钙在植物生长发育过程中发挥重要作用,是植物生长发育的必需营养元素之一[1],主要行使营养功能和信使功能[2]。钙在植物中作为单一营养元素维持植物细胞壁和细胞膜结构完整性,同时通过膜内外钙离子流动维持胞内环境的稳定,增强植物的抗性[3-4],从20 世纪80 年代开始,钙被作为“第二信使”的研究越来越广泛,主要参与植物激素调控过程和光调节过程[5-6]。Ca2+行使第二信使功能时通常与CaM 结合直接或间接调控植物体内生理生化过程[7-8]。植株缺钙使其顶芽、侧芽和根尖等分生组织发生卷曲变形,叶缘开始变黄并逐渐坏死[9],缺钙导致多种生理病害,如辣椒、番茄脐腐病[10-11]及苹果苦痘病[12]。外源喷施200 mmol·L-1纳米碳酸钙,增加芍药茎中的钙含量,增强茎秆强度[13];喷施150 mg·L-1 CaCl2,可使小白菜的产量与品质显著提高[14];向烟草叶片喷施20 mmol·L-1 CaCl2可有效提高超氧化物歧化酶(SOD)、抗坏血酸过氧化物酶(APX)、过氧化氢酶(CAT)等抗氧化酶活性[15];培养液中添加外源钙离子可显著降低水稻中茉莉酸含量,延缓叶片衰老[16];在黄瓜水培营养液中外源施加2 mmol·L-1的CaCl2可显著促进植株的净光合速率(Pn)、蒸腾速率(Tr)、气孔导度(Gs)、胞间CO2浓度(Ci)、光系统Ⅱ实际光化学量子效率(FPSⅡ)的提升[17]。但高浓度钙也会对植株生长发育有抑制作用,高钙基质(1 g·kg-1 CaCl2)栽培的玉米株高、生物量、光合速率、蒸腾速率及气孔导度显著下降[18],高钙营养液[360 mg·L-1的Ca(NO3)2]中甜瓜叶片叶绿素含量和类胡萝卜素含量显著降低[19]。蓝莓(Blueberry)是杜鹃花科(Ericaceae)越橘属(Vaccinium)植物,被认为是嫌钙植物[20-21],但也有研究显示土壤施加硫酸钙可增加叶片钙含量,提高果实硬度[22-23];叶面喷施0.5%的钙制剂显著增加蓝莓果实中K、Ca、Mg含量,此外对果实中的多酚和维生素C含量具有显著影响[24]。然而,施加不同浓度的外源钙是否通过影响蓝莓生理指标进而影响蓝莓生长状况,这一关键科学问题一直未得到较好的回答。因此,笔者利用水培技术,以3月生的大果蓝金幼苗为试验材料,设计不同钙离子供给水平,研究其对蓝莓幼苗生长发育的影响,为蓝莓钙素营养供给提供理论参考。
1 材料和方法
1.1 试验设计
试验于2020年7—11月在辽宁省大连市金普新区华家街道大连森茂现代农业有限公司温室大棚进行。以3 月生的大果蓝金扦插幼苗为试验材料,采用水培方式培养,营养液以Hoagland 和Arnon 营养液为基础(减钙),微量元素参照其他通用配方。营养液用去离子水配置,利用硫酸调整pH 为4.0~4.5。试验首先将长势一致的蓝莓幼苗转移到普通水培营养液中进行2 周的适应性转水培养,在此基础上再次挑选长势一致的幼苗进行下一步正式试验。试验以CaSO4的最高溶解度为上限,设定6 个Ca2+浓度:0(CK)、2.9(Ca-1)、5.8(Ca-2)、8.7(Ca-3)、11.6(Ca-4)及14.5(Ca-5)mmol·L-1,钙由CaSO4 提供。幼苗培养前期7 d更换1次营养液,生长旺盛期4 d 更换1 次。试验所用水培容器为高15 cm、直径8 cm、容量900 mL 的玻璃广口瓶,外部用黑色塑料袋包裹,避免透光影响根系生长。
分别于试验处理后0、40 d测定株高、基径、根体积、茎钙含量、叶片钙含量;在试验处理后0 d(7 月28日)、10 d(8月8日)、20 d(8月18日)、30 d(8月28日)、40 d(9月8日)测定光合参数;在试验处理40 d时,取样,测定叶片含水率、茎含水率、地上生物量、总生物量、根系活力、SOD活性、CAT活性。
1.2 分析测定方法
分别用卷尺(精确到1 mm)、游标卡尺(精确到0.02 mm)测量株高和基径。采用LI-6400/XT 光合测定系统于上午8:30—11:00测定叶片光合参数(净光合速率、蒸腾速率、胞间CO2浓度和气孔导度),气温在18~19 ℃,叶温23 ℃,光强为1000 μmol·m-2·s-1,测定部位为从顶部往下数第4片叶,每组处理随机测3 株,3 次重复。根体积、根系活力、SOD 和CAT活性、钙含量分别采用排水法、TTC 法[25]、丙酮比色法[26]、碘化钾法[27]、火焰原子吸收分光光度法[28]测定。采用Excel2016 软件整理原始数据,用SPSS Statistics 26对整理好的所有数据进行单因素方差分析(ANOVA),其中p <0.05表示处理组之间差异显著,数据均为3 次重复的平均植±标准误差,最后将分析好的数据利用Excel2016制图。
2 结果与分析
2.1 钙对蓝莓幼苗叶片气体交换参数的影响
较低浓度钙离子对蓝莓幼苗的光合速率具有一定的促进作用,随施钙时间的增加呈上升趋势,Ca-1处理组在20 d与40 d时的光合速率与对照相比分别增加了6.47%和10.4%。而钙离子浓度高于Ca-3时,40 d 时植株的光合速率与对照相比显著降低(p <0.05),且光合速率在测定周期内变化趋势平缓(图1-A);胞间CO2浓度随施钙时间的增加呈先上升后下降的趋势,当钙离子浓度低于Ca-4 时,在施钙20 d时胞间CO2浓度达最高之后下降,Ca-4和Ca-5处理组在施钙10 d后胞间CO2浓度就开始下降(图1-B);不同钙水平处理对蓝莓叶片的气孔导度和蒸腾速率影响的变化与光合速率相似,呈上升趋势,施钙浓度低于Ca-4时,叶片的气孔导度和蒸腾速率在40 d时显著高于对照(p <0.05);而高于Ca-3时,叶片的气孔导度和蒸腾速率在20 d 后受到抑制,40 d时差异显著(p <0.05)。因此说明,低浓度的钙处理促进蓝莓幼苗的气孔开放和蒸腾作用,而浓度过高时会对其产生抑制作用(图1-C、1-D)。
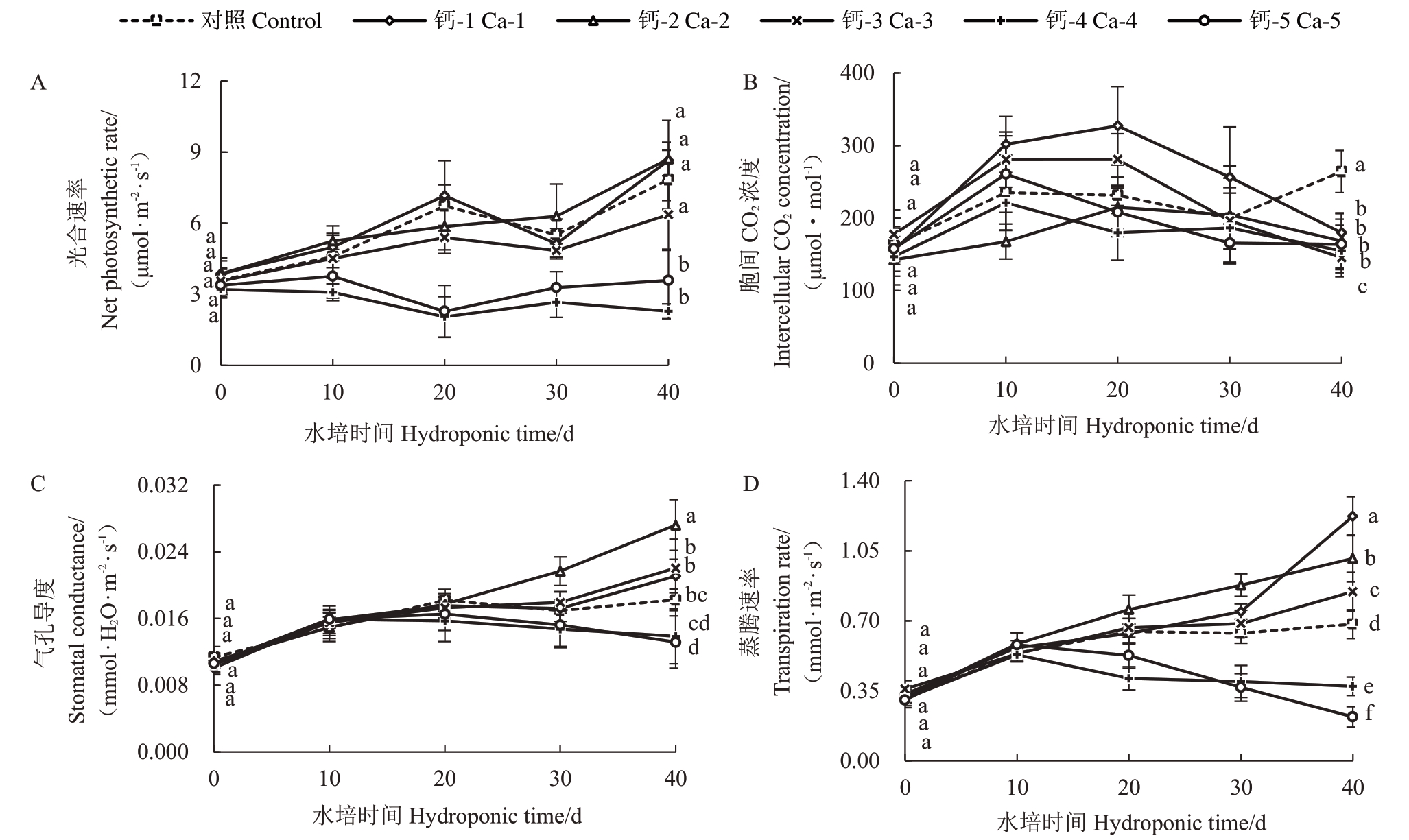
图1 钙处理对蓝莓幼苗叶片净光合速率(Pn)、胞间CO2浓度(Ci)、气孔导度(Gs)和蒸腾速率(Tr)的影响
Fig.1 Effects of calcium application on net photosynthetic rate(Pn),the intercellular CO2 concentration(Ci),stomatal conductance(Gs)and transpiration rate(Tr)of blueberry seedings
不同小写字母表示在p <0.05 差异显著。下同。
Different small letters indicate significant difference at p <0.05.The same below.
2.2 钙对蓝莓幼苗生长的影响
随钙离子浓度的升高各项测定指标数植呈现先上升后下降的趋势,Ca-1、Ca-2处理组可促进蓝莓幼苗的株高、基径、根体积等各项指标的提升,其中根体积在Ca-2 与Ca-3 处理组中分别是对照的3.23 与2.84 倍,差异显著(p <0.05)(表1);较高浓度的钙对蓝莓幼苗的各项指标产生一定的抑制作用,其中基径、地上生物量与总生物量在钙离子浓度高于Ca-3 时明显小于对照,差异显著(p <0.05),Ca-4、Ca-5 处理对植株各项生长指标均有不同程度的抑制作用。
表1 钙处理对蓝莓幼苗株高、基径、根体积、地上生物量及总生物量的影响
Table 1 Effects of calcium application on plant height,basal diameter,root volume,above-ground biomass and total biomass of blueberry seedlings
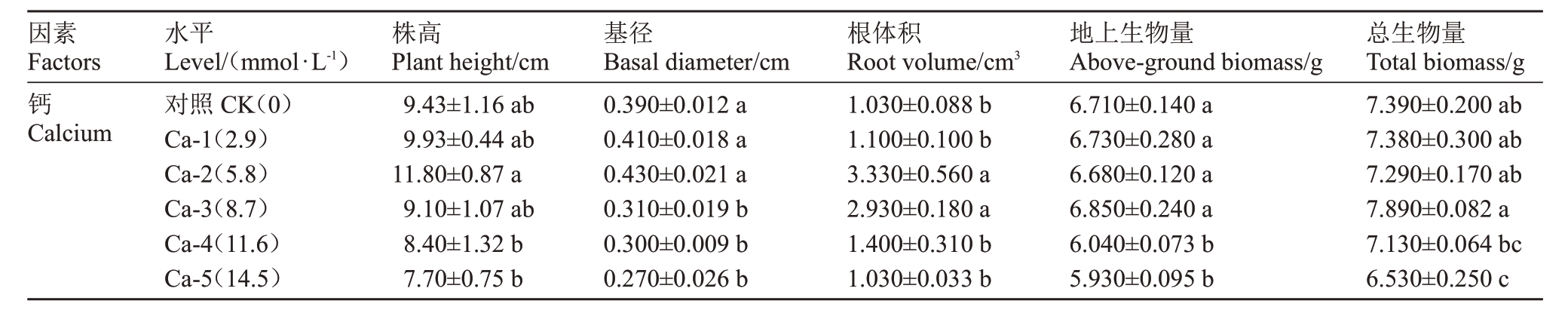
因素Factors钙Calcium水平Level/(mmol·L-1)对照CK(0)Ca-1(2.9)Ca-2(5.8)Ca-3(8.7)Ca-4(11.6)Ca-5(14.5)株高Plant height/cm 9.43±1.16 ab 9.93±0.44 ab 11.80±0.87 a 9.10±1.07 ab 8.40±1.32 b 7.70±0.75 b基径Basal diameter/cm 0.390±0.012 a 0.410±0.018 a 0.430±0.021 a 0.310±0.019 b 0.300±0.009 b 0.270±0.026 b根体积Root volume/cm3 1.030±0.088 b 1.100±0.100 b 3.330±0.560 a 2.930±0.180 a 1.400±0.310 b 1.030±0.033 b地上生物量Above-ground biomass/g 6.710±0.140 a 6.730±0.280 a 6.680±0.120 a 6.850±0.240 a 6.040±0.073 b 5.930±0.095 b总生物量Total biomass/g 7.390±0.200 ab 7.380±0.300 ab 7.290±0.170 ab 7.890±0.082 a 7.130±0.064 bc 6.530±0.250 c
2.3 钙对蓝莓幼苗茎和叶片含水率的影响
植株组织中的含水率是植物生理特征的重要指标。试验中发现,叶片含水率随钙离子浓度的增加呈现先上升后下降的趋势,各处理组中茎含水率较对照无显著变化,叶片含水率在浓度为Ca-1时显著增加,Ca-5 时显著降低(p <0.05)。说明适宜浓度的钙离子可提高叶片含水率,较高浓度的钙离子抑制叶片含水率(图2)。
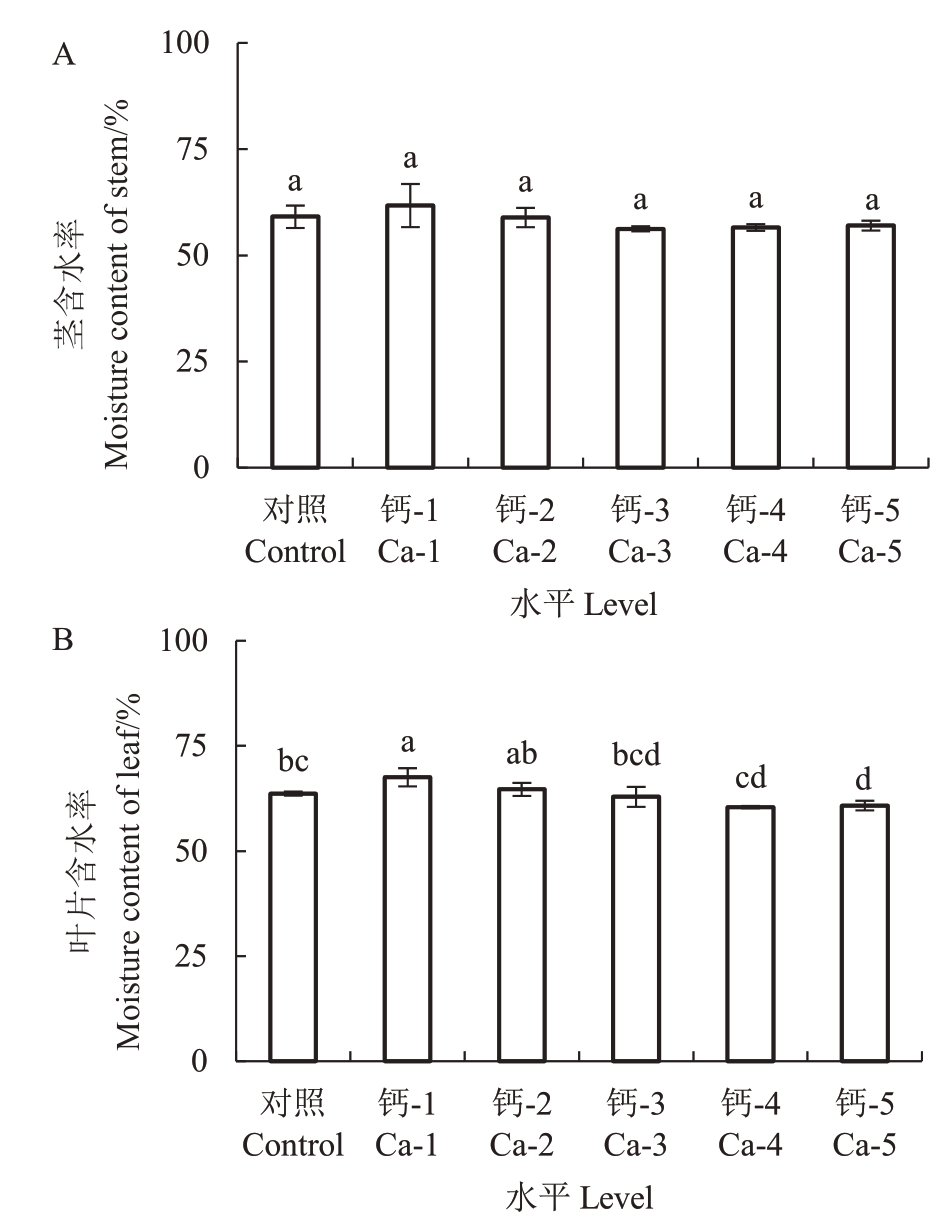
图2 钙处理对蓝莓茎、叶片含水率的影响
Fig.2 Effects of calcium application on stem,leaf moisture content of blueberry seedings
2.4 钙对蓝莓幼苗茎和叶片钙含量的影响
蓝莓幼苗地上各部位钙含量由高到低依次为叶片、茎。水培前各处理组中茎、叶片钙含量均无显著差异,向营养液中施加不同浓度的硫酸钙,培养40 d 后各处理组中茎和叶片的钙含量虽无显著差异,但随着钙离子浓度的增加叶片钙含量呈先上升后下降的趋势,其中Ca-3 钙含量达最高,Ca-5 最低,即饱和浓度的硫酸钙对蓝莓叶片的钙含量积累有抑制作用(图3)。
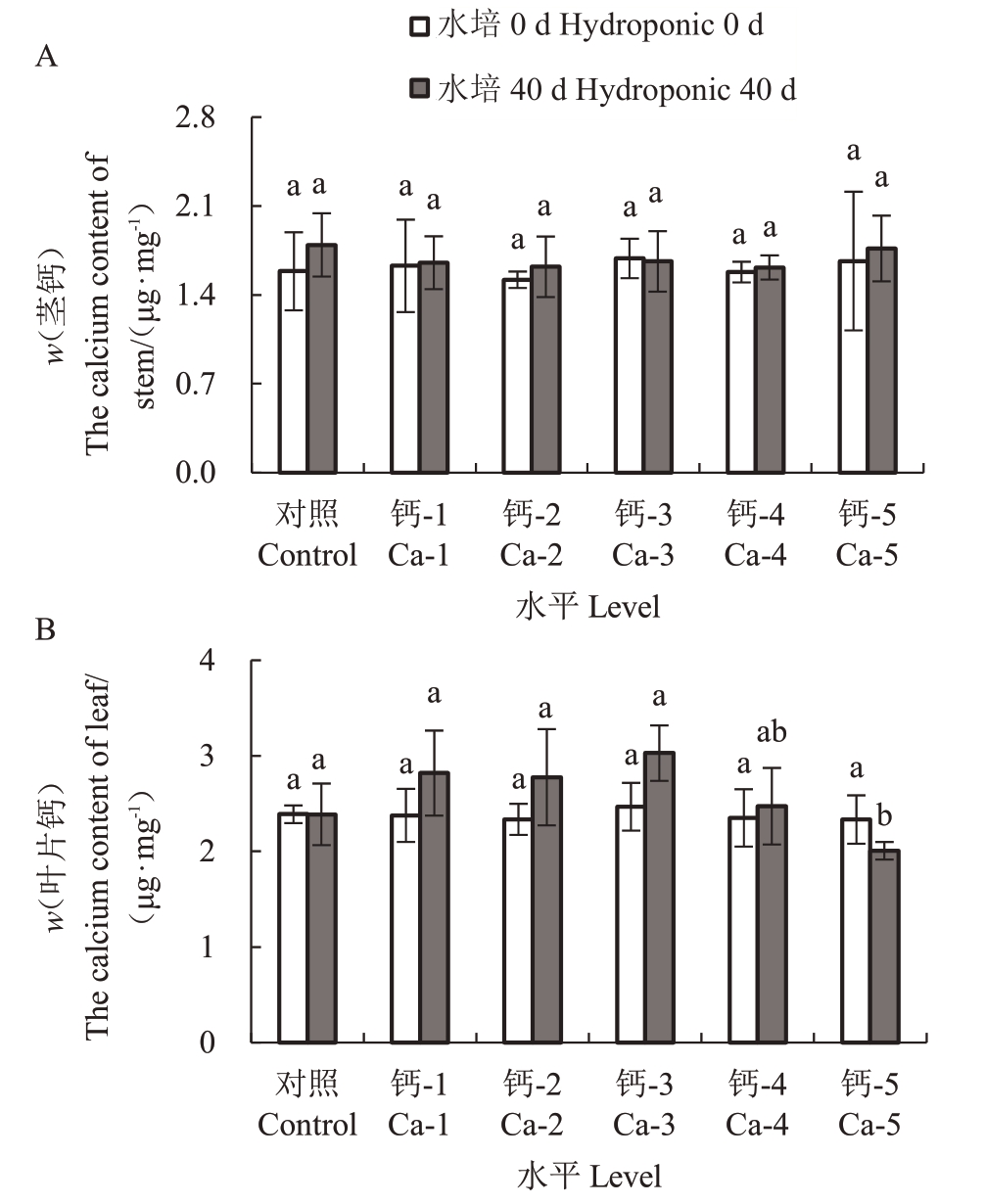
图3 钙处理对蓝莓茎、叶片钙含量的影响
Fig.3 Effects of calcium application on stem,leaf calcium content of blueberry seedings
2.5 钙对蓝莓根系活力的影响
四氮唑还原强度是检测植物根系活力的重要指标,钙浓度在Ca-3 和Ca-4 时,蓝莓幼苗的根系活力明显高于对照组;而钙离子浓度为Ca-1、Ca-2、Ca-5时,蓝莓幼苗的根系活力明显低于对照,不适宜蓝莓根系的生长(图4)。说明蓝莓根系活力的保持需要中高浓度的钙离子,而较低浓度与过高浓度钙均会降低根系活力,影响蓝莓根系的生长。
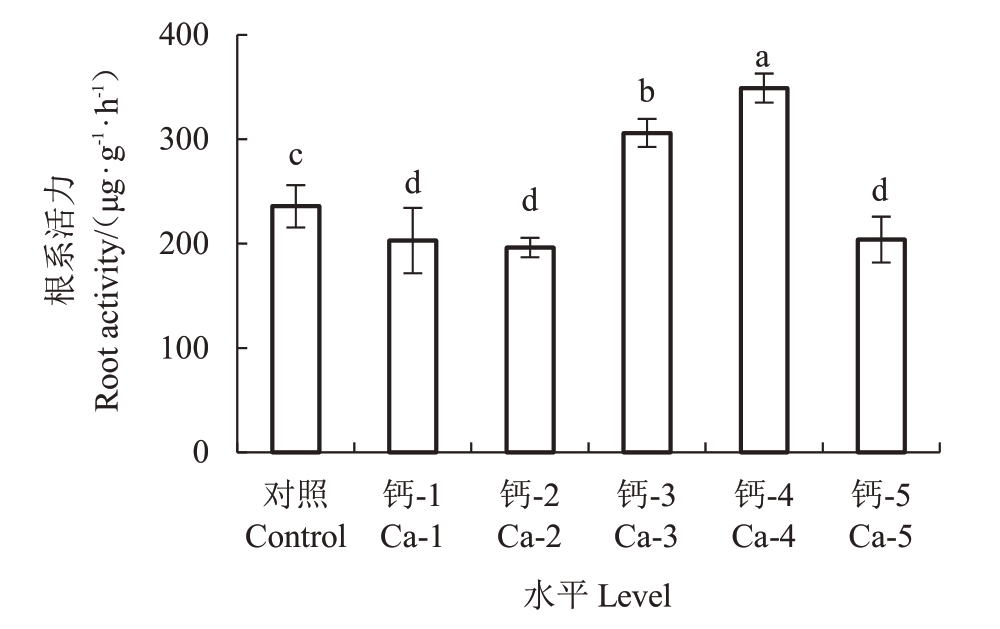
图4 钙处理对蓝莓幼苗根系活力的影响
Fig.4 Effects of calcium application on root activity of blueberry seedings
2.6 钙对蓝莓叶片SOD、CAT活性的影响
外源施加钙离子对叶片SOD、CAT活性均有积极影响。钙离子浓度为Ca-2时SOD活性达最大,差异显著(p <0.5),随钙离子浓度增加其活性呈现先上升后下降的趋势,当钙离子浓度范围在Ca-1~Ca-3 时,SOD 活性明显高于对照处理(图5-A);钙离子处理的蓝莓幼苗叶片CAT 活性较对照差异显著(p <0.05),随钙离子浓度的增加CAT 活性呈上升趋势,钙离子浓度为Ca-5时,其活性最高(图5-B)。
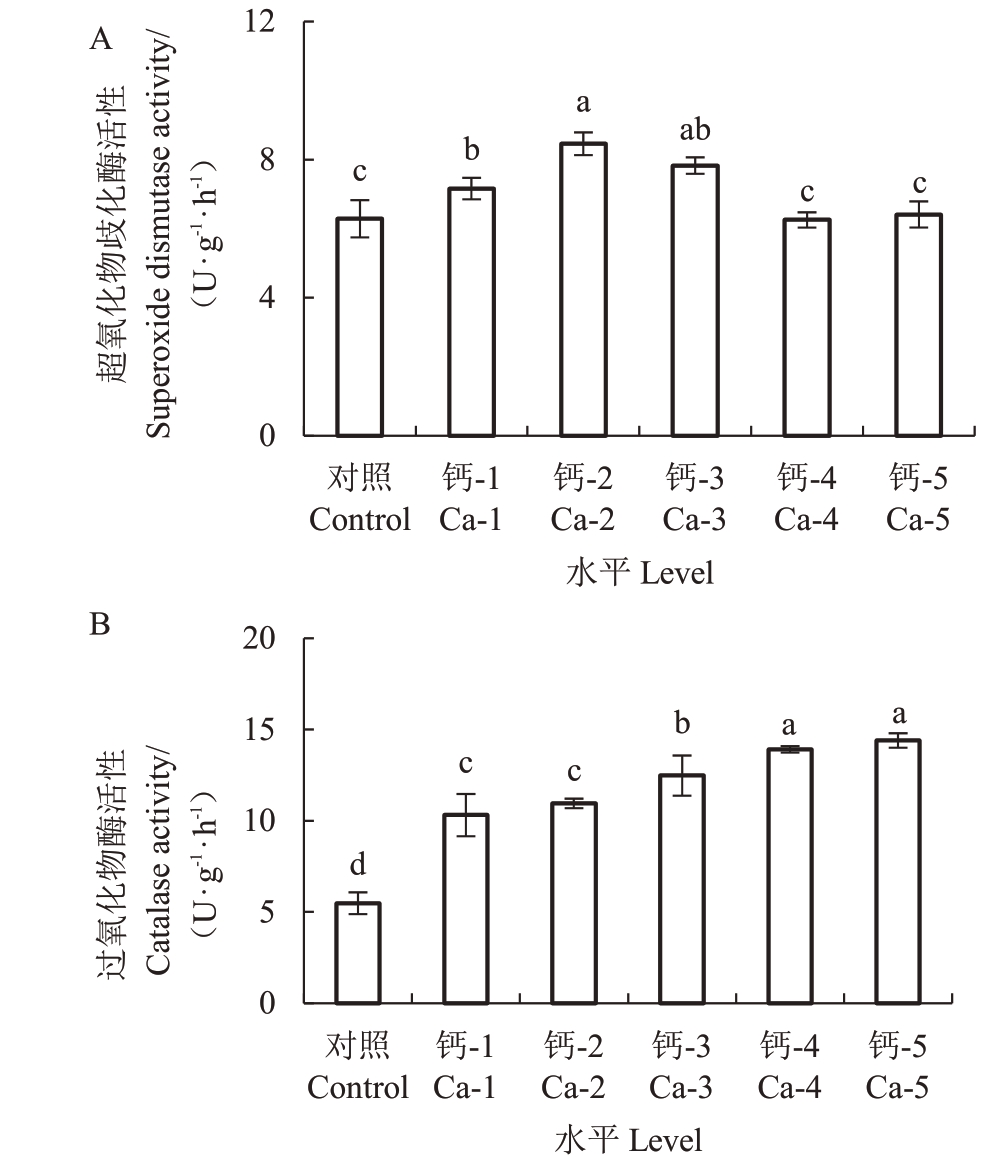
图5 钙处理对蓝莓叶片SOD 和CAT 活性的影响
Fig.5 Effects of calcium application on the activity of SOD and CAT leaf
3 讨 论
钙是植物生长所需的必要营养元素,参与植株体内的光合作用、细胞骨架维持、离子吸收与激素调控等系列生理生化过程,有效促进植株生长[6,29]。越橘属植物(包括蓝莓)长期以来被认为是嫌钙植物,叶片中钙浓度低且适宜在少钙土壤中生长[30-31]。但在本研究中发现Ca-1 和Ca-2 处理使得蓝莓幼苗株高分别增高了5.41%与25.13%,基径分别增粗了5.13%与10.26%,叶片钙含量分别增加了17.99%与16.32%。在水培20 d 时,Ca-1 和Ca-2 处理的胞间CO2浓度达到最高,20 d 后气孔导度与蒸腾作用显著高于对照,且在40 d 时的光合速率分别比对照组增加了6.47%和10.4%。这与钙在玉米、拟南芥和大蒜等的研究中结果相似,钙信号不仅参与叶片气孔的开闭[32-34],同时对胞间CO2浓度、蒸腾作用均有促进作用[35-36]。但过高浓度的钙会抑制植物的生长代谢,导致信号紊乱,严重时引起细胞死亡[37-38]。本试验中较高浓度钙(Ca-4、Ca-5)处理抑制了蓝莓幼苗株高和基径的生长,叶片含水率降低了11.76%,植株的光合速率、胞间CO2浓度、气孔导度、蒸腾速率、生物量与总生物量显著低于对照组。说明适宜浓度钙可促进蓝莓幼苗的生长,蓝莓并非是嫌钙植物,可能是由于适宜蓝莓生长的土壤环境为透水性、通气性及排水性良好,有机质含量丰富,尤为关键的是pH 需控制在4.5~5.5 之间,若pH 过高,造成缺铁失绿,生长不良,产量降低甚至植株死亡[39-40]。但通常未改良土壤中钙大多为碳酸钙和氯化钙,pH近于中性或呈微碱性[41-42],不符合蓝莓栽培条件。而本试验利用营养液水培法,通过施加不同浓度硫酸钙,控制pH,发现外源施钙促进气孔的开放,一方面,提高了叶片的蒸腾速率,有利于水分和无机营养的吸收;另一方面,增加了胞间CO2浓度,促进叶片的光合碳同化,为蓝莓植株的生长提供了更多的有机营养[43-44]。
根系是重要的营养器官,其形态大小与活力程度影响植物对水分与矿质营养的吸收与利用,外源钙的施加促进根的生长[45]。Ca2+通过参与调控IAA合成过程诱导黄瓜不定根的形成[46];拟南芥根毛长度与培养基中Ca2+浓度相关,适宜浓度的Ca2+(0.3~3 mmol·L-1)促进根毛生长[47];Ca2+通过促进蚕豆根部GA 和IAA 的积累进而影响根部生长[48]。蓝莓属于浅根性须根系植物,钙离子对于蓝莓根系的影响结果各不相同。Austin等[49]研究发现蓝莓土壤中钙离子浓度较高时,降低其根系活力,但在Hanson等[22]的研究中发现外源土壤施加硫酸钙对蓝莓植株无害。本试验中Ca-2 和Ca-3 处理组的根体积分别是对照组的3.23 和2.84 倍,Ca-3 和Ca-4 处理组中,蓝莓幼苗的根系活力明显高于对照组。推测是因为外源施加钙并没有改变适宜蓝莓幼苗生长的水培营养液环境,适宜浓度钙离子(Ca-3)促进蓝莓根体积生长和根系活力的提高。钙对植物细胞膜的结构和功能具有稳定作用[50],Zhou 等[51],Afiyanti 等[52]和王博伟等[53]研究发现,外源施钙可有效提高番茄、苜蓿、生菜叶片SOD、POD与CAT等抗氧化酶的活性,提高其抗逆适应能力。本研究中发现,Ca-1~Ca-3范围浓度的钙离子可以促进蓝莓SOD活性的升高,CAT活性随钙离子浓度的升高而升高。说明适宜浓度的钙处理,可以促进蓝莓SOD、CAT 等抗氧化酶的活性,可能提高其对逆境胁迫的适应能力。
4 结 论
适宜的外源钙可以有效调节蓝莓幼苗叶片气孔开放,促进光合和蒸腾作用,有利于蓝莓植株的叶片、根系等器官的生长,此外,还可提高SOD、CAT等抗氧化酶活性;但较高浓度的钙处理会明显抑制蓝莓的生长发育。笔者在本研究中初步探讨了钙对蓝莓生长发育的影响,以期为蓝莓栽培中外源施钙应用提供一定理论参考。
[1] BURSTROM H G. Calcium and plant growth[J]. Biological Reviews,1968,43(3):287-316.
[2] WHITE,P J,BROADLEY M R.Calcium in plants[J].Annals of Botany,2003,92(4):487-511.
[3] 刘剑锋,唐鹏,彭抒昂.采后浸钙对梨果实不同形态钙含量及生理生化变化的影响[J]. 华中农业大学学报,2004,23(5):560-562.LIU Jianfeng,TANG Peng,PENG Shu’ang. Effects of calcium dipping after harvest on content of calcium in different forms and physio-chemical characteristics of pear[J]. Journal of Huazhong Agricultural University,2004,23(5):560-562.
[4] GILLIHA M,DAYOD M,HOCKING,XU B,CONN S J,KAISER B N,LEIGH R A,TYERMAN S D.Calcium delivery and storage in plant leaves:exploring the link with water flow[J].Journal of Experimental Botany,2011,62(7):2233-2250.
[5] 龚明,李英,曹宗巽.植物体内的钙信使系统[J].植物学通报,1990,7(3):21-31.GONG Ming,LI Ying,CAO Zongxun. Calcium messenger system in plants[J].Chinese Bulletin of Botany,1990,7(3):21-31.
[6] HEPLER P K. Calcium:A central regulator of plant growth and development[J].The Plant Cell,2005,17(8):2142-2155.
[7] POOVAIAH B W,MCFADDEN J J,REDDY A S N.The role of calcium ions in gravity signal perception and transduction[J].Physiologia Plantarum,2010,71(3):401-407.
[8] EDEL K H,MARCHADIER E,BROWNLEE C,KUDLA J,HETHERINGTON A M.The evolution of calcium-based signalling in plants[J].Current Biology,2017,27(13):667-679.
[9] 周卫,汪洪.植物钙吸收、转运及代谢的生理和分子机制[J].植物学通报,2007,24(6):762-778.ZHOU Wei,WANG Hong. The physiological and molecular mechanisms of calcium uptake,transport,and metabolism in plants[J].Chinese Bulletin of Botany,2007,24(6):762-778.
[10] REITZ N F,SHACKEL K A,MITCHAM E J. Differential effects of excess calcium applied to whole plants vs. excised fruit tissue on blossom-end rot in tomato[J]. Scientia Horticulturae,2021,290.doi:10.1016/j.scienta.2021.110514.
[11] MAYORGA-GOMEZ A,NAMBEESAN S U,COOLONG T,DÍAZ-PÉREZ J C. Temporal relationship between calcium and fruit growth and development in bell pepper (Capsicum annuum L.)[J].HortScience,2020,55(6):1-8.
[12] SINGH S,GAMRASNI D,PARIMI P,KOCHANCK B,NASCHITZ S,ZEMACH H,FRIEDMAN H. Postharvest calcium treatment of apple fruit increased lenticel breakdown and altered cuticle structure[J]. Postharvest Biology and Technology,2021,171:111331.
[13] 汤寓涵,夏星,陈德伟,赵大球,陶俊.芍药CIPK 基因克隆及其响应钙调控的表达水平研究[J]. 植物生理学报,2018,54(8):1316-1324.TANG Yuhan,XIA Xing,CHEN Dewei,ZHAO Daqiu,TAO Jun.Cloning of herbaceous peony CIPK gene and its expression level analysis in response to calcium regulation[J].Plant Physiology Journal,2018,54(8):1316-1324.
[14] 袁伟玲,陈磊夫,刘志雄,吴金平,袁尚勇.外源钙对小白菜酶活性、钙含量及其产量和品质的影响[J]. 中国土壤与肥料,2020(6):254-261.YUAN Weiling,CHEN Leifu,LIU Zhixiong,WU Jinping,YUAN Shangyong. Effects of exogenous CaCl2 on the enzyme activity,leaf calcium concentrations,yield and quality of cabbage[J].Soil and Fertilizer Sciences in China,2020(6):254-261.
[15] TAN W,MENG Q W,BRESTIC M,OLSOVSKA K,YANG X H. Photosynthesis is improved by exogenous calcium in heatstressed tobacco plants[J]. Journal of Plant Physiology,2011,168(17):2063-2071.
[16] CHEN S J,KAO C H. Methyl jasmonate,ammonium,and leaf senescence in rice[J]. Journal of Plant Physiology,1998,152(4/5):353-357.
[17] HE L,YU L,DU N S,GUO S R.The effect of exogenous calcium on cucumber fruit quality,photosynthesis,chlorophyll fluorescence,and fast chlorophyll fluorescence during the fruiting period under hypoxic stress[J]. BMC Plant Biology,2018,18(1):1-10.
[18] 冉琼,钟章成,杜道林.严重钙胁迫下接种AM 真菌无益于缓解玉米苗期的钙毒害[J].土壤与作物,2020,9(4):388-395.RAN Qiong,ZHONG Zhangcheng,DU Daolin. Inoculation of AM fungi may not relieve calcium toxicity of maize seedling under severe calcium stress[J]. Soils and Crops,2020,9(4):388-395.
[19] LONG M H,TANG X F,YU W J,LIAO Y,HUANG W H,QIN R Y. Effects of different calcium levels on photosynthesis and protective enzyme activities of melon leaves[J]. Guihaia,2005,25(1):77-82.
[20] KORACK R F. Variation in nutrient requirements of blueberries and other calcifuges[J].HortScience,1989,24(4):573-578.
[21] 刘红弟,宋杨,张红军.越橘矿质营养元素缺乏症及矫治措施研究进展[J].中国果树,2018(5):57-62.LIU Hongdi,SONG Yang,ZHANG Hongjun.Research development of mineral nutrient deficiency symptoms and rectification measures of cowberry[J].China Fruits,2018(5):57-62.
[22] HANSON E J,BERKHEIMER S F.Effect of soil calcium applications on blueberry yield and quality[J]. Small Fruits Review,2004,3(1/2):133-139.
[23] LOBOS T E,RETAMALES B R,HANSON E J. Early preharvest calcium sprays improve postharvest fruit quality in‘Liberty’highbush blueberries[J]. Scientia Horticulturae,2021,277:109790.
[24] OCHMIAN I D.The impact of foliar application of calcium fertilizers on the quality of highbush blueberry fruits belonging to the 'Duke' cultivar[J]. Notulae Botanicae Horti Agrobotanici Cluj-Napoca,2012,40(2):163-169.
[25] 朱秀云,梁梦,马玉. 根系活力的测定(TTC 法)实验综述报告[J].广东化工,2020,416(6):219-220.ZHU Xiuyun,LIANG Meng,MAYu.Areview report on the experiments for the determination of root activity by TTC method[J].Guangdong Chemical Industry,2020,416(6):219-220.
[26] 潘百明,苏辉兰,梁昌祥,覃晓婷.紫茄超氧化物歧化酶的提取及其活性测定[J].食品工业,2020,281(2):149-152.PAN Baiming,SU Huilan,LIANG Changxiang,QIN Xiaoting.SOD extraction and activity determination from purple eggplant[J].The Food Industry,2020,281(2):149-152.
[27] 董娜,张爱菊,张小林.基于碘化钾-淀粉显色光度法测定过氧化氢酶活性[J].中国食品添加剂,2019,30(8):150-153.DONG Na,ZHANG Aiju,ZHANG Xiaolin. Determination of catalase activity by potassium iodode-starch spectrophotometric method[J].China Food Additives,2019,30(8):150-153.
[28] 张济宇.火焰原子吸收分光光度法测定水中微量钙[J].环境监测管理与技术,2001,13(4):46.ZHANG Jiyu. Determination of trace calcium in water by flame atomic absorption spectrophotometry[J]. The Administration and Technique of Environmental Monitoring,2001,13(4):46.
[29] BUSH,DOUGLAS S. Calcium regulation in plant cells and its role in signaling[J].Annual Review of Plant Physiology&Plant Molecular Biology,1995,46(1):95-122.
[30] WRIGHT G C,PATTEN K D,DREW M C.Salinity and supplemental calcium influence growth of rabbiteye and southern highbush blueberry[J]. Journal of American Society for Horticultural Science,1992,117(5):749-756.
[31] KORCAK R F. Variation in nutrient requirements of blueberries and other calcifuges[J].HortScience,1989,24(4):573-578.
[32] AMNON S,NITZA I,GRANTZ D A.Calcium effects on stomatal movement in Commelina communis L.[J].Plant Physiology,1988,87(3):583-587.
[33] YANG D L,SHI Z Y,BAO Y M,YAN J P,YANG Z Y,YU H Y,LI H Y,GOU M Y,WANG S,ZOU B H,XU D C,MA Z Q.JITAE KIM,HUA J.Calcium pumps and interacting BON1 protein modulate calcium signature,stomatal closure,and plant immunity[J].Plant Physiology,2017,175(1):424-437.
[34] SCHULZE S,DUBEAUX G,CECILIATO P H,MUNEMASA S,NUHKAT D.YARMOLINSKY J,AGUILAR R D,DIAZ R,TAMAR T A,STEINHORST L,OFFENBORN J N,KUDLA J,KOLLIST H,JULIAN I. A role for calcium-dependent protein kinases in differential CO2-and ABA-controlled stomatal closing and low CO2-induced stomatal opening in Arabidopsis[J]. New Phytologist,2021,229(5):2765-2779.
[35] 张浩,郑云普,叶嘉,高伟,乔雅君,戴川景,赵雨欣,石少婕.外源钙离子对盐胁迫玉米气孔特征、光合作用和生物量的影响[J].应用生态学报,2019,30(3):923-930.ZHANG Hao,ZHENG Yunpu,YE Jia,GAO Wei,QIAO Yajun,DAI Chuanjing,ZHAO Yuxin,SHI Shaojie. Effects of exogenous Ca2 + on stomatal traits,photosynthesis,and biomass of maize seedings under salt stress[J]. Chinese Journal of Applied Ecology,2019,30(3):923-930.
[36] SALAZAR L,PAEZ-VACAS M,KESSLER M,KLUGE J,HOMEIER J.Variation of foliar calcium and magnesium in six fern species at different elevations[J]. Earth and Environmental Science,2021,690(1):012056.
[37] HU X L,JIANG M Y,ZHANG J H,ZHANG A Y,LIN F,TAN M P. Calcium-calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize(Zea mays)plants[J].New Phytologist,2007,173(1):27-38.
[38] YANG T B,POOVAIAH B W. Calcium/calmodulin-mediated signal network in plants[J]. Trends in Plant Science,2003,8(10):505-512.
[39] PRODORUTTI D,PERTOT I,GIONGO L,GESSLER C.Highbush blueberry:cultivation,protection,breeding and biotechnology[J].The European Journal of Plant Science and Biotechnology,2007,1(1):44-56.
[40] OCHMIAN I,JAROSZEWSKA A,MALINOWSKI R,KOZOS K. Chemical and enzymatic changes of different soils during their acidification to adapt them to the cultivation of highbush blueberry[J].Agronomy,2020,11(44):1-25.
[41] 陶漉,马东豪,张丛志,陈林,张佳宝.石灰性土壤团聚体中钙形态特征及其与有机碳含量的关系[J]. 土壤,2021,53(4):715-722.TAO Lu,MA Donghao,ZHANG Congzhi,CHEN Lin,ZHANG Jiabao.Distribution characteristics of calcium forms and their relations with organic carbon content in calcareous soil aggregates[J].Soils,2021,53(4):715-722.
[42] MOSES E,THLIZA B A,SHANU B U. Nanotechnical production and optimization of biofuel (biodiesel) from an edible seed oil (Palm-Olein)[J]. Annual Research & Review in Biology,2020,6:20-31.
[43] STAEL S. Chloroplast calcium signalling quenches a thirst[J].Nature Plants,2019,5(6):559-560.
[44] CHIGRI F,SOLL J,VOTHKNECHT U C. Calcium regulation of chloroplast protein import[J].The Plant Journal,2005,42(6):821-831.
[45] 李贺,刘世琦,刘中良,冯磊,刘景凯,陈祥伟,王越.钙对大蒜生理特性及主要矿质元素吸收的影响[J]. 中国农业科学,2013,46(17):3626-3634.LI He,LIU Shiqi,LIU Zhongliang,FENG Lei,LIU Jingkai,CHEN Xiangwei,WANG Yue.Effects of calcium on physiological characteristics and main mineral elements absorption of garlic[J].Scientia Agricultura Sinica,2013,46(17):3626-3634.
[46] LANTERI M L,PAGNUSSAT G C,LAMATTINA L. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber[J].Journal of Experimental Botany,2006,57(6):1341-51.
[47] SCHIEFELBEIN J W,SHIPLEY A,ROWSE P. Calcium influx at the tip of growing root-hair cells of Arabidopsis thaliana[J].Planta,1992,187(4):455-459.
[48] HASAN H A H.Gibberellin and auxin production by plant rootfungi and their biosynthesis under salinity-calcium interaction[J].Rostlinna Vyroba,2002,48(3):101-106.
[49] AUSTIN M E,GAINES T P,MOSS R E. Influence of soil pH on soil nutrients,leaf elements,and yield of young rabbiteye blueberries[J].HortScience,1986,21(3):443-445.
[50] PARK J,FAN Z,DENG C X. Effects of shear stress cultivation on cell membrane disruption and intracellular calcium concentration in sonoporation of endothelial cells[J]. Journal of Biomechanics,2011,44(1):164-169.
[51] ZHOU B,GUO Z. Calcium is involved in the abscisic acid-induced ascorbate peroxidase,superoxide dismutase and chilling resistance in Stylosanthes guianensis[J]. Biologia Plantarum,2009,53(1):63-68.
[52] AFIYANTI M,CHEN H J.Catalase activity is modulated by calcium and calmodulin in detached mature leaves of sweet potato[J].Journal of Plant Physiology,2014,171(2):35-47.
[53] 王博伟,陈艳丽,朱国鹏,王旭,杨雨,刘金伟.叶面喷施氯化钙对海南高温季节水培生菜生长生理的影响[J].中国瓜菜,2021,34(4):94-98.WANG Bowei,CHEN Yanli,ZHU Guopeng,WANG Xu,YANG Yu,LIU Jinwei. Effect of foliar spraying calcium chloride on the growth and physiology of hydroponic lettuce in Hainan province in hot season[J]. China Cucurbits and Vegetables,2021,34(4):94-98.