成花起始标志着植物由营养生长阶段向生殖生长阶段的转变。在成花诱导条件下,成花素基因FLOWERING LOCUS T(FT)在叶脉筛管中的伴胞细胞表达,其编码的蛋白被FT-INTERACTING PROTEIN 1(FTIP1)蛋白通过韧皮部转运至茎尖分生组织[1-2]。在茎尖分生组织中,FT 与细胞质内的14-3-3 蛋白形成FAC(Florigen Activation Complex)复合体,并进入细胞核,与亮氨酸拉链(basic leucine zipper,bZIP)蛋白FLOWERING LOCUS D(FD)结合,共同激活下游花分生组织身份基因LEAFY(LFY)和APETALA1(AP1),起始花分生组织分化[3-6]。FT 的转录有两种调控机制:由CONSTANT(CO)介导的光周期调控机制和由PcG 介导的表观遗传沉默调控机制。在长日照诱导条件下,锌指蛋白转录因子CO促进FT的转录[7]。在表观遗传沉默机制中,营养生长阶段抑制型组蛋白H3K27me3 在FT染色质中沉积,以抑制其转录。在黄昏前和黑夜FT 转录处于低水平时,由EMBRYONIC FLOWER1(EMF1)、LIKE HETEROCHROMATIN PROTEIN1(LHP1)和组蛋白H3K4 甲基化酶组成的PcG 复合体,维持FT 染色质的抑制状态[8-9]。被子植物中FT编码的氨基酸序列比较保守,但其生物学功能则因物种而异[10]。如在拟南芥(Arabidopsis thaliana)[11]、百合(Lilium longiflorum)[12]、木薯(Manihot esculenta)[13]、杨树(Populus nigra var. italica)[14]等物种中,FT 促进开花。而水稻中的研究表明,FT 还可参与分蘖[15]。在洋葱(Allium cepa)中,FT 调控开花时间和鳞茎形成[16]。随着生物技术的不断发展,越来越多FT同源基因的生物学功能逐渐被揭示。
板栗(Castanea mollissima)为我国重要经济林树种,适于山地栽植。但板栗童期长,开花不稳定,易产生“二次花”和“大小年”现象,严重阻碍了其产业发展。因此开展板栗成花调控机制的研究对于童期短、开花稳定板栗品种的培育及栽培措施的制定具有重要意义。前期的研究发现,生长素、细胞分裂素和赤霉素均可影响板栗的开花[17-19]。但其调控机制仍不清楚。Chen等[20]的研究认为赤霉素(GA3)可能是通过上调miR156,降低板栗成花相关SQUAMOSA PROMOTER- BINDING PROTEIN- LIKE(SPL)家族成员CmSPL9 和CmSPL16 的表达量,参与花芽分化调控。近期板栗基因组测序的完成,为板栗成花调控分子机制研究奠定了重要基础[21]。张煜等[22]从板栗基因组中鉴定出了5个FT/TFL-like基因,并分析了其在板栗一次花和二次花发育中的表达情况。该研究表明CmFT 在2 次花发育中均有较高表达,并且可能参与雄花序和雌花簇形态建成的调控,但未进行基因功能验证。同时CmFT 是否是板栗中的成花素基因,尚不清楚。笔者在本研究中对板栗CmFT 基因进行克隆,通过表达模式分析和异源过表达植株表型分析明确其生物学功能。研究结果有助于解析板栗成花调控的分子机制,并为缩短育种周期和板栗高产稳产提供实践依据。
1 材料和方法
1.1 材料
试验材料为生长健壮、长势一致的20年生板栗品种燕山红栗(Yanshanhongli),选取3株作为3个生物学重复。取板栗2020年4月7日混合芽和5月25日结果母枝上部新梢的完全展开叶片、茎尖、雄花序,液氮速冻后置于-80 ℃冰箱保存,用于CmFT的组织特异性表达检测。分别于2020 年4 月28 日、5月25日、6月17日、7月15日、8月17日和9月5日取板栗结果母枝上部新梢的完全展开叶片,液氮速冻后置于-80 ℃冰箱保存,用于检测不同时期叶片中CmFT的表达量。
野生型拟南芥(A.thaliana)材料保存于课题组实验室。
1.2 方法
1.2.1 总RNA 提取及反转录 取0.1 g 植物组织,用液氮速冻后存于-80 ℃,以供RNA提取。用艾德莱植物RNA 提取试剂盒,按照说明书提取总RNA。用Nano Drop 2000 检测总RNA 的浓度和纯度,用琼脂糖凝胶电泳检测总RNA的完整性。利用TaKaRa公司的反转录试剂盒6110A,按照其说明书合成cDNA第一链,-20 ℃保存备用。
1.2.2 板栗CmFT编码区序列克隆 根据板栗基因组测序数据(http://gigadb.org/dataset/100643)设计板栗开花素基因CmFT 编码区序列(CDS)全长引物(表1)。根据1.2.1 的方法获得板栗叶片总RNA 的反转录产物,并以此为模板进行CmFT 基因CDS 的扩增。PCR产物经1.5%琼脂糖凝胶电泳检测后,利用TAKARA 胶回收试剂盒回收目的条带。将回收产物连接至pBM21(pBM21 Topsmart Cloning Kit)载体上,转化大肠杆菌(Escherichia coli)DH5α,在含氨苄霉素的LB 培养基上,37 ℃培养16 h。挑取阳性单克隆进行测序。
表1 PCR 引物信息
Table 1 Primers information for PCR
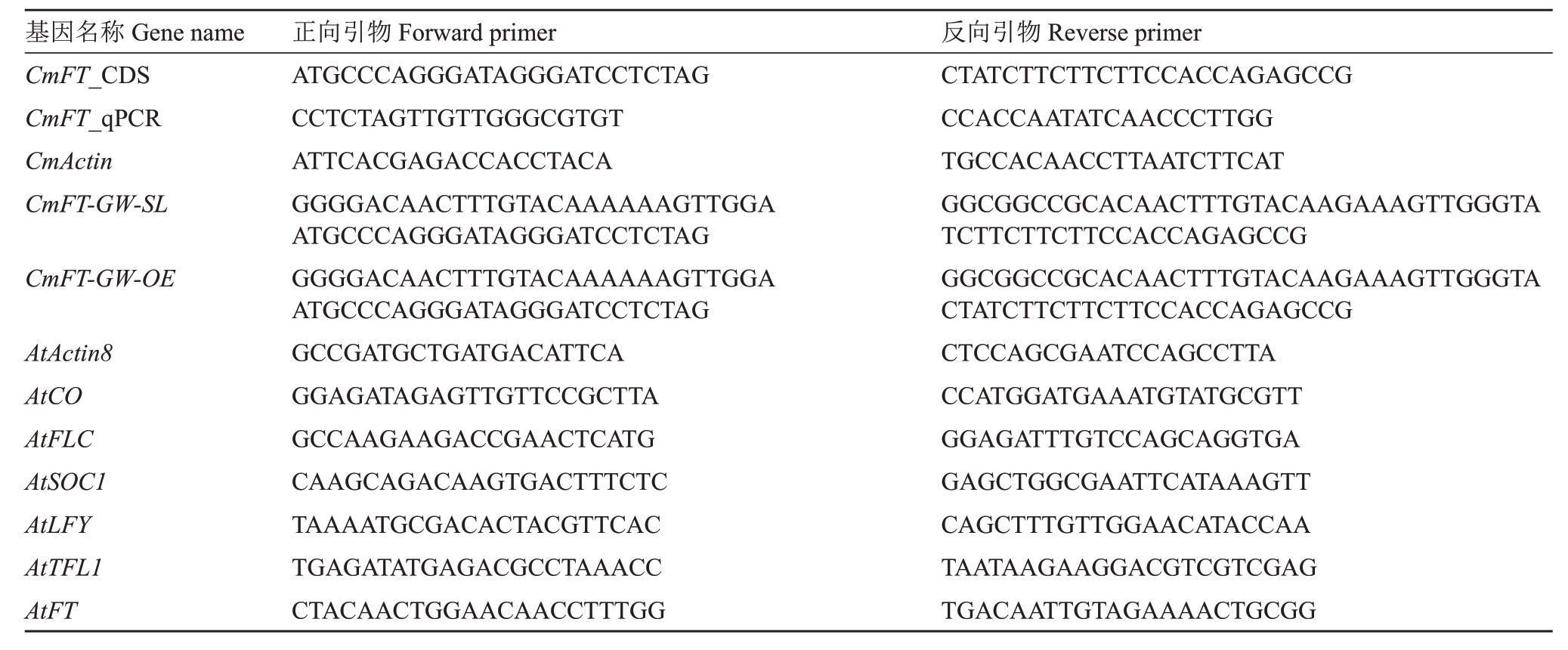
基因名称Gene name CmFT_CDS CmFT_qPCR CmActin CmFT-GW-SL CmFT-GW-OE AtActin8 AtCO AtFLC AtSOC1 AtLFY AtTFL1 AtFT正向引物Forward primer ATGCCCAGGGATAGGGATCCTCTAG CCTCTAGTTGTTGGGCGTGT ATTCACGAGACCACCTACA GGGGACAACTTTGTACAAAAAAGTTGGA ATGCCCAGGGATAGGGATCCTCTAG GGGGACAACTTTGTACAAAAAAGTTGGA ATGCCCAGGGATAGGGATCCTCTAG GCCGATGCTGATGACATTCA GGAGATAGAGTTGTTCCGCTTA GCCAAGAAGACCGAACTCATG CAAGCAGACAAGTGACTTTCTC TAAAATGCGACACTACGTTCAC TGAGATATGAGACGCCTAAACC CTACAACTGGAACAACCTTTGG反向引物Reverse primer CTATCTTCTTCTTCCACCAGAGCCG CCACCAATATCAACCCTTGG TGCCACAACCTTAATCTTCAT GGCGGCCGCACAACTTTGTACAAGAAAGTTGGGTA TCTTCTTCTTCCACCAGAGCCG GGCGGCCGCACAACTTTGTACAAGAAAGTTGGGTA CTATCTTCTTCTTCCACCAGAGCCG CTCCAGCGAATCCAGCCTTA CCATGGATGAAATGTATGCGTT GGAGATTTGTCCAGCAGGTGA GAGCTGGCGAATTCATAAAGTT CAGCTTTGTTGGAACATACCAA TAATAAGAAGGACGTCGTCGAG TGACAATTGTAGAAAACTGCGG
1.2.3 板栗CmFT 生物信息学分析 利用TBtool(https://github.com/CJ-Chen/TBtools)工具从板栗基因组数据库中获取CmFT基因组序列。用在线软件GSDS(http://gsds.gao-lab.org)绘制CmFT 结构示意图。用NCBI 网站CDD-tools(https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)查找克隆得到的CmFT 序列的保守结构域。从Phytozome 数据库下载拟南芥(A.thaliana)、春兰(Cymbidium goeringii)、葡萄(Vitis vinifera)、小桐子(Jatropha curcas)、牡丹(Paeonia suffruticosa)、钻天杨(Populus nigra)、黄瓜(Cucumis sativus)、大豆(Glycine max)、油茶(Camellia oleifera)、莴苣(Lactuca sativa)、欧洲油菜(Bras-sica napus)、漏斗菜(Aquilegia coerulea)的FT 氨基酸序列,并与CmFT氨基酸序在MEGAX软件中,用邻接法(Neighbor-Joining)构建进化树,校验参数为bootsrap=1000。
1.2.4 CmFT在板栗不同组织和不同时期叶片中的表达分析 以板栗不同组织和不同时期叶片cDNA为模板,利用MX3000P 荧光定量PCR 仪,检测基因的表达量。CmFT 荧光定量PCR 引物为CmFT_qPCR(表1)。以CmActin基因为内参。反应体系按照SYBR Premix Ex TaqTMII(TaKaRa)试 剂 要 求 配制。反应程序为:95 ℃预变性30 s;95 ℃变性5 s,60 ℃延伸30 s,40 个循环。每个样品设3 个技术重复,用2-△△Ct法计算基因相对表达量。
1.2.5 载体构建 根据试剂盒(Life technologies,Carlsbad,CA,USA)说明书分别设计亚细胞定位载体和过表达载体引物。亚细胞定位引物不含终止密码子,为CmFT-GW-SL(表1)。过表达载体引物为CmFT-GW-OE(表1)。利用PCR扩增得到含有载体接头的CmFT 基因,并将其克隆至pDNOR201 中间载体上。将中间载体上不含终止密码子的CmFT基因和完整CmFT 基因分别重组至pEarleyGate103 和pMDC32载体上,得到含35S::CmFT-GFP亚细胞定位载体和35S::CmFT过表达载体。
1.2.6 亚细胞定位 根据Miao 等[23]的方法将构建好的35S::CmFT-GFP 亚细胞定位载体转化拟南芥原生质体,并以35S::GFP转化拟南芥原生质体作对照。用激光共聚焦显微镜(Leica TCS SPII)观察荧光信号。
1.2.7 CmFT过表达载体转化拟南芥的获取及表型分析 将含有目的基因的过表达载体通过冻融法转入GV31001 农杆菌感受态细胞。参照Clough 等[16]报道的花粉管侵染法转化拟南芥。用50 mg·L-1的潮霉素筛选抗性植株。将长出真叶的拟南芥幼苗移栽到花盆中置于光照培养箱培养,培养条件为光照16 h(24 ℃),黑暗8 h(20 ℃)。选择表型变化明显的5个株系继续培养至T2代。每株系10株,以花薹为2~5 cm时所用时间及莲座叶数量为指标[25],分析开花表型。
1.2.8 转基因拟南芥开花关键基因表达分析 选取3 个转基因拟南芥株系(L1、L2、L3)作为3 个重复,每重复5 株,对叶片进行混合取样。以叶片cDNA为模板,利用实时荧光定量RT-PCR,以AtActin8 为内参,测定开花关键基因的表达量。引物见表1。
2 结果与分析
2.1 CmFT基因的克隆及分析
利用TBtools在板栗基因组数据库中检索到FT同源基因BUA.CMHBY204722,命名为CmFT。根据全长转录组数据获取CmFT 基因5’UTR 和3’UTR序列。以起始密码子上游2000 bp为启动子区域,利用GSDS 2.0 绘制基因结构图(图1-A)。利用PCR克隆CmFT 基因CDS 全长(图1-B)。结果显示,CmFT 基因CDS 全长525 bp,5’UTR 长75 bp,3’UTR长219 bp,包含3个内含子。克隆得到的CmFT编码174个氨基酸,具有保守的PEBP结构域。氨基酸序列比对分析结果显示,在板栗(C.mollissima)、拟南芥(A.thaliana)、春兰(C.goeringii)、葡萄(V.vinifera)、小桐子(J.curcas)、牡丹(P.suffruticosa)、钻天杨(P. nigra)和黄瓜(C. sativus)中的相似度为87.36%(图1-C)。利用MEGAX 将上述物种与大豆(G. max)、油茶(C. oleifera)、莴苣(L. sativa)、欧洲油菜(B.napus)、耧斗菜(A.coerulea)的FT同源蛋构建系统进化树。结果显示,板栗与黄瓜FT同源蛋白亲缘关系最近,杨树次之(图2)。
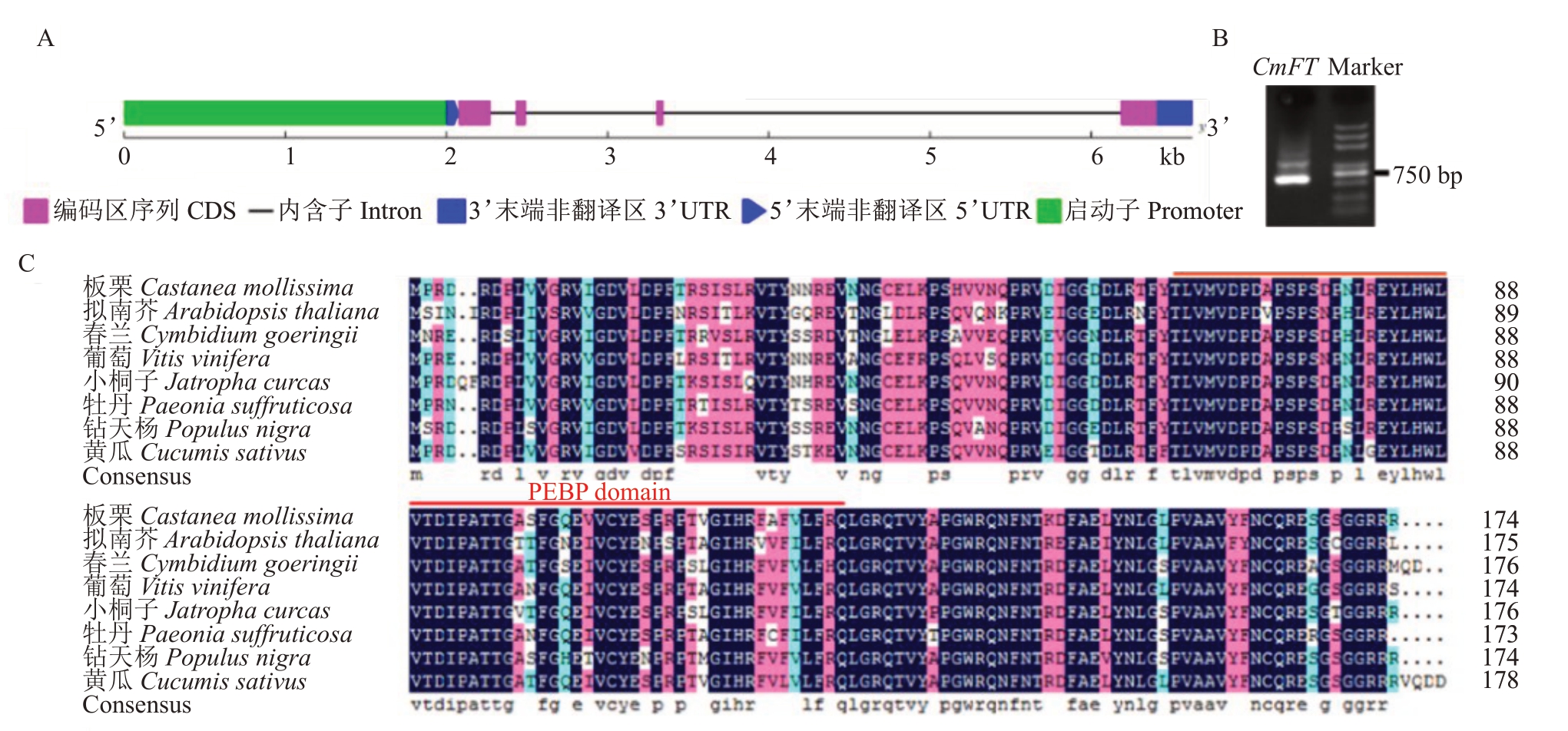
图1 板栗CmFT 基因克隆及序列分析
Fig.1 Cloning and sequence analysis of CmFT
A. 板栗CmFT 基因结构示意图;B.CmFT 同源克隆PCR 产物琼脂糖电泳图;C. 不同物种FT 氨基酸序列比对。
A.Gene structure of CmFT;B.The amplification of CmFT;C.Amino acid sequence alignment of FT in different species.

图2 CmFT 与其他物种FT 同源蛋白系统发育树
Fig.2 Phylogenetic tree analysis of CmFT with homology FT proteins in other species
2.2 CmFT时空表达分析
基因的表达具有时空特异性。利用实时荧光定量PCR对板栗不同组织中CmFT的表达量进行了测定。结果表明,CmFT 在茎尖、叶片、混合芽和雄花序中均有表达,其中茎尖和叶片中CmFT 的表达量较高,其次为雄花序和混合芽(图3-A)。FT 为开花素基因,受光周期等成花信号的诱导而在叶片中表达。叶片中FT 的高表达标志着植物从营养生长向生殖生长的转变。因此检测不同时期叶片中CmFT的表达量有助于从分子水平明确板栗成花规律。本研究的检测结果显示,4月开始叶片中CmFT的表达水平逐渐升高,于7 月15 日达到顶峰,随后迅速降低,并在9月5日再次升高(图3-B)。板栗花期一般为5月中旬至6月下旬。7月15日板栗花期已过,叶片中FT表达量增高,说明此时期为板栗成花诱导的关键时期。
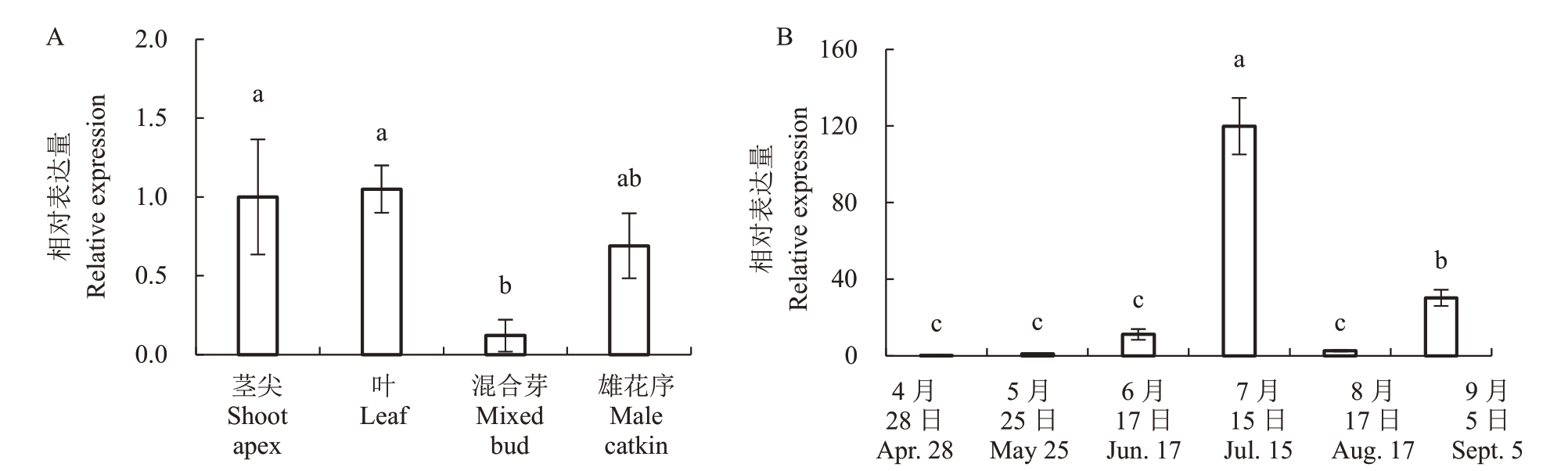
图3 CmFT 在板栗不同组织(A)及不同时期叶片(B)中的表达分析
Fig.3 Expression analysis of CmFT in different tissues(A)and leaves at different periods(B)of C.mollissima
用LSD 测试法分析不同组间的差异显著性。数据为(平均植±标准误),n=3。不同小写字母表示差异显著(p <0.05)。下同。
Least significant difference (LSD) tests were used to determine significant differences between groups. Data are(mean ± SE), n = 3. Different small letters indicate significant difference(p <0.05).The same below.
2.3 CmFT的亚细胞定位分析
通过构建CmFT-GFP 融合表达载体,瞬时转化拟南芥叶片原生质体,观察GFP荧光信号的方法确定CmFT在细胞中的定位信息。GFP荧光信号观察结果显示,经35S::CmFT-GFP重组表达载体转化的拟南芥叶片原生质体中的绿色荧光仅分布在细胞核中。该结果说明CmFT蛋白定位于细胞核(图4)。
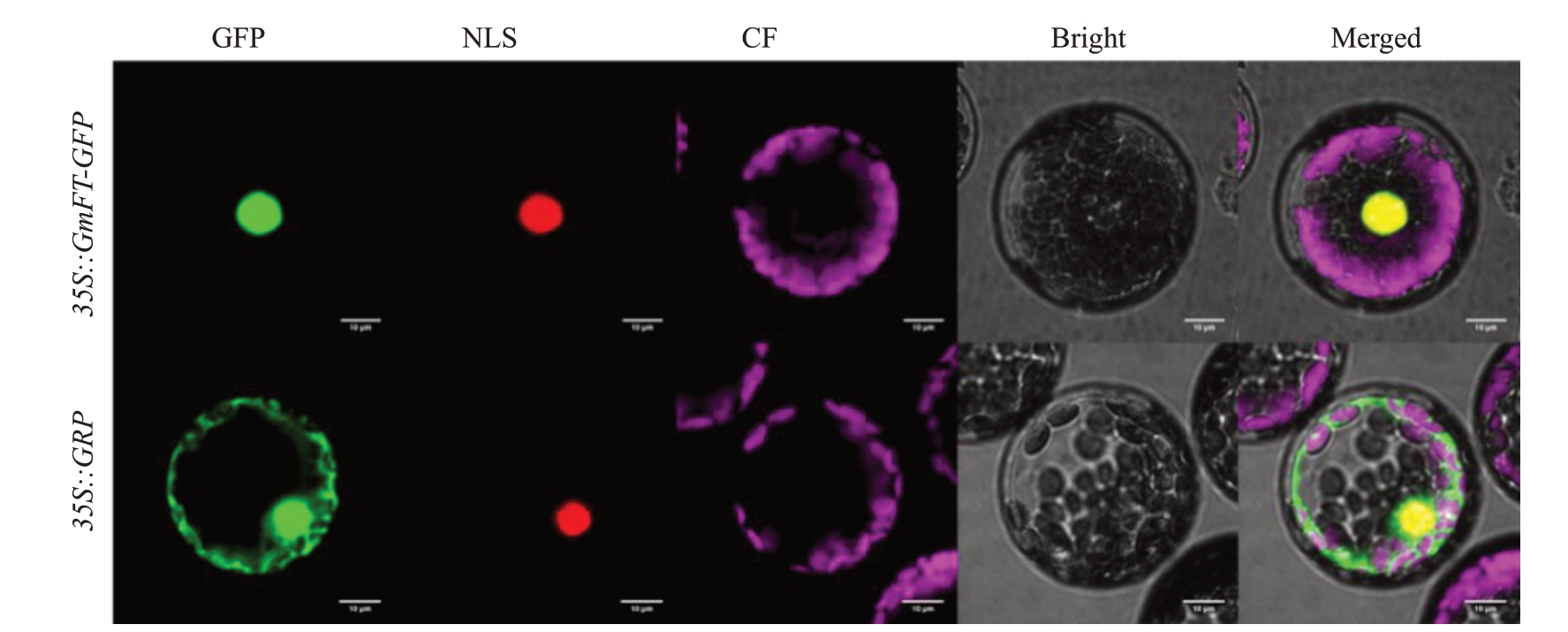
图4 CmFT-GFP 在拟南芥原生质体中的亚细胞定位
Fig.4 Subcellular localization of CmFT-GFP in A.thaliana
GFP 为绿色荧光信号,NLS 为RedDotTM1 核定位信号,CF 为叶绿体荧光信号,Bright 为明场,Merged 为叠加场。
GFP represents green fluorescence signal,NLS represents nuclear localization signal of RedDotTM1,CF represents chloroplast fluorescence signal,Bright represents bright field,and Merged field represents superposition field.
2.4 过量表达CmFT促进拟南芥开花
为了进一步研究CmFT 的生物学功能,将其在野生型拟南芥中异源过量表达,观察转基因植株的开花表型,分析该基因在植物开花中的作用。选取5 个CmFT 转基因拟南芥株系进行开花表型分析。L1、L2、L3、L4、L5的开花时间与野生型相比分别提前了16.2、14.2、14.9、15.2和12.4 d,均达到显著水平(p <0.05)(图5-A)。5个CmFT转基因拟南芥株系花薹为2~5 cm时莲座叶的数量依次为6.3、7.2、6.3、6.0和8.2,均显著(p <0.05)低于野生型拟南芥11.8(图5-B)。该结果说明过表达CmFT 显著促进拟南芥开花(图5-C)。
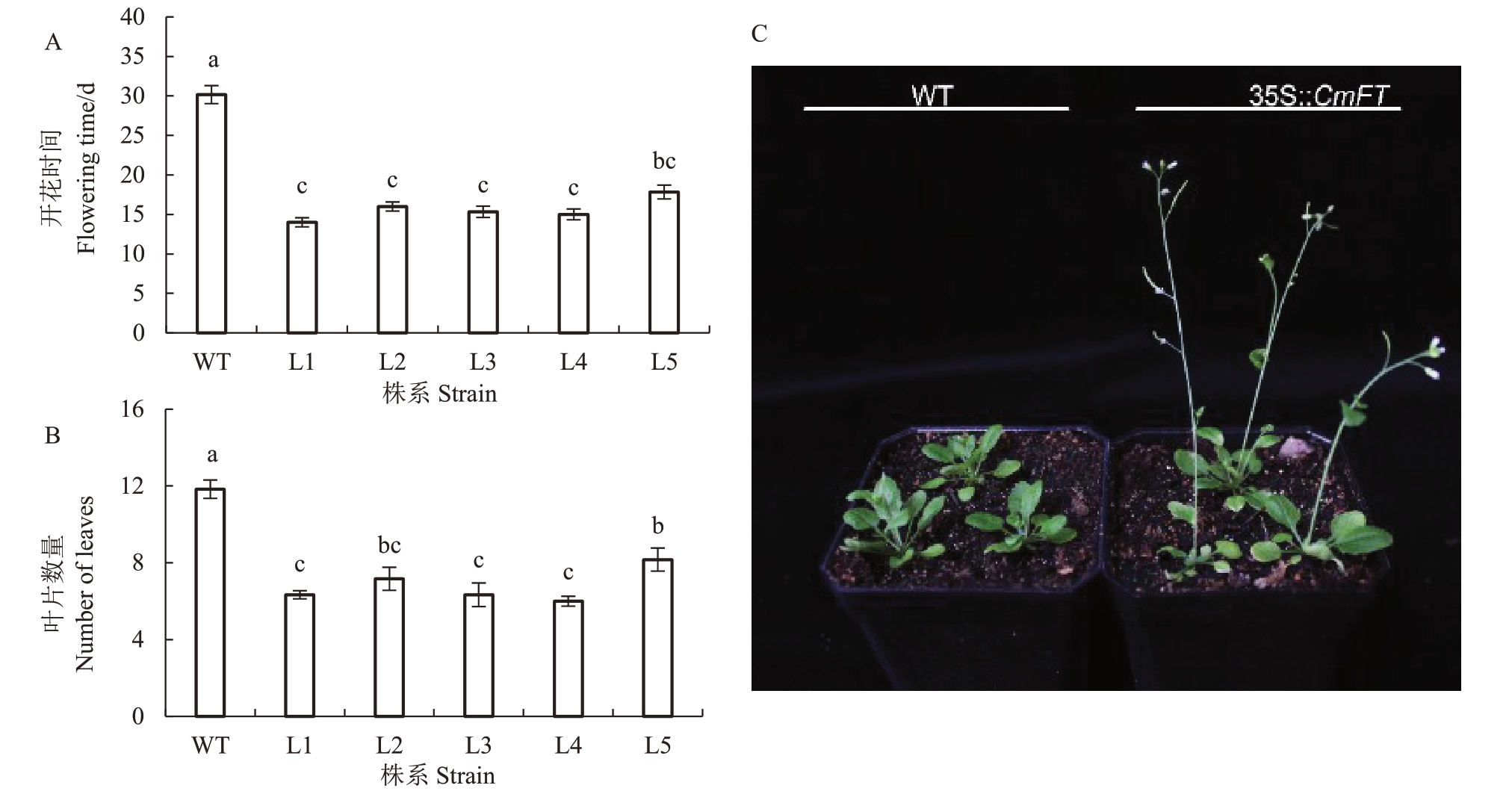
图5 CmFT 转基因拟南芥开花表型分析
Fig.5 Flowering phenotype analysis of CmFT transgenic A.thaliana
利用荧光定量PCR 检测不同时期转基因拟南芥中开花关键基因AtFT、AtTFL1、AtFLC、AtCO、At-SOC和AtLFY的表达量。结果(图6)显示,8叶期与4 叶期相比,野生型拟南芥和转基因拟南芥叶片中成花素基因AtFT 及其抑制基因AtTFL1、光周期通路基因AtSOC1表达水平均明显增高,其中转基因拟南芥的达显著水平(p <0.05)。而开花先锋基因AtLFY在两个时期内的表达水平变化不显著,但在8叶期转基因拟南芥叶片中AtLFY 的表达水平显著(p <0.05)高于对照。另外,开花抑制基因AtFLC在转基因拟南芥中的表达水平在4 叶期显著(p <0.05)高于野生型,但不同时期的表达水平差异不显著。而光周期通路基因AtCO 在8 叶期的表达水平则显著(p <0.05)低于4叶期,但转基因拟南芥和野生型拟南芥间无显著差异。
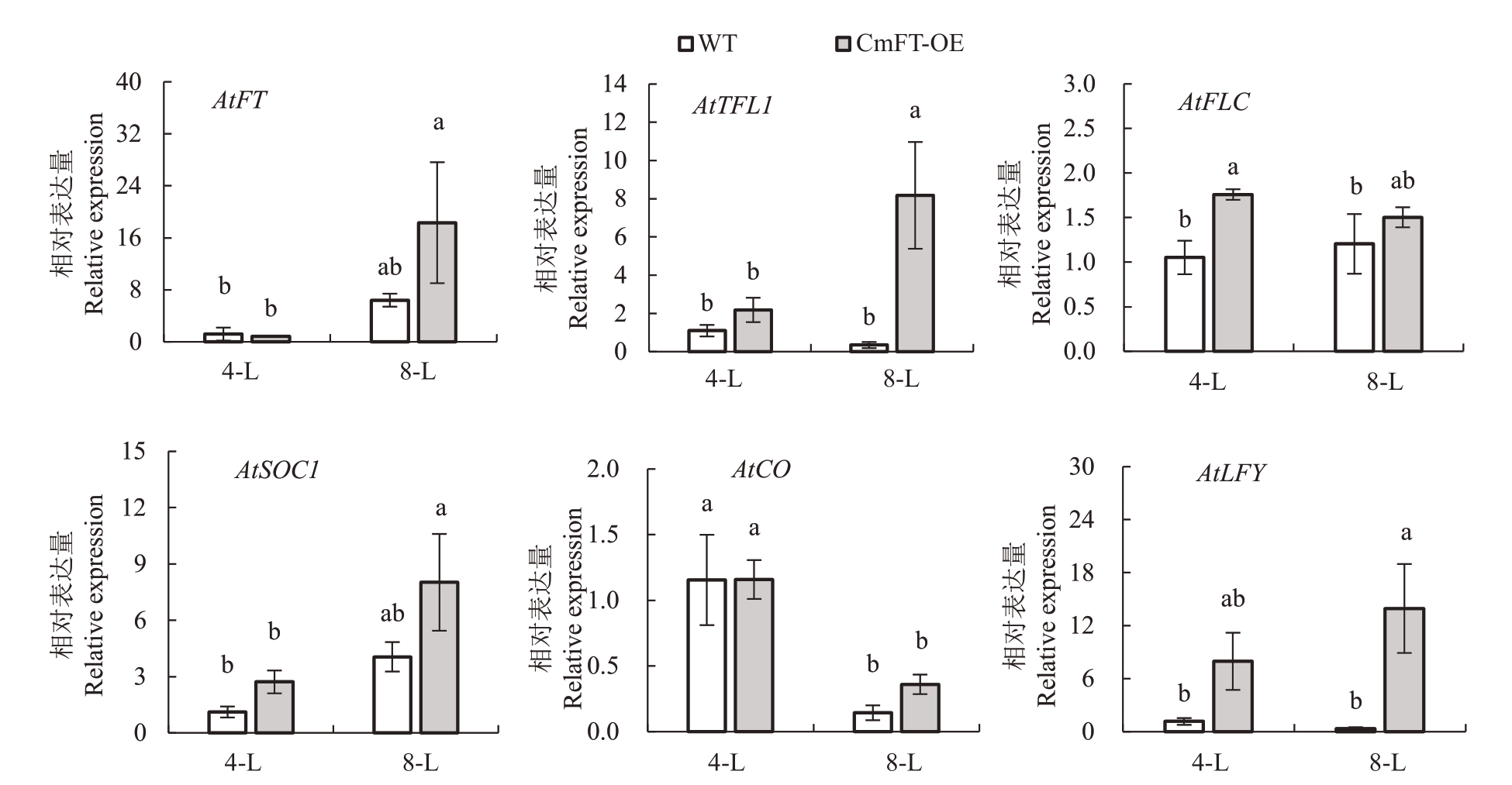
图6 拟南芥开花关键基因表达分析
Fig.6 Expression analysis of key flowering genes in A.thaliana
WT 代表野生型拟南芥,CmFT-OE 代表CmFT 过表达转基因拟南芥。4-L.4 叶期;8-L.8 叶期。
WT stands for wild type Arabidopsis plants,and CmFT-OE stands for overexpression CmFT transgenic Arabidopsis plants.4-L.4 leaves stage;8-L.8 leaves stage.
3 讨 论
成花素基因FT是植物感受开花信号,起始成花的关键基因。本研究中,在板栗基因组数据库检索到的FT 同源基因CmFT 与拟南芥、竹子、杨树等物种相似,均具有4个外显子,且编码区(CDS)序列长度相似。经克隆得到的CmFT基因,CDS全长525 bp,编码174个氨基酸,具有保守的PEBP结构域。氨基酸序列比对结果显示,板栗、拟南芥、春兰、葡萄等8个物种的FT同源基因编码173~178个氨基酸,相似度为87.36%。该结果进一步证实了FT氨基酸序列在不同物种中高度保守[10,26]。亚细胞定位结果表明CmFT 为核定位基因,与其在细胞核中发挥激活下游开花基因的功能一致[5-6]。
FT基因在叶脉筛管伴胞中表达,其编码的蛋白被FT-INTERACTING PROTEIN 1(FTIP1)蛋白通过韧皮部转运至茎尖分生组织[1-2]。板栗叶片CmFT的表达水平在7月中旬达到峰植。该结果与板栗6—8月份进行成花启动的生物学特性一致,暗示CmFT为板栗成花素基因,响应外界环境,促进成花起始。CmFT组织表达分析结果显示,CmFT在叶片和茎尖中均有较高表达量,而在雄花序和混合芽中表达较低。张煜等[22]的研究结果为CmFT 在雌、雄花序中的表达最高,其次为叶片和花芽。该结果与本研究结果的不同可能是由取样部位和时期不同造成的。不同物种FT同源基因的组织表达模式存在差异,如荔枝[27](Litchi chinensis)LcFT1和LcFT2基因只在叶片中表达,枣[28](Ziziphus jujuba)ZjFT 基因在不同生长阶段的各器官中均有表达,苹果[29](Malus domestica)MdFT1和MdFT2基因主要表达部位分别为结果母枝顶芽和生殖器官。上述结果与近年来FT 生物学功能多样性的研究结论一致。在水稻(Oryza sativa)中的研究表明,FT 可参与分蘖调控[30]。在马铃薯(Solanum tuberosum)中过表达水稻FT 同源基因Hd3a,能够使短日型马铃薯在长日照条件下产生块茎[31]。在苹果中过表达梨(Pyrus communis)的PcFT2 基因,可促进营养生长,延迟休眠和叶片衰老[32]。葡萄(V. vinifera)的VvFT 基因[33]和猕猴桃(Actinidia chinensis)的AcFT基因[34]也均参与休眠过程调控。在毛竹(Phyllostachys edulis)中的研究表明PheFT9、PheTFL2 和PheTFL8 参 与 休 眠 的 激活[35]。
将CmFT 在野生型拟南芥中过量表达,以进一步验证其生物学功能。研究结果显示,CmFT 过表达拟南芥株系具有极早开花的表型。该结果表明板栗与拟南芥、百合、土豆、棉花、杨树等物种的FT 同源基因均具有促进成花的生物学功能[10,26]。梨[32]、荔枝[36]、甜樱桃[37](Prunus avium)、柑橘[38](Citrus unshiu)等果树的FT同源基因过表达也可导致早花现象[39]。上述研究表明不同物种FT 同源基因具有保守的促进成花的生物学功能。对转基因株系和野生型拟南芥叶片中开花基因的表达分析表明,过表达CmFT 能促进内源开花促进基因AtFT、AtSOC1 和AtLFY 基因的增加。其中仅AtFT 的直接下游基因AtLFY 在8 叶期显著高于野生型拟南芥。上述结果暗示CmFT对拟南芥开花的促进作用是通过直接增加AtLFY 的表达量实现的。这与拟南芥中AtFT 在细胞核中与FD结合共同激活下游成花基因LFY的研究结果一致[3]。
植得注意的是,CmFT 过表达株系中开花抑制基因AtFLC 和AtFT 拮抗基因AtTFL1 的表达量分别在4叶期和8叶期显著高于野生型拟南芥。其中At-FLC 位于AtFT 上游,在营养生长阶段抑制AtFT 的表达。本研究中,AtFLC 在4 叶期转基因拟南芥中表达高于野生型拟南芥,可能是其表达能受CmFT的反馈调节,但其具体原因需进一步研究。而AtTFL1 与AtFT 均为PEBP 家族基因,共同调控营养生长和生殖生长的转变。TFL1 与FT 竞争结合FD 蛋白,FT-FD复合体可启动植物成花,而TFL1-FD复合体则抑制成花[5]。近期的研究表明,TFL1 基因在成花转变期间被显著特异上调,以维持花序顶端分生组织的无限生长[40]。本研究中,转基因拟南芥在8叶期已进入花期,而野生型拟南芥尚未进行成花转变,这可能是该时期转基因拟南芥植株中AtTFL1基因表达量高于野生型拟南芥的原因。上述结果暗示CmFT可能与AtFT均具有调控花期和顶端形态建成的生物学功能,需进一步研究验证。
4 结 论
从燕山红栗中克隆得到CmFT 基因,与其他物种的同源基因在基因结构和编码的氨基酸序列方面高度保守。CmFT 为核定位基因,在叶片和茎尖中表达,并在7月中旬的叶片中表达量最高,具有促进开花的生物学功能。
[1] ABE M,KOBAYASHI Y,YAMAMOTO S,DAIMON Y,YAMAGUCHI A,IKEDA Y,ICHINOKI H,NOTAGUCHI M.,GOTO K.,ARAKI T.FD,a bzip protein mediating signals from the floral pathway integrator FT at the shoot apex[J]. Science,2005,309(5737):1052-1056.
[2] YOO S C,CHEN C,ROJAS M,DAIMON Y,HAM B K,ARAKI T,LUCAS W J.Phloem long-distance delivery of FLOWERING LOCUS T (FT) to the apex[J]. Plant Journal,2013,75(3):456-468.
[3] ZHU Y,KLASFELD S,JEONG C W,JIN R,GOTO K,YAMAGUCHI N,WAGNER D.Terminal Flower 1-FD complex target genes and competition with FLOWERING LOCUS T[J]. Nature Communications,2020,11(1):5118.
[4] ZHU Y,LIU L,SHEN L S,YU H. NaKR1 regulates long-distance movement of FLOWERING LOCUS T in Arabidopsis[J].Nature Plants,2016,2(6):16075.
[5] SONG Y H,LEE I,LEE S Y,IMAIZUMI T,HONG J C.CONSTANS and ASYMMETRIC LEAVES 1 complex is involved in the induction of FLOWERING LOCUS T in photoperiodic flowering in Arabidopsis[J].Plant Journal,2012,69(2):332-342.
[6] JING Y J,GUO Q,ZHA P,LIN R C. The chromatin-remodelling factor PICKLE interacts with CONSTANS to promote flowering in Arabidopsis[J]. Plant Cell and Environment,2019,42(8):2495-2507.
[7] LUO X,GAO Z,WANG Y Z,CHEN Z J,ZHANG W J,HUANG J R,YU H,HE Y H.The NUCLEAR FACTOR-CONSTANS complex antagonizes polycomb repression to de-repress FLOWERING LOCUS T expression in response to inductive long days in Arabidopsis[J].Plant Journal,2018,95(1):17-29.
[8] JIN S,NASIM Z,SUSILA H,AHN J H.Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants[J].Seminars in Cell&Developmental Biology,2021,109(5):20-30.
[9] KARDAILSKY I,SHUKLA V K,AHN J H,DAGENAIS N,CHRISTENSEN S K,NGUYEN J T,CHORY J,HARRISON M J,WEIGEL D.Activation tagging of the floral inducer FT[J].Science,1999,286(5446):1962-1965.
[10] LEE,J H,LEE J S,AHN J H.Ambient temperature signaling in plants:An emerging field in the regulation of flowering time[J].Journal of Plant Biology,2008,51(5):321-326.
[11] ODIPIO J,GETU B,CHAUHAN R D,ALICAI T,BART R,NUSINOW D A,TAYLOR N J. Transgenic overexpression of endogenous FLOWERING LOCUS T-like gene MeFT1 produces early flowering in cassava[J].PLoS One,2020,15(1):e0227199.
[12] IGASAKI T,WATANABE Y,NISHIGUCHI M,KOTODA N.The FLOWERING LOCUS T/TERMINAL FLOWER 1 family in lombardy poplar[J].Plant Cell and Physiology,2008,49(3):291-300.
[13] KOMIYA R,IKEGAMI A,TAMAKI S,YOKOI S,SHIMAMOTO K.Hd3a and RFT1 are essential for flowering in rice[J].Development,2008,135(4):767-774.
[14] LEE R,BALDWIN S,KENEL F,MCCALLUM J,MACKNIGHT R. FLOWERING LOCUS T genes control onion bulb formation and flowering[J]. Nature Communications,2013,4:2884.
[15] MIAO Y S,JIANG L W.Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells[J]. Nature Protocols,2007,2(10):2348-2353.
[16] CLOUGH S J,BENT A F. Floral dip:A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana[J].The Plant Journal,1998,16(6):735-743.
[17] 吕守芳,闫爱玲,王贵禧.植物生长调节剂对板栗生长、性别分化和结蓬的影响[J].林业科学研究,2003,16(5):642-645.LÜ Shoufang,YAN Ailing,WANG Guixi. Effect of plant growth regulators on growth, sex differentiation and fructification of Chinese chestnut[J]. Forest Research,2003,16(5):642-645.
[18] 程华,李琳玲,袁红慧,徐向阳,王燕,程水源.内源激素含量变化与板栗花芽分化关系研究[J].北方园艺,2013(22):5-9.CHENG Hua,LI Linling,YUAN Honghui,XU Xiangyang,WANG Yan,CHENG Shuiyuan. Study on the relationship of changes of the endogenous hormone content in Castanea mollissima and bud differentation[J]. Northern Horticuture,2013(22):5-9.
[19] 陈澍燕,邢宇,王宗义,曹庆芹,张卿,秦岭.内源激素及其受体在板栗雌花分化过程中的变化[J].北京农学院学报,2016,31(1):9-14.CHEN Shuyan,XING Yu,WANG Zongyi,CAO Qingqin,ZHANG Qing,QIN Ling. Study on the changes of the hormones and recpetors in female flower differentiation of chestnut(Castanea mollissima)[J]. Journal of Beijing University of Agriculture,2016,31(1):9-14.
[20] CHEN G S,LI J T,LIU Y,ZHANG Q,GAO Y R,FANG K F,CAO Q Q,QING L,XING Y. Roles of the GA-mediated SPL gene family and miR156 in the floral development of Chinese chestnut (Castanea mollissima)[J]. International Journal of Molecular Sciences,2019,20(7):1577.
[21] XING Y,LIU Y,ZHANG Q,NIE X H,SUN Y M,ZHANG Z Y,LI H C,FANG K F,WANG G P,HUANG H W,BISSELING T,CAO Q Q,QIN L.Hybrid de novo genome assembly of Chinese chestnut (Castanea mollissima)[J]. GigaScience,2019,8(9):giz112.
[22] 张煜,刘阳,于文杰,房克凤,张卿,曹庆芹,邢宇,秦岭. FT/TFL1-like 基因在板栗一次花和二次花发育中的表达分析[J].分子植物育种,2020,20(4):8.ZHANG Yu,LIU Liuyang,YU Wenjie,FANG Kefeng,ZHANG Qin,CAO Qingqin,XING Yu,QIN Ling.Expression analysis of FT/TFL1-like genes in primary and secondary flowering of Chinese chestnut[J].Molecular Plant Breeding,2020,20(4):8.
[23] SONG Y H,SMITH R W,TO B J,MILLAR A J,IMAIZUMI T.FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering[J]. Science,2012,336(6084):1045-1049.
[24] WICKLAND D P,HANZAWA Y.The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family:Functional evolution and molecular mechanisms[J].Molecular Plant,2015,8(7):983-997.
[25] CHEN Q G,PAYYAVULA R S,CHEN L,ZHANG J,ZHANG C K,TURGEON R. FLOWERING LOCUS T mRNA is synthesized in specialized companion cells in Arabidopsis and Maryland Mammoth tobacco leaf veins[J].Proceedings of the National Academy of Sciences of the United States of America,2018,115(11):2830-2835.
[26] LIU L,LIU C,HOU X L,XI W Y,SHEN L S,TAO Z,WANG Y,YU H. FTIP1 is an essential regulator required for florigen transport[J].PLoS Biology,2012,10(4):e1001313.
[27] 丁峰,彭宏祥,何新华,李冬波,朱建华,秦献泉,李鸿莉,罗聪,曹辉庆.荔枝FLOWERING LOCUST(FT)同源基因cDNA 全长克隆及其表达[J].果树学报,2012,29(1):75-80.DING Feng,PENG Hongxiang,HE Xinhua,LI Dongbo,ZHU Jianhua,QIN Xianquan,LI Hongli,LUO Cong,CAO Huiqing.Cloning and expression analysis of the FLOWERING LOCUS T(FT)homologous gene cDNA from Litchi chinensis[J]. Journal of Fruit Science,2012,29(1):75-80.
[28] 李翔,侯璐,康亚璇,庞晓明,李颖岳.‘冬枣’FT 同源基因克隆及表达分析[J].果树学报,2017,34(11):1374-1384.LI Xiang,HOU Lu,KANG Yaxuan,PANG Xiaoming,LI Yingyue. Cloning and expression analysis of FT homologous gene in Ziziphus jujuba Mill.‘Dongzao’[J].Journal of Fruit Science,2017,34(11):1374-1384.
[29] KOTODA N,HAYASHI H,SUZUKI M,IGARASHI M,HATSUYA-MA Y,KIDOU S I,IGASAKI T,NISHIGUCHI M,YANO K,SHIMIZU T,TAKAHASHI S,IWANAMI H,MORIYA S,ABE K. Molecular characterization of FLOWERING LOCUS T like genes of apple (Malus×dometica Borkh.)[J]. Plant Cell and Physiology,2010,51(4):561-575.
[30] TSUJI H,TACHIBANA C,TAMAKI S,TAOKA K,KYOZUKA J,SHIMAMOTO K. Hd3a promotes lateral branching in rice[J].Plant Journal,2015,82(2):256-266.
[31] NAVARRO C,ABELENDA J A,CRUZ-ORÓ E,CUÉLLAR C A,TAMAKI S,SILVA J,SHIMAMOTO K,PRAT S.Control of flowering and storage organ formation in potato by FLOWERING LOCUS T[J].Nature,2011,478(7367):119-122.
[32] FREIMAN A,GOLOBOVITCH S,YABLOVITZ Z,BELAUSOV E,DAHAN Y,PEER R,AVRAHAM L,FREIMAN Z,EVENOR D,REUVENI M,SOBOLEV V,EDELMAN M,SHAHAK Y,SAMACH A,FLAISHMAN MA. Expression of FLOWERING LOCUS T2 transgene from Pyrus communis L.delays dormancy and leaf senescence in Malus × domestica Borkh.,and causes early flowering in tobacco[J].Plant Science,2015,241:164-76.
[33] VERGARA R,NORIEGA X,PARADA F,DBORA D,FRANCISCO P. Relationship between endodormancy,FLOWERING LOCUS T and cell cycle genes in Vitis vinifera[J].Planta,2016,243(2):411-419.
[34] VARKONYI-GASIC E,MOSS A,VOOGD C,WANG C,PUTTERILL J,HELLENS P. Homologs of FT,CEN and FD respond to developmental and environmental signals affecting growth and flowering in the perennial vine kiwifruit[J]. New Phytologist,2013,198(3):732-746.
[35] ZHAO J W,GAO P J,LI C L,LIN X C,GUO X Q,LIU S K.PhePEBP family genes regulated by plant hormones and drought are associated with the activation of lateral buds and seedling growth in phyllostachys edulis[J]. Tree Physiology,2019,39(8):1387-1404.
[36] DING F,ZHANG S W,CHEN H B,SU Z X,ZHANG R,XIAO Q S,LI H. Promoter difference of LcFT1 is a leading cause of natural variation of flowering timing in different litchi cultivars(Litchi chinensis Sonn.)[J].Plant Science,2015,241:128-137.
[37] YARUR A,SOTO E,GABRIEL L,AND ALMEIDA A. The sweet cherry (Prunus avium) FLOWERING LOCUS T gene is expressed during floral bud determination and can promote flowering in a winter-annual Arabidopsis accession[J]. Plant Reprodution,2016,29(4):311-322.
[38] ENDO T,SHIMADA T,FUJII H,KOBAYASHI Y,ARAKI T,OMU M.Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange [Poncirus trifoliata(L).Raf.][J].Transgenic Research,2005,14(5):703-712.
[39] 范志毅,罗聪,余海霞,王金英,何新华.园艺植物FT 基因研究进展[J].分子植物育种,2020,18(24):8099-8108.FAN Zhiyi,LUO Cong,YU Haixia,WANG Jinying,HE Xinhua. Research progress of horticulture plants Flowering Locus T gene[J].Molecular Plant Breeding,2020,18(24):8099-8108.
[40] GORETTI D,SILVESTRE M,COLLANI S,LANGENECKER T,MÉNDEZ C,MADUEÑO F,SCHMID M. TERMINAL FLOWER1 functions as a mobile transcriptional cofactor in the shoot apical meristem[J]. Plant Physiology,2020,182(4):2081-2095.