甲氧基吡嗪(methoxypyrazines,MPs)是一类六元杂环化合物,具有挥发性,与红葡萄酒中带有不愉悦的生青味有关。目前大多数分析和研究在葡萄和葡萄酒中进行[1-4],且聚集在3-异丁基-2-甲氧基吡嗪(IBMP)、3-异丙基-2-甲氧基吡嗪(IPMP)和3-仲丁基-2-甲氧基吡嗪(SBMP)[5]。它们主要存在于赤霞珠[6]、美乐[7]、品丽珠[8]、佳美娜[9]、长相思[10]、霞多丽、黑比诺[11]和赛美蓉等品种中。MPs感官阈植极低,在水中仅为1~2 ng·L-1,而葡萄酒中也只要2~16 ng·L-1就可使葡萄酒带有强烈的青草、青椒和青豌豆气味[12]。IBMP是果实中含量最高的MPs,被认为对葡萄酒的青椒类香气具有明显作用,IPMP则有时被描述为泥土气味[13]。有研究认为当白葡萄酒中MPs类物质含量适中时,对葡萄酒香气具有协调作用;但在红葡萄酒中含量过高,则会带给消费者不良的感官体验[14]。
葡萄酒中MPs 含量与浆果中的含量高度相关,因此,葡萄栽培研究者正在逐步探索一些新的栽培方法来影响葡萄收获时的MPs 水平。葡萄果实MPs 在葡萄转色前大量积累,随着果实成熟而被逐渐降解,推迟采收可降低果实中MPs 含量[15]。良好的土壤墒情和高密度定植均会提高赤霞珠果实IBMP 含量[16],但不同N 素供应水平对葡萄果实IBMP 含量没有影响[17]。转色前对葡萄果际施加光照可明显降低果实MPs含量,但转色后进行光照处理则无此作用[18]。在转色前高温处理葡萄果实可以下调VVOMT3基因表达,从而抑制甲氧基转移酶活性,减少IBMP 的合成[19]。目前,通过转色之前摘除赤霞珠果穗周围叶片和疏除枝蔓来降低MPs 含量已被应用于生产实践[20]。有研究指出对葡萄植株进行适度的水分胁迫,虽然会影响植株的长势,使果实体积变小,但明显提高了葡萄果实中酚类和单宁聚合物的含量,同时对于水分的利用率最高,可以节约栽培成本[21]。但水分胁迫下葡萄果实中MPs种类构成和含量变化的研究还未见报道。
目前关于水分胁迫的研究,主要集中在水分胁迫时期和水分胁迫程度。因此研究植株在哪一时期施加哪种水分胁迫程度,对于提高植株的产果率、生长发育以及节约成本具有重要意义。笔者从葡萄植株坐果期至转色期和从转色期至采收期分别采用不同程度水分胁迫对果实发育过程中5种甲氧基吡嗪含量的影响,了解不同水分胁迫下葡萄发育过程中甲氧基吡嗪含量的变化规律,结合其果实品质差异,以获得科学高效的水分调控技术,有利于生产优质的葡萄酒。
1 材料和方法
1.1 试验地概况
于2017 年5—9 月在宁夏玉泉营农场国家现代葡萄产业技术体系水分生理与节水栽培岗位试验点进行(38°14′25″N,106°01′43″E)。试验地位于中温带干旱区,全年光照充足,降雨量少,且昼夜温差大,年有效积温可达到3400~3800 ℃。
1.2 材料
选用9年生赤霞珠(Vitis Vinifera‘Cabernet Sauvignon’葡萄,东西行向定植,“厂”字整形,植株株行距为0.6 m×3 m,按照田间常规水肥管理。各处理均选取生长势良好的植株60 株,3 次重复,共180株。其盛花期为2017 年5 月25 日,记为花后0 d(0 DAA)。
1.3 试验设计
采取完全随机设计,在葡萄生长初期(萌芽期—开花期)正常灌溉,从坐果期即花后20 d,至转色期,对葡萄植株采用轻度(-0.2 MPa≥Ψb≥-0.4 MPa)、中度(-0.4 MPa≥Ψb≥-0.6 MPa)和 重 度(Ψb≤-0.6 MPa)水分胁迫程度,葡萄果实转色后至采收期(120 DAA),对每一个胁迫程度再进行轻度、中度和重度3个不同处理。如表1所示,该试验共设9个处理,其中第一组为对照组,以黎明前葡萄叶片基础水势植来反映水分胁迫强度。
表1 各处理黎明前叶片水势值范围
Table 1 Range of leaf water potential before dawn for each treatment
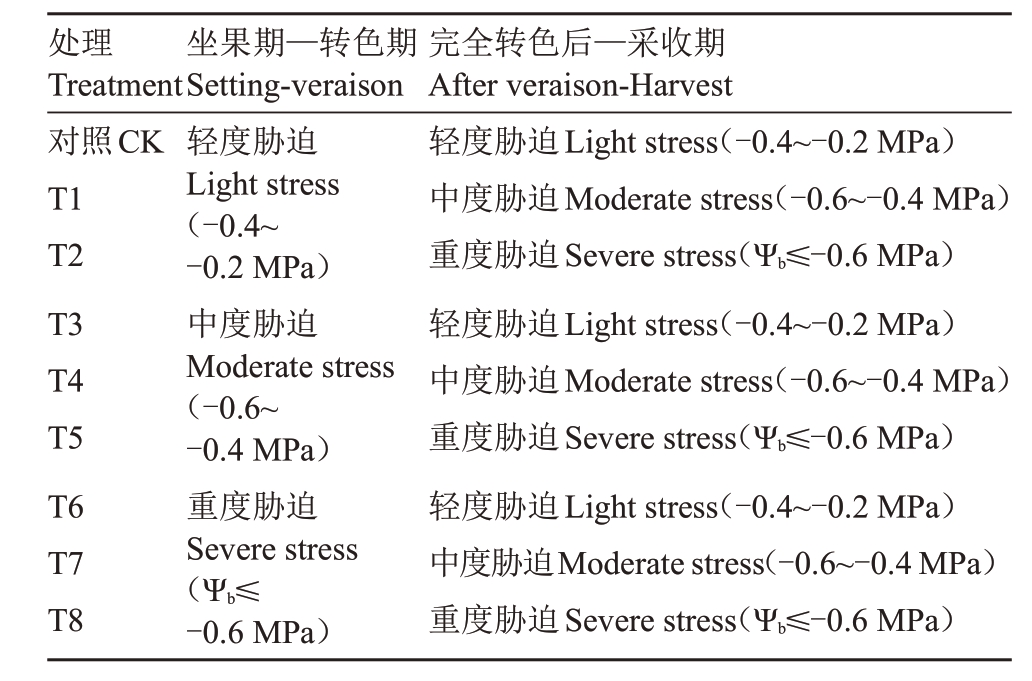
处理Treatment对照CK T1 T2 T3 T4 T5 T6 T7 T8坐果期—转色期Setting-veraison轻度胁迫Light stress(-0.4~-0.2 MPa)中度胁迫Moderate stress(-0.6~-0.4 MPa)重度胁迫Severe stress(Ψb≤-0.6 MPa)完全转色后—采收期After veraison-Harvest轻度胁迫Light stress(-0.4~-0.2 MPa)中度胁迫Moderate stress(-0.6~-0.4 MPa)重度胁迫Severe stress(Ψb≤-0.6 MPa)轻度胁迫Light stress(-0.4~-0.2 MPa)中度胁迫Moderate stress(-0.6~-0.4 MPa)重度胁迫Severe stress(Ψb≤-0.6 MPa)轻度胁迫Light stress(-0.4~-0.2 MPa)中度胁迫Moderate stress(-0.6~-0.4 MPa)重度胁迫Severe stress(Ψb≤-0.6 MPa)
灌水方式采用滴灌设备,流速为0.6 L·h-1,每个处理行两端安装控水阀门,根据降雨量及水分蒸发量及时调整灌水时间,进而控制灌水量,使不同处理的植株叶片黎明前水势植保持在相应的范围内。2017 年玉泉营试验基地葡萄生育期内气温变化和日降雨量见图1。
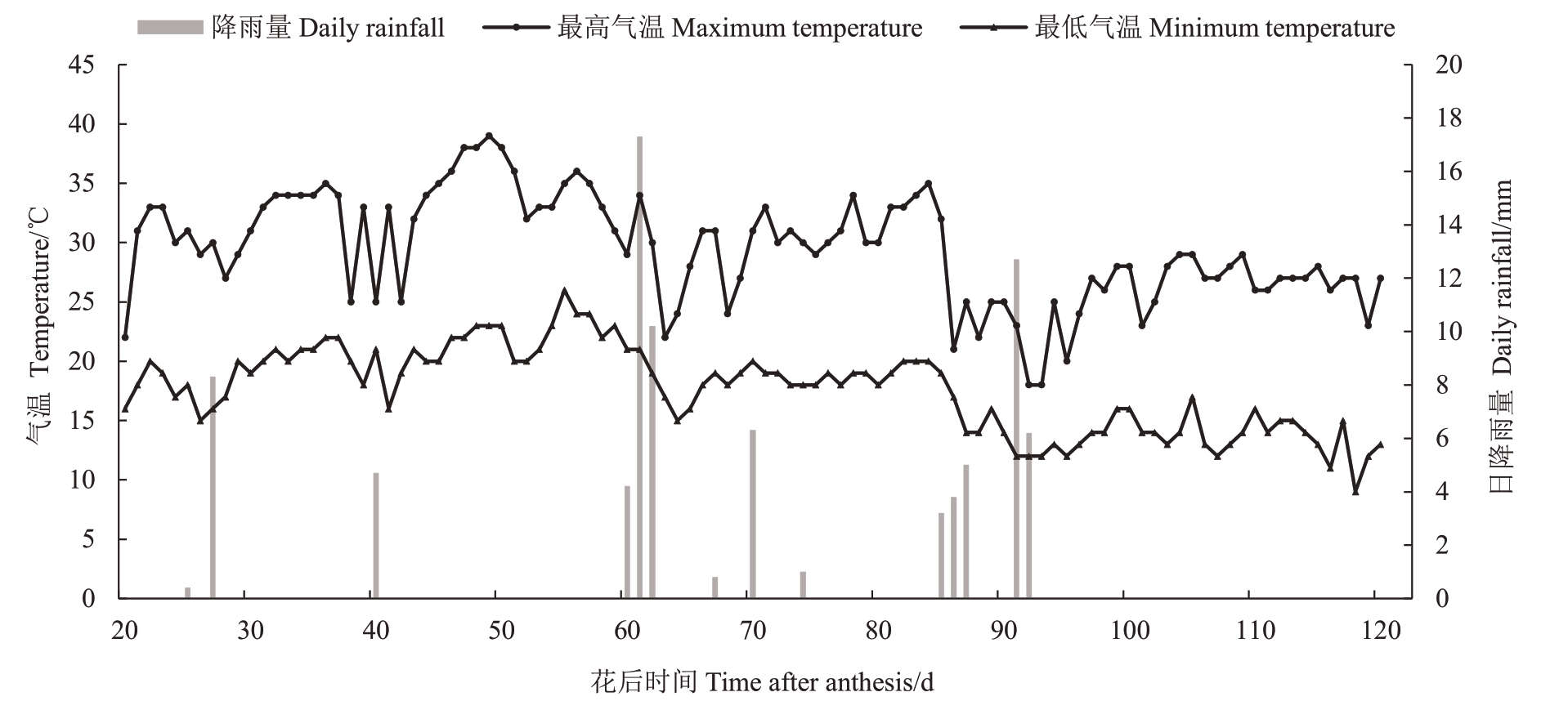
图1 试验期间气温和日降雨量
Fig.1 Air temperature and daily rainfall during experiment
1.4 样品采集
于2017年6月15日(20 DAA)开始采样至采收期(120 DAA),每隔10 d 对各水分胁迫处理的赤霞珠葡萄果实进行采样。每次样品采集时间为早上8:00—9:00,在各处理不同植株向光面、背光面果穗的各部位随机取样共500粒。混匀后,300粒用于果实基础理化指标测定。其他样品液氮速冻后置于-80 ℃冰箱。
1.5 测定指标与方法
1.5.1 黎明前叶片水势测定 于黎明前摘取各处理葡萄植株新梢中部健康的功能叶放入自封袋中带回,立即将叶片正确放置于植物水势压力室中(美国Soil Moisture Equipment 公司),加压直至叶片叶柄处出现小水珠,立即读取表盘示数,每个处理9次重复。
1.5.2 果实品质的测定 百粒质量采用天平称重法。可溶性固形物含量用手持糖度折光仪测定,可滴定酸含量用酸碱滴定法测定。总酚含量用福林酚法、单宁含量用福林-丹尼斯法[22]、总花色苷含量用盐酸-甲醇法[23]测定。每个处理3次重复。
1.5.3 甲氧基吡嗪含量测定——气相色谱法 甲氧基吡嗪标准品母液和梯度混合标准液配置参考姜文广等[24]的方法。称取-80 ℃贮藏的各水分胁迫处理葡萄果实30 g,分别置于粉碎机中,加入1%CaCl2,避免葡萄汁氧化,4 ℃环境中放置将果实解冻。4 ℃,5000 r·min-1条件下离心5 min。准确移取上清液5 mL 至15 mL 深棕色顶空样品瓶中,加2.0 g NaCl和转子,盖上顶空瓶盖。
选用CAR/PDMS 萃取纤维作为萃取介质插入样品顶空瓶,在30 ℃下固相微萃取装置中预热5 min,然后遮光环境中搅拌萃取3 h。然后取出立刻置于进样口解吸附5 min,开始检测。气相色谱柱为J&W DB Wax(50 m×0.25 mm×0.25 μm)。GCFID 温度设定参考文献[24]。采用外标法对甲氧基吡嗪进行定性和定量分析。
1.6 数据处理及分析
数据采用Microsoft office excel 2019 和DPS V15.10 进行数据记录和统计分析,用Excel 2019 作图,LSD多重检验样本间的差异显著性(p <0.05)。
2 结果与分析
2.1 不同水分胁迫对赤霞珠果实品质的影响
2.1.1 水分胁迫下葡萄植株黎明前叶片水势值(Ψb)及灌水量 水分胁迫试验期间,调节各处理组的灌水量,根据黎明前叶片水势测定结果反映水分胁迫程度。由图2可知,40 DAA后水分胁迫处理整体控制较好,符合试验中不同水分胁迫黎明前叶片水势植设定范围。在85~90 DAA 由于降雨导致各处理水势植有所上升,但各处理的水势植均在所设定的试验范围内波动。
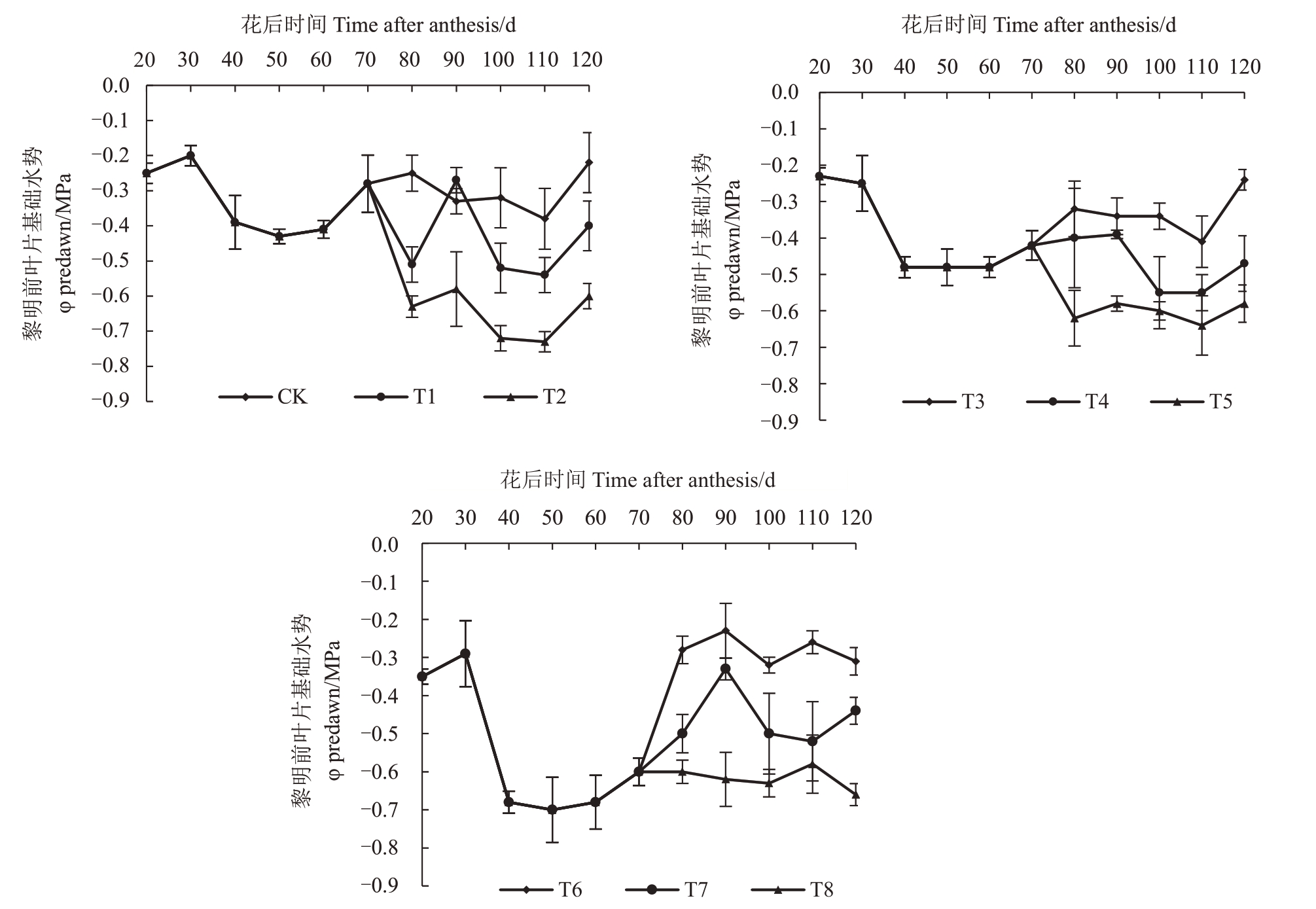
图2 葡萄植株黎明前叶片水势值
Fig.2 Leaf water potential values of grapevine before dawn
2.1.2 水分胁迫下采收期葡萄品质测定 由表2可知,水分胁迫对各个果实品质指标均有影响。在采收期对照果实百粒质量最高,为110.70 g,与T3 和T4 没有显著差异,其余各处理均低于对照;坐果期期至转色期采用的水分胁迫程度对果实百粒质量影响更大,此时期内重度水分胁迫严重降低果实百粒质量。采收期,T4 处理果实TSS 含量最高,为22.09%,T3、T4、T5和对照无显著差异;T1、T2和T8处理含量显著低于对照,坐果期至转色期采用中度水分胁迫处理,其含量较轻度和重度高。水分胁迫使可滴定酸含量降低,各处理均低于对照;T1-T4与对照可滴定酸含量差异不显著,T5-T8 显著低于对照,分别降低了20.51%、16.87%、22.40%和25.78%;转色期至采收期采用不同水分胁迫使果实中可滴定酸含量差异显著。采收期,各处理果实总酚含量均高于对照,T4、T2、T6、T3和T8处理总酚含量显著高于对照,分别提高了36.02%、36%、31.23%、27.97%和27.44%。除T6 和T8 外,所有处理果实单宁含量均高于对照,T5和T4、T1和T3处理差异性显著,与对照相比分别提高了20.80%、17.00%、11.93%和10.85%。水分胁迫提高了果实花色苷含量,其中T5、T8和T2最高,即转色期至采收期采用重度胁迫处理显著提高其含量,比对照提高了31.37%、28.15%和22.25%。
表2 不同水分胁迫的采收期赤霞珠葡萄果实品质
Table 2 Grape quality of Cabernet Sauvignon under water stress at harvest
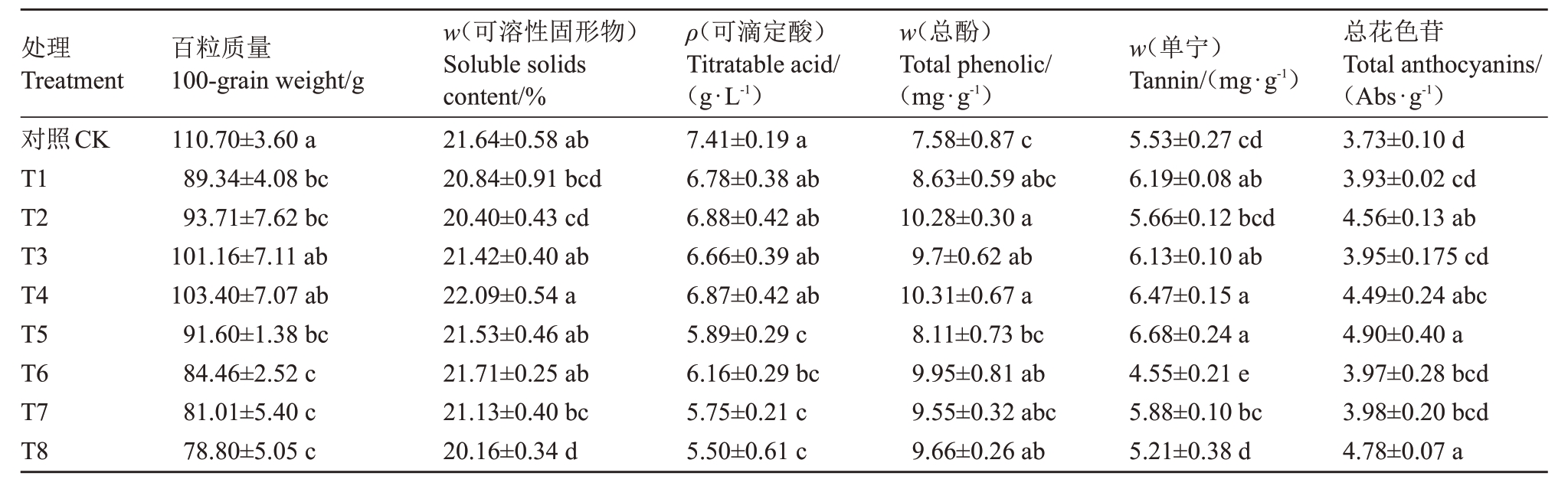
注:不同小写字母表示差异显著(p <0.05)。
Note:Different small letters indicates significant difference at p <0.05.
总花色苷Total anthocyanins/(Abs·g-1)3.73±0.10 d 3.93±0.02 cd 4.56±0.13 ab 3.95±0.175 cd 4.49±0.24 abc 4.90±0.40 a 3.97±0.28 bcd 3.98±0.20 bcd 4.78±0.07 a处理Treatment对照CK T1 T2 T3 T4 T5 T6 T7 T8 w(单宁)Tannin/(mg·g-1)5.53±0.27 cd 6.19±0.08 ab 5.66±0.12 bcd 6.13±0.10 ab 6.47±0.15 a 6.68±0.24 a 4.55±0.21 e 5.88±0.10 bc 5.21±0.38 d ρ(可滴定酸)Titratable acid/(g·L-1)7.41±0.19 a 6.78±0.38 ab 6.88±0.42 ab 6.66±0.39 ab 6.87±0.42 ab 5.89±0.29 c 6.16±0.29 bc 5.75±0.21 c 5.50±0.61 c百粒质量100-grain weight/g 110.70±3.60 a 89.34±4.08 bc 93.71±7.62 bc 101.16±7.11 ab 103.40±7.07 ab 91.60±1.38 bc 84.46±2.52 c 81.01±5.40 c 78.80±5.05 c片w(可溶性固形物)Soluble solids content/%21.64±0.58 ab 20.84±0.91 bcd 20.40±0.43 cd 21.42±0.40 ab 22.09±0.54 a 21.53±0.46 ab 21.71±0.25 ab 21.13±0.40 bc 20.16±0.34 d叶w(总酚)Total phenolic/(mg·g-1)7.58±0.87 c 8.63±0.59 abc 10.28±0.30 a 9.7±0.62 ab 10.31±0.67 a 8.11±0.73 bc 9.95±0.81 ab 9.55±0.32 abc 9.66±0.26 ab
2.2 不同水分胁迫下葡萄果实5种甲氧基吡嗪含量的差异
2.2.1 水分胁迫下葡萄果实3-异丁基-2-甲氧基吡嗪(IBMP)含量 水分胁迫下赤霞珠葡萄果实IBMP含量变化如图3所示。坐果至转色期(转色前)采用不同程度胁迫时,各处理均在40 DAA,IBMP 含量达到最大,轻度、中度和重度胁迫下IBMP 含量(ρ)依次为0.27 μg·L-1>0.21 μg·L-1>0.11 μg·L-1,且转色至成熟期(转色后)不同胁迫下IBMP降解速度不同。在转色前采用轻度胁迫时(图3-A1和3-B1),转色后中度和重度胁迫单粒果实IBMP含量显著低于轻度胁迫(T1和T2显著低于对照)。转色前采用中度胁迫时(图3-A2 和3-B2),采收期单粒果实中T4(中度胁迫)IBMP含量最低。转色前采用重度胁迫时(图3-A3和3-B3),采收期在单粒果实中轻度和中度处理下含量显著低于重度(T6 和T7 显著低于T8)。
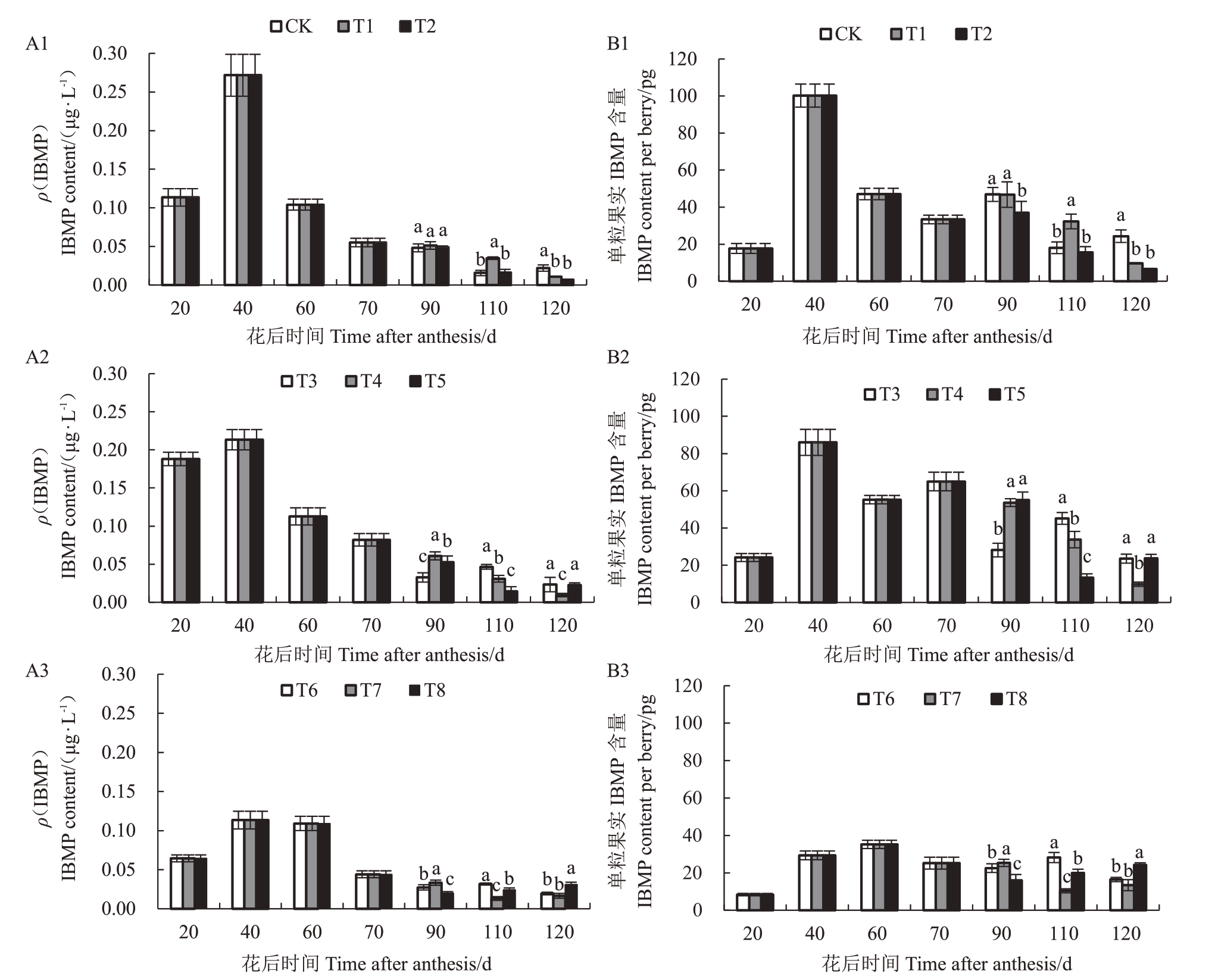
图3 水分胁迫下葡萄果实IBMP 含量
Fig.3 IBMP level in berries under water stress
A1、A2 和A3 分别为果实生长发育期内IBMP 含量变化;B1、B2 和B3 分别为单粒果实中IBMP 含量变化。
A1,A2 and A3 in the figure are the changes of IBMP content during fruit growth and development respectively,B1,B2 and B3 are the changes of IBMP content in per berry.
转色后轻度胁迫下(对照、T3 和T6),转色前重度处理含量最低(T6);转色后中度胁迫下(处理T1、T4 和T7),转色前中度胁迫处理最低(T4);转色后重度胁迫下(处理T2、T5和T8),转色前重度胁迫下IBMP 含量大于中度大于轻度(T8 大于T5 大于T2)。110 DAA,T5和T7处理,赤霞珠果实IBMP含量均低于对照;120 DAA,T5、T7 和T8 处理,果实IBMP含量反而有较大幅度升高。
在采收期,与对照相比各处理(除T4 和T8 外)均降低了果实中IBMP 含量,降低幅度为3.21%~72.82%。
2.2.2 水分胁迫下葡萄果实中3-异丙基-2-甲氧基吡嗪(IPMP)含量 葡萄浆果成熟过程中,各梯度水分胁迫IPMP 含量均呈逐渐降低的趋势(图4)。坐果期至转色期(转色前)采用不同程度水分胁迫时,20 DAA,各处理IPMP含量达到最大,单粒葡萄果实中IPMP含量先升高后降低。转色前采用轻度胁迫时(图4-A1 和4-B1),转色后重度胁迫可明显降低IPMP 含量(T2 显著低于T1 和对照)。在转色前采用中度胁迫时(图4-A2和4-B2),采收期在单粒果实中中度胁迫处理(T5)IPMP 含量最低。在转色前采用重度胁迫时(图4-A3和4-B3),采收期在单粒果实中重度IPMP 含量小于中度胁迫小于轻度胁迫(T8含量小于T7小于T6)。
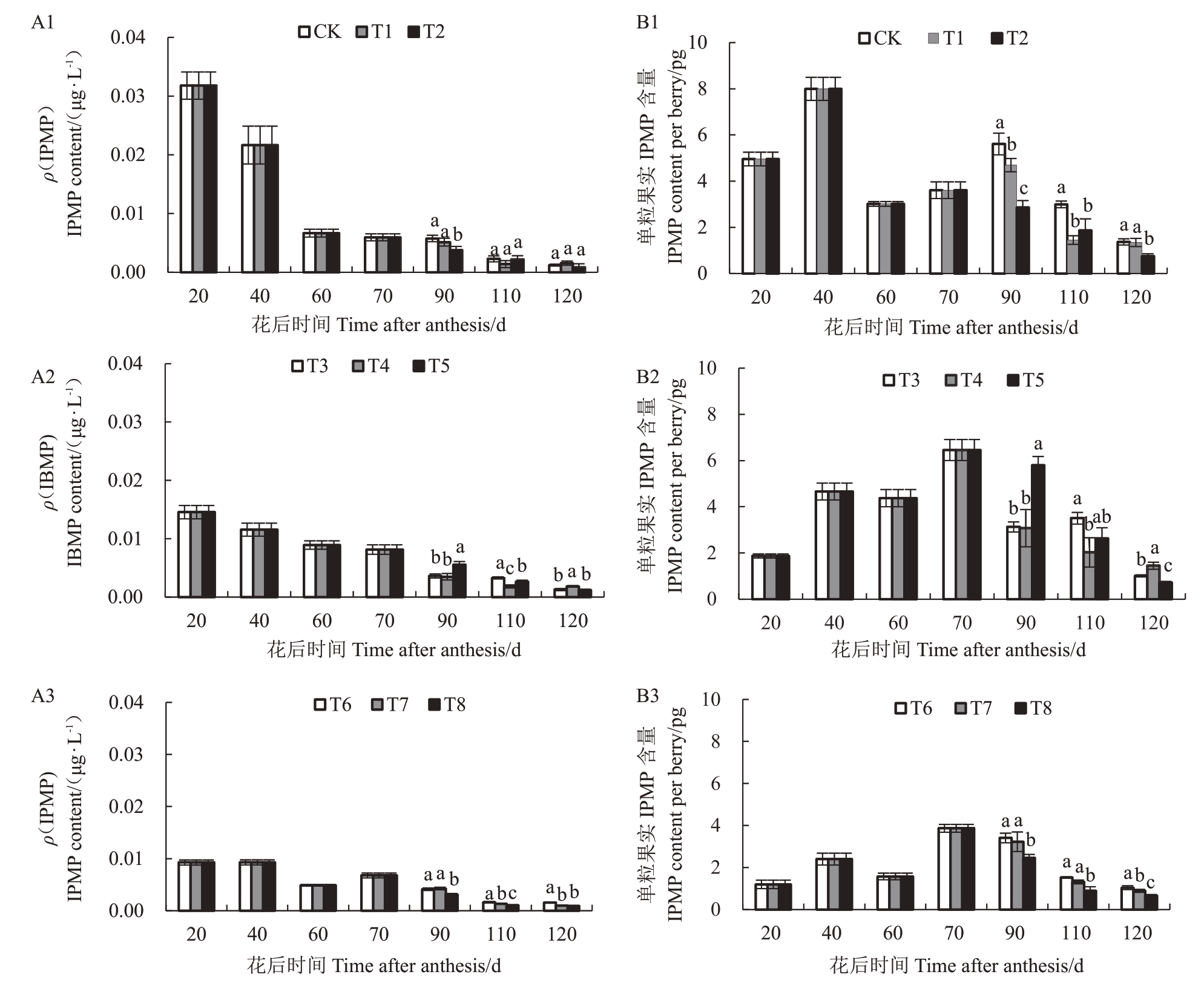
图4 水分胁迫下葡萄果实IPMP 含量
Fig.4 IPMP level in berries under water stress
A1、A2 和A3 分别为果实生长发育期内IPMP 含量变化;B1、B2 和B3 分别为单粒果实中IPMP 含量变化。
A1,A2 and A3 in the figure are the changes of IPMP content during fruit growth and development respectively,B1,B2 and B3 are the changes of IPMP content in per berry.
转色后轻度胁迫下(处理对照、T3 和T6),转色前采用中度胁迫处理含量最低(T3);转色后中度胁迫下(处理T1、T4和T7),转色前采用重度胁迫处理(T7)最低;转色后重度胁迫下(处理T2、T5 和T8),转色前采用重度胁迫小于中度胁迫小于轻度胁迫(T8含量小于T5小于T2)。
在采收期,与对照相比各处理(除T4外)均降低了果实中IPMP含量,降低幅度为2.19%~53.85%。
2.2.3 水分胁迫下萄果实中3-仲丁基-2-甲氧基吡嗪(SBMP)含量 在赤霞珠浆果生长过程中,各水分胁迫下SBMP含量均呈逐渐降低的趋势。在坐果至转色期(转色前)采用不同程度水分胁迫时,20 DAA,各处理SBMP 含量达到最大植,轻度胁迫、中度胁迫和重度胁迫依次为0.91 μg·L-1>0.87 μg·L-1>0.70 μg·L-1,同时单粒葡萄果实中SBMP含量均先升高后降低。
在转色前采用轻度水分胁迫时(图5-A1 和5-B1),转色后采用重度胁迫SBMP含量小于中度胁迫含量小于轻度胁迫含量(T2 小于T1 小于对照)。转色前采用中度胁迫时(图5-A2和5-B2),采收期在单粒果实中中度胁迫(T4)SBMP含量最低。转色前采用重度胁迫时(图5-A3和5-B3),采收期在单粒果实中重度胁迫和中度胁迫含量显著大于轻度(T8和T7显著大于T6)。
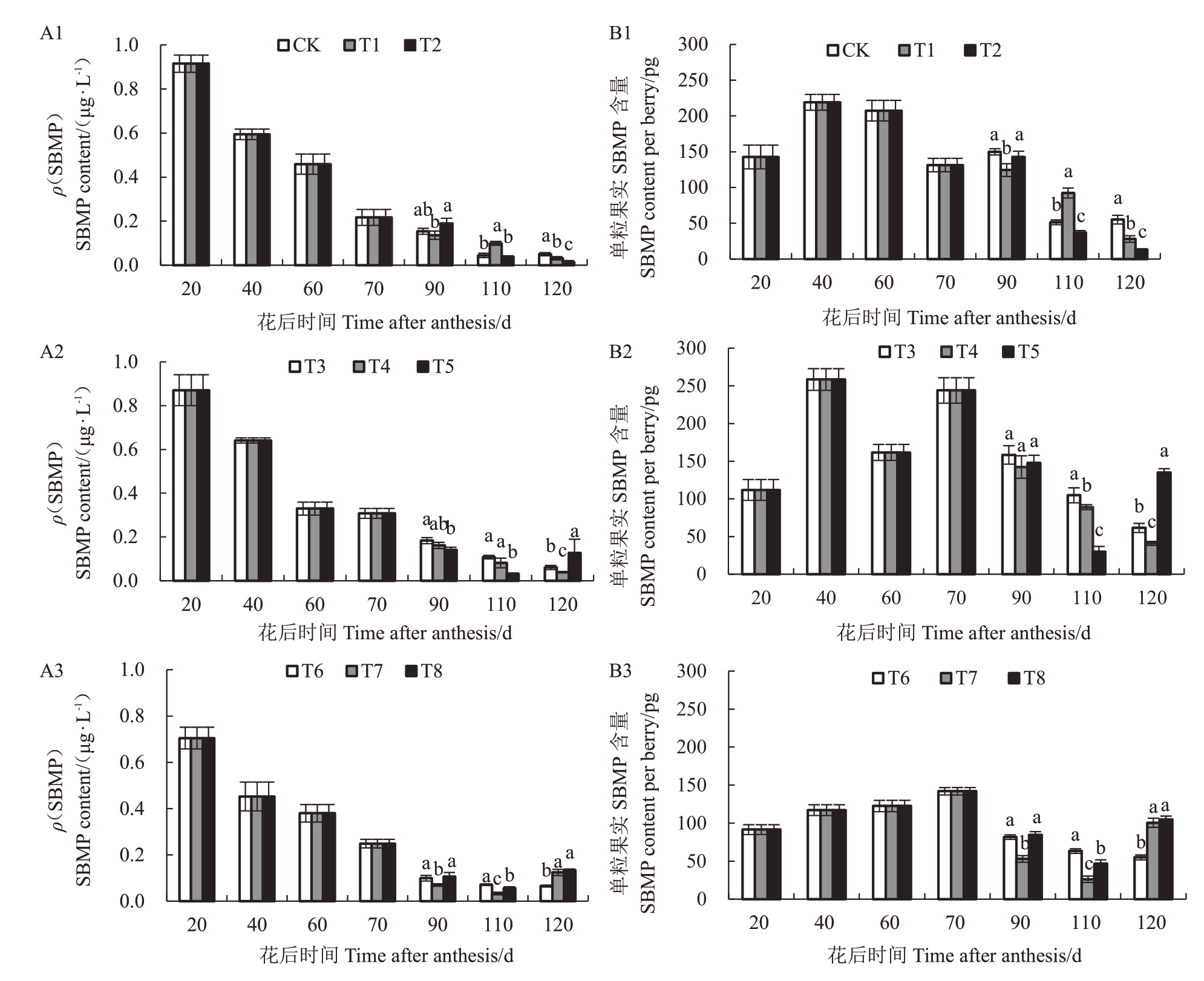
图5 水分胁迫下葡萄果实SBMP 含量差异
Fig.5 SBMP level in berries under water stress
A1、A2 和A3 分别为果实生长发育期内SBMP 含量变化;B1、B2 和B3 分别为单粒果实中SBMP 含量变化。
A1,A2 and A3 in the figure are the changes of SBMP content during fruit growth and development respectively,B1,B2 and B3 are the changes of SBMP content in per berry.
转色后轻度胁迫下(处理对照、T3 和T6),转色前采用重度处理含量(T6)最低;转色后中度胁迫和重度胁迫下,110 DAA,T2、T5 和T7 处理含量低于其他处理;120 DAA,T5、T7 和T8 葡萄果实SBMP含量反而升高。
在采收期,与对照相比各处理(除T4 和T8 外)均降低了果实中SBMP 含量,降低幅度为1.82%~76.36%。
2.2.4 水分胁迫下葡萄果实中2-甲氧基吡嗪(MOMP)含量 图6表明,在不同的物候期,各处理果实MOMP 含量存在差异,且均呈下降趋势。20 DAA,各处理MOMP 含量均达到最大植;60~70 DAA,各处理MOMP 快速下降,仅有轻度处理MOMP 含量可被检测出;20~70 DAA,MOMP 含量均为对照>中度胁迫>重度胁迫;70~120 DAA,各处理均未检测出MOMP。40~60 DAA,各处理单粒果实中MOMP含量最大,均为对照>中度胁迫>重度胁迫,每粒果实含量最大植分别为59.32 pg、49.59 pg和33.79 pg,水分胁迫可降低果实发育过程中MOMP 含量,完全转色到收获期,均未检测到MOMP。
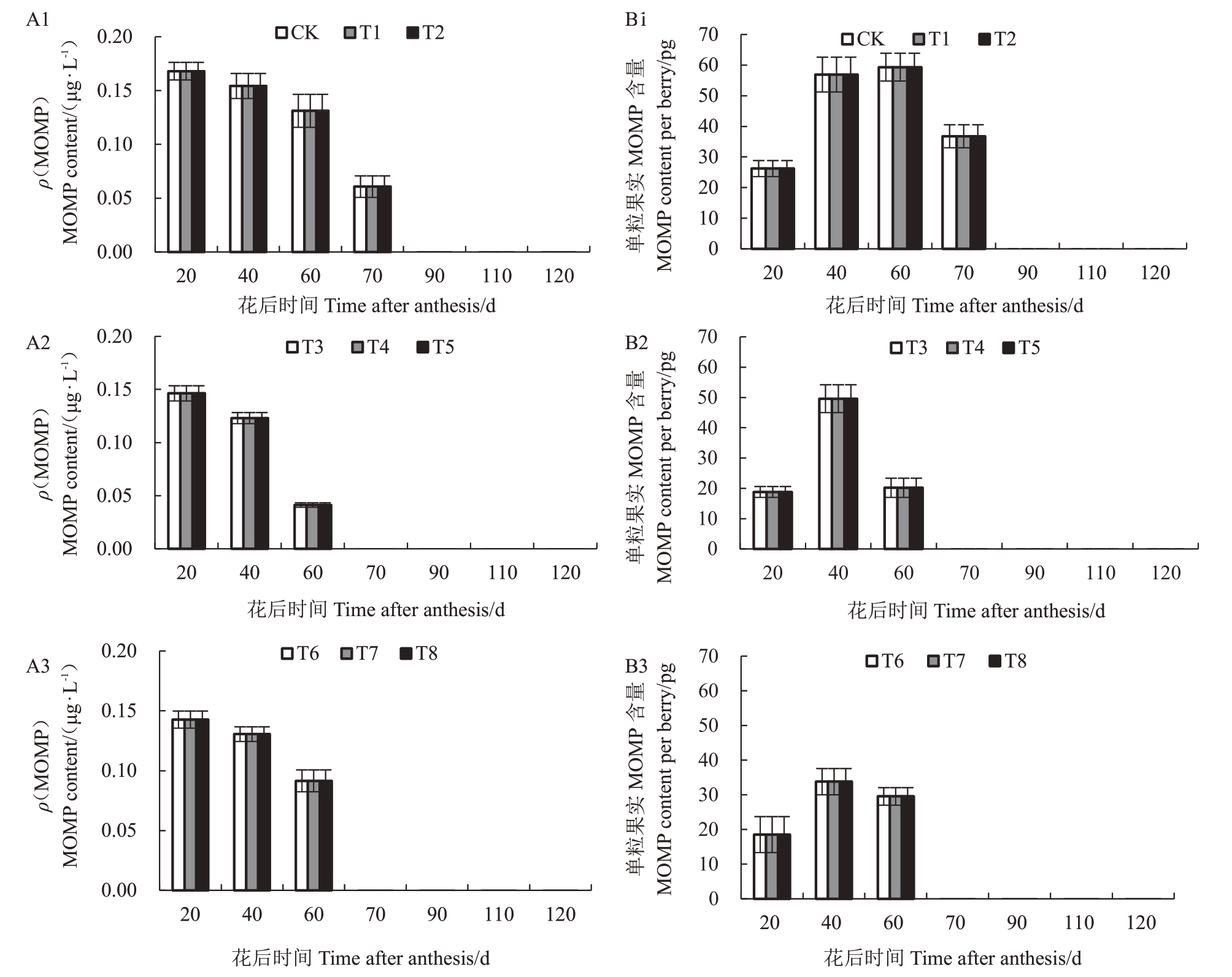
图6 水分胁迫下葡萄果实MOMP 含量
Fig.6 MOMP level in berries under water stress
A1、A2 和A3 分别为果实生长发育期内MOMP 含量变化;B1、B2 和B3 分别为单粒果实中MOMP 含量变化。
A1,A2 and A3 in the figure are the changes of MOMP content during fruit growth and development respectively,B1,B2 and B3 are the changes of MOMP content in per berry.
2.2.5 水分胁迫下葡萄果实中3-甲基-2-甲氧基吡嗪(MEMP)含量 赤霞珠葡萄果实MEMP 不同水分胁迫下含量变化如图7 所示。在不同的物候期,各处理果实MEMP 含量存在差异,且均呈下降趋势。各处理果实MEMP 含量在20 DAA 最大;60 DAA,MEMP含量为中度胁迫>重度胁迫>轻度胁迫;70 DAA,仅重度胁迫处理MEMP 含量可检测出,为0.067 μg·L-1。随着浆果的成熟,赤霞珠单粒葡萄果实中MEMP 含量均呈先升高后降低的变化趋势;40~70 DAA,各处理单果MEMP 含量达到最大,为重度胁迫>中度胁迫>对照,每粒果实MEMP 含量最大植分别为38.37、36.68 和31.46 pg;70~90 DAA内快速下降,此后未检测到其含量。
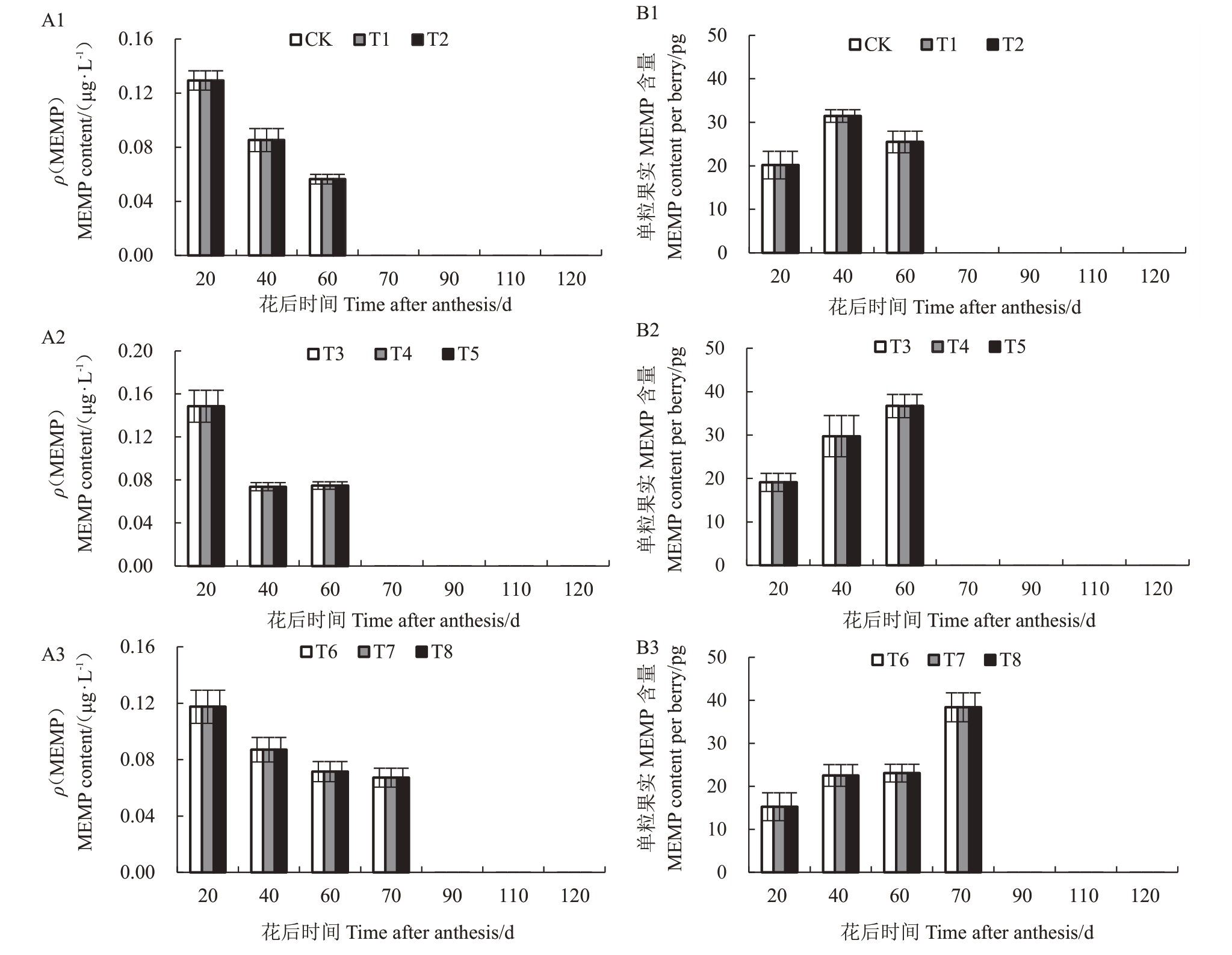
图7 水分胁迫下葡萄果实MEMP 含量
Fig.7 MEMP level in berries under water stress
A1、A2 和A3 分别为果实生长发育期内MEMP 含量变化;B1、B2 和B3 分别为单粒果实中MEMP 含量变化。
A1,A2 and A3 in the figure are the changes of MEMP content during fruit growth and development respectively,B1,B2 and B3 are the changes of MEMP content in per berry.
3 讨 论
3.1 水分胁迫对葡萄果实品质的影响
果实中的含糖量决定了葡萄酒的潜在酒精度,酸含量的高低影响葡萄酒的口感、香气、色泽及稳定性,单宁决定了葡萄酒的风味和结构,而花色苷是呈现葡萄酒颜色的主要物质。在葡萄不同物候期进行水分胁迫可以不同程度地改善葡萄浆果品质[21]。Acevedo-Opazo等[25]研究表明,水分胁迫可降低葡萄浆果质量,但是能提高果实TSS。本研究中,葡萄果实百粒质量随水分胁迫程度的增加而降低,转色前和转色后的水分胁迫均会显著减小成熟期果实质量和体积,尤其是转色期前进行水分胁迫对成熟期果实的质量影响最大。可能是由于水分不足限制了果实细胞快速分裂和增殖,导致浆果质量呈现不可逆的减小。Ju 等[26]研究表明,调亏灌溉会导致可溶性固形物含量上升,可滴定酸含量下降,pH植升高;本研究发现,适当的水分处理可加速葡萄浆果的成熟,转色后适当的水分处理会提高葡萄果实可溶性固形物含量,对总酸的降解有促进作用,但重度胁迫会阻碍葡萄果实有机酸降解。史晓敏等[27]研究发现适度的水分胁迫有助于赤霞珠葡萄叶片叶绿素的合成与积累,但持续的重度水分胁迫则会加速叶绿素的分解,光合作用降低,最终导致果实糖分积累的差异。可滴定酸含量的下降主要是由于浆果呼吸作用引起苹果酸代谢,以及果实体积的增加导致酸浓度的稀释。
Cáceres-Mella 等[28]研究表明,多酚类物质对水分胁迫也有着显著反应,水分胁迫处理可有效提高单宁含量,但不会降低葡萄果实的品质。Yang 等[29]利用转录组技术研究表明适度水分调亏灌溉上调了花青素生物合成途径中7 个相关基因的表达水平,也增加了部分代谢物的含量。Ju 等[26]研究表明,调亏灌溉会调控果实中花青素代谢,显著增加花色苷的含量。李云飞等[30]对紫叶矮樱进行干旱胁迫发现,重度胁迫降低了叶片中的花青素含量。本研究中发现,转色前中度水分胁迫处理的花色苷含量均高于重度胁迫处理且总体高于对照,表明中度水分胁迫处理可使葡萄果实转色提前,同时可能是水分胁迫促进了花青素相关基因的表达。因此适当的水分胁迫处理有利于总花色苷含量的积累,长期过度的重度胁迫处理使得葡萄浆果在成熟后期发生皱缩,使总花色苷浓度增加。水分胁迫对葡萄果实单宁的影响与之一致。综上所述,水分胁迫对葡萄果实品质的影响与水分胁迫施加的时间和强度均密切相关。
3.2 水分胁迫对葡萄果实甲氧基吡嗪含量的影响
甲氧基吡嗪(MPs)在葡萄果实和葡萄酒中含量适中时对葡萄酒香气有协调作用,但含量过高会降低葡萄酒的品质[31]。果实中MPs于葡萄转色前大量积累,随着果实成熟而被逐渐降解,且不同种类MPs降解速率不同,这与Ryona等[8]研究结果一致。本试验发现,中度和重度水分胁迫可降低赤霞珠葡萄果实中MPs 含量,有利于减少生青味。其中,MOMP和MEMP含量在单粒果实中转色期前逐渐积累,在70 DAA 之后均未被检测出,说明MOMP 和MEMP在葡萄采收期全部降解,可能转化为其他物质,对果实和葡萄酒中生青气味没有影响,这可能是葡萄果实中MOMP和MEMP鲜见报道的原因。
前期对IPMP、SBMP 和IBMP 在果实中的含量变化研究最多[32],其中IBMP 最为重要而且报道最多[8]。IBMP在水和白葡萄酒中阈植为0.5~2 ng·L-1,在红葡萄酒中为10~16 ng·L-1,SBMP在水中1 ng·L-1时即可被人感知,而IPMP 在水中的感官检测阈植为2 ng·L-1[17]。水分胁迫导致各处理浆果体积差异较大,计算了每种化合物的绝对含量(pg·浆果-1)更能说明甲氧基吡嗪在果实中的合成和降解[6, 10]。本研究发现单粒果实中IPMP、SBMP 和IBMP 均在转色前积累,在转色后含量持续下降,直至葡萄采收期,说明转色期是吡嗪代谢的关键节点,这与前人研究结果一致[8]。在采收期SBMP含量最高,SBMP>IBMP>IPMP,但这三种吡嗪含量均高于阈植,可被感官检测到,是生青气味的主要来源[33]。其中,转色前水分胁迫抑制了吡嗪合成,重度水分胁迫下IPMP、SBMP和IBMP含量显著降低,但轻度和中度水分胁迫差异较小。前人研究不同灌溉量和种植密度下葡萄酒中MPs含量发现灌溉使浆果中IBMP含量明显高于非灌溉植物果实[17]。
本研究发现,水分胁迫条件下赤霞珠葡萄果实中甲氧基吡嗪峰植出现于转色前或转色期内,其中重度水分胁迫处理其含量最低。可能是由于转色期前进行水分胁迫对MPs 合成相关基因表达具有抑制作用,从而降低了MPs 相关合成酶活性,最终抑制了MPs 合成与积累。120 DAA,T5 和T8 处理果实中SBMP 和IBMP 含量反而上升,可能是由于转色期后长期严重的水分胁迫降低了MPs 降解相关酶活性,抑制了葡萄果实MPs 降解。另外,有文献报道在甲氧基吡嗪生物合成最后一步,将没有挥发性的羟基吡嗪在甲氧基转移酶的作用下形成甲氧基吡嗪,其过程可逆[33]。因此,在后熟期,果实中吡嗪可能发生相互转化,使甲氧基吡嗪含量反而上升。
4 结 论
甲氧基吡嗪在葡萄果实转色前合成,转色后开始降解,水分胁迫可抑制其积累。转色前中度水分胁迫,转色后重度水分胁迫可提高采收期赤霞珠葡萄果实品质,同时显著降低甲氧基吡嗪含量。
[1] BOTEZATU A,PICKERING G J. Determination of ortho- and retronasal detection thresholds and odor impact of 2,5-dimethyl-3-methoxypyrazine in wine[J]. Journal of Food Science,2012,77(11):S394-398.
[2] SADOUGHI N,SCHMIDTKE L M,ANTALICK G,BLACKMAN J W,STEEL C C. Gas chromatography-mass spectrometry method optimized using response surface modeling for the quantitation of fungal off-flavors in grapes and wine[J]. Journal of Agriculture and Food Chemistry,2015,63(11):2877-2885.
[3] CALLEJÓN R M,UBEDA C,RÍOS-REINA R,MORALES M L,TRONCOSO A M. Recent developments in the analysis of musty odour compounds in water and wine:A review[J]. Journal of Chromatogr A,2016,1428:72-85.
[4] HjELMELAND A K,WYLIE P L,EBELER S E.A comparison of sorptive extraction techniques coupled to a new quantitative,sensitive,high throughput GC-MS/MS method for methoxypyrazine analysis in wine[J].Talanta,2016,148:336-345.
[5] 吕佳恒,管雪强,孙玉霞,王恒振,王世平,王俊芳.葡萄和葡萄酒中甲氧基吡嗪的研究进展[J].食品工业,2017,38(9):261-266.LÜ Jiaheng,GUAN Xueqiang,SUN Yuxia,WANG Hengzhen,WANG Shiping,WANG Junfang. Process of methoxypyrazine in grapes and wine[J].The Food Industry,2017,38(9):261-266.
[6] DUNLEVY J D,SOOLE K L,PERKINS M V,NICHOLSON E L,MAFFEI S M,BOSS P K. Determining the methoxypyrazine biosynthesis variables affected by light exposure and crop level in Cabernet Sauvignon[J].American Journal of Enology and Viticulture,2013,64:450-458.
[7] SIVILOTTI P,HERRERA J C,LISjAK K,BAšA H,SABBATINI P,PETERLUNGER E,CASTELLARIN S D. Impact of leaf removal,applied before and after flowering,on anthocyanin,tannin,and methoxypyrazine concentrations in 'Merlot' (Vitis vinifera L.) grapes and wines[J]. Journal of Agriculture and Food Chemistry,2016,64(22):4487-4496.
[8] RYONA I,LECLERC S,SACKS G L.Correlation of 3-isobutyl-2-methoxypyrazine to 3-isobutyl-2-hydroxypyrazine during maturation of bell pepper (Capsicum annuum) and wine grapes (Vitis vinifera)[J]. Journal of Agriculture and Food Chemistry,2010,58(17):9723-9730.
[9] ANDREA B,EDUARDO A. Methoxypyrazines in grapes and wines of Vitis vinifera cv. Carmenere[J]. American Journal of Enology&Viticulture,2007,58:462-469.
[10] GREGAN S M,JORDAN B. Methoxypyrazine accumulation and O-methyltransferase gene expression in Sauvignon Blanc grapes:the role of leaf removal,light exposure,and berry development[J].Journal of Agriculture and Food Chemistry,2016,64(11):2200-2208.
[11] KÖGEL S,BOTEZATU A,HOFFMANN C,PICKERING G.Methoxypyrazine composition of Coccinellidae-tainted Riesling and Pinot noir wine from Germany[J]. Journal of Science and Food Agricuture,2015,95(3):509-514.
[12] 刘文忠.葡萄酒中甲氧基吡嗪类物质的研究进展[J].中外葡萄与葡萄酒,2014(5):60-62.LIU Wenzhong. Research progress of methoxypyrazines in wine[J].Sino-Overseas Grapevine&Wine,2014(5):60-62.
[13] SIDHU D,LUND J,KOTSERIDIS Y,SAUCIER C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes,musts,and wines[J].Critical Reviews in Food Science and Nutrition,2015,55(4):485-502.
[14] PARR W V,GREEN J A,WHITE K G,SHERLOCK R R. The distinctive flavour of New Zealand Sauvignon blanc:sensory characterisation by wine professionals[J].Food Quality and Preference,2007,18(6):849-861.
[15] GREGAN S M,WARGENT J J,LIU L,SHINKLE J,HOFMANN R,WINEFIELD C,TROUGHT M,JORDAN B.Effects of solar ultraviolet radiation and canopy manipulation on the biochemical composition of Sauvignon Blanc grapes[J]. Australian Journal of Grape and Wine Research,2012,18(2):227-238.
[16] KOCH A,EBELER S E,WILLIAMS L E,MATTHEWS M A.Fruit ripening in Vitis vinifera:light intensity before and not during ripening determines the concentration of 2-methoxy-3-isobutylpyrazine in Cabernet Sauvignon berries[J]. Physiologia Plantarum,2012,145(2):275-285.
[17] SALA C,BUSTO O,GUASCH J,ZAMORA F. Contents of 3-alkyl-2-methoxypyrazines in musts and wines from Vitis vinifera variety Cabernet Sauvignon:influence of irrigation and plantation density[J]. Journal of the Science of Food and Agriculture,2005,85(7):1131-1136.
[18] HELWI P,HABRAN A,GUILLAUMIE S,THIBON C,HILBERT G,GOMES E,DELROT S,DARRIET P,LEEUWEN C V. Vine nitrogen status does not have a direct impact on 2-methoxy-3-isobutylpyrazine in grape berries and wines[J]. Journal of Agriculture and Food Chemistry,2015,63(44):9789-9802.
[19] MILDNER- SZKUDLARZ S,SIGER A,SZWENGIEL A,PRZYGOŃSKI K,WOJTOWICZ E,ZAWIRSKA- WOJTASIAK R. Phenolic compounds reduce formation of N(epsilon)-(carboxymethyl)lysine and pyrazines formed by Maillard reactions in a model bread system[J]. Food Chemistry,2017,231:175-184.
[20] 吕佳恒,管雪强,孙玉霞,王世平,王恒振.摘叶处理对‘赤霞珠’葡萄3-异丁基-2-甲氧基吡嗪积累的影响[J]. 北方园艺,2017,(11):23-28.LÜ Jiaheng,GUAN Xueqiang,SUN Yuxia,WANG Shiping,WANG Hengzhen. Effects of leaf removal on 3-isobutyl-2-methoxypyrazine accumulation in‘Cabernet Sauvignon’grape[J].Northern Horticulture,2017(11):23-28.
[21] 胡宏远.水分胁迫对赤霞珠葡萄生长、生理及品质的影响[D].银川:宁夏大学,2016.HU Hongyuan. Effects of water stress on growth,physiology and quality of Cabernet Sauvignon grape[D].Yinchuan:Ningxia University,2016.
[22] WILSON F M. Comparison of tannin levels in developing fruit buds of two orchard pear varieties using two techniques,folindenis and protein precipitation assays[J]. Journal of Chemical Ecology,1984,10:493-498.
[23] GIUSTI M. M,WROLSTAD R. E. Characterization and measurement of anthocyanins by UV-visible spectroscopy[J]. Food Analytical Chemistry, 2001, https://doi.org/10.1002/0471142913.faf0102s00.
[24] 姜文广,李记明,范文来,徐岩,于英.蛇龙珠葡萄成熟过程中6 种甲氧基吡嗪分析[J].食品工业科技,2012,33(4):163-167.JIANG Wenguang,LI Jiming,FAN Wenlai,XU Yan,YU Ying.Analysis of trace six 3-alkyl-2-methoxypyrazines evolution of Cabernet Gernischt during ripening[J]. Science and Technology of Food Industry,2012,33(4):163-167.
[25] ACEVEDO-OPAZO C,ORTEGA-FARIAS S,FUENTES S.Effects of grapevine (Vitis vinifera L.) water status on water consumption,vegetative growth and grape quality:An irrigation scheduling application to achieve regulated deficit irrigation[J].Agricultural Water Management,2010,97(7):956-964.
[26] JU Y L,YANG B H,HE S,TU T Y,MIN Z,FANG Y L,SUN X Y. Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in Cabernet Sauvignon(Vitis vinifera L.)grapes and wines[J].Plant Physiology and Biochemistry,2019,135:469-479.
[27] 史晓敏,刘竞择,张艳霞,陈祖民,郭帅奇,王振平.水分胁迫对‘赤霞珠’不同叶龄叶片光合特性的影响[J]. 果树学报,2021,38(1):50-63.SHI Xiaomin,LIU Jingze,ZHANG Yanxia,CHEN Zumin,GUO Shuaiqi,WANG Zhenping.Effects of water stress on photosynthetic characteristics of‘Cabernet Sauvignon’at different leaf ages[J].Journal of Fruit Science,2021,38(1):50-63.
[28] CÁCERES-MELLA A,TALAVERANO M I,VILLALOBOSGONZÁLEZ L,RIBALTA-PIZARRO C,PASTENES C. Controlled water deficit during ripening affects proanthocyanidin synthesis,concentration and composition in Cabernet Sauvignon grape skins[J]. Plant Physiology Biochemistry,2017,117:34-41.
[29] YANG B H,HE S,LIU Y,LIU B C,JV Y L,KANG D Z,SUN X Y,FANG Y L.Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries[J]. Food Chemistry,2020,314:126170.
[30] 李云飞,李彦慧,王中华,关楠,冯晨静,杨建民.土壤干旱胁迫对紫叶矮樱叶片呈色的影响[J]. 生态学报,2009,29(7):3678-3684.LI Yunfei,LI Yanhui,WANG Zhonghua,GUAN Nan,FENG Chenjing,YANG Jianmin. Effect of soil drought stress on leaf coloration-emerging of Prunus cistenena cv. Pissardii[J]. Acta Ecologica Sinica,2009,29(7):3678-3684.
[31] CRISTINA S,OLGA B,JOSEP G,FERNANDO Z.Influence of vine training and sunlight exposure on the 3-alkyl-2-methoxypyrazines content in musts and wines from the Vitis vinifera variety Cabernet Sauvignon[J]. Journal of Agriculture and Food Chemistry,2004,52(11):3492-3497.
[32] KOCH A,DOYLE C L,MATTHEWS M A,WILLIAMS L E,EBELER S E. 2-Methoxy-3-isobutylpyrazine in grape berries and its dependence on genotype[J]. Phytochemistry,2010,71(17/18):2190-2198.
[33] ZHAO X F,JU Y L,WEI X F,DONG S,SUN X Y,FANG Y L.Significance and transformation of 3-alkyl-2-methoxypyrazines through grapes to wine:Olfactory properties,metabolism,biochemical regulation,and the HP-MP cycle[J].Molecules,2019,24(24):4598.