哈密瓜(Cucumis melo L.)原产于新疆,含糖量在15%左右,口感爽脆、果肉甘甜,营养丰富,有“瓜中之王”的美称。哈密瓜是典型的呼吸跃变型果实,果实具有糖分高和水分多等特点,极易在销售运输过程中由于生理代谢加速,使果实温度增高而加速腐烂,也易受到病原菌侵染引起品质下降及腐烂[1]。低温冷藏可有效抑制果实采后腐烂和品质下降,但哈密瓜果实对温度敏感,若温度过低则易发生冷害,造成品质下降甚至失去商品价值[2-3],故通常采用外用浸泡杀菌剂处理的方法来防治果实腐烂[4-7]。目前对水果霉菌病害的防治主要利用化学杀菌剂,但易出现药物残留超标和病原菌产生抗药性等问题,因此有针对性地开发低毒性的无公害杀菌剂十分必要。有研究表明,碳酸氢钠(NaHCO3)和硼酸等对多种水果的采后病害防治十分有效,且NaHCO3和硼酸的价格便宜,使用浓度低而被广泛使用[8-11]。纳他霉素是一种多烯大环类抗生素,广泛用于食品防腐和不同果蔬的采后保鲜[12-13]。近年来,绍兴地区也开始利用大棚种植哈密瓜,笔者在田间调查发现,有些果实在成熟期和采收后的果蒂周围极易出现病害,引起果实腐烂,但病因还未有报道。因此,笔者首先分离引起哈密瓜果实腐烂的病原菌,结合形态学观察和分子分类方法,确定病原菌的种类;明确该病菌生物学特性,筛选不同药剂进行室内防效试验并探讨可能的抑菌机制,以期为防治由真菌引起的哈密瓜果实腐烂提供有效的方法。
1 材料和方法
1.1 病原菌的分离纯化及对不同水果的致病性鉴定
从浙江绍兴地区大棚种植的哈密瓜(西州蜜)采集果蒂附近果肉出现病害的样本,取病健交界处的果肉,采用组织分离法进行病原菌的分离。病原菌经75%乙醇表面消毒2 min 后,再经0.1%的NaClO消毒5 min,无菌水漂洗2次。置于马铃薯葡萄糖琼脂(potato dextrose agar, PDA)培养基上,30 ℃黑暗条件下倒置培养。经多次纯化后,用消毒的接种针在果实上刺出伤口,在伤口处接种孢子悬液20 μL,接种到不同种类的水果以检测该病原菌对其他水果的致病性[14]。每个果实上接种3处,其中2处伤口接种菌液,另一处伤口接种清水为对照。30 ℃黑暗条件下倒置培养,接种不同时间后观察果实表面是否有菌落生长,以从果实表面明显看到菌斑为准,每种水果设置3次重复,每种处理至少用9个果实。
1.2 病原菌的形态观察与分子鉴定
挑取生长良好的菌丝及孢子,用显微镜观察其形态。用北京索莱宝科技有限公司的真菌基因组DNA 提取试剂盒提取病原菌基因组DNA,以基因组DNA 为模板采用引物(ITS5:F 5’-GGAAGTAAAAGTCGTAACAAGG-3’,ITS4:R 5’-TCCTCCGCTTATTGATATGC-3’)扩增真菌28SrDNA 的ITS区域(ITS1、5.8S、ITS2)[15]。采用引物(EF-1:F 5’-ATGGGTAAGGAGGACAAGAC- 3’和EF-2:R 5’-GGAAGTACCAGTGATCATGTT- 3’)对真菌DNA的EF-1α进行扩增[16],采用引物(H3-1a:F 5’-ACTAAGCAGACCGCCCGCAGG-3’和H3-1b:R 5’-GCGGGCGAGCTGGATGTCCTT- 3’)对真菌DNA 的Histone 3进行扩增[17]。PCR具体反应条件参照北京天根公司试剂盒进行,反应程序:95 ℃预变性5 min;94 ℃变性30 s,57 ℃退火30 s,72 ℃延伸1 min,共32个循环;最后72 ℃延伸10 min。PCR扩增产物用1.2%琼脂糖凝胶电泳观察,PCR扩增产物送至上海生工公司测序,所得序列在GenBank 内用BLAST 进行同源性比较,下载同源序列,再用邻接法在MEGA 5.05X件中构建系统发育进化树。
1.3 病原菌生物学特性
参照郜海燕等[18]的方法,将病原菌置于15~40 ℃条件下黑暗培养以确定最适培养温度;在pH值为4~11的培养基上培养,以确定最适pH值;以察氏培养基为基础,用等碳(C)质量的D-果糖等代替蔗糖以确定最适的C源;用等氮(N)质量牛肉膏、蛋白胨及尿素等代替NaNO3以确定最适N源。24 h光照和24 h黑暗培养,以确定光照对生长的影响;除温度和光照处理外,其他培养均在30 ℃黑暗培养1~4 d,测量菌落直径。在容量为250 mL 的锥形瓶里分别加入50、100、150 以及200 mL 的PDB 培养基,用封口膜密封在转速为30 r·min-1的摇床里30 ℃黑暗培养4 d,用纱布过滤测菌落湿质量,以确定氧气含量对菌落生长的影响。每种处理设置5 个平行对照,结果取平均值。
1.4 不同药剂对病原菌生长的影响
参照赵宁等[19]的方法,在PDA 培养基中分别加入不同浓度和种类的药剂,并以空白的PDA培养基为对照,其中NaHCO3 质量分数为0.2%、0.4%、0.6%、0.8%、1.0%;硼酸质量分数为0.2%、0.4%、0.6%、0.8%、1.0%、1.2%;咪鲜胺锰盐50%可湿性粉剂(施保功)质量分数为0.1%、0.05%、0.03%和0.025%;苯甲·嘧菌酯(325 g·L- 1)质量分数为0.065%、0.033%、0.022%、0.016%;纳他霉素质量浓度为20、25、30、35、40 mg·L-1。30 ℃黑暗培养1~4 d,测量菌落大小。
1.5 不同药剂处理对病原菌细胞结构完整性的影响
基于罗丹明123可结合于功能良好的线粒体膜上;健那绿B 能体现线粒体膜电位的高低及其功能的完整性,将有活性的线粒体染成蓝绿色;线粒体红色荧光探针可透过活细胞与线粒体结合,但不能透过死细胞,其积累依靠膜电位的高低;溴化丙锭(PI)不能透过细胞膜完整的活细胞,只能与死细胞内DNA结合。分别在含有1.0%NaHCO3、1.2%硼酸和30 mg·L-1纳他霉素的PDA培养基上挑取适量菌落,参考前人的方法,分别用罗丹明123(100 μg·mL-1)染色30 min;PI染色剂(100 μg·mL-1)染色2 h;健那绿B(50 μg·mL-1)和线粒体红色荧光探针(20 μg·mL-1)染色10 min后立即观察,染色结束后用无菌水清洗菌丝和孢子表面残存的染料3 次,置于荧光显微镜下观察孢子与菌丝染色结果,通过荧光亮度确定细胞的完整性[20-21]。
1.6 室内防效
取健康的哈密瓜用75%乙醇进行表面消毒,用打孔针果实表面刺出3个小孔,分别接入病原菌、病原菌和20 mg·L-1纳他霉素、病原菌和30 mg·L-1纳他霉素,置于30 ℃黑暗培养1~4 d,观察发病情况,实验设置3个重复。
1.7 数据处理与分析
数据结果用(平均值±标准差)表示,采用SPSS 14.0软件进行方差分析,并利用Duncan多重比较进行差异显著性分析。
2 结果与分析
2.1 病原菌的分离纯化、鉴定及对不同水果的致病性
哈密瓜果实染病后产生白色绒毛状菌丝(图1-A)。在PDA 培养基上时,菌落圆形,产生大量绒状气生菌丝。开始生长时呈白色,随着培养时间延长,菌丝变为黄褐色(图1-B),培养基背面中间部位为米黄色。菌丝有横隔和分枝,表面光滑(图1-C~D)。孢子为白色镰刀状,两端逐渐变细,具4~5个分隔,长度为25.4~35.5 μm,宽为3.3~5.4 μm(图1-D),分生孢子梗在气生菌丝上顶端形成,顶端可产生大型分生孢子。
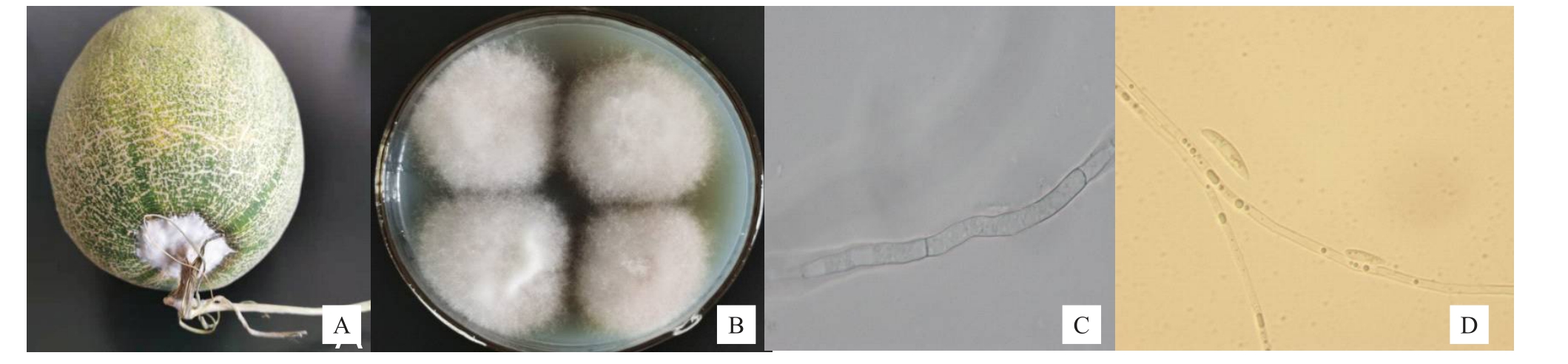
图1 病原菌分离与形态学观察
Fig.1 Isolation and morphological characteristics of Fusarium incarnatum from Hami melon fruit
A.病害果实;B.菌落正面;C. 菌丝结构;D.菌丝及孢子结构。
A.Disease fruit;B.Colony;C.Hypha;D.Hypha and spore.
提取病原菌DNA 后,分别用对应的引物PCR对rDNA-ITS、EF-1α和Histone 3的基因序列进行扩增,获得的片段大小分别为555、688 和504 bp。将rDNA-ITS 序列与GenBank 上的序列进行Blast 比对,发现与Fusarium incarnatum(MH979697.1)、Fusarium incarnatum(MG694577.1)和Fusarium incarnatum(MH290471)的相似率均在99%以上。基于IST 序列与GenBank 中Fusarium 属的相关序列构建系统发育树,发现与Fusarium incarnatum(MH979697.1)、Fusarium incarnatum(MG694577.1)和Fusarium incarnatum(MG694577.1)聚于同一分支(图2)。
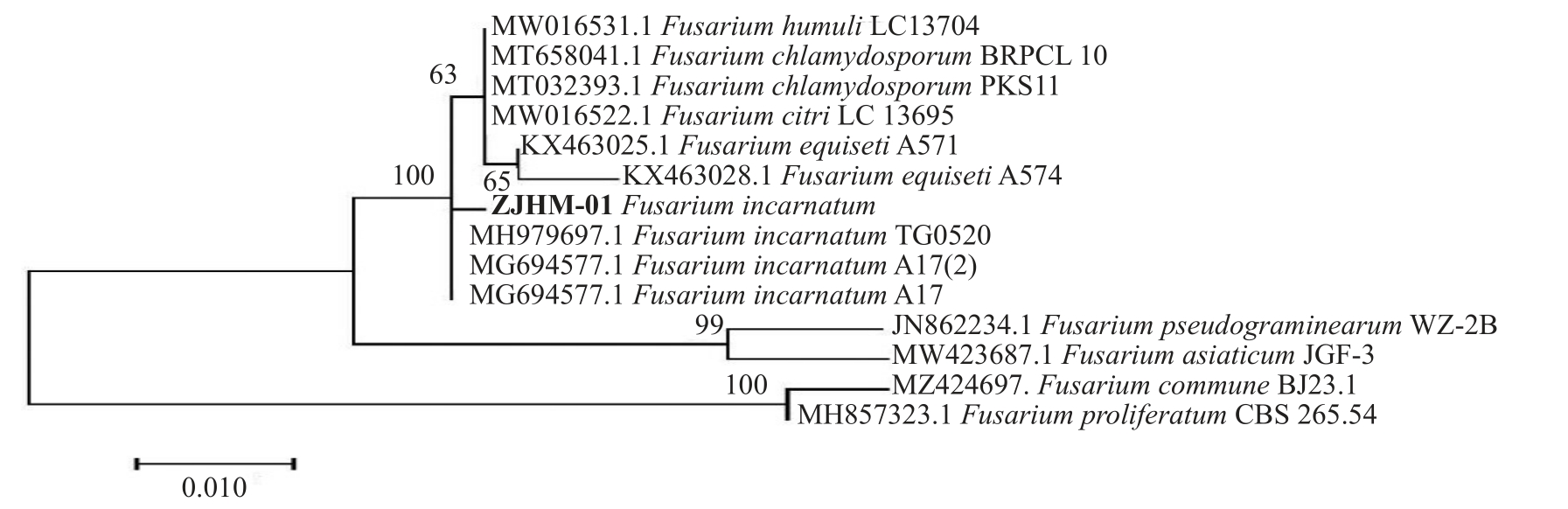
图2 基于IST 基因序列构建的系统发育树
Fig.2 Phylogenetic tree based on IST sequences
基于EF-1α序列与GenBank中Fusarium属的相关序列构建系统发育树,其与Fusarium incarnatum(KY785018.1)、Fusarium incarnatum(JX269001.1)和Fusarium incarnatum(MN233577.1)聚于同一分支(图3)。
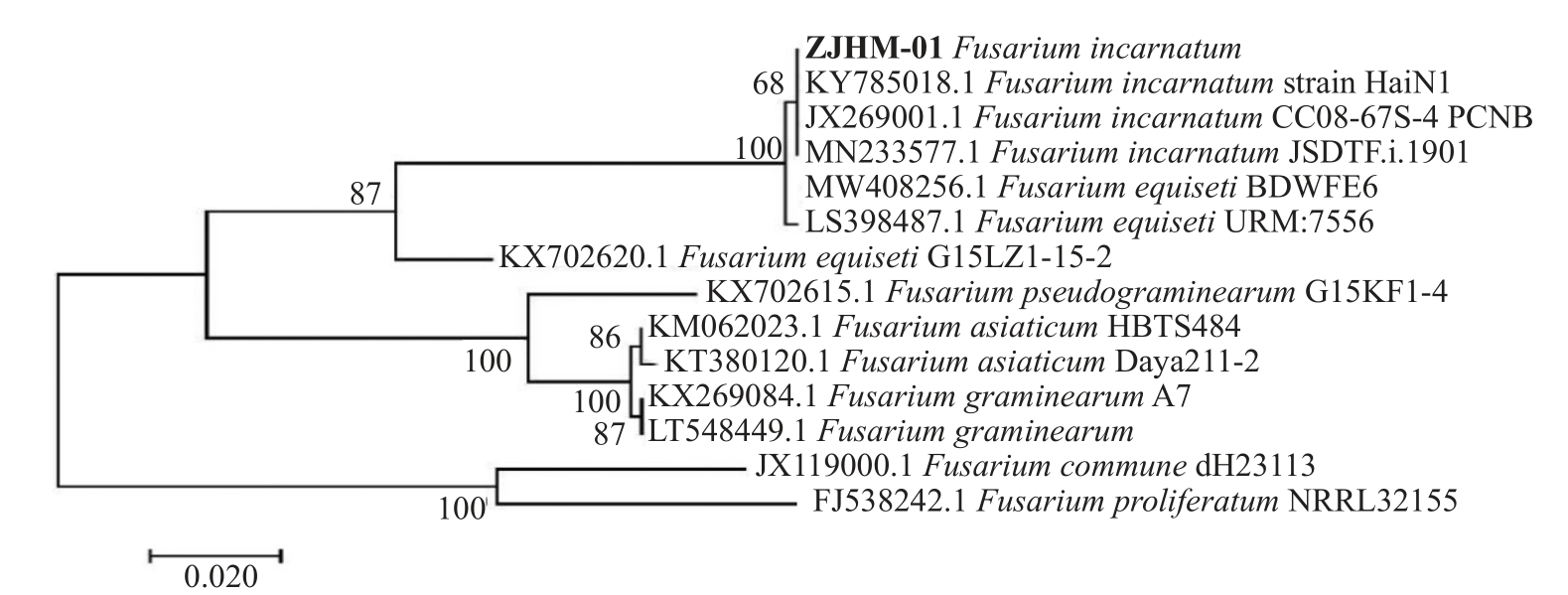
图3 基于EF-1α 基因序列构建的系统发育树
Fig.3 Phylogenetic tree based on EF-1α sequences
基于Histone3 序列与GenBank 中Fusarium 属8个种的基因序列构建系统发育树,其与Fusarium incarnatum(MN233578.1)、Fusarium incarnatum(MW-034438.1)和Fusarium incarnatum(MW159142.1)聚于同一分支,达100%支持率,远离Fusarium 属的其他种(图4)。
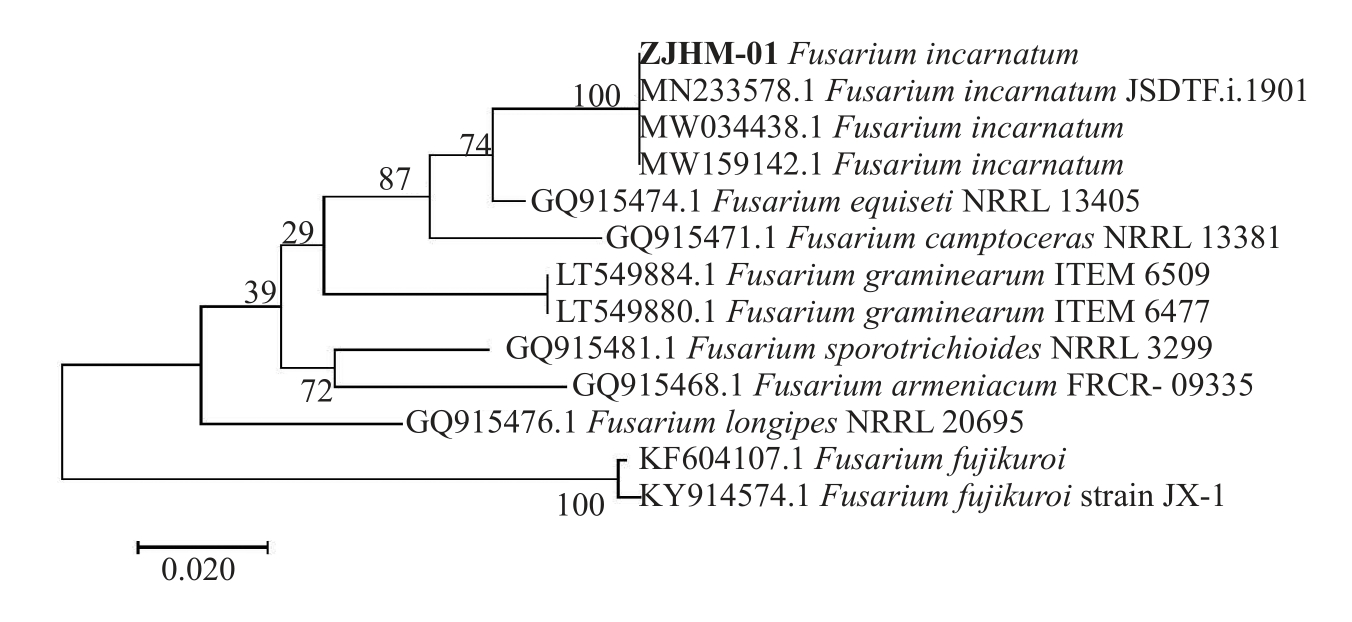
图4 基于Histone 3 基因序列构建的系统发育树
Fig.4 Phylogenetic tree based on Histone 3 sequences
因此基于形态鉴定及3 种分子鉴定结果,病原菌为哈密瓜果腐变红镰刀菌(Fusarium incarnatum,编号ZJHM-01)。进一步通过对病原菌宿主检测发现,接菌后1 d,除西瓜、黄瓜外,其余水果接种点附近都出现了大小不一的褐色病斑;4 d 时,所有果实均出现明显病斑,且其症状与自然发病的症状一致,而清水处理的果实则未发病。其中番茄、杏、冬枣腐烂最为严重,黄瓜病害较轻,表明该菌株对10 种水果均有致病力。
2.2 病原菌生物学特性
菌株在10~30 ℃条件下培养4 d 后,发现30 ℃时菌落直径最大,可达36.09 mm,25 ℃时为35.06 mm,20 ℃时菌落直径为32.28 mm,10 ℃时菌落直径为22.09 mm,35 ℃时菌落则停止生长,说明过低和过高的温度均不适合菌株生长(图5-A)。
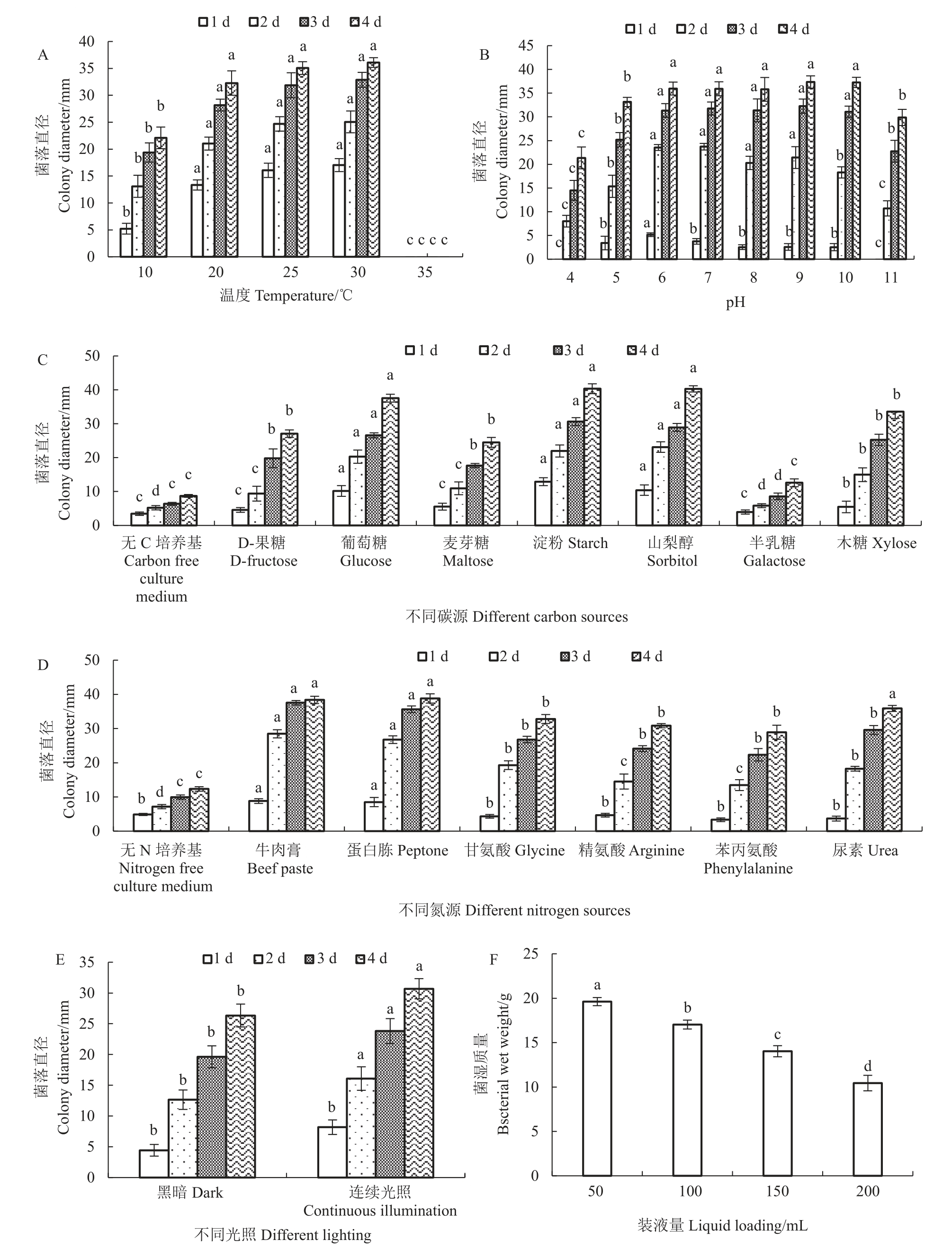
图5 病原菌生物学特性
Fig.5 Biological characteristics of the pathogen
不同小写字母表示在p <0.05 差异显著。下同。
Different small letters indicate significant difference at p <0.05.The same below.
菌株培养4 d 时发现pH 为4~11 时菌落均可生长,以pH 为6~9 时生长最好,菌落直径分别为35.96、35.92、35.79、37.33 mm。pH 为4 时菌落直径最小,仅有21.35 mm,比pH 为11 时的29.88 mm更小(p <0.05),说明强酸和强碱环境均不利于菌落生长(图5-B);菌株在7 种C 源培养基上均能生长,以淀粉、山梨醇和葡萄糖为C 源时生长最好,其次是木糖、果糖与麦芽糖,半乳糖为C 源时生长最差,菌落直径仅有12.58 mm(图5-C);菌株在7 种N源上均能生长,以蛋白胨、牛肉膏为N源时菌落直径最大,菌落直径可达38.79 和38.38 mm,其次是尿素、甘氨酸和精氨酸,苯丙氨酸为N源时菌落直径最小,仅有28.92 mm(图5-D)。试验还发现,菌株在无C 源和无N 源培养基上也能生长,说明菌株可利用其他物质来代替C源和N源,但生长缓慢,菌落直径分别为8.72 mm和12.33 mm。病原菌在光照和黑暗条件下均能生长,菌落直径分别为30.69 mm 和26.34 mm,但以光照条件下生长更好,两者间达显著差异(p <0.05);菌株对于生长环境的氧气含量有要求,在含氧量高的环境中生长迅速,在装液量为50 mL时,菌落湿质量为19.62 g,而在含氧量低的环境条件下生长缓慢,装液量为200 mL 时,菌落湿质量仅有10.44 g(图5-F),两者间达显著差异(p <0.05)。
此外,生长在不同N 源和C 源中的菌落形态和颜色却不同,如以D-果糖为C源的培养基上菌落颜色呈微黄色,而以葡萄糖为C 源的培养基上菌落颜色呈乳白色;以牛肉膏为N源,菌落颜色较对照更加透明,说明不同的N 源或C 源不但影响菌落的生长速率,也影响菌落的形态与色泽(图6),导致这种差异的原因还需要深入研究。
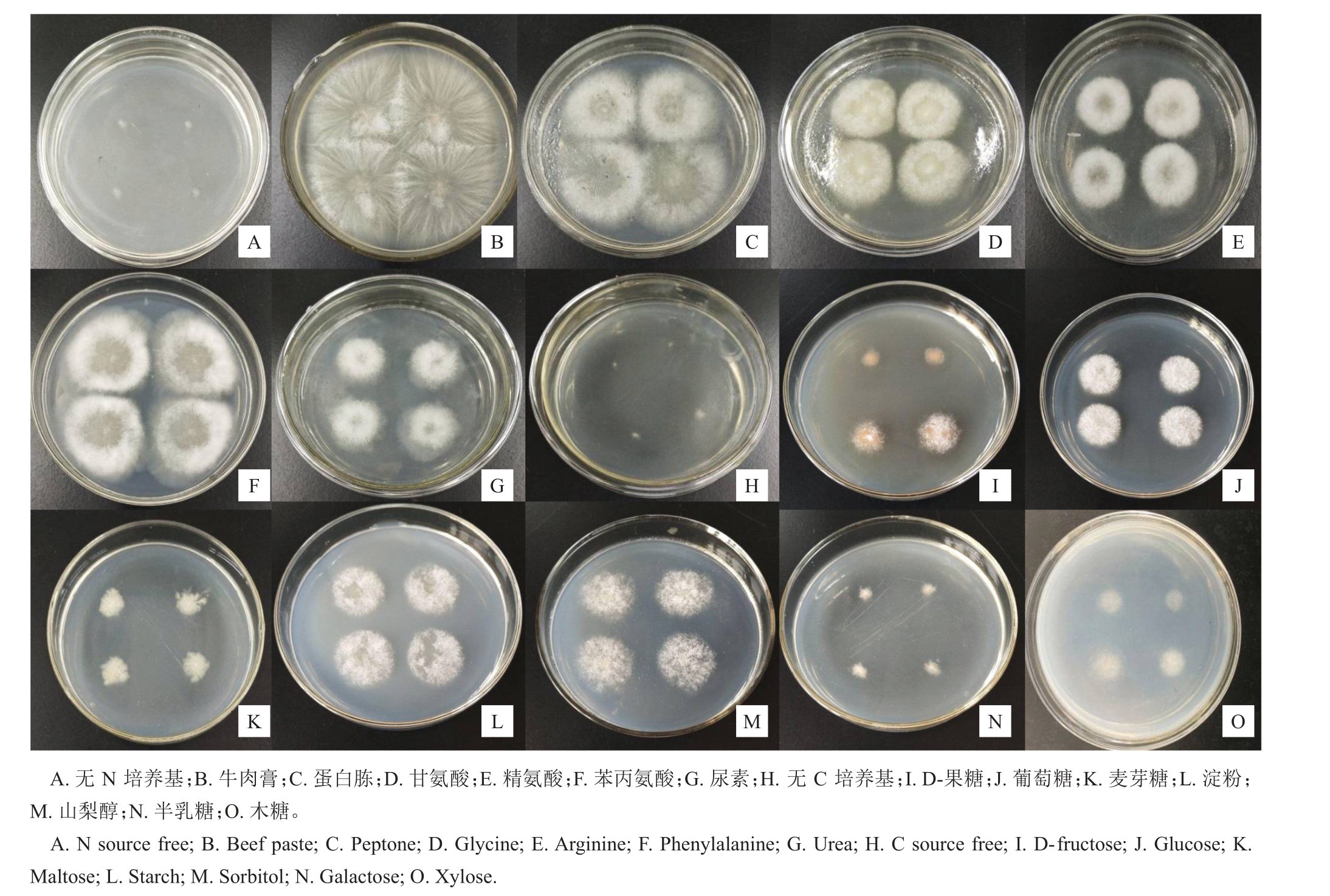
图6 不同N 源(3 d)和C 源(2 d)对菌落形态的影响
Fig.6 Effect of different nitrogen sources(3 d)and different carbon sources(2 d)on colonial morphology
2.3 不同浓度NaHCO3、硼酸、施保功、苯甲·嘧菌酯和纳他霉素对菌落生长的影响
与对照相比,且随着NaHCO3处理浓度增加,对菌落生长的抑制效果加强。当浓度(w)达1.0%时抑制效果最好,如培养4 d 菌落直径仅为对照的42.88%,达显著差异(p <0.05),但仍不能完全抑制菌落生长(图7-A)。与对照相比,0.2%~0.6%的硼酸没有抑菌作用,且0.2%的硼酸反而促进菌落的生长(p >0.05),当浓度高于0.6%时,则具有明显的抑菌效果,浓度达到1.2%时抑菌效果最强,菌落的直径最小,在培养4 d 菌落直径仅为对照的30.61%(p <0.05)(图7-B)。经纳他霉素处理4 d后发现,随着纳他霉素浓度升高抑菌效果也增强,如在20、25、30和35 mg·L-1 质量浓度下,菌落直径分别为对照的82.22%、59.26%、48.89%、23.70%,均达显著性差异(p <0.05),当浓度达40 mg·L-1时则完全抑制菌落生长(图7-C)。
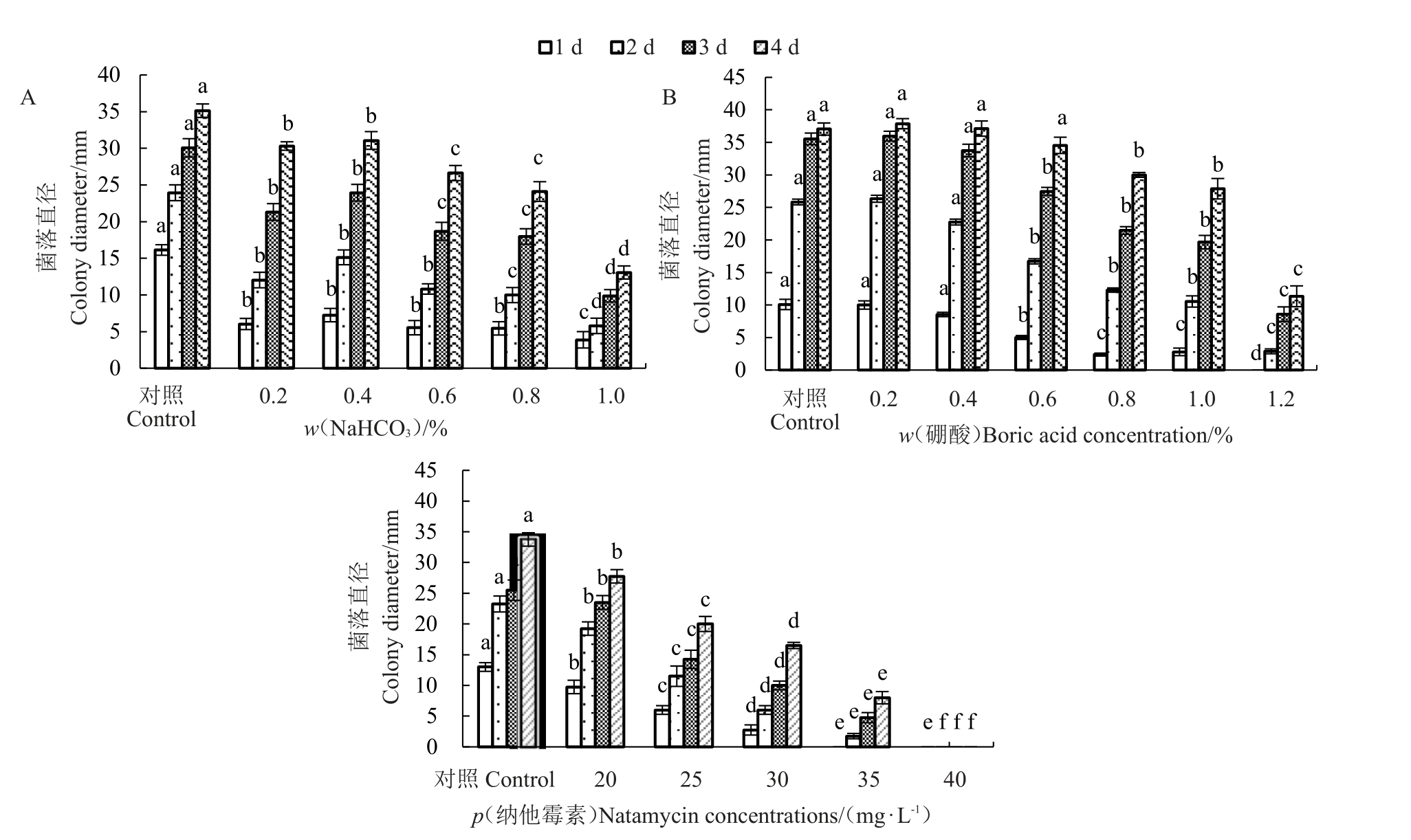
图7 NaHCO3、硼酸和纳他霉素处理对菌落生长影响
Fig.7 Effect of NaHCO3,boric acid and natamycin treatments on colony growth
在施保功质量分数为0.1%、0.05%、0.03%和0.025%以及苯甲·嘧菌酯使质量分数为0.065%、0.033%、0.022%和0.016%的PDA 培养基中培养4 d后均无菌落生长,对照处理的菌落已长满整个培养皿,说明病原菌对这两种药剂非常敏感,极低的使用浓度就能完全抑制菌落生长(图8)。
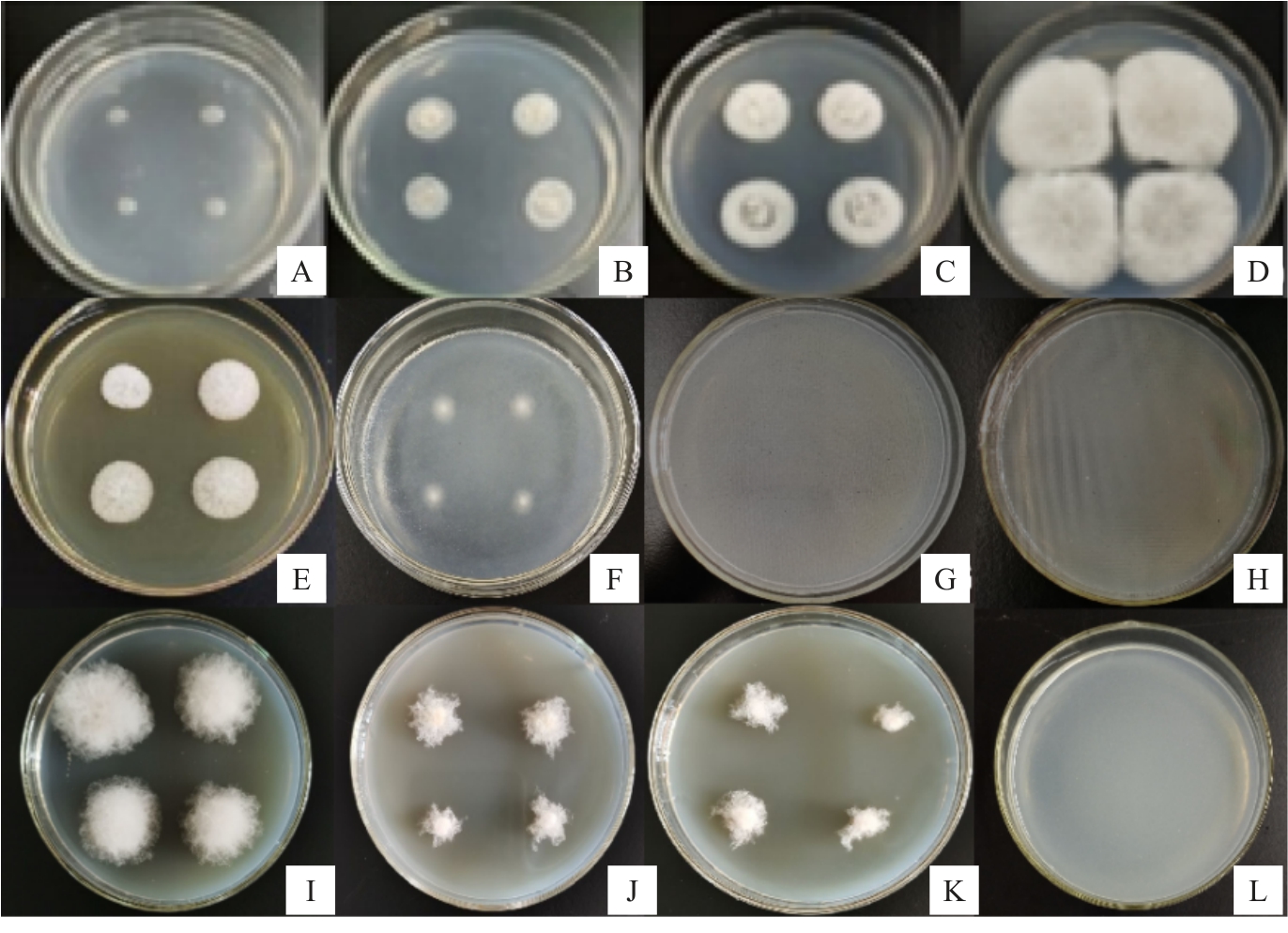
图8 NaHCO3、硼酸、施保功、苯甲·嘧菌酯和纳他霉素的抑菌效果
Fig.8 Antifungal effects of NaHCO3,boric acid,prochloraz,difenoconazole azoxystrobine and natamycin
A.对照(1 d);B.对照(2 d);C.对照(3 d);D.对照(4 d);E.1.0%NaHCO3(4 d);F.1.2%硼酸(4 d);G.施保功(4 d);H.苯甲·嘧菌酯(4 d);I.20 mg·L-1纳他霉素(4 d);J.25 mg·L-1纳他霉素(4 d);K.35 mg·L-1纳他霉素(4 d);L.40 mg·L-1纳他霉素(4 d)。
A.Control(1 d);B.Control(2 d);C.Control(3 d);D.Control(4 d);E.1.0%NaHCO3(4 d);F.1.2%boric acid(4 d);G.Prochloraz(4 d);H.Difenoconazole azoxystrobine(4 d);I.20 mg·L-1 natamycin(4 d);J.25 mg·L-1 natamycin(4 d);K.35 mg·L-1 natamycin(4 d);L.40 mg·L-1 natamycin(4 d).
2.4 NaHCO3、硼酸和纳他霉素处理对病原菌细胞结构完整性的影响
由于极低浓度的施保功和苯甲·嘧菌酯处理就能完全抑制菌落生长,因此无法获得相应的试验材料用于染色。而NaHCO3、硼酸和纳他霉素是公认的无公害药剂,并对笔者分离到的病原菌有较好地抑菌效果,所以用这3 种无公害的药剂处理后再用不同染料对病原菌进行染色,以分别从不同的层次来探讨其抑菌机制。结果发现,经罗丹明123 染色后,对照处理的菌丝(图9-A,B)染色较深,经1.0%NaHCO3、1.2%硼酸和30 mg·L-1纳他霉素处理的菌丝染色较浅(图9-C~H);经健那绿B染色后,与对照相比(图9-I~J),1.0% NaHCO3、1.2%硼酸和纳他霉素处理的菌丝染色明显变浅(图9-K~P),说明其线粒体的膜电位形成已受到破坏。
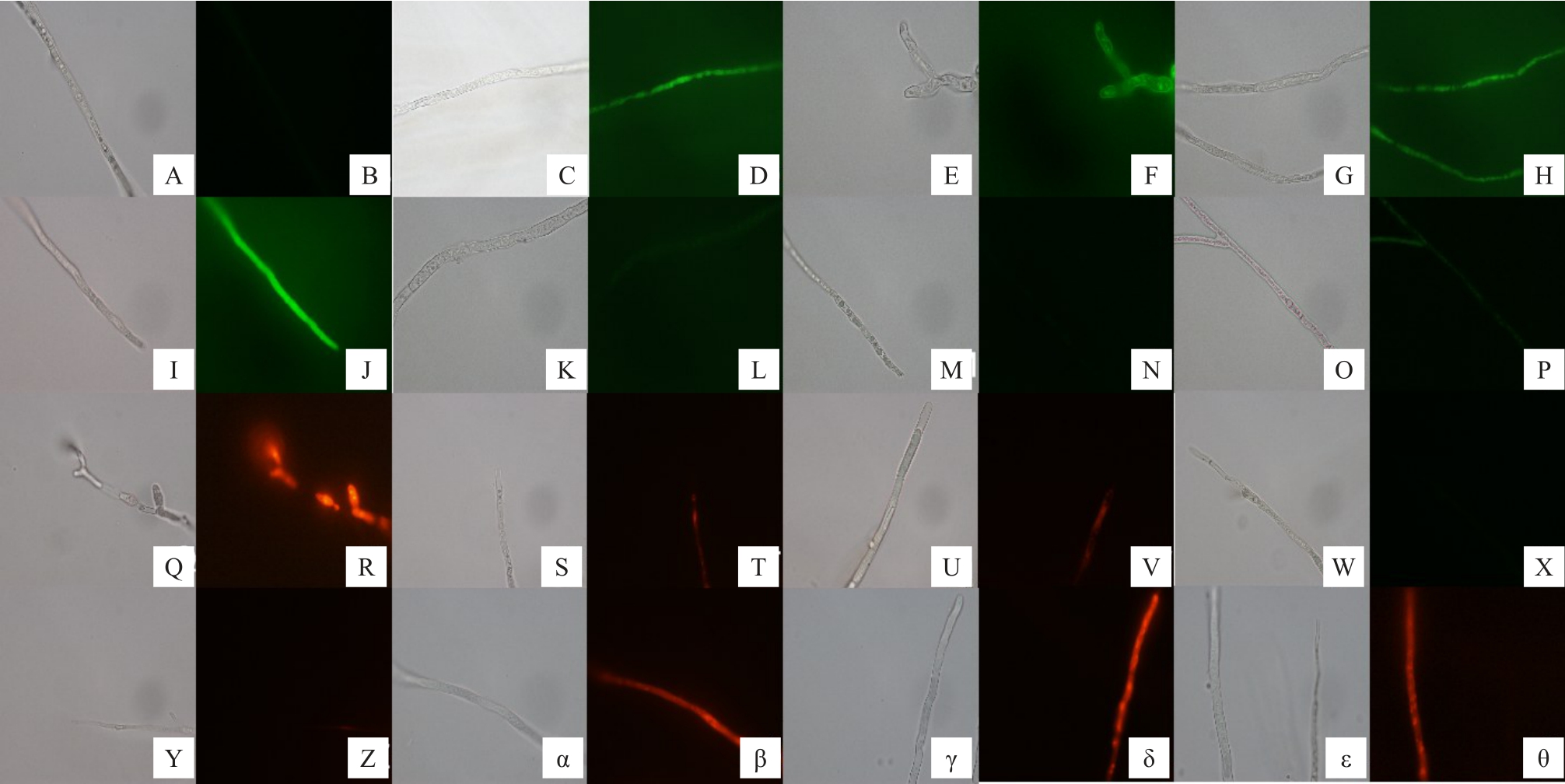
图9 罗丹明123、健那绿B、线粒体红色荧光探针和PI 对NaHCO3、硼酸和纳他霉素处理后菌丝染色结果
Fig.9 Staining results by Rhodamine 123,Ganus Green B,Mito TrackerTM Red CMXRos and PI of mycelial after treated with NaHCO3,boric acid and natamycin
A~H 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后的菌丝经罗丹明123 染色结果;I~P 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后健那绿B 染色结果;Q~X 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后线粒体红色荧光探针染色结果;Y~θ 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后PI 染色结果。AB 等两个对应图之间分别是白光和荧光结果。
A-H are the rhodamine staining results treated by control,NaHCO3,boric acid and natamycin respectively;I-P are the Ganus Green B staining results treated by control, NaHCO3, boric acid and natamycin; Q-X are the Mito TrackerTM Red CMXRos staining results treated by control, NaHCO3,boric acid and natamycin treatment;Y-θ are the results PI staining results treated by control,NaHCO3,boric acid and natamycin.Two corresponding diagrams are white light and fluorescence results respectively.
线粒体红色荧光探针(Mito TrackerTM Red CMXRos)能与活细胞中的线粒体结合,染色越深,说明线粒体活性越强。实验结果表明,经线粒体红色荧光探针染色后,对照处理的菌丝染色荧光强度最强(图9-Q~R),经NaHCO3、硼酸和纳他霉素处理后的菌丝红色荧光明显减弱(图9-S~X),说明线粒体活性明显受到抑制;经PI 染色后,对照处理的染色较浅(图9-Y~Z),1.0% NaHCO3、1.2%硼酸以及30 mg·L-1 纳他霉素处理的菌株染色较深(图9-α~θ),说明细胞膜受到的破坏最严重。4 种染料染色结果均说明1.0%NaHCO3、1.2%硼酸和30 mg·L-1纳他霉素均能破坏菌丝的细胞膜并抑制其线粒体活性。
罗丹明123 对孢子染色后发现,对照组无明显荧光(图10-A~B),经1.0%NaHCO3、1.2%硼酸以及30 mg·L-1纳他霉素处理后孢子的荧光较强,与菌丝染色结果一致(图10-C~H)。对照组孢子经健那绿染色后发出蓝绿色荧光(图10-I~J),且集中在隔膜部位,说明其线粒体功能良好,而且在隔膜部位活性较强。而经1.0%NaHCO3、1.2%硼酸以及30 mg·L-1纳他霉素处理的孢子几乎不发荧光(图10-K~P),说明线粒体活性受到显著抑制。用Mito TrackerTM Red CMXRos 对孢子染色发现,经1.0% NaHCO3、1.2%硼酸以及30 mg·L-1纳他霉素处理的孢子荧光强度明显比对照组弱(图10-Q~X),表明药剂处理抑制孢子线粒体的活性。对照组的孢子经PI 染色无明显荧光(图10-Y~Z),而经1.0%NaHCO3、1.2%硼酸以及30 mg·L-1纳他霉素处理的孢子经染色后发出强烈的红色荧光(图10-α~θ)。PI染色结果也表明1.0%NaHCO3、1.2%硼酸以及30 mg·L-1纳他霉素处理破坏了病原菌孢子细胞膜结构的完整性,而相对于菌丝的线粒体荧光强度,孢子的荧光强度普遍偏弱,说明孢子中的线粒体活性不如菌丝的线粒体活性强。
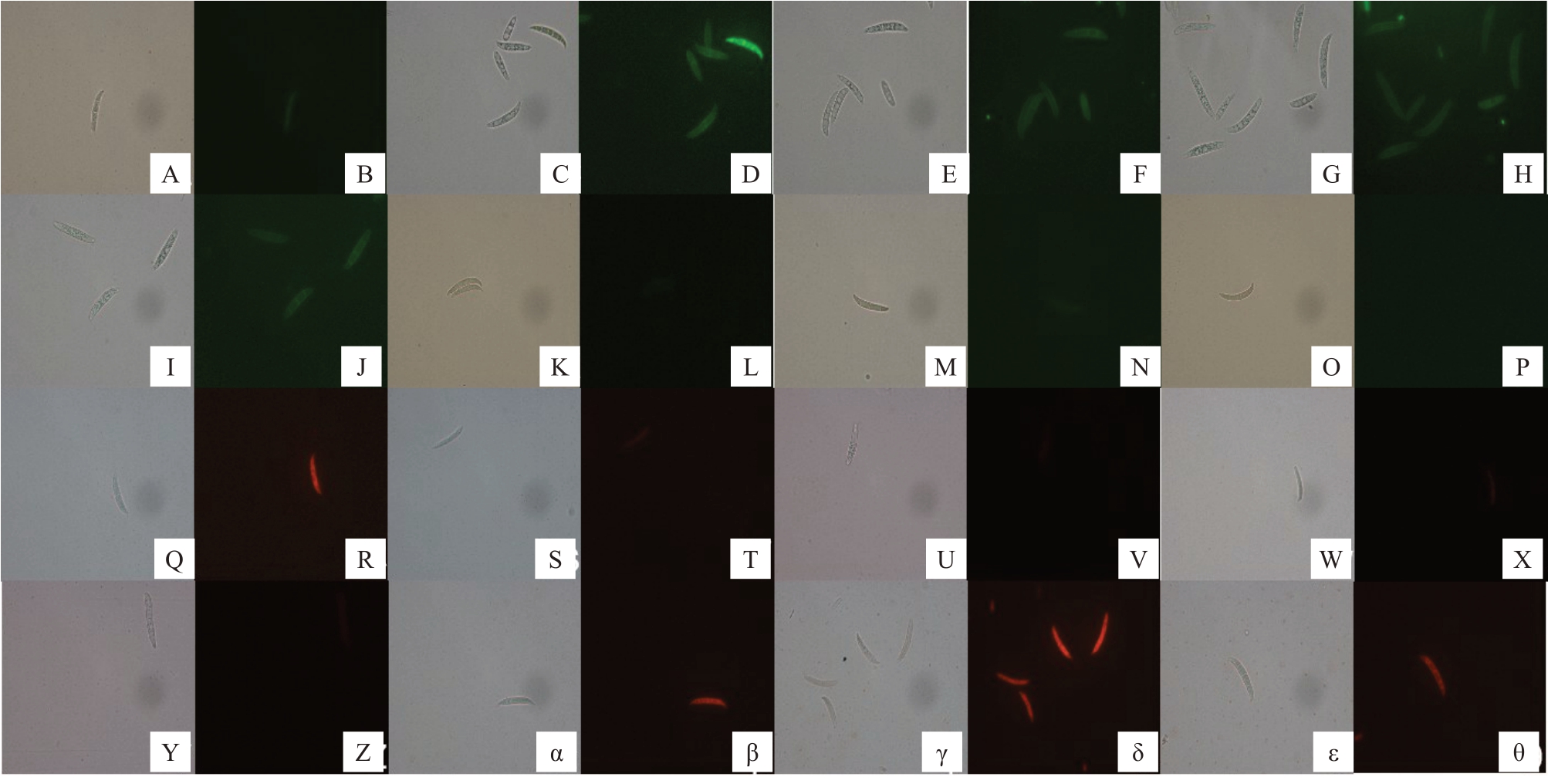
图10 分别经罗丹明123、健那绿B、线粒体红色荧光探针和PI 对NaHCO3、硼酸和纳他霉素处理后的孢子染色结果
Fig.10 Staining results by Rhodamine123,Ganus Green B,Mito TrackerTM Red CMXRos and PI of spore after treated with NaHCO3,boric acid and natamycin
A~H 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后的孢子经罗丹明123 染色结果;I~P 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后的孢子经健那绿B 染色结果;Q~X 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后的孢子经线粒体红色荧光探针染色结果;Y~θ 分别是对照、1.0%NaHCO3、1.2%硼酸和30 mg·L-1 纳他霉素处理后的孢子经PI 染色结果(AB等两个对应图之间分别是白光和荧光结果)。
A-H are the rhodamine staining results treated by control,NaHCO3,boric acid and natamycin respectively;I-P are the Ganus Green B staining results treated by control, NaHCO3, boric acid and natamycin; Q-X are the Mito TrackerTM Red CMXRos staining results treated by control, NaHCO3,boric acid and natamycin treatment;Y-θ are the results PI staining results treated by control,NaHCO3,boric acid and natamycin(Two corresponding diagrams are white light and fluorescence results respectively).
2.5 纳他霉素的室内防效
健康的哈密瓜接种哈密瓜镰刀菌果腐病后,对照处理在接种后第2 天时果实表面出现白色菌丝,加入20 mg·L-1纳他霉素处理的果实到第3天才出现少量菌丝,加入40 mg·L-1纳他霉素时,则到第4天果实的表面才出现菌丝。在接菌4 d后,对果实纵切后测量发现,对照处理的果肉已腐烂变软,病斑直径最大,达29.12 mm;经20 mg·L-1纳他霉素处理的病斑直径为19.32 mm;经40 mg·L-1纳他霉素处理的病斑直径最小,仅为10.43 mm 左右(图11),各处理间的病斑直径均达显著差异(p <0.05)。说明适宜浓度的纳他霉素处理能有效抑制哈密瓜镰刀菌果腐病原菌引起的病害。
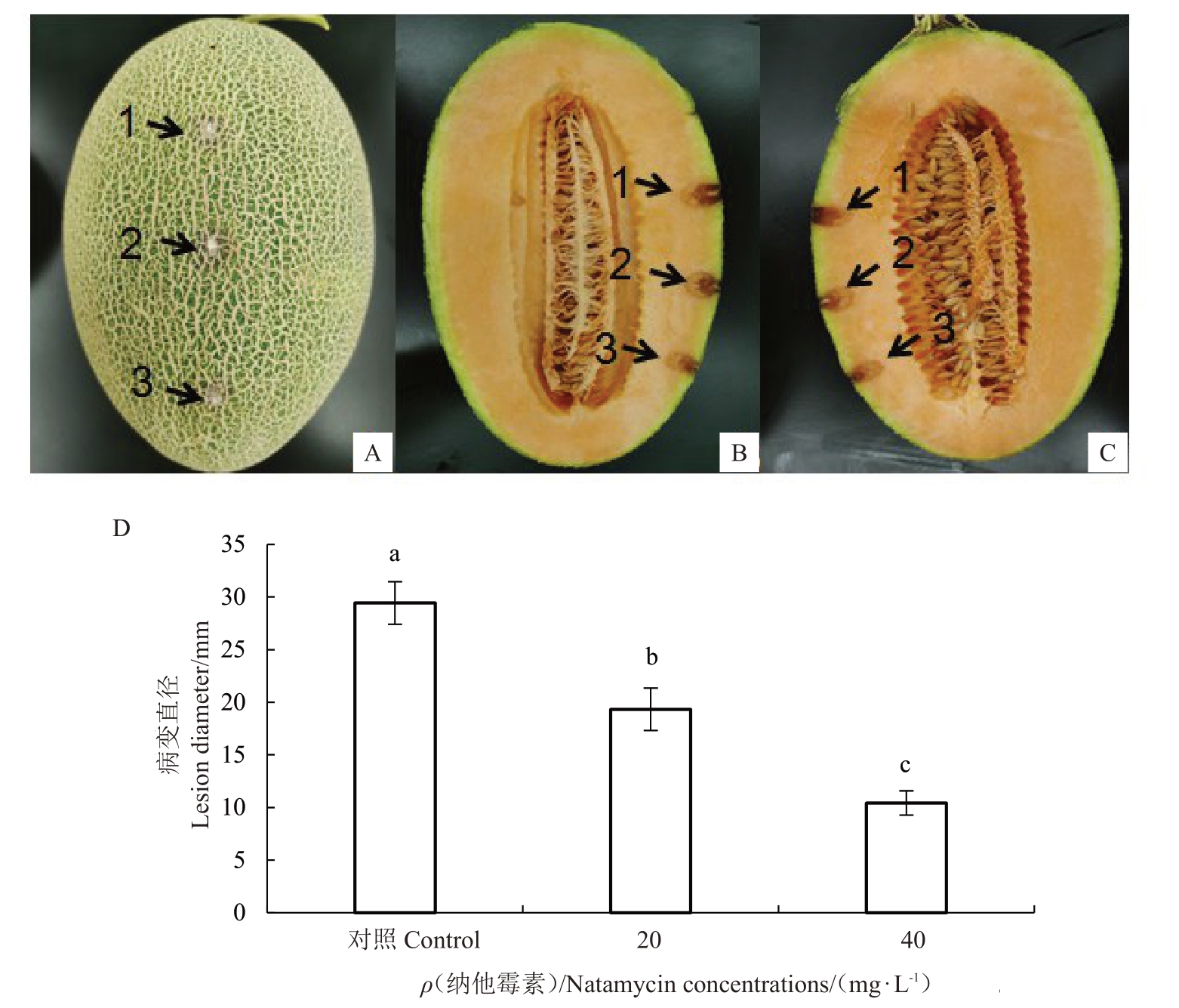
图11 纳他霉素处理对接种哈密瓜镰刀菌果腐果实的防效
Fig.11 Antifungal effect of natamycin treatment on Hami melon fruit after inoculated with Fusarium incarnatum
A.接菌4 d 后哈密瓜;B、C 为哈密瓜纵切图;D.不同浓度纳他霉素处理后病斑直径。图A、B、C 中,1 为只接种哈密瓜镰刀菌菌斑,2 为接种哈密瓜镰刀菌与20 mg·L-1 纳他霉素菌斑,3 为接种哈密瓜镰刀菌与40 mg·L-1 纳他霉素菌斑。
A.Hami melon after 4d incubation;Figure B,C Hami melon cross-cutting diagram;D.Lesion diameter after treated by natamycin.The 1,2,3 in figures A, B, C disease spot in Hami melon infected by Fusarium incarnatum, Fusarium incarnatum and 20 mg·L-1 natamycin,Fusarium incarnatum and 40 mg·L-1 natamycin.
3 讨 论
3.1 病原菌培养条件及寄主的探明为病害的防治提供新的参考
前人通过大量研究发现,镰刀菌是寄主极广的丝状病原真菌,对各种植物均可造成严重危害[22]。如有研究发现,在甜瓜连作时由尖孢镰刀菌引起的枯萎病更是一种毁灭性病害,甚至导致绝收[23]。且当人类食用受镰刀菌侵染的食品后,可诱发皮肤病、甲癣和角膜炎等[24-25],故需要加强对镰刀菌的有效防控。笔者在本研究中根据形态学和分子鉴定确定病原菌为哈密瓜果腐变红镰刀菌。在培养基pH 4~11内该菌株均可生长,最适pH 是7~9,强酸或强碱对病原菌生长均有显著的抑制作用;最适生长温度为30 ℃,35 ℃以上或低于10 ℃时均不利于该菌株的生长;淀粉、山梨醇和葡萄糖是最适C 源,蛋白胨和牛肉膏是最适N 源,这与耿丽华等[26]发现尖孢镰刀菌甜瓜专化型Fusarium oxysporum f. sp. melonis 所需的C 源和N 源相似。笔者也发现,在不同C 源和N源培养基上,菌落形态和颜色也不同,这与前人在其他菌株得到的结果类似[27];而且光照促进菌落生长,这与光照促进甜瓜镰刀菌果腐病病原菌(Fusarium incarnatum)[28]和欧李褐腐菌(Monilinia fructicola)[29]的生长相似,但与莫亿伟等[30]发现光照抑制桃形李丝衣霉菌的生长结果不同。所以不同病原菌所需最佳生长条件不同[31]。如王军节等[32]发现甜瓜粉霉病菌(Trichothecium roseum)所需的最适碳源则为果胶,且在最适pH、C源和N源时不但菌丝生长快,而且孢子产量及孢子萌发率也最高。所以建议在采后贮藏时应该避开病原菌适宜生长条件,如适当地降低贮藏温度等。鉴于该菌能对多种水果致病,在贮藏与销售时应防止与易感病的水果共运输或贮藏,以免感染其他水果。
3.2 药剂处理使病原菌细胞膜完整性受破坏并降低线粒体活性从而达到抑菌效果
本研究表明,NaHCO3、硼酸、纳他霉素、施保功和苯甲·嘧菌酯处理均能显著抑制哈密瓜果腐变红镰刀菌的生长,这与赵宁等[19]对桃形李青霉菌防控效果相似。且随着处理药剂使用浓度增加,抑菌效果也加强,并显著地抑制菌丝生长和孢子萌发,这与前人发现硼酸可抑制青霉菌生长的结果一致[33-34]。抑制生长可能是NaHCO3 改变了培养基pH,随着NaHCO3浓度增加,培养基碱性增强,菌落生长受到抑制;也有研究表明CO32-和HCO3-对菌丝和孢子的生长有抑制作用[35-36];硼酸抑菌机制可能与硼酸使病原菌活性氧代谢发生紊乱、线粒体功能受损及蛋白质氧化受损等有关[37]。如有研究发现,低浓度硼酸即可显著抑制绿霉病菌孢子萌发以及菌丝和芽管生长,引起该菌对糖吸收障碍[38]。适宜浓度的纳他霉素能有效抑制哈密瓜果腐变红镰刀菌的生长,对哈密瓜果实病害有显著的防效,这与陈方圆等[13]发现纳他霉素能显著抑制柑橘青霉菌的生长,降低病害发生的结果相似。究其原因,纳他霉素可能与甾醇结合后形成抗生素-甾醇复合物,从而破坏细胞质膜的渗透性,细胞膜受损导致细胞内含物大量流出,最终造成细胞死亡有关,但对哈密瓜果腐变红镰刀菌是否也通过相似的抑菌机制还需要将来深入研究。
前人研究发现,当线粒体跨膜电位崩溃时,经罗丹明123 染色后的线粒体发出很强的黄绿色荧光[39];细胞中由于存在细胞色素氧化酶,经健那绿B染色后能保持蓝绿色,当细胞受损或者发生细胞凋亡时,细胞色素c氧化酶活性受到抑制,蓝绿色荧光减弱甚至消失,而正常细胞中线粒体在细胞内分布均匀,当细胞受损后会造成线粒体聚集在某个区域,导致线粒体活性降低和荧光强度明显减弱[40]。本研究中哈密瓜果腐变红镰刀菌的菌丝和孢子经1.0%NaHCO3、1.2%硼酸及30 mg·L-1纳他霉素处理后,再用罗丹明123、健那绿B、线粒体红色荧光探针和PI染色,结果表明,NaHCO3、硼酸和纳他霉素处理后病原菌的菌丝和孢子线粒体跨膜电位的形成均受到显著抑制,线粒体上的细胞色素c 氧化酶的活性被破坏。与NaHCO3和硼酸处理相比,低浓度纳他霉素处理对线粒体的破坏更大,这与刘佳怡等[41]的研究结果相似。为了进一步探讨不同药剂处理对细胞膜完整性的影响,经PI染色以及NaHCO3、硼酸和纳他霉素处理均能破坏菌丝及孢子的细胞膜,这与van Leeuwen 等[42]的研究结果相似。此外,笔者发现在菌丝开始形成分支点及孢子开始形成时,PI 的荧光强度最大,说明在初始形成的细胞膜的完整性最易受到破坏,从而抑制菌丝分支和生长。
本研究表明,NaHCO3、硼酸、纳他霉素、施保功和苯甲·嘧菌酯对哈密瓜果腐变红镰刀菌均有良好抑菌效果。NaHCO3具有较高安全性和抑菌效果强已用于多种果实病害的防治,低浓度的硼酸也对多种细菌和霉菌都有极好的抑制作用[43]。施保功和苯甲·嘧菌酯虽属低毒性的杀菌剂,且已广泛用于田间杀菌防腐,但毕竟还是一种低毒的农药,若在采后使用可能会造成药物残留问题[44]。而纳他霉素是安全性较高的食品防腐剂且能抑制多种真菌病害[13],本研究发现,极低浓度纳他霉素即可显著抑制哈密瓜果腐变红镰刀菌的生长,降低果实腐烂率。所以,建议将施保功和苯甲·嘧菌酯这两种药剂在采收前用于防治哈密瓜镰刀菌果腐病;在采收后,可用安全性较高的NaHCO3、硼酸和纳他霉素浸泡处理以降低哈密瓜镰刀菌果腐病病害的发生率。基于极低浓度的纳他霉素就有很好的抑菌效果,本研究建议将纳他霉素作为哈密瓜采后镰刀菌果腐病防治的首选药剂。
4 结 论
从哈密瓜果实分离到的病原菌为哈密瓜果腐变红镰刀菌(Fusarium incarnatum,编号ZJHM-01),该菌生长最适pH 为7~9,最适温度是30 ℃,最适C 源为淀粉、山梨醇和葡萄糖;最适N源为蛋白胨和牛肉膏,宿主具有广谱性。施保功、苯甲·嘧菌酯、纳他霉素、NaHCO3、硼酸和纳他霉素均具有良好抑菌效果。但施保功和苯甲·嘧菌酯属于低毒性的杀菌剂,建议在采收前使用。在采收后,可用安全性较高的NaHCO3、硼酸和纳他霉素浸泡处理降低哈密瓜镰刀菌果腐病病害的发生率,建议将纳他霉素作为哈密瓜采后镰刀菌果腐病防治的首选药剂。
[1] 杜娟,廖新福,再吐娜,热比古丽,张敏,姚军.杀菌剂处理对采后甜瓜品质的影响[J].天津农业科学,2015,21(6):11-14.DU Juan,LIAO Xinfu,ZAI Tuna,RE Biguli,ZHANG Min,YAO Jun. Effect of fungicide treatments on postharvest quality of melon[J].Tianjin Agricultural Sciences,2015,21(6):11-14.
[2] ZHANG Q,SHAN C H,NING M,ZHAO X X,DU H F,CAI W C,TANG F X. Transcriptome profiling of gold queen hami melons under cold stress[J]. Russian Journal of Plant Physiology,2020,67(5):888-897.
[3] 刘同业,张婷,车凤斌,訾王贝,晁翔翔,徐斌.不同贮藏温度下西州密25 号哈密瓜果实冷害生理的研究[J].新疆农业科学,2015,52(1):26-32.LIU Tongye,ZHANG Ting,CHE Fengbin,ZI Wangbei,CHAO Xiangxiang,XU Bin. Studies on chilling injury physiology of Xizhoumi No.25 hami melon fruits at different storage temperatures[J].Xinjiang Agricultural Sciences,2015,52(1):26-32.
[4] 赵洪薇. 壳寡糖、水杨酸处理提高甜瓜耐贮性及其机理研究[D].乌鲁木齐:新疆农业大学,2016.ZHAO Hongwei.Studies on the mechanism of improving the resistance of postharvest muskmelons with chitosan oligosaccharide and salicylic acid[D]. Urumqi:Xinjiang Agricultural University,2016.
[5] 王静,李学文,刘媛,廖新福,杨军,吴芳.嘧菌酯处理对哈密瓜采后抗病性的影响[J].新疆农业科学,2012,49(3):440-447.WANG Jing,LI Xuewen,LIU Yuan,LIAO Xiaofu,YANG Jun,WU Fang. Effect of azoxystrobin treatments on postharvest disease resistance of Hami melon fruit[J]. Xinjiang Agricultural Sciences,2012,49(3):440-447.
[6] 李学文.1-MCP 和MeJA 对哈密瓜采后品质调控及其机理研究[D].南京:南京农业大学,2011.LI Xuewen. Study on 1-MCP and MeJA regulation and mechanism of quality of Hami melon fruit after harvest[D]. Nanjing:Nanjing Agricultural University,2011.
[7] 卞生珍,王彦章,杨世忠. 虫胶膜剂对甜瓜采后生理的影响[J].新疆农业大学学报,2000,23(2):38-41.BIAN Shengzhen,WANG Yanzhang,YANG Shizhong. Influence of shellac coating on post-harvest physiology in Hami melon[J]. Journal of Xinjiang Agricultural University,2000,23(2):38-41.
[8] USALL J,SMILANICK J,PALOU L,DENIS- ARRUE N,TEIXIDÓ N,TORRES R,VIÑAS I.Preventive and curative activity of combined treatments of sodium carbonates and Pantoea agglomerans CPA-2 to control postharvest green mold of citrus fruit[J].Postharvest Biology and Technology,2008,50(1):1-7.
[9] ALVINDIA D G,NATSUAKI K T. Control of crown rot-causing fungal pathogens of banana by inorganic salts and a surfactant[J].Crop Protection,2007,26(11):1667-1673.
[10] LARSEN B,PETROVIC M,DE SETA F. Boric acid and commercial organoboron products as inhibitors of drug-resistant candida albicans[J].Mycopathologia,2018,183(2):349-357.
[11] PALOU L,SMILANICK J L,USALL J,VIÑAS I. Control of postharvest blue and green molds of oranges by hot water,sodium carbonate,and sodium bicarbonate[J]. Plant Disease,2001,85(4):371-376
[12] SHEN W H,WANG D H,WEI L L,ZHANG Y.Fungal elicitorinduced transcriptional changes of genes related to branched-chain amino acid metabolism in Streptomyces natalensis HW-2[J].Applied Microbiology and Biotechnology,2020,104(10):4471-4482.
[13] 陈方圆,戴久竣,徐家延,张爽,陈蒙恩,班兆军,王立军,吴元锋.纳他霉素抑菌机制及在食品保鲜中的应用研究进展[J].食品科技,2021,46(9):47-51.CHEN Fangyuan,DAI Jiujun,XU Jiayan,ZHANG Shuang,CHEN Mengen,BAN Zhaojun,WANG Lijun,WU Yuanfeng.Research progress of antimicrobial mechanism of natamycin and its application in food preservation[J]. Food Science and Technology,2021,46(9):47-51.
[14] 朱迎迎,高兆银,李敏,陈亮,胡美姣.火龙果镰刀菌果腐病病原菌鉴定及生物学特性研究[J].热带作物学报,2016,37(1):164-171.ZHU Yingying,GAO Zhaoyin,LI Min,CHEN Liang,HU Meijiao. Identification and biological characteristics of dragon fruit(Hylocereus undatus Britt.) fusarium rot pathogen[J]. Chinese Journal of Tropical Crops,2016,37(1):164-171.
[15] 臧威,孙翔,孙剑秋,于文喜,王雪松,尹军霞,宋瑞清.南方红豆杉内生真菌的多样性与群落结构[J].应用生态学报,2014,25(7):2071-2078.ZANG Wei,SUN Xiang,SUN Jianqiu,YU Wenxi,WANG Xuesong,YIN Junxia,SONG Ruiqing.Diversity and community structure of endophytic fungi from Taxus chinensis var. mairei[J].Chinese Journal of Applied Ecology,2014,25(7):2071-2078.
[16] PALMORE T N,SHEA Y R,CHILDS R W,SHERRY R M,WALSH T J. Fusarium proliferatum soft tissue infection at the site of a puncture by a plant:Recovery,isolation,and direct molecular identification[J].Journal of Clinical Microbiology,2010,48(1):338-342.
[17] GLASS N L,DONALDSON G C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J].Applied and Environmental Microbiology,1995,61(4):1323-1330.
[18] 郜海燕,肖尚月,陈杭君,黎云龙,刘瑞玲,吴伟杰.蓝莓采后主要病原真菌的分离鉴定与生物学特性研究[J].农业机械学报,2017,48(5):327-334.GAO Haiyan,XIAO Shangyue,CHEN Hangjun,LI Yunlong,LIU Ruiling,WU Weijie.Identification and biological characteristics of fungal pathogens in blueberry during postharvest storage[J].Transactions of the Chinese Society for Agricultural Machinery,2017,48(5):327-334.
[19] 赵宁,宋昱辉,陈莉,莫亿伟.嵊州桃形李采后橘青霉分离鉴定及室内抑菌试验[J].福建农业学报,2018,33(4):407-412.ZHAO Ning,SONG Yuhui,CHEN Li,MO Yiwei. Isolation,Identification and antibacterial tests on Penicillium citrinum for postharvest Shengzhou plums[J]. Fujian Journal of Agricultural Sciences,2018,33(4):407-412.
[20] 吴永明,夏欣一,潘连军,邵永,金保方,黄宇烽,王修来.罗丹明/碘化吡啶双染法检测精子线粒体膜功能的研究[J].中华男科学杂志,2006,12(9):803-806.WU Yongming,XIA Xinyi,PAN Lianjun,SHAO Yong,JIN Baofang,HUANG Yufeng,WANG Xiulai. Evaluation of sperm mitochondrial function using Rh123/PI dual fluorescent staining[J].National Journal of Andrology,2006,12(9):803-806.
[21] 杨琼,邓继辉,王振华,俞宁.台盼蓝、次甲基蓝及健那绿在猪精子活率测定中的比较研究[J].四川畜牧兽医,2019,46(3):29-32.YANG Qiong,DENG Jihui,WANG Zhenhua,YU Ning. Comparison of trypan blue,methylene blue and janus green in the determination of sperm viability[J]. Sichuan Animal & Veterinary Sciences,2019,46(3):29-32.
[22] SHARMA M,GULERIA S,SINGH K,CHAUHAN A,KULSHRESTHA S. Mycovirus associated hypovirulence,a potential method for biological control of Fusarium species[J].Virusdisease,2018,29:134-140.
[23] 阿衣加玛丽·库都热提,张以和.甜瓜连作障碍及其消减技术研究进展[J].中国瓜菜,2020,33(5):1-5.Ayijianmali·Kudureti,ZHANG Yihe.Research progress on continuous cropping obstacle and its reductiontechniques of muskmelon[J].China Cucurbits and Vegetables,2020,33(5):1-5.
[24] MUNKVOLD G P.Fusarium species and their associated mycotoxins[J].Mycotoxigenic Fungi,2017,1542:51-106.
[25] ROSA P D,HEIDRICH D,CORRÊA C,SCROFERNEKER M L,VETTORATO G,FUENTEFRIA A M,GOLDANI L Z. Genetic diversity and antifungal susceptibility of Fusarium isolates in onychomycosis[J].Mycoses,2017,60(9):616-622.
[26] 耿丽华,王建设,马建,杨洋,史越,宋顺华.一种甜瓜新症状病害的病原鉴定及其生物学特性[J].中国瓜菜,2021,34(3):15-20.GENG Lihua,WANG Jianshe,MA Jian,YANG Yang,SHI Yue,SONG Shunhua. Identification and biological characteristics of the pathogen causing anew symptom disease of melon[J]. China Cucurbits and Vegetables,2021,34(3):15-20.
[27] 刘瑜,王海,王艳丹,孙文怡,郭雪霞,冉国伟,张慧媛.枸杞鲜果霉变菌种分离鉴定及其生物学特性[J]. 农业工程学报,2017,33(S1):374-380.LIU Yu,WANG Hai,WANG Yandan,SUN Wenyi,GUO Xuexia,RAN Guowei,ZHANG Huiyuan. Isolation,identification and biological characteristics of pathogenic fungus from Chinese wolfberry fruit[J]. Transactions of the Chinese Society of Agricultural Engineering,2017,33(S1):374-380.
[28] 王燕,王春伟,王琳,李志远,张曦倩,刘峥,陈二毛,王美琴,张作刚,王建明.甜瓜镰刀菌果腐病新病原菌Fusarium incarnatum 的鉴定及生物学特性[J].园艺学报,2019,46(3):529-539.WANG Yan,WANG Chunhui,WANG Lin,LI Zhiyuan,ZHANG Xiqian,LIU Zheng,CHEN Ermao,WANG Meiqin,ZHANG Zuogang,WANG Jianming. Identification and biological characteristics of a novel pathogen Fusarium incarnatum causing muskmelon fruit fusarium rot[J].Acta Horticulturae Sinica,2019,46(3):529-539.
[29] 郝晓娟,高莹,李新凤,王建明,李生才,王美琴,李俊林.欧李褐腐病病原菌生物学特性及其寄主范围[J].果树学报,2014,31(1):101-104.HAO Xiaojuan,GAO Ying,LI Xinfeng,WANG Jianming,LI Shengcai,WANG Meiqin,LI Junlin. Biological characteristics and host range of the pathogen causing brown rot of Chinese dwarf cherry[J].Journal of Fruit Science,2014,31(1):101-104.
[30] 莫亿伟,林海雁,郑梦琪,王海,杨国,王玮.桃形李果实丝衣霉菌的分离、鉴定、生物学特性及其防治[J].农业生物技术学报,2019,27(12):2227-2237.MO Yiwei,LIN Haiyan,ZHENG Mengqi,WANG Hai,YANG Guo,WANG Wei. Isolation,identification,physicochemical parameters and antibacterial characteristics of Byssochlamys sp.from Shengzhou Nane (Prunus salicina var. taoxingli) fruit[J].Journal of Agricultural Biotechnology,2019,27(12):2227-2237.
[31] ZHOU M S,LI P Z,WU S Y,ZHAO P Y,GAO H Y. Bacillus subtilis CF-3 volatile organic compounds inhibit Monilinia fructicola growth in peach fruit[J].Frontiers in Microbiology,2019,10:1804.
[32] 王军节,赵鲁迺克,范春霞,张琇,王鹏,穆风花.甜瓜粉霉病病原菌生物学特性及其寄主范围测定[J].中国瓜菜,2021,34(10):20-25.WANG Junjie,ZHAO Lunaike,FAN Chunxia,ZHANG Xiu,WANG Peng,MU Fenghua. Biological characteristics and host range of the pathogen causing pinkrot of muskmelon[J]. China Cucurbits and vegetables,2021,34(10):20-25.
[33] CAO B H,LI H,TIAN S P,QIN G Z. Boron improves the biocontrol activity of Cryptococcus laurentii against Penicillium expansum in jujube fruit[J]. Postharvest Biology and Technology,2012,68:16-21.
[34] LAI T F,BAI X L,WANG Y,ZHOU J Y,SHI N N,ZHOU T.Dissecting inhibitory effect of exogenous sodium bicarbonate on developmentand pathogenicity of postharvest disease Penicillium expansum[J].Scientia Horticulturae,2015,187:108-114.
[35] PALOU L,SMILANICK J L,USALL J,VIÑAS I. Control of postharvest blue and green molds of oranges by hot water,sodium carbonate,and sodium bicarbonate[J]. Plant Disease,2001,85(4):371-376.
[36] 潘寒姁,谢芳,王姣,袁树枝,曹建康,赵玉梅.碳酸氢钠与抗坏血酸复合处理对鲜切苹果褐变和品质的影响[J].中国农业大学学报,2015,20(2):114-123.PAN Hanju,XIE Fang,WANG Jiao,YUAN Shuzhi,CAO Jiankang,ZHAO Yumei.Effect of the treatment of sodium bicarbonate combined with ascorbic acid on browning and quality of fresh-cut apples[J]. Journal of China Agricultural University,2015,20(2):114-123.
[37] LAI T F,WANG Y,BAI X L,QI Q Q,XU M J,ZHOU T. Dissecting inhibitory effect of boric acid on virulence and patulin production of Penicillium expansum[J]. Postharvest Biology and Technology,2016,117:187-196.
[38] 罗锦,王小红,叶必顺,闫芝茜,刘颖,周丽琬,周婷.外源硼酸抑制柑橘采后绿霉病害Penicilliun digitatum 的效果研究[J].杭州师范大学学报(自然科学版),2018,17(2):159-165.LUO Jin,WANG Xiaohong,YE Bishun,YAN Zhiqian,LIU Ying,ZHOU Liwan,ZHOU Ting. Inhibitory effect of exogenous boric acid postharvest disease Penicillium digitatum of citrus[J].Journal of Hangzhou Normal University(Natural Science Edition),2018,17(2):159-165.
[39] LONG H Y,HUANG Q X,YU Y Y,ZHANG Z B,YAO Z W,CHEN H B,FENG J W. Dehydrocostus lactone inhibits in vitro gastrinoma cancer cell growth through apoptosis induction,sub-G1 cell cycle arrest,DNA damage and loss of mitochondrial membrane potential[J]. Archives of Medical Science,2019,15(3):765-773.
[40] SANTIANI A,UGARELLI A,EVANGELISTA-VARGAS S.Characterization of functional variables in epididymal alpaca(Vicugna pacos) sperm using imaging flow cytometry[J]. Animal Reproduction Science,2016,173:49-55.
[41] 刘佳怡,王嘉欣,宋海超,张正科,徐祥彬,吉训聪,史学群.纳他霉素对芒果采后胶孢炭疽菌的抑菌效果及机理[J].植物学报,2019,54(4):455-463.LIU Jiayi,WANG Jiaxin,SONG Haichao,ZHANG Zhengke,XU Xiangbin,JI Xuncong,SHI Xuequn.Antifungal activity and mechanisms of natamycin against colletotrichum gloeosporioides in postharvest mango fruit[J]. Bulletin of Botany,2019,54(4):455-463.
[42] VAN LEEUWEN M R,GOLOVINA E A,DIJKSTERHUIS J.The polyene antimycotics nystatin and filipin disrupt the plasma membrane,whereas natamycin inhibits endocytosis in germinating conidia of Penicillium discolor[J].Journal of Applied Microbiology,2009,106(6):1908-1918.
[43] JULISKA P,LEONIE B,ADAM S,GLADYS S,RICK S,THOMAS M,JÖRG R. Ecotoxicity of boric acid in standard laboratory tests with plants and soil organisms[J]. Ecotoxicology,2017,26(4):471-481.
[44] ZHU X Y,LIN H Z,SI Z W,XIA Y H,CHEN W X,LI X P.Benzothiadiazole-mediated induced resistance to colletotrichum musae and delayed ripening of harvested banana fruit[J].Journal of Agricultural and Food Chemistry,2016,64(7):1494-1502.