杧果(Mangifera indica L.)是中国重要的热带果树,广泛种植分布于海南、广东、广西、云南等热带、南亚热带冬春干旱少雨区域。成花诱导对木本果树果实产量形成至关重要,杧果成花诱导始于叶芽休眠,止于花芽开始形态分化,低温、干旱和赤霉素合成抑制剂多效唑(PBZ)、烯效唑喷施等可以诱导杧果成花[1-2]。在中国海南产区,常采用土壤淋施PBZ 结合叶面喷布诱导杧果反季节开花,实现产期提前;在广东雷州半岛和广西百色等杧果产区,由于冬春低温不足,花量少,生产中也常采用PBZ 控梢促花增加杧果花量。然而,长期使用PBZ 或土施PBZ用量控制不当容易导致土壤残留、树势衰退,影响产量和果实品质[3-4]。
前人研究证实了PBZ 可以抑制赤霉素生物合成促进杧果开花[5]。除PBZ外,烯效唑、调环酸钙和缩节胺等也是赤霉素合成抑制剂,它们已被证明可以促进苹果、杨梅和葡萄等果树成花[6-10]。烯效唑、调环酸钙能抑制赤霉素生物合成中贝壳杉烯氧化酶(KO)的活性,阻碍赤霉素的生物合成;缩节胺通过抑制GA信号转导诱导成花[11-13]。然而,这些药剂对杧果开花调节的效果与作用机制尚未得到评估。
多年生果树中的一些特定的代谢物参与了成花诱导。然而,这些研究主要集中在叶片和花芽中碳水化合物和内源激素在成花诱导期间的变化[14-15]。前人研究还评估了葡萄糖、蔗糖和黄腐酸等代谢物与PBZ 对成花诱导的影响[16-18]。早期报道表明,其他代谢物(如氨基酸和酚类)也参与了杧果成花诱导[19]。Osuna-Enciso 等[20]报道杧果花蕾中的氨基酸含量高于营养芽,表明开花期间增加了对氨基酸的需求。在拟南芥中,茎分生组织中磷脂酰胆碱(PC)的增加加速了开花,而其减少延迟了开花[21-22]。
代谢组学是继基因组学、转录组学和蛋白质组学之后的新兴组学科学。前人基于超高效液相色谱串联质谱(UPLC-MS/MS)的广泛靶向代谢组学,在各种植物物种中进行大规模代谢物分析[23-24]。然而,通过代谢组学方法确定的杧果成花诱导不同阶段的代谢产物变化尚未有报道。
笔者在本研究中评估了烯效唑、调环酸钙和缩节胺组成的复配药剂SPD 对杧果开花率和产量的影响,并观察了SPD/清水处理后不同时间(30、60、80、100、120 d)的花蕾形态特征。此外,还进行了UPLC-MS/MS分析,以获得杧果顶芽全面和动态的代谢谱,并探索杧果成花诱导期间响应SPD处理的关键代谢物和代谢途径。该研究结果将为亚热带地区杧果开花管理提供一种潜在的方法,并为应用SPD 调控杧果开花的内在机制的揭示提供理论依据。
1 材料和方法
1.1 植物材料与处理
于2019—2021 年在中国热带农业科学院南亚热带作物研究所(广东湛江)的试验果园(110°16′E,21°10′N)随机选择15年生、长势树龄等一致的台农1号杧果树18株。2019年9月下旬和2020年9月下旬,第二次抽梢叶片变绿时,对9株杧果树喷洒自制外源赤霉素抑制剂SPD组合(以ρ计)(缩节胺:2 g·L-1,调环酸钙:100 mg·L-1,烯效唑:300 mg·L-1;经前期正交试验筛选获得),喷洒至药剂混合液凝而不滴,另对9 株杧果树喷洒清水作为对照(CK)。试验采用随机区组设计,每组处理3次重复,每次重复3株树。
2020 年10 月—2021 年1 月,基于形态特征变化,收集3个阶段的顶芽(SPD/清水处理30、80、100 d后)。并给样品命名(T-30:TA;T-80:TB;T-100:TC;C-30:A;C-80:B;C-100:C)。每组样品设3 个生物学重复。每个生物学重复取自3株树的12个芽。收集的顶芽样品立即放入液氮中,并储存在-80 ℃的冰箱中。在SPD/水处理140 d 后(盛花期),通过每株树开花的枝条数量与每株树的总枝条数量的比率,计算花芽率。当杧果达到商业收获成熟时,统计每株树的杧果产量。
1.2 代谢谱检测
代谢物提取:对芽样品如前所述进行预处理和提取[25]。将上述冷冻顶芽样品冻干,并在研磨仪(MM 400,Retsch)以30 Hz 的频率研磨1.5 min,直至样品为粉末状。然后,将100 mg的粉末在1.2 mL 70%甲醇提取液中充分溶解。每30 min 涡旋1 次,每次持续30 s,共涡旋6次,样本置于4 ℃冰箱过夜;然后以12 000 r·min-1的转速离心10 min,吸取上清液,用0.22 μm 孔径的微孔滤膜过滤后加入进样瓶中,用于UPLC-MS/MS分析。
液相条件主要包括:顶芽提取物用超高效液相色 谱(ultra performance liquid chromatography,UPLC,SHIMADZU Nexera X2,https://www.shimadzu.com.cn/)和串联质谱(Tandem mass spectrometry,MS/MS,Applied Biosystems 4500 QTRAP,http://www.appliedbiosystems.com.cn/)系 统 进 行 分析。首先,将等分试样注入UPLC 系统中的Agilent SB-C18 色谱柱(1.8 m,2.1 mm×100 mm)。在样品分析过程中,流动相的组成和比例略有变化。使用流动相溶剂A(含0.1%甲酸的超纯水)和溶剂B(含0.1%甲酸的乙腈)以及如下梯度程序实现分离:0 min:95%A,5%B;9 min:5%A,95%B;维持1 min,10.00~11.10 min,B 相比例降为5%,并以5%平衡至14 min;在40 ℃的柱温下,流速为0.35 mL·min-1。
质谱条件主要包括:流出物交替连接至AB4500 QTRAP UPLC/MS/MS系统,配备ESI Turbo离子喷雾接口,在正负离子模式下运行,并由分析软件控制(Analyst 1.6.3 软件控制)。操作参数与之前的研究相似[26]。使用550 ℃的离子源温度和5500 V(正离子模式)/-4500 V(负离子模式)的离子喷雾电压;离子源气体I(GSI),气体II(GSII)和帘气(CUR)分别设定在0.35、0.41和0.17 MPa。最后,根据在此期间洗脱的代谢物,对每个时期监测1 组特定的MRM离子对。
1.3 代谢物定性和定量分析
定性分析:质谱数据基于自建的纯化代谢物标准数据库和与MWDB(中国武汉迈特维尔生物技术有限公司)合作的公共代谢物数据库进行分析。将准确的前体离子(Q1)和产物离子(Q3)植、保留时间和碎片模式与标准进行比较,以分析主要和次要的质谱信息,然后去除少量重复信号[27]。
定量分析:采用三重四极杆质谱(QQQ)的MRM 模式进行定量分析,初步排除其他分子量物质对应的离子以避免干扰,并搜索MRM 模式下目标物质的母离子,结果的精密度和重复性更高。
1.4 统计分析
所有样本的代谢物数据标准化由R(v3.63,http://www.r-project.org)中的scale函数计算,使用prcomp函数进行主成分分析(PCA),由pheatmap 包(v1.0.12)中的pheatmap 函数进行聚类分析(HCA)。通过R 中的MetaboAnalystR(v1.0.1)包进行正交偏最小二乘判别分析(OPLS-DA)[28]。OPLSDA 中变量重要性投影(VIP)和差异倍数(FC)被用来鉴定差异代谢物(VIP≥1 和log2FC|foldchange|≥1)。将注释的代谢物比对到KEGG 数据库(https://www.genome.jp/kegg/pathway.html)以确定关键通路。对差异代谢物进行KEGG 富集分析;p <0.05的途径被认为显著富集。
2 结果与分析
2.1 SPD处理对杧果开花率和产量的影响
连续2 a(2020—2021 年)统计喷施SPD 和清水处理杧果树的开花率和单株产量(图1)。2020年和2021年,SPD处理的开花率和单株杧果产量显著高于对照,连续2 a 开花率超过80%。2021 年,SPD 处理的单株产量较2020年略有下降,可能与其他客观因素有关。结果证实了SPD 对促进杧果成花诱导有效。
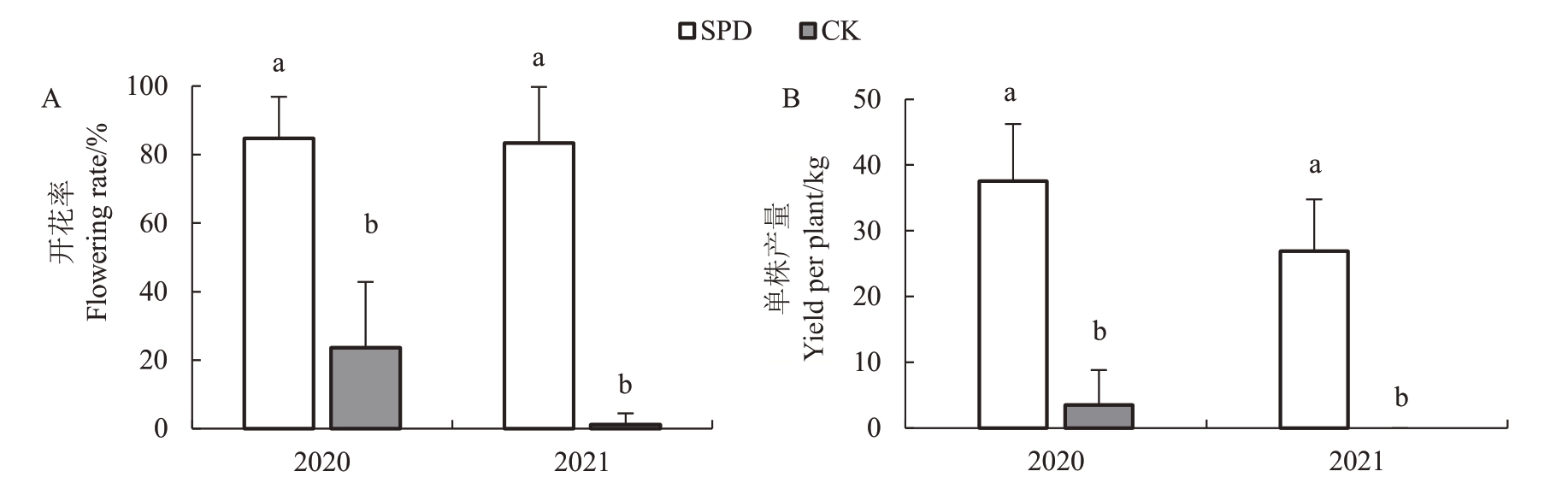
图1 2020 年和2021 年开花率和单株产量比较
Fig.1 Comparison of flowering rate and yield per tree in 2020 and 2021
2.2 SPD处理后顶芽的形态特征
环境诱导的休眠芽和生长芽都能发育成花芽[29]。杧果顶芽的生长点被绿色或褐色鳞片覆盖,这与一些多年生热带常绿植物的芽不同,如荔枝的芽是裸露的[30]。随着第二趟梢的成熟,用SPD/清水处理30 和60 d 后,休眠芽瘦小,被紧密的绿色鳞片相互覆盖,顶芽被隐藏其中。然后,芽在SPD 处理80 d后开始膨胀并暴露出内叶原基,但用水处理后没有明显变化。在SPD处理100 d后,鳞片分离,顶芽出现类似于毛笔头(该特征在生产中有形成花序的希望)的外观形态,清水处理100 d后的芽,鳞片略有松动,没有毛笔头的出现。此后,花穗在SPD 处理120 d后开始发育,可以观察到第一个花原基,而用清水处理过的芽鳞片轻度分离,尚未出现毛笔头(图2)。
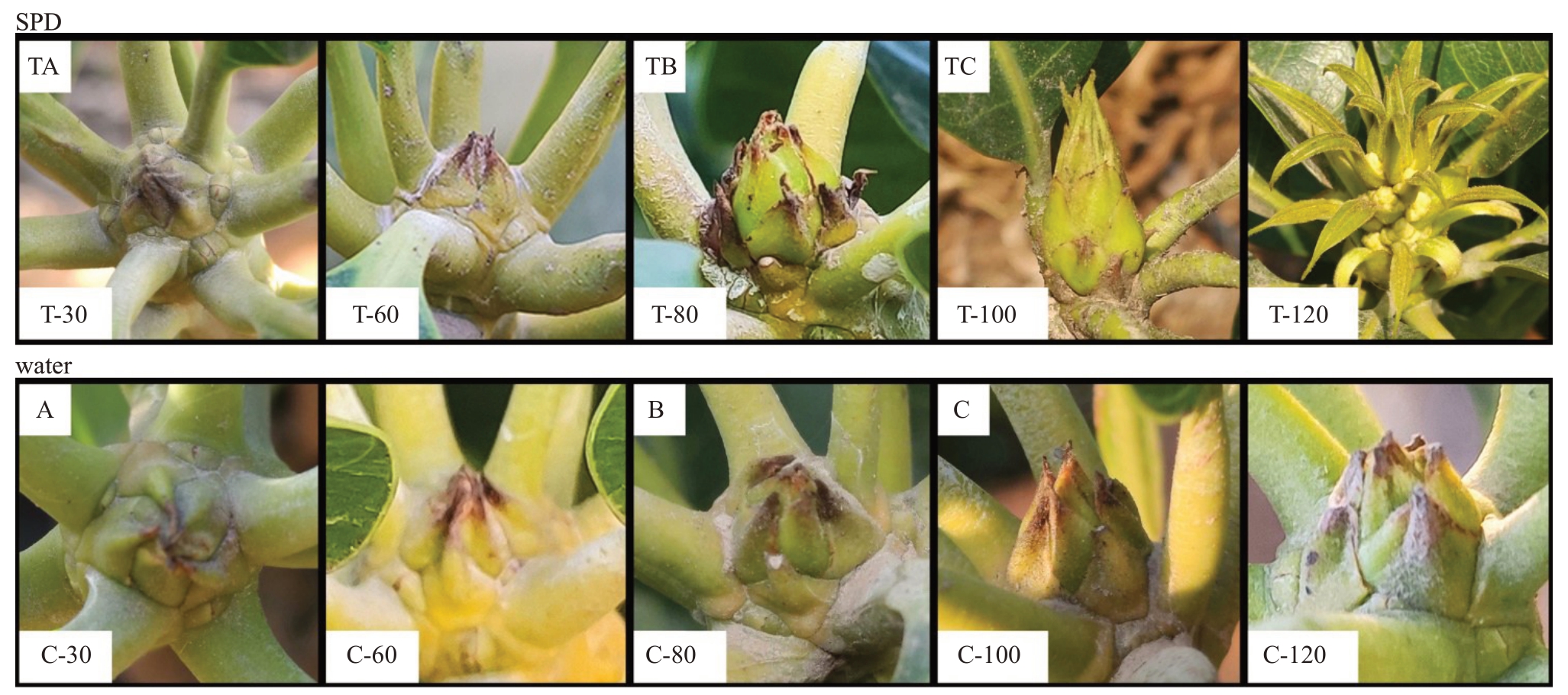
图2 杧果成花芽诱导和分化不同阶段的形态变化
Fig.2 Morphological changes of mango buds at different stages of floral induction and initiation
TA,TB,TC,A,B,C. 样品名称;T-30/C-30. 在SPD/清水处理30 d 后杧果顶芽;T-60/C-60. 在SPD/清水处理60 d 后杧果顶芽;T-80/C-80. 在SPD/清水处理80 d 后杧果顶芽;T-100/C-100. 在SPD/清水处理100 d 后杧果顶芽;T-120/C-120. 在SPD/清水处理120 d 后杧果顶芽。
TA,TB,TC,A,B,C.Sample name;T-30/C-30.Mango stem apex after SPD/water treatment for 30 days;T-60/C-60.Mango stem apex after SPD/water treatment for 60 days;T-80/C-80. Mango stem apex after SPD/water treatment for 80 days;T-100/C-100. Mango stem apex after SPD/water treatment for 100 days;T-120/C-120.Mango stem apex after SPD/water treatment for 120 days.
2.3 SPD处理不同时间顶芽的代谢特征分析
在SPD/清水处理30、80、100 d 后,总共从杧果顶芽中检测出582 种代谢物。对582 种代谢物进行主成分分析(PCA),PC1 和PC2 分别解释了总变异的39.62%和19.31%。18 个顶芽样品分布在不同的簇中,这意味着在SPD/清水处理30、80、100 d 后顶芽之间的代谢特征不同,且表型特征的变化一致(图3-A)。此外,生物重复的相关系数大于0.97,而不同样品之间的相关性较低,表明样品重复性相对较好(图3-B)。此外,将每个代谢物峰面积进行lg转换,以消除数植对代谢模式的影响,并进行聚类分析(HCA)。该分析揭示了分别与TA、TB、TC、A、B 和C 相关的6 个不同组(图4)。PAC 和HCA 说明3 个阶段顶芽样品中代谢产物有明显的差异,且样品重复性较好,适合进一步分析。
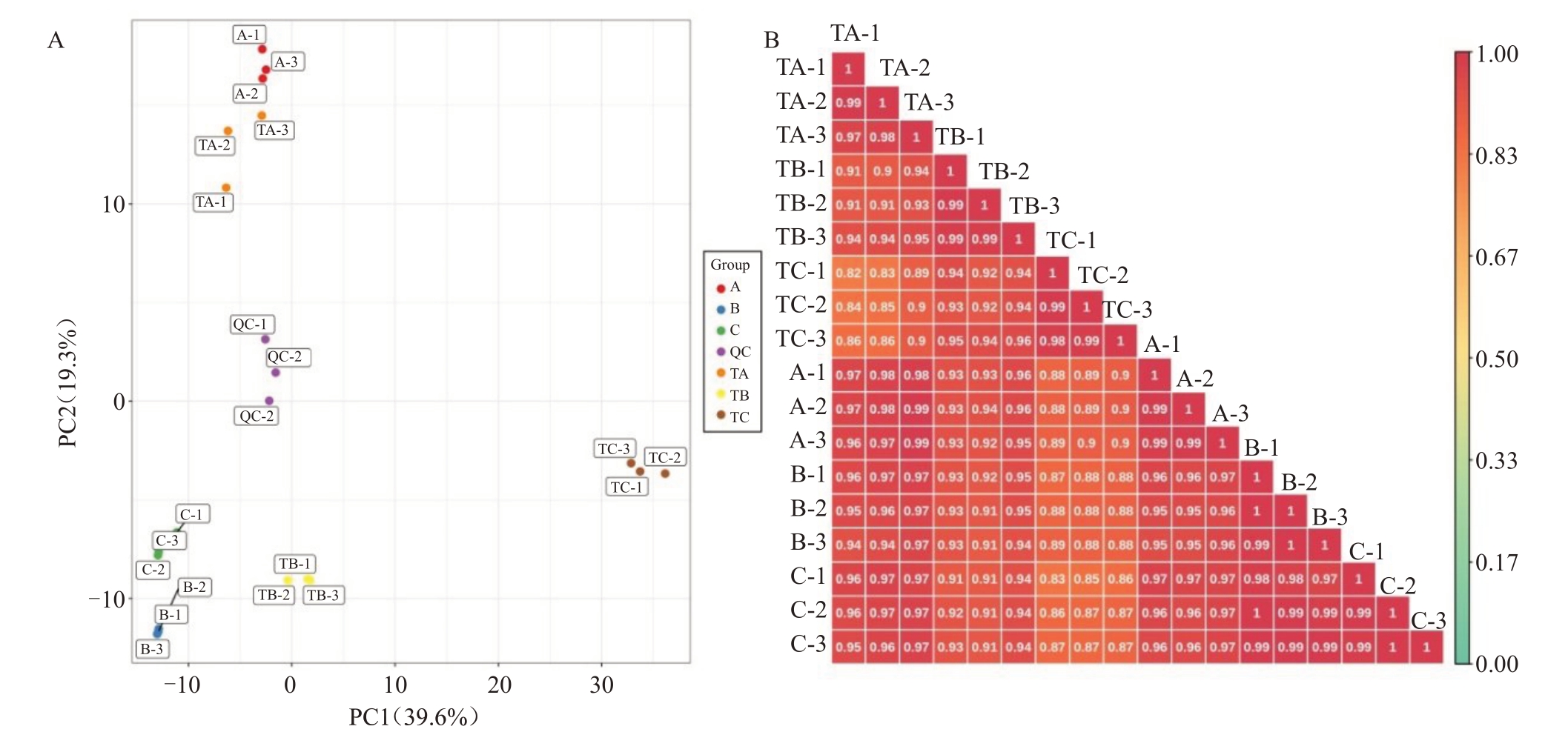
图3 杧果顶芽代谢物的主成分(A)及代谢物含量的相关性分析(B)
Fig.3 Principal component analysis of mango apical bud metabolites(A)and sample correlation analysis of metabolite contents(B)
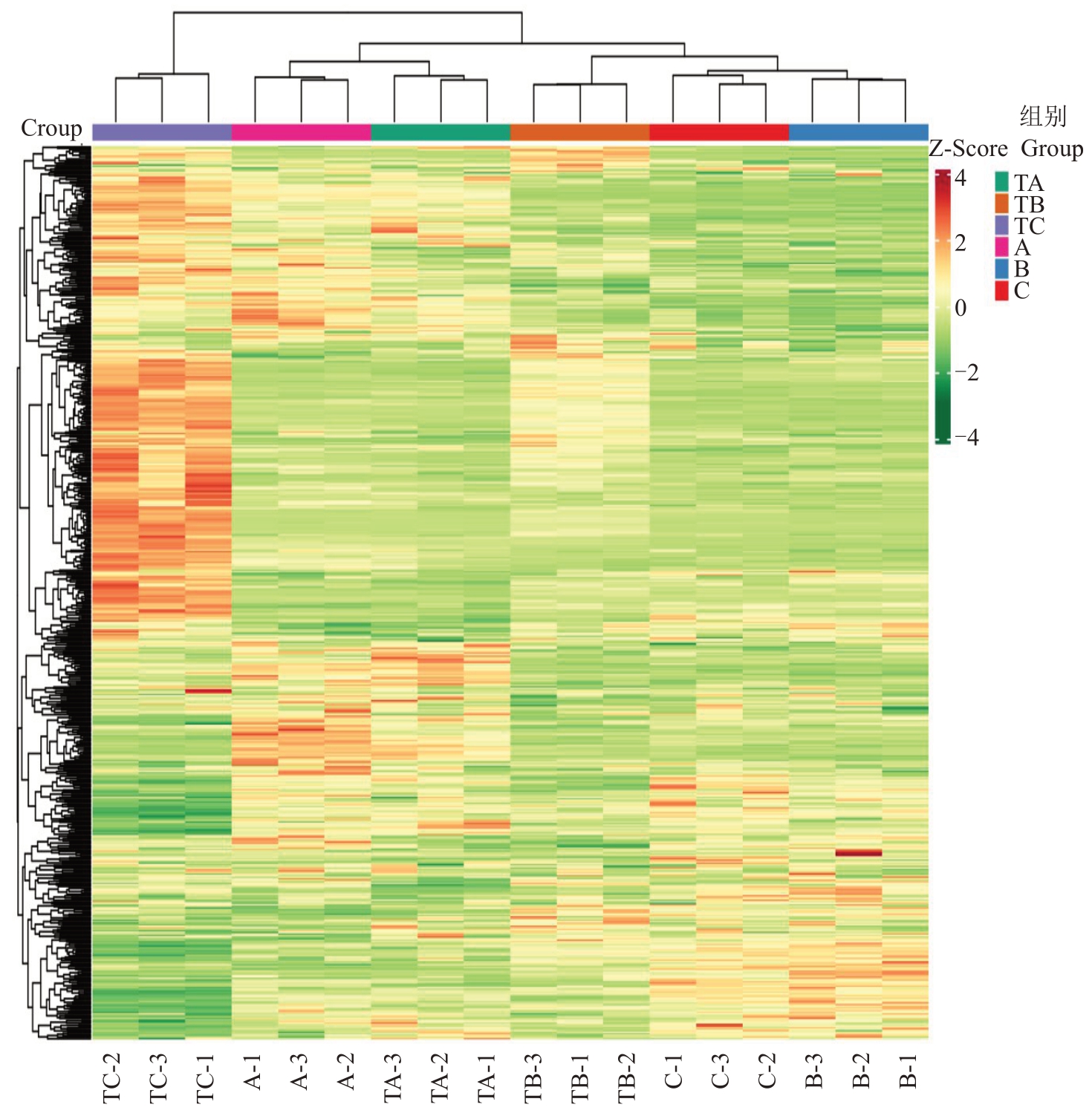
图4 杧果顶芽代谢物聚类分析
Fig.4 Cluster analysis of metabolites from mango stem apex
进一步在A vs B、A vs C、B vs C、TA vs TB、TA vs TC、TB vs TC、A vs TB、B vs TB和C vs TC中筛选差异代谢物,首先基于OPLS-DA 模型的VIP 植(VIP≥1)进行初步筛选,然后选择FC≥2(上调)或≤0.5(下调)的代谢物作为差异代谢物进行进一步分析,以鉴定SPD/清水处理不同时间后杧果顶芽之间或不同处理下顶芽之间的差异代谢物。从9组比较中共鉴定出372 种代谢物(图5)。结果显示SPD 处理后,随着成花诱导进程,差异代谢物数量逐渐增加,而对照数量逐渐减少,且同时期比较数量增加,此结果与其形态特征一致。
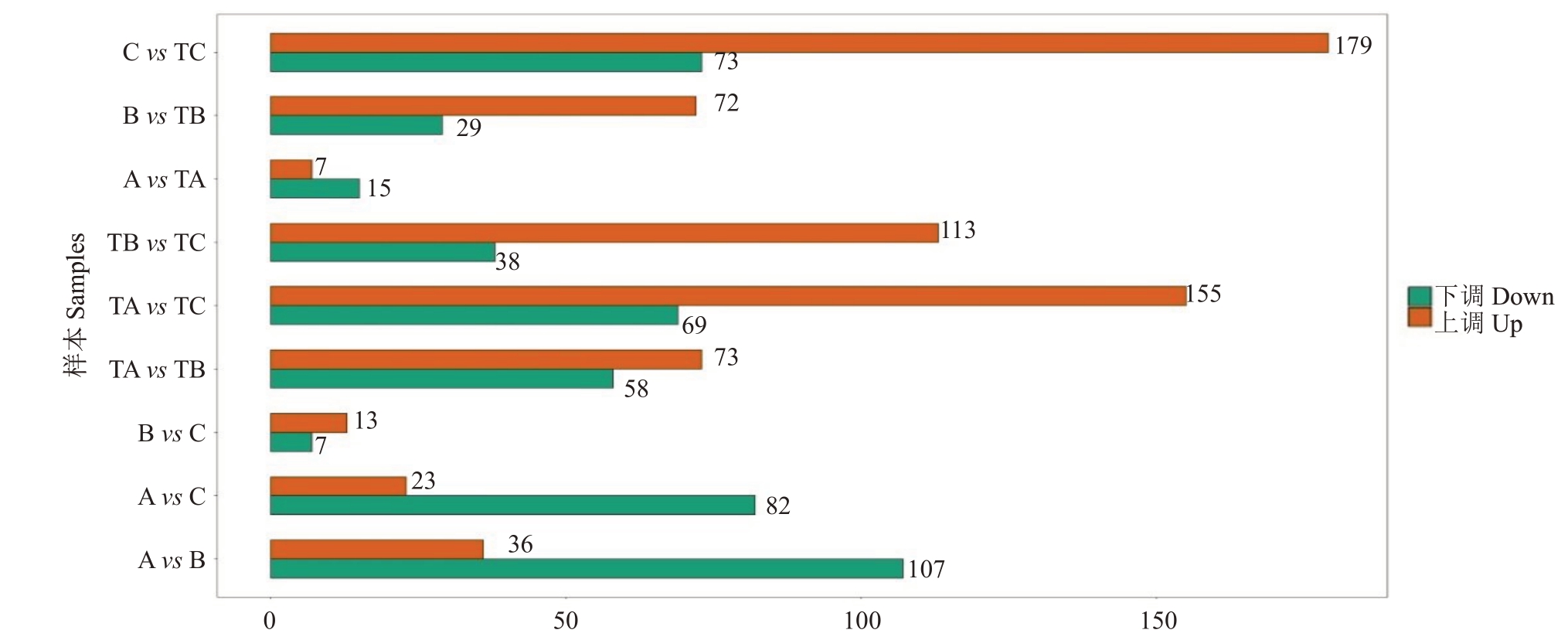
图5 不同样品间代谢产物的差异
Fig.5 Differential metabolites between different sample
差异代谢物数量Number of differential metabikutes
2.4 差异代谢物的KEGG富集分析
将差异代谢物(A vs TA:22,B vs TB:101,C vs TC:252)比对到KEGG 数据库。结果表明,大多数代谢途径与次生代谢产物的生物合成有关。少量代谢产物与氨基酸的生物合成有关。只有最少比例的代谢物比对到ABC转运蛋白、2-氧代羧酸代谢和氨酰-tRNA生物合成等。对差异代谢物的KEGG富集分析,以确定A vs TA、B vs TB、C vs TC代谢途径的差异。富集分析(图6)表明,甘氨酸、丝氨酸和苏氨酸代谢、半胱氨酸和蛋氨酸代谢、玉米素生物合成、氨基酸生物合成、甘油脂类代谢、色氨酸代谢、苯丙氨酸酪氨酸和色氨酸生物合成、光合生物的固碳作用和脯氨酸代谢在B vs TB,C vs TC中显著富集(P植<0.05)。

图6 差异代谢物的KEGG 富集分析
Fig.6 KEGG enrichment analysis of differential metabolites
2.5 SPD处理后成花诱导期间脂质、酚酸和氨基酸的鉴定与分析
重点关注对SPD 处理作出反应且成花诱导期间具有显著差异的代谢物,通过SPD和水处理比较它们在不同时间后的积累模式。在成花诱导期间鉴定了53 种代谢物(图7-A),这些代谢物在SPD 处理后,在TA vs TB 和TB vs TC 均发生显著变化,且其积累模式与对照不同(图7-B),包括20种脂质、18种酚酸、7 种氨基酸及其衍生物、5 种核苷酸及其衍生物、2 种有机酸和1 种其他。结果表明,这些代谢产物,尤其是脂质类、酚酸类和氨基酸类,可能在杧果顶芽对SPD 处理的响应中起关键作用。脂质(20)中的溶血磷脂酰乙醇胺(12,LPE)、溶血磷脂酰胆碱(7,LPC)和游离脂肪酸(1,FA),在SPD 处理80~100 d后显著增加,但在清水处理后无明显变化。结果表明,SPD处理作用于杧果FA的分解和合成。同时,18 种酚酸中有12 种在SPD 处理30~80 d 后显著增加,80~100 d 持续增加,但在清水处理30~80 d 后显著增加,80~100 d无明显变化。其中,4种单宁类化合物(1,4,6-三-O-没食子酰-β-D-葡萄糖,1,3,6-三-O-没食子酰-β-D-葡萄糖,2,4,6-三-O-没食子酰-D-葡萄糖,1,2,3,6-四-O-没食子酰-β-D-葡萄糖)30~100 d持续升高,且SPD处理后各阶段相对含量均高于其他酚酸。此外,7 种氨基酸在SPD 处理后随成花诱导显著增加,而在清水处理的各个阶段均无显著变化;在SPD 处理下,L-高丝氨酸(乙烯合成的前体物质)、L-丝氨酸和L-苏氨酸相对含量最高。此外,脯氨酸及其生物合成的前提物质在SPD处理30~100 d后呈现持续增加,然而在清水处理30~100 d 后没有发生显著变化(图7-C)。

图7 成花诱导关键代谢物的鉴定和分析
Fig.7 Identification and analysis of key metabolites in floral induction
A.TA vs TB,TB vs TC 差异代谢物的韦恩图;B.成花诱导3 个阶段53 种差异代谢物热图;C.成花诱导3 个时期杧果顶芽脯氨酸生物合成途径中代谢物的表达水平。
A.Venn diagram of differential metabolites of TA vs TB and TB vs TC;B.Heatmap of amounts of 53 differential metabolite content at three stages of floral induction;C.Levels of metabolites in proline biosynthetic pathway in mango stem apex at three stages of floral induction.
2.6 SPD 处理后成花诱导期间碳水化合物和维生素的鉴定与分析
7种维生素和11种糖类及醇类物质在SPD处理30~100 d 后发生显著的变化,并且它们与对照组的积累模式不同(图8-A)。在11 种糖类及醇类物质中,10 种碳水化合物(包括D-果糖-6-磷酸酯、D-果糖-1,6-二磷酸酯、葡萄糖-1-磷酸酯、山梨醇-6-磷酸酯和D-葡萄糖6-磷酸酯等)在SPD处理30~100 d急剧增加,但清水处理30~100 d 后没有显著变化。在7 种维生素中,相对含量最高的抗坏血酸和左旋抗坏血酸在SPD处理30~100 d后显著增加,清水处理30~100 d 后没有显著变化。此外,SPD 处理的成花诱导的不同阶段,有14 种代谢物变化倍数超过10倍,清水处理后不同阶段均没有显著变化(图8-B);这一组包括L-同型半胱氨酸(30~80 d,30~100 d)、L-组氨酸(30~100 d)和L-同型蛋氨酸(30~100 d,80~100 d)等。
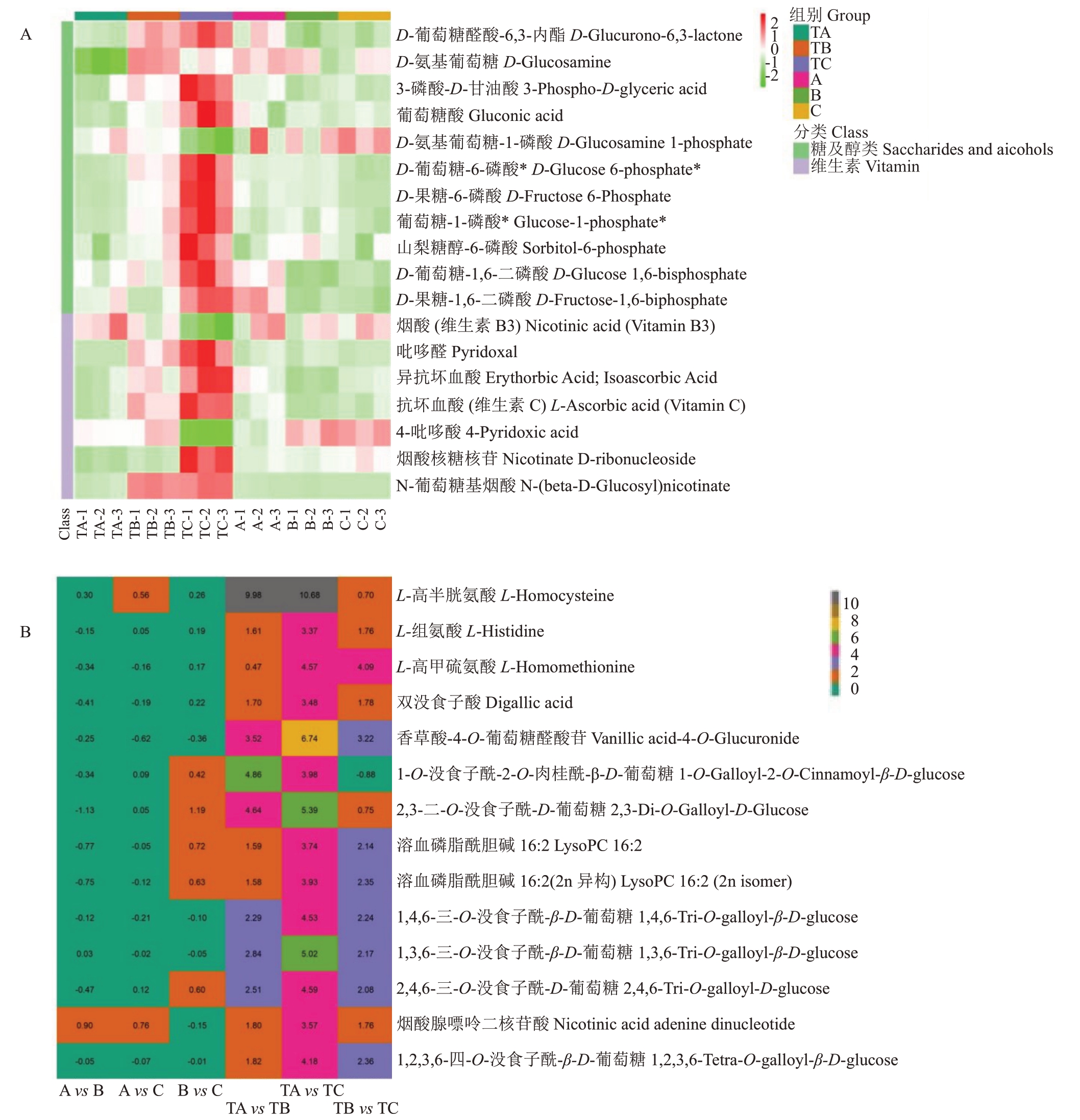
图8 成花诱导3 个阶段18 种差异代谢物含量热图(A)和相对含量变化超过10 倍的代谢物(B)
Fig.8 Heatmap of amounts of 18 differential metabolite content at three stages of floral induction(A)and metabolites with relative abundance changed more than 10 times(B)
3 讨 论
最近,温带水果花芽休眠的代谢调控引起了许多研究者的关注[31-32]。杧果的成花诱导伴随着顶芽的休眠解除,并受低温调节,类似于温带果树花芽休眠的调节[33]。然而,关于杧果顶芽成花诱导期间代谢物变化尚未见报道。本研究连续2 a 评估SPD 诱导杧果成花率以及果实产量,证实了SPD对促进杧果成花诱导有效。然后利用基于UPLC-MS/MS 的广泛靶向代谢组学来了解杧果顶芽初生代谢物在成花诱导3个具有代表性阶段的总体动态。共鉴定出582 种代谢物,372 种在成花诱导的3 个阶段差异积累。代谢物中脂质、酚酸、氨基酸、碳水化合物和维生素被进一步分析。
脂质是细胞膜的组成部分,在调节各种生物途径中起着至关重要的作用[34]。植物中的脂质类可分为几类,包括甘油磷脂(GP)、甘油脂(GL)和FA,GP主要包括LPC、LPE 和PC。欧洲梨顶芽休眠期间,磷脂随着低温积累而显著增加[31]。细菌侵染和低温导致拟南芥叶片中LPC 和LPE 含量显著升高。同时,在黑醋栗顶芽休眠解除时,检测到PC 生物合成的前体物质磷酸胆碱增加[35]。在本研究中,18 种脂质在SPD 处理后发生了显著变化,LPE 17:1 和LPE 15:1 在SPD 处理80~100 d 后出现了10 倍以上的变化,说明它们在杧果成花诱导中对SPD处理作出了响应。有趣的是,Nakamura 等[21-22]在拟南芥中的生化分析表明,FLOWERING LOCUS T(FT)与PC结合以促进开花,这表明PC 在开花时间中起作用。然而,SPD处理后,杧果成花诱导期间脂质代谢需要通过整合相关基因转录水平来进一步研究。
酚酸类化合物在调节植物的生长和发育中起着至关重要的作用[36]。酚酸是带有酚羟基的有机酸。酚羟基与自由基形成稳定的苯氧基,以清除体内自由基。酚酸含量较高的野生草莓和栽培草莓均具有较强的抗自由基活性[37]。Oliveira 等[38]从细胞学角度证明了花芽启动前酚类物质在杧果顶芽的显著积累。在本研究中,酚酸占代谢物的很大比例,SPD处理30、80、100 d 后,酚酸类代谢物发生了显著的变化。大多数含量持续增高,可能与清除自由基有关。同时,有研究证明单宁及相关化合物,包括香豆素、反式肉桂酸和许多酚类化合物,是赤霉素拮抗剂[39]。因此,上调的单宁类化合物也可能拮抗成花诱导过程中的赤霉素,从而促进开花。
顶芽休眠的解除伴随着脯氨酸的积累[40]。此外,游离脯氨酸的积累是植物对各种胁迫的典型反应,以维持细胞质和液泡之间的渗透平衡。脯氨酸可以去除胁迫下产生的活性氧,并作为渗透剂保护亚细胞结构[41]。研究表明柳枝稷中低水平的脯氨酸延迟了开花。此外,脯氨酸参与开花信号。在长日照和短日照条件下,过表达的Δ1-Pyrroline-5-carboxylatesynthetase(AtP5CS1)的转基因拟南芥开花较早,且积累了大量脯氨酸[42]。AtP5CS2 是一个多等位基因的AtP5CS基因,是CONSTANS(CO)的早期靶点,参与花的形成[43]。本研究中,许多差异代谢物与氨基酸生物合成有关,在成花诱导期间响应SPD,L-脯氨酸及其前体持续增加,每个阶段的相对含量较高。这些结果表明,杧果顶芽休眠解除伴随着脯氨酸积累。
植物生长发育需要碳水化合物提供能量来维持芽顶休眠期间的生长,并将糖信号与开花相关的途径联系起来[44]。Jesús发现[45]巴旦杏花芽中的D-果糖-2-磷酸和D-果糖-2,6-二磷酸在接近内胚层释放的阶段增加。本研究中,SPD 处理30~100 d 积累的糖类主要是与淀粉和蔗糖代谢相关的物质。这些发现表明在SPD处理后,糖类物质(D-果糖6-磷酸、D-果糖-1,6-二磷酸、葡萄糖-1-磷酸和D-葡萄糖6-磷酸)的积累在杧果花芽发育中可能非常重要[14]。只有D-氨基葡萄糖1-磷酸减少。先前的研究表明,氨基葡萄糖6-磷酸可能代谢为果糖6-磷酸,以增加糖酵解通量[46]。在红米种子中,休眠解除与糖酵解增加和获得能量有关[47]。这些发现与笔者对杧果顶芽的研究结果一致,表明了碳水化合物在成花诱导中的作用。
此外,抗坏血酸和异抗坏血酸相对含量较高,并且在SPD处理30~100 d后持续积累,但是在清水处理后没有观察到显著变化。抗坏血酸是合成少量激素的重要抗氧化剂和辅助因子[48]。它还通过复杂的信号转导网络将开花时间与对病原体的反应联系起来[49]。在日本梨和甜樱桃中,活性氧在内胚层形成过程中增加,在内胚层释放后减少[47,50]。此外,研究表明,抗坏血酸水平随着黄酮醇的增加而增加,这与葡萄叶片中过氧化氢的降解有关[51]。说明SPD对杧果顶芽产生了持续的胁迫,抗坏血酸作为抗氧化剂可以清除自由基以响应胁迫。
除了上述物质,还有一些代谢物,包括L-半胱氨酸、L-组氨酸和L-高甲硫氨酸等,也可能在某些特定阶段发挥重要作用。在SPD处理后的特定阶段,这些代谢物的相对含量变化超过10 倍。这些代谢物在成花诱导期间相对含量显著变化以响应SPD 处理,表明了它们在杧果成花诱导中起作用。
4 结 论
研究证实了SPD 在调节杧果开花中有积极的作用,并评价了SPD对杧果代谢的影响。基于广泛靶向的代谢组学分析鉴定出582种代谢物。PCA和聚类分析显示在SPD/清水处理30、80、100 d后杧果顶芽代谢特征的差异。此外,鉴定出372 种差异代谢物,并根据积累模式将其分为六类:脂类(78种)、酚酸(125种)、核苷酸及其衍生物(36种)、氨基酸及其衍生物(52种)、有机酸(42种)和其他(39种)。在SPD处理30~80 d后,然后至100 d,检测到代谢物如脂质、单宁、脯氨酸、抗坏血酸和糖类的含量发生显著变化,这表明它们在成花诱导期间对响应SPD处理起着重要作用。本研究揭示了杧果开花过程中对SPD 反应的新代谢产物,为利用SPD 诱导杧果开花提供了理论依据。
[1] DAVENPORT T L. Reproductive physiology of mango[J]. Brazilian Journal of Plant Physiology,2007,19(4):363-376.
[2] GUEVARA E,JIMÉNEZ V M,BANGERTH F K. Response of endogenous hormone concentrations to two floral inductive treatments,viz. KNO3 and PBZ,in mango cv.‘Tommy Atkins’growing under tropical conditions[J]. Tropical Plant Biology,2012,5(3):253-260.
[3] SINGH V K,BHATTACHERJEE A K. Genotypic response of mango yield to persistence of paclobutrazol in soil[J]. Scientia Horticulturae,2005,106:53-59.
[4] COELHO E F.Flowering and fruit set of mango in different doses of paclobutrazol (PBZ)[J]. Enciclopédia Biosfera,2014,10(19):1117-1123.
[5] UPRETI K K,REDDY Y T N,PRASAD S R S,BINDU G V,JAYARAM H L,RAJAN S. Hormonal changes in response to paclobutrazol induced early flowering in mango cv. Totapuri[J].Scientia Horticulturae,2013,150:414-418.
[6] 曹尚银,张秋明,张俊畅,江爱华.喷施烯效唑对苹果顶芽激素水平和花芽分化的影响[J].植物生理与分子生物学学报,2003,29(5):375-379.CAO Shangyin,ZHANG Qiuming,ZHANG Junchang,JIANG Aihua. Effects of uniconazole spray on flower bud differentiation and endogenous hormone level in terminal bud of apple trees[J]. Journal of Plant Physiology and Molecular Biology,2003,29(5):375-379.
[7] CHRISTOPHER D,JOHN A C.Ethephon and prohexadione-calcium influence the flowering,early yield,and vegetative growth of young‘Northern Spy’apple trees[J]. Scientia Horticulturae,2013,151:128-134.
[8] RAHIM A O A,ELAMIN O M,BANGERTH F K. Effects of growth retardants,paclobutrazol (PBZ) and prohexadione-Ca on floral induction of regular bearing mango(Mangifera indica L.)[J].Journal of Agricultural and Biological Science,2011,6(3):18-26.
[9] 吴振旺,唐征,熊自力. 烯效唑对荸荠种杨梅控梢促花的效应[J].中国南方果树,2001,30(1):30-31.WU Zhenwang,TANG Zheng,XIONG Zili. Effects of uniconazole on controlling shoots and promoting flowers of Myrica rubra‘Biqizhong’[J].South China Fruits,2001,30(1):30-31.
[10] 郑辉艳,赵玉华,杨国顺.缩节胺和不同的修剪方式对夏黑无核二次成花的影响[J].湖南农业科学,2016(3):21-24.ZHENG Huiyan,ZHAO Yuhua,YANG Guoshun. Effects of mepiquat chloride and pruning pattern on inflorescence formation of Summer Black seedless grape’s second crop[J]. Hunan Agricultural Sciences,2016(3):21-24.
[11] NAGAO M A,HO-A E B,YOSHIMOTO J M. Uniconazole retards growth and increases flowering of young macadamia trees[J].HortScience,1999,34(1):104-105.
[12] KIM H,LEE H,KANG J,HWANG S. Prohexadione-calcium application during vegetative growth affects growth of mother plants,runners,and runner plants of maehyang strawberry[J].Agronomy,2019,9(3):155.
[13] WU Q,DU M,WU J,WANG N,WANG B,LI F,TIAN X,LI Z. Mepiquat chloride promotes cotton lateral root formation by modulating plant hormone homeostasis[J]. BMC Plant Biology,2019,19:573-589.
[14] UPRETI K K,PRASAD S S,REDDY Y,RAJESHWARA A N.Paclobutrazol induced changes in carbohydrates and some associated enzymes during floral initiation in mango (Mangifera indica L.) cv. Totapuri[J]. Indian Journal of Plant Physiology,2014,19(4):317-323.
[15] XING L,ZHANG D,LI Y,SHEN Y,ZHAO C,MA J,AN N,HAN M.Transcription profiles reveal sugar and hormone signaling pathways mediating flower induction in apple(Malus domestica Borkh.)[J]. Plant & Cell Physiology,2015,56(10):2052-2068.
[16] DU L S,QI S Y,MA J J,XING L B,FAN S,ZHANG S W,LI Y M,SHEN Y W,ZHANG D,HAN M Y. Identification of TPS family members in apple (Malus×domestica Borkh.) and the effect of sucrose sprays on TPS expression and floral induction[J].Plant Physiology and Biochemistry,2017,120:10-23.
[17] DOS SANTOS SILVA L,DE SOUSA K Â O,PEREIRA E C V,ROLIM L A,DA CUNHA J G,SANTOS M C,DA SILVA M A,CAVALCANTE Í H L. Advances in mango‘Keitt’production system:PBZ interaction with fulvic acids and free amino acids[J].Scientia Horticulturae,2021,277:109787.
[18] 张昕. 葡萄糖促进富士苹果花芽孕育的基因调控网络研究[D].杨凌:西北农林科技大学,2018.ZHANG Xin. Research on gene regulatory network of glucose inducing flower bud initiation[D]. Yangling:Northwest A & F University,2018.
[19] TIWARI D K,PATEL V,PANDEY A. Floral induction in mango:Physiological,biochemical and molecular basis[J]. International Journal of Chemical Studies,2018,6(1):252-259.
[20] COLEGIO D P,OSUNA-ENCISO T,BECERRIL-ROMÁN A E,MOSQUEDA-VÁZQUEZ R,VILLARREAL-ROMERO M,CASTILLO-MORALES A. Promotores de floracion y concentracion de almidon y aminoacidos en yemas de mango[J].Revista Chapingo Serie Horticultura,2001,7(2):209-215.
[21] NAKAMURA Y,ANDRÉS F,KANEHARA K,LIU Y,DÖRMANN P,COUPLAND G. Arabidopsis florigen FT binds to diurnally oscillating phospholipids that accelerate flowering[J].Nature Communications,2014,5:45-53.
[22] NAKAMURA Y,LIN Y,WATANABE S,LIU Y,KATSUYAMA K,KANEHARA K,INABA K.High-resolution crystal structure of Arabidopsis FLOWERING LOCUS T illuminates its phospholipid-binding site in flowering[J]. iScience,2019,21:577-586.
[23] WANG D,LIU X X,LI T T,LI Q,LIU Y,GONG G S,YANG H. First report of cucumber mosaic virus infection in kiwifruit(Actinidia chinensis) in China[J]. Plant Disease,2018,102(6):1180.
[24] SHICHENG Z,JINCHENG W,MUHAMMAD Q S,YEHUA H,SHUNQUAN L,ZHENHUA L,XIANGHUI Y. Identification of key taste components in loquat using widely targeted metabolomics[J].Food Chemistry,2020,323:126822.
[25] DANDAN W,LIANGXIAO Z,XIAORONG H,XIAO W,RUINAN Y,JIN M,XUEFANG W,XIUPIN W,QI Z,PEIWU L.Identification of nutritional components in black sesame determined by widely targeted metabolomics and traditional Chinese medicines[J].Molecules,2018,23(5):1180.
[26] ZHANG J,QIU X,TAN Q,XIAO Q,MEI S.A comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.)[J]. Journal of Agricultural and Food Chemistry,2020,68(49):14463-14470.
[27] ZHENG B,ZHAO Q,WU H,WANG S,ZOU M. A comparative metabolomics analysis of guava (Psidium guajava L.) fruit with different colors[J]. ACS Food Science & Technology,2021,1(1):96-106.
[28] THÉVENOT E A,ROUX A,XU Y,EZAN E,JUNOT C.Analysis of the human adult urinary metabolome variations with age,body mass index,and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses[J]. Journal of Proteome Research,2015,14(8):3322-3335.
[29] DJ B,CA M. Floral induction in growing buds of lychee (Litchi chinensis) and mango (Mangifera indica)[J]. Functional Plant Biology,1995,22(5):783-791.
[30] HUIFENG Z,HUA L,BIAO L,HAOQIANG X,HUI-CONG W,XUMING H. Morphological characterization and gene expression profiling during bud development in a tropical perennial,Litchi chinensis Sonn.[J].Frontiers in Plant Science,2016,7:1517-1537.
[31] GABAY G,FAIGENBOIM A,DAHAN Y,IZHAKI Y,ITKIN M,MALITSKY S,ELKIND Y,FLAISHMAN M A. Transcriptome analysis and metabolic profiling reveal the key role of αlinolenic acid in dormancy regulation of European pear[J]. Journal of Experimental Botany,2019,70(3):1017-1031.
[32] YANG Q,GAO Y,WU X,MORIGUCHI T,BAI S,TENG Y.Bud endodormancy in deciduous fruit trees:Advances and prospects[J].Horticulture Research,2021,8:139-150.
[33] ROBERTO N,THOMAS L D. Effect of leaf age,duration of cool temperature treatment,and photoperiod on bud dormancy release and floral initiation in mango[J]. Scientia Horticulturae,1995,62:63-73.
[34] QUARTACCI M F,PINZINO C,SGHERRI C,NAVARI-IZZO F. Lipid composition and protein dynamics in thylakoids of two wheat cultivars differently sensitive to drought[J].Plant Physiology,1995,108(1):191-197.
[35] JUNG K,FASTOWSKI O,POPLACEAN I,ENGEL K.Analysis and sensory evaluation of volatile constituents of fresh blackcurrant (Ribes nigrum L.) fruits[J]. Journal of Agricultural and Food Chemistry,2017,65:9475-9487.
[36] HALBWIRTH H,PUHL I,HAAS U,JEZIK K,TREUTTER D,STICH K. Two-phase flavonoid formation in developing strawberry (Fragaria×ananassa) fruit[J]. Journal of Agricultural and Food Chemistry,2006,54:1479-1485.
[37] LIDIJA J,MATO D,VLADIMIR J,MARIJAN . Phenolic acids,flavonols,anthocyanins and antiradical activity of“Nero”,“Viking”,“Galicianka”and wild chokeberries[J].Scientia Horticulturae,2012,147:56-63.
[38] MOACIR B O,MARIA G F F,MARLON C T P,MARIA A D C M,LEONARDO M R,MARIA O M.Structural and cytological aspects of mango floral induction using paclobutrazol[J].Scientia Horticulturae,2020,262:109057.
[39] CORCORAN M R,GEISSMAN T A,PHINNEY B O. Tannins as gibberellin antagonists[J].Plant Physiology,1972,49(3):323-330.
[40] MOHAMED A S E,SAMIR A S E,MOSTAFA M R. Exogenous dormancy-breaking substances positively change endogenous phytohormones and amino acids during dormancy release in‘Anna’apple trees[J].Plant Growth Regulation,2014,72(3):211-220.
[41] ZHUANG W,GAO Z,WANG L,ZHONG W,NI Z,ZHANG Z.Comparative proteomic and transcriptomic approaches to address the active role of GA4 in Japanese apricot flower bud dormancy release[J]. Journal of Experimental Botany,2013,64(16):4953-4966.
[42] MATTIOLI R,BIANCUCCI M,LONOCE C,COSTANTINO P,TROVATO M. Proline is required for male gametophyte development in Arabidopsis[J]. BMC Plant Biology,2012,12:263-579.
[43] ALON S,HITOSHI O,SCOTT E G,GARY S D,ZSUZSANNA S,MARTIN F Y,GEORGE C. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis[J]. Science,2000,288(5471):1613-1616.
[44] CHEN X,QI S,ZHANG D,LI Y,AN N,ZHAO C,ZHAO J,SHAH K,HAN M,XING L. Comparative RNA-sequencingbased transcriptome profiling of buds from profusely flowering‘Qinguan’and weakly flowering‘Nagafu No. 2’apple varieties reveals novel insights into the regulatory mechanisms underlying floral induction[J].BMC Plant Biology,2018,18:370-391.
[45] GUILLAMÓN J G,PRUDENCIO Á S,YUSTE J E,DICENTA F,SÁNCHEZ P R. Ascorbic acid and prunasin,two candidate biomarkers for endodormancy release in almond flower buds identified by a nontargeted metabolomic study[J]. Horticulture Research,2020,7:203-216.
[46] XING D,FENG W,NÖT L G,MILLER A P,ZHANG Y,CHEN Y,MAJID-HASSAN E,CHATHAM J C,OPARIL S.Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury[J].American Journal of Physiology-Heart and Circulatory Physiology,2008,295(1):335-342.
[47] ALBERTO G,FRANCA F,PAOLO B,ANTONELLA Z,PRIMETTA F,LUIGI C,GIAMPIERO V,CHIARA B. Seed dormancy involves a transcriptional program that supports early plastid functionality during imbibition[J]. Plants,2018,7(2):35-85.
[48] BARTH C,De TULLIO M,CONKLIN P L. The role of ascorbic acid in the control of flowering time and the onset of senescence[J]. Journal of Experimental Botany,2006,57(8):1657-1665.
[49] DU D F,GAO X,GENG J,LI Q Y,LI L Q,LV Q,LI X J.Identification of key proteins and networks related to grain development in wheat (Triticum aestivum L.) by comparative transcription and proteomic analysis of allelic variants in TaGW2-6A[J].Frontiers in Plant Science,2016,7:922.
[50] SUSANNE B,THOMAS H,SUSANNE N,FRANK M C,KLAUS-PETER G,KRISTIN G,GERD H,GETRUD E M,HARSHADRAI M R,HELEN D S. Selected plant metabolites involved in oxidation-reduction processes during bud dormancy and ontogenetic development in sweet cherry buds (Prunus avium L.)[J].Molecules,2018,23(5):1197.
[51] FRANCISCO J P,DANIEL V,NILO M.Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves[J].Phytochemistry,2002,60(6):573-580.