 郭祥泉.用膜透性、SOD 酶活性和膜脂肪酸成分探讨林木抗寒性[J].西南林业大学学报,2012,32(4):6-11.GUO Xiangquan.Cold-resistance assessment of eucalyptus dunnii by SOD enzyme activity,membrane permeability and membrane-fat acid content[J].Journal of Southwest Forestry College,2012,32(4):6-11.
[10] 张文. 抗溃疡病枸橼C05 杂交后代的获得及分子标记鉴定[D]. 长沙:湖南农业大学,2013.ZHANG Wen. The hybrids obtainment of citro C05 resistant to cank disease and the identification by molecular markers[D].Changsha:Hunan Agricultural University,2013.
[11] 李益,马先锋,唐浩,李娜,江东,龙桂友,李大志,牛英,韩瑞玺,邓子牛.柑橘品种鉴定的SSR 标记开发和指纹图谱库构建[J].中国农业科学,2018,51(15):2969-2979.LI Yi,MA Xianfeng,TANG Hao,LI Na,JIANG Dong,LONG Guiyou,LI Dazhi,NIU Ying,HAN Ruixi,DENG Ziniu. SSR markers screening for identification of citrus cultivar and construction of DNA fingerprinting library[J]. Scientia Agricultura Sinica,2018,51(15):2969-2979.
[12] 陈竹生,江东,崔野韩,李喜庆,郭天池,卢志红,沈丽娟,张戈壁.NY/T 2435—2013 植物品种特异性、一致性和稳定性测试指南柑橘[S].北京:中华人民共和国农业部,2013.CHEN Zhusheng,JIANG Dong,CUI Yehan,LI Xiqing,GUO Tianchi,LU Zhihong,SHEN Lijuan,ZHANG Gebi. NY/T 2435—2013 Guidelines for the conduct of tests for distinctness,uniformity and stability:Citrus(Citrus L.)[S]. Beijing:Ministry of Agriculture and Rural Affairs of the people’s Pepublic of China,2013.
[13] 林媚.柑橘鲜果检验方法进展[J].浙江柑橘,2014,31(2):27-29.LIN Mei.Progress in inspection methods of fresh citrus fruits[J].Zhejiang Citrus,2014,31(2):27-29.
[14] 帅焕丽,杨途熙,魏安智,王佳,李晓,张莹.杏花芽石蜡切片方法的改良[J].果树学报,2011,28(3):536-539.SHUAI Huanli,YANG Tuxi,WEI Anzhi,WANG Jia,LI Xiao,ZHANG Ying.A modified technique of paraffin section for apricot flower buds[J]. Journal of Fruit Science,2011,28(3):536-539.
[15] 徐新娟,李勇超.2 种植物相对电导率测定方法比较[J].江苏农业科学,2014,42(7):311-312.XU Xinjuan,LI Yongchao. Comparison of relative conductivity measurement methods of two plants[J].Jiangsu Agricultural Sciences,2014,42(7):311-312.
[16] 李娜,李大志,何建明,龙紫薇,黄婷,龙立长,邓子牛,文跃伟,龙桂友. 柑橘新品种‘黔阳冰糖脐橙’的选育[J]. 果树学报,2020,37(1):140-143.LI Na,LI Dazhi,HE Jianming,LONG Ziwei,HUANG Ting,LONG Lichang,DENG Ziniu,WEN Yuewei,LONG Guiyou.A new citrus cultivar‘Qianyang Bingtang Navel Orange’[J].Journal of Fruit Science,2020,37(1):140-143.
[17] 周嘉,夏绪平,胡双双,夏茜,唐帅,张秋明,邓子牛,李大志.柑橘新品种‘阿香2 号’的选育[J].果树学报,2019,36(4):529-532.ZHOU Jia,XIA Xuping,HU Shuangshuang,XIA Xi,TANG Shuai,ZHANG Qiuming,DENG Ziniu,LI Dazhi. Breeding report of a new tangerine cultivar‘Axiang No.2’[J]. Journal of Fruit Science,2019,36(4):529-532.
[18] 陈雪峰,景晨娟,赵习平,武晓红.植物叶片组织结构在抗逆研究中的应用进展[J].河北农业科学,2018,22(3):50-53.CHEN Xuefeng,JING Chenjuan,ZHAO Xiping,WU Xiaohong. Advances in application of plant leaf tissue structure in the research of stress tolerance[J]. Journal of Hebei Agricultural Sciences,2018,22(3):50-53.
[19] 吴林,刘海广,刘雅娟,林东慧,李亚东.越橘叶片组织结构及其与抗寒性的关系[J].吉林农业大学学报,2005,27(1):48-50.WU Lin,LIU Haiguang,LIU Yajuan,LIN Donghui,LI Yadong.Studies on leaf tissue structure and its relations to cold resistance of blueberry[J]. Journal of Jilin Agricultural University,2005,27(1):48-50.
[20] 马翠兰,刘星辉,胡又厘. 柚品种间的耐寒性差异及其机理[J].福建农业大学学报,1998,27(2):160-165.MA Cuilan,LIU Xinghui,HU Youli. Differences of cold tolerance of pomelo cultivars and their mechanisms[J].Journal of Fujian Agricultural University,1998,27(2):160-165.
[21] SOLOKLUI A A G,ERSHADI A,FALLAHI E. Evaluation of cold hardiness in seven Iranian commercial pomegranate (Punica granatum L.) cultivars[J]. Hortscience,2012,47(12):1821-1825.
[22] KUSHAD M M,YELENOSKY G.Evaluation of polyamine and proline levels during low temperature acclimation of citrus[J].Plant Physiology,1987,84(3):692-695.
[23] YELENOSKY G. Accumulation of free proline in citrus leaves during cold hardening of young trees in controlled temperature regimes[J].Plant Physiology,1979,64(3):425-427.
[24] 徐燕,薛立,屈明. 植物抗寒性的生理生态学机制研究进展[J].林业科学,2007,43(4):88-94.XU Yan,XUE Li,QU Ming.Physiological and ecological mechanisms of plant adaptation to low temperature[J].Scientia Silvae Sinicae,2007,43(4):88-94.
[25] 冯昌军,罗新义,沙伟,王凤国. 低温胁迫对苜蓿品种幼苗SOD、POD 活性和脯氨酸含量的影响[J].草业科学,2005,22(6):29-32.FENG Changjun,LUO Xinyi,SHA Wei,WANG Fengguo. Effect of low temperature stress on SOD,POD activity and proline content of alfalfa[J].Pratacultural Science,2005,22(6):29-32.
[26] 刘兴禄,王红平,孙文泰,董铁,牛军强,马明.5 个砧木苹果枝条的抗寒性评价[J].果树学报,2021,38(8):1264-1274.LIU Xinglu,WANG Hongping,SUN Wentai,DONG Tie,NIU Junqiang,MA Ming. Cold resistance evaluation of the shoots of 5 apple rootstocks[J]. Journal of Fruit Science,2021,38(8):1264-1274.