桃树连作障碍是指在原桃园定植新苗后,引起植株生长减缓、产量品质下降、病虫害发生加剧的现象[1-2]。发生原因有致病线虫增加、根际微生物群落破坏、化感自毒物质作用等[3-5]。前人研究表明,土壤线虫群落由食真菌、食细菌、捕食线虫和植物寄生线虫(又称致病线虫)四类组成,前三者统称为自由生活线虫,对植物危害不大,还作为衡量土壤健康水平的重要依据[6]。但是致病线虫往往生活在植物根际周围的土壤或寄生在根系中,使根系发育受阻、损伤、产生根结,从而影响作物产量和品质[7-8]。据《植物线虫志》[9]报道,桃的寄生线虫有裸矮化线虫(Tylenchorhynchus maxbnus)、双宫螺旋线虫(Helicotylenchus dihystera)、秃尾盘旋线虫(Rotylenchus phaliurus)、南方根结线虫(Meloidogyne incognita)、弓形拟滑刃线虫(Aphelenchoides cyrtus)和球头小环线虫(Criconemella sphaerocephala)等超过12 个属40多个种,其中螺旋属有15种。
桃(Amygdalus persica)是浙江省主栽果树之一,种植历史悠久,经济效益稳定。但由于高温多湿的气候条件,导致桃树寿命较短,一般10 a左右需要轮换新种,在土地资源紧缺的背景下,由线虫危害导致的连作障碍时常发生。但是目前浙江省对线虫研究主要集中在境外输入线虫鉴定和抗线虫果树品种选育上,对果树致病线虫鉴定和物理及生物防治方法的研究有所欠缺,对桃树致病线虫研究更未见报道,浙江地区老桃园主要的致病线虫类型有待明确。为了解决以上问题,特对浙江浙北地区经济桃园的土壤致病线虫进行鉴定,并对不同的土壤改良方式进行对比,以筛选最佳土壤改良方式,为桃树连作研究和浙江桃产业发展提供技术指导。
1 材料和方法
1.1 试验基地概况
试验于浙江省湖州市长兴县一经济桃园(119°93′E,30°84′N)进行,该地属于浙北地区,亚热带季风气候,四季分明,平均气温15.6 ℃,平均降水量1306 mm,年均日照时数1 810.3 h(中国天气网:http://www.weather.com.cn)。该园区连作10 a,经检测土壤理化性质为:有机质含量(w)15.80 g·kg-1,土壤pH值5.02,电导率6.35 mS·m-1。
1.2 试验设计
试验地块将老树刨除后清理残枝和根系,用翻耕机深度翻耕松土,并起长宽为75 m×3 m 的垄,垄间留深40 cm排水沟。
试验使用2种消毒材料构成4个消毒方式(棉隆熏蒸、棉隆菌棒联合熏蒸、菌棒熏蒸和不消毒)和2种菌剂构成4 个菌剂施用方式(哈茨木霉、哈茨木霉+芽孢杆菌、芽孢杆菌和不施用菌剂),共16 个处理(表1),每个处理小区占地100 m2。消毒材料和菌剂用量是在前一年预备试验的基础上筛选出来的。
表1 试验设计
Table 1 Experimental design
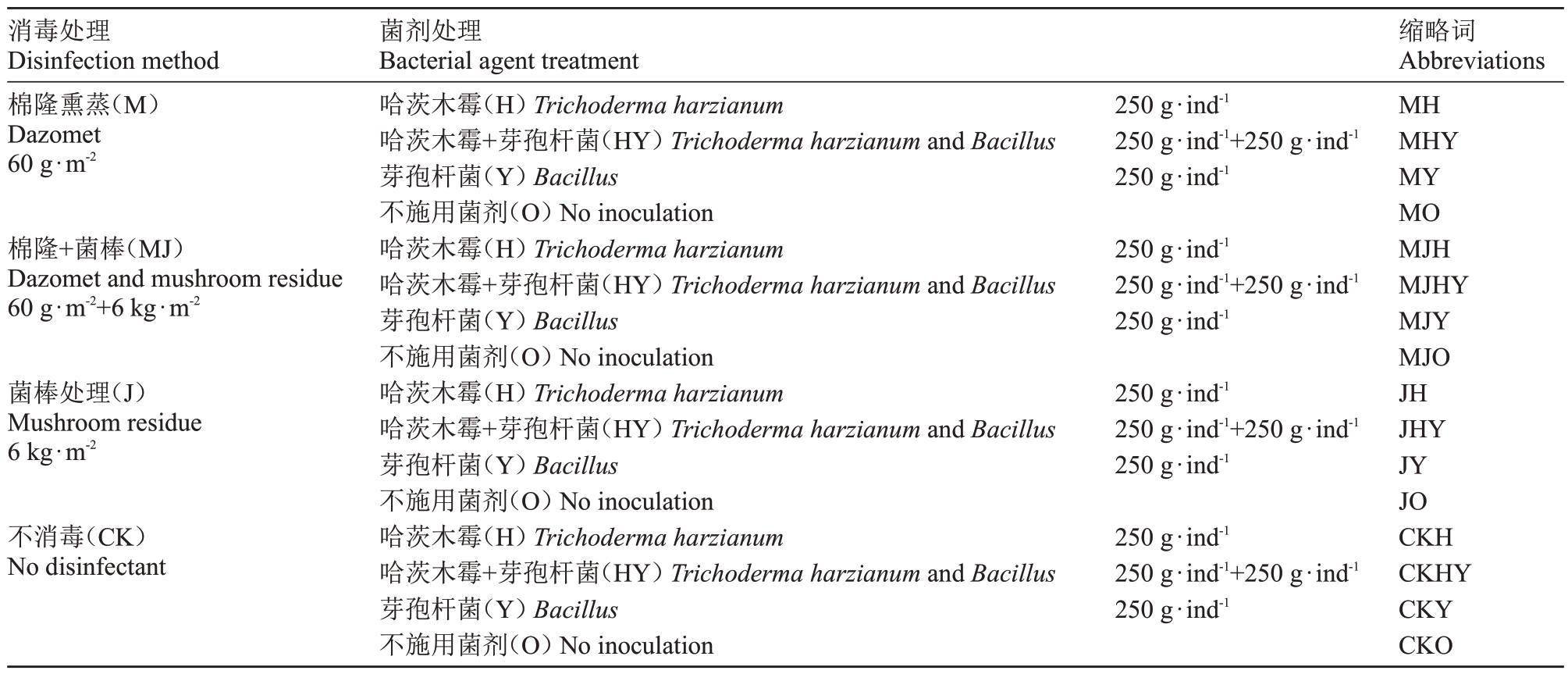
注:250 g·ind-1 全称为250 g·individual-1,表示每株桃树幼苗施用250 g 菌剂。
Note:The full name of 250 g·ind-1 is 250 g·individual-1,which means that 250 grams of microbial agent should be applied to each peach tree seedling.
消毒处理Disinfection method棉隆熏蒸(M)Dazomet 60 g·m-2棉隆+菌棒(MJ)Dazomet and mushroom residue 60 g·m-2+6 kg·m-2菌棒处理(J)Mushroom residue 6 kg·m-2不消毒(CK)No disinfectant菌剂处理Bacterial agent treatment哈茨木霉(H)Trichoderma harzianum哈茨木霉+芽孢杆菌(HY)Trichoderma harzianum and Bacillus芽孢杆菌(Y)Bacillus不施用菌剂(O)No inoculation哈茨木霉(H)Trichoderma harzianum哈茨木霉+芽孢杆菌(HY)Trichoderma harzianum and Bacillus芽孢杆菌(Y)Bacillus不施用菌剂(O)No inoculation哈茨木霉(H)Trichoderma harzianum哈茨木霉+芽孢杆菌(HY)Trichoderma harzianum and Bacillus芽孢杆菌(Y)Bacillus不施用菌剂(O)No inoculation哈茨木霉(H)Trichoderma harzianum哈茨木霉+芽孢杆菌(HY)Trichoderma harzianum and Bacillus芽孢杆菌(Y)Bacillus不施用菌剂(O)No inoculation 250 g·ind-1 250 g·ind-1+250 g·ind-1 250 g·ind-1 250 g·ind-1 250 g·ind-1+250 g·ind-1 250 g·ind-1 250 g·ind-1 250 g·ind-1+250 g·ind-1 250 g·ind-1 250 g·ind-1 250 g·ind-1+250 g·ind-1 250 g·ind-1缩略词Abbreviations MH MHY MY MO MJH MJHY MJY MJO JH JHY JY JO CKH CKHY CKY CKO
土壤熏蒸消毒于2020年10月底进行,施用消毒剂后用旋耕机搅拌均匀并浇透水,用塑料膜封实50 d,揭膜晾晒15 d 以上。菌剂施用于定植后进行,使用哈茨木霉(杭州欣禧农林科技有限公司:3亿CFU·g-1)和芽孢杆菌(浙江归野农业科技有限公司:有效活菌数≥200 亿·g-1)溶于水浇灌在树盘周围和原定植沟内。
使用桃赤月1 年生嫁接苗(砧木为毛桃),选择长势一致、无明显病虫害的植株,株行距为2.5 m×5 m。
1.3 土壤取样及线虫调查鉴定方法
土壤取样,于2021 年4 月1 日采取老龄果园和仅翻耕施肥的新植果园距离树体主干25 cm和50 cm处,10~<20 cm、20~<30 cm、30~40 cm深土样各1 kg,将每个土层的样品充分混匀,剔除杂质用塑封袋封口运回实验室,并于4 ℃冰箱保存待用,测定土壤线虫群落空间分布和致病线虫种类。并于7 月1 日植株旺盛生长季在每个试验小区内随机挑选5株长势一致的桃树幼苗,采取距离树干25 cm 处、20 cm 深的土样1 kg,以相同方法保藏处理,测定不同处理对土壤线虫群落的影响。所有土样均于土壤线虫提取时设置3个生物学重复。
线虫分离提取方法为过筛法[10]和浅盘法[11]结合,即将200 g新鲜土壤用流水冲洗过筛后的残渣置于装有面巾纸的网筛中浸水过夜,再过38 μm网筛,获取过滤物于离心管4 ℃保存备用。将离心管中的过滤物于倒置显微镜下观察鉴定计数。将初步鉴定为致病线虫的,挑取到光学显微镜下拍照,并使用系统自带软件和ImageJ绘图工具进行测量。
线虫分子鉴定使用AB28(5’-ATATGCTTAAGTTCAGCGGGT-3’)和TW81(5’-GTTTCCGTAGGTGAACCTGC-3’)作为引物,参考赵雷等[12]方法和扩增程序进行试验,致病线虫DNA 提取:挑取单条线虫于PCR 管,加入6 μL ddH2O,液氮浸没2 min 后于85 ℃保藏2 min,3 次重复后加入2 μL 10×PCR Buffer和2 μL蛋白酶K,65 ℃培育1 h后置于95 ℃下10 min。PCR 扩增体系:10×PCR Buffer 2.5 μL、dNTP 2 μL、Taq 酶0.25 μL,上下游引物(10 μmol·L-1)各1 μL,cDNA 3 μL,ddH2O 补充至25 μL。PCR 扩展程序:94 ℃预变性4 min,94 ℃变性1 min,56 ℃退火90 s,72 ℃延伸2 min,35 个循环;72 ℃延伸10 min。所得ITS 基因纯化产物送往杭州有康生物科技有限公司进行测序。
1.4 数据处理
所获数据使用Excel进行数据收集、整理、制表,并且使用IBM SPSS Statistics 进行方差分析。于国家生物技术信息中心官网(https://www.ncbi.nlm.nih.gov/blast/treeview)绘制系统发育树状图。
2 结果与分析
2.1 桃园致病线虫鉴定
本试验果园中鉴定出的自由生活线虫占多数,致病线虫有螺旋属、垫刃属、针属、短体线虫属和小环属,其中螺旋属和垫刃属线虫居多(图1)。螺旋属特征为:线虫虫体固定后呈螺旋状,头部缢缩,唇突出。口针强壮,口针基球大,食道基球较明显,尾部呈现半月球形,肠道和阴门清晰可见,尾部呈透明或半透明状;L=769.46 μm,W=27.12 μm,a=28.37,b=5.71,c=43.20,c’=1.20,V=63.94,St=26.77 μm。经鉴定确定其为螺旋属线虫,属于桃树致病线虫的一种。垫刃属特征为:线虫整体呈细长蠕虫形,有环纹,口针较短,口针基球较小,食道和肠道明显,尾部呈现透明细丝状,食道基球较模糊,阴门位于尾部透明区以上;L=476.79 μm,W=27.12 μm,a=30.77,b=6.21,c=5.49,V=62.77,spear=8.78 μm。经鉴定确定该线虫属于垫刃属线虫,是植物致病线虫之一。
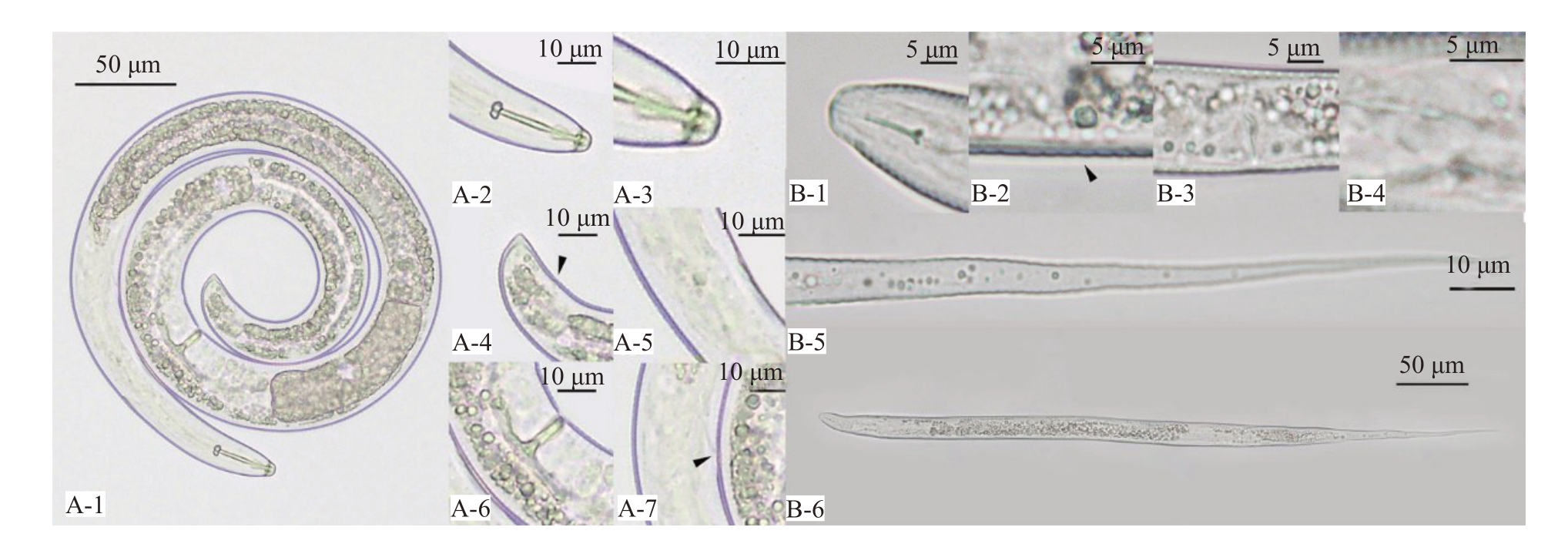
图1 桃园主要致病线虫
Fig.1 The main pathogenic nematodes in peach orchard
螺旋属致病线虫:A-1. 虫体;A-2. 口针;A-3. 头部唇区;A-4. 尾部透明区;A-5. 食道球;A-6. 阴门;A-7. 排泄孔。垫刃属致病线虫:B-1. 口针及头部;B-2. 排泄孔;B-3. 阴门;B-4. 食道球;B-5. 尾部透明区;B-6. 虫体。
Helicotylenchus:A-1.Body;A-2.Stylet;A-3.Head;A-4.Tail;A-5.Esophageal ball;A-6.Vulva;A-7.Excretion hole.Tylenchus:B-1.Head;B-2.Excretion hole;B-3.Vulva;B-4.Esophageal ball;B-5.Tail;B-6.Body.
所得扩增序列长度为1054 bp,将测序结果于NCBI上进行BLAST比对,并绘制系统发育树(图2),结果显示,目标序列与登录号为JX207113的双宫螺旋线虫聚在同一个分支上,目标序列(target squence)与双宫螺旋线虫(Helicotylenchus dihystera,登录号:JX207113,ITS序列长度1206 bp)序列重合度为100%,可以得出螺旋属线虫的鉴定结果为双宫螺旋线虫。垫刃属致病线虫分子鉴定还未出结果,需要进一步试验确定该线虫种类。
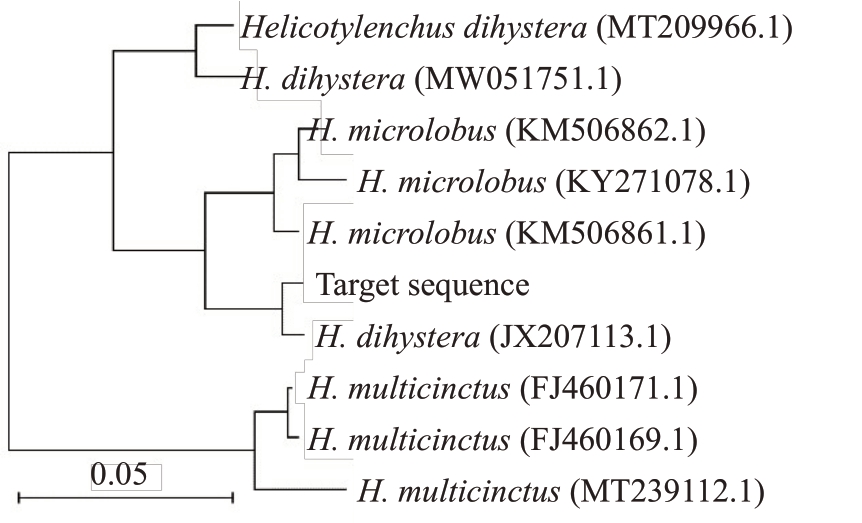
图2 螺旋属线虫系统发育树状
Fig.2 Clustering dendrogram of Helicotylenchus
2.2 老桃园土壤改良前后线虫数量对比
本试验桃园不同土层线虫数量结果(表2)显示,在老龄桃园中,距离树体主干25 cm 处,自由生活线虫和致病线虫的数量在10~<30 cm 土层显著高于30~40 cm 土层;而距离树体主干50 cm 处,10~<20 cm 深度的2 类线虫显著高于20~40 cm 深的土层。新改良桃园2种线虫分布未呈现出明显规律,自由生活线虫在距离树体25 cm处显著高于50 cm处的土层,致病线虫则在距离树体25 cm、深30~40 cm,距离树体50 cm、10~<30 cm 土层分布显著高于其他土层。且无论改良前后,距离树体25 cm处的土壤自由生活线虫均高于50 cm处。改良新植桃园的自由生活线虫和致病线虫数量均高于老龄桃园,但是致病线虫在土壤线虫群落的比例却低于老龄桃园。
表2 新老桃园土壤线虫群落结构
Table 2 Soil nematode community structure in new and old peach orchards
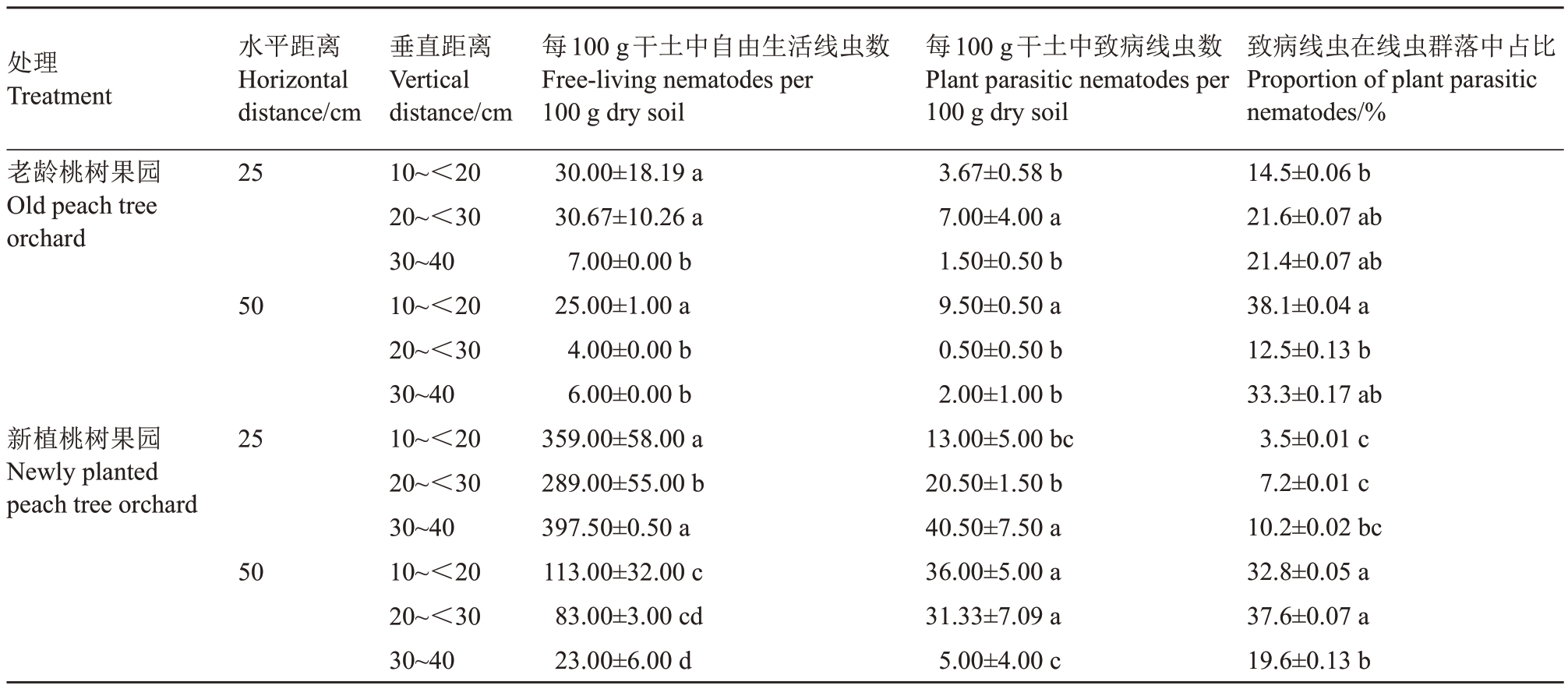
注:同列不同小写字母表示同一果园中在p <0.05 差异显著。
Note:Different lowercase letters after the number indicate that the numbers in the same column in the same orchard have significant differences at p <0.05.
处理Treatment老龄桃树果园Old peach tree orchard新植桃树果园Newly planted peach tree orchard水平距离Horizontal distance/cm 25 50 25 50垂直距离Vertical distance/cm 10~<20 20~<30 30~40 10~<20 20~<30 30~40 10~<20 20~<30 30~40 10~<20 20~<30 30~40每100 g干土中自由生活线虫数Free-living nematodes per 100 g dry soil 30.00±18.19 a 30.67±10.26 a 7.00±0.00 b 25.00±1.00 a 4.00±0.00 b 6.00±0.00 b 359.00±58.00 a 289.00±55.00 b 397.50±0.50 a 113.00±32.00 c 83.00±3.00 cd 23.00±6.00 d每100 g干土中致病线虫数Plant parasitic nematodes per 100 g dry soil 3.67±0.58 b 7.00±4.00 a 1.50±0.50 b 9.50±0.50 a 0.50±0.50 b 2.00±1.00 b 13.00±5.00 bc 20.50±1.50 b 40.50±7.50 a 36.00±5.00 a 31.33±7.09 a 5.00±4.00 c致病线虫在线虫群落中占比Proportion of plant parasitic nematodes/%14.5±0.06 b 21.6±0.07 ab 21.4±0.07 ab 38.1±0.04 a 12.5±0.13 b 33.3±0.17 ab 3.5±0.01 c 7.2±0.01 c 10.2±0.02 bc 32.8±0.05 a 37.6±0.07 a 19.6±0.13 b
2.3 不同土壤改良方式对土壤线虫群落的影响
不同处理对土壤自由生活线虫数量的影响见表3。在HY组合和O组合中,自由生活线虫数量表现为M>MJ>J>CK,处理间均达到显著水平;而在H和Y 组合中则表现为MJ>J、CK>M。而对消毒组合进行分析,在M组合中,显示结果为O、HY>Y>H;在MJ 组合中,4 种菌剂处理方式间无显著差异;在J 组合中,Y 处理自由生活线虫数量最高,O 和H处理其次,HY处理最低;在CK组合中,显示结果为H>O、HY>Y。
表3 不同土壤改良方式处理后每100 g 干土中自由生活线虫数量
Table 3 Number of free-living nematodes per 100 g dry soil in different soil improvement methods
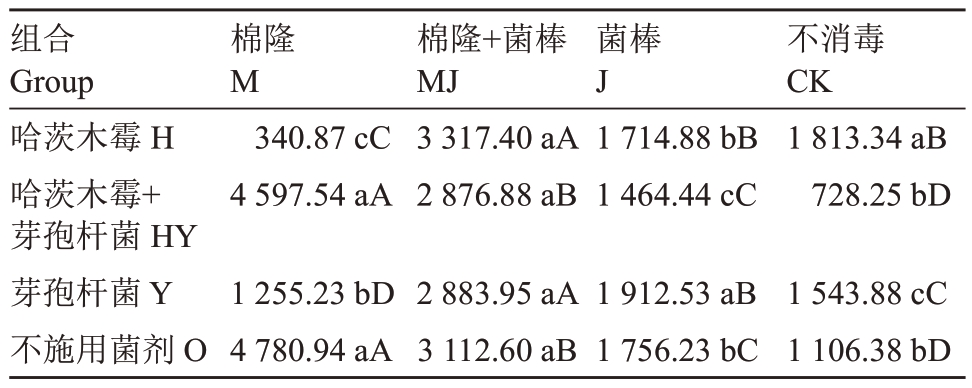
注:不同小写字母表示同列数字在p <0.05 差异显著,不同大写字母表示同行数字在p <0.05 差异显著。下同。
Note: Different lowercase letters after the number indicate that the numbers in the same column have significant differences at p <0.05,and different uppercase letters indicate that the numbers in the same row have significant differences at p <0.05.The same below.
组合Group哈茨木霉H哈茨木霉+芽孢杆菌HY芽孢杆菌Y不施用菌剂O棉隆M 340.87 cC 4 597.54 aA 1 255.23 bD 4 780.94 aA棉隆+菌棒MJ 3 317.40 aA 2 876.88 aB 2 883.95 aA 3 112.60 aB菌棒J 1 714.88 bB 1 464.44 cC 1 912.53 aB 1 756.23 bC不消毒CK 1 813.34 aB 728.25 bD 1 543.88 cC 1 106.38 bD
不同处理对螺旋属致病线虫的影响见表4,在H组合中,J、CK 的螺旋属致病线虫数量显著高于M、MJ处理;在HY组合中,J处理显著高于同组的其他3 个处理;在Y 组合中显示为CK>J>MJ 和M 处理。在O组合中,CK处理显著高于同组其他3个处理,且3个处理间无显著差异。M组合中,O处理的螺旋属线虫显著高于H、HY 处理,并显著高于Y 处理;与MJ组合结果一致。在J组合中,H处理后螺旋线虫数量显著高于同组其他处理,其他3 个处理无显著性差异。CK组合中螺旋属线虫数量表现为O>Y>H>HY。
表4 不同土壤改良方式处理后每100 g 干土中螺旋属致病线虫数量
Table 4 Number of Helicotylenchus nematodes per 100 g dry soil in different soil improvement methods
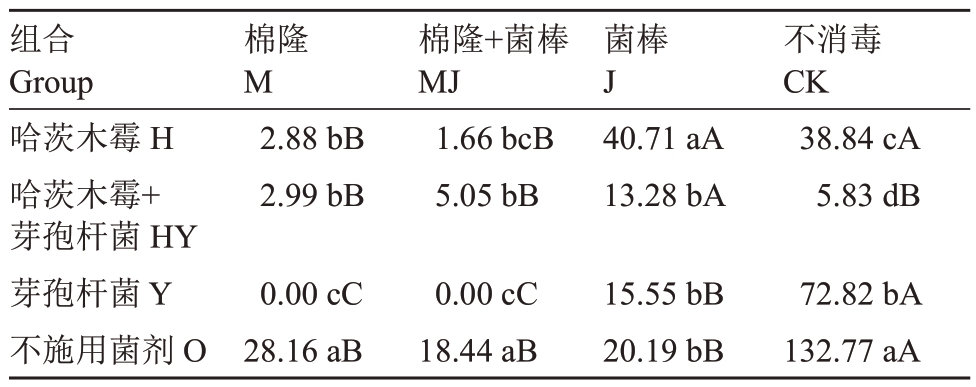
组合Group哈茨木霉H哈茨木霉+芽孢杆菌HY芽孢杆菌Y不施用菌剂O棉隆M 2.88 bB 2.99 bB 0.00 cC 28.16 aB棉隆+菌棒MJ 1.66 bcB 5.05 bB 0.00 cC 18.44 aB菌棒J 40.71 aA 13.28 bA 15.55 bB 20.19 bB不消毒CK 38.84 cA 5.83 dB 72.82 bA 132.77 aA
不同处理对垫刃属致病线虫的影响见表5,在H组合中,MJ组合的土壤垫刃属致病线虫数量显著高于J、CK,并显著高于M 处理。在HY 组合中,J 和CK处理显著高于MJ和M处理。在Y组合中,则显示出J>CK>MJ、M;在O 组合显示出一致的结果。在M组合中,4个处理并未显示出显著性差异,但O 处理的垫刃属线虫高于同组其他处理。在MJ组合中,H 处理的垫刃属线虫数量最高,O 处理其次,施用HY 和Y 处理最低。J 组合中表现为O>Y>H、HY处理。在CK中,O、Y处理的垫刃属致病线虫数量显著高于H、HY处理。
表5 不同土壤改良方式处理后每100 g 干土中垫刃属致病线虫数量
Table 5 Number of Tylenchus nematodes per 100 g dry soil in different soil improvement methods

组合Group哈茨木霉H哈茨木霉+芽孢杆菌HY芽孢杆菌Y不施用菌剂O棉隆M 0.00 aC 0.00 aB 1.99 aC 11.65 aC棉隆+菌棒MJ 42.34 aA 2.02 cB 6.56 cC 19.67 bC菌棒J 25.82 cB 14.94 cA 82.93 bA 953.81 aA不消毒CK 24.27 bB 15.54 bA 50.98 aB 47.94 aB
在该园区土壤线虫群落中,垫刃属和螺旋属占致病线虫的大部分,因此2 种致病线虫总和可以代表土壤致病线虫总数。综合以上结果,土壤总致病线虫数量表现为棉隆组合低于其他3 个消毒组合,哈茨木霉+芽孢杆菌组合相对低于其他3 个菌剂处理组合。
3 讨 论
3.1 土壤线虫分布及土壤改良对线虫群落的影响
根据试验结果,在改良前后的果园中,土壤中线虫群落分布的趋势是根际25 cm处的数量多于50 cm,即主要分布在桃树根际周围。纵向看,老果园土壤自由生活线虫和致病线虫主要分布在10~<30 cm的浅层土壤中,即浅层土壤中线虫数量多于深层土壤,这与颜秀娟等[13]和潘凤娟等[14]的研究结果基本一致;而新植桃园分布无显著规律。这可能与生产操作习惯有关,浙北地区桃园的施肥除草,一般都在表土层的10~<20 cm 进行,浅层土壤透气性良好,土壤生物活性较好,线虫数量多;而新定植果园土壤要经过深翻改土,土层原有结构改变,且各土层透气性增强,线虫数量分布没有明显规律。可见,深翻改土可以打破线虫群落结构,使土壤线虫群落快速重塑。
在高飞等[6]的研究中,随着连作年限的增加,土壤中自由生活线虫(食细菌和捕食类)的数量显著减少,致病线虫数量会增加。本试验中,连作10 a的老龄桃园自由生活线虫数量显著少于新改良土壤,虽然寄生性线虫数量也少于新改良桃园,但是致病线虫数量在线虫群落中占比却比新改良果园增加10%~30%。改良后的土壤线虫群落表现出自由生活线虫和致病线虫数量增加,而致病线虫比例下降的原因可能是土壤改良后土壤有机质含量、透气性和孔隙度增加促进线虫发生,但土壤改良可以达到降低致病线虫的比例,使土壤线虫群落向更有利于植物生长的方向改变。从本试验园改土前后的土壤理化特性上看出,改土前土壤有机质含量(w)为23.73 g·kg-1,棉隆菌棒、菌棒改土后分别提升至34.73 g·kg-1和29.08 g·kg-1;经过棉隆、棉隆菌棒和菌棒改土,土壤孔隙度较改土前提升了11.36%、11.01%和7.45%,结果表明,土壤理化性质与土壤线虫数量有关。由此可见,在老龄果园改造过程中,对果园土壤进行合理的深翻改良是十分必要的。
3.2 消毒方式对土壤线虫群落的影响
《植物线虫志》[9]记载,棉隆是一种效果良好的杀虫剂;同时增肥覆膜熏蒸可以培育更好的土壤环境,有利于线虫天敌微生物的发育,使线虫错过盛发期,不能完成生活史,以达到防治致病线虫的目的。大量研究发现,棉隆化学熏蒸后植物受土壤致病性线虫侵染减轻的同时,土壤微生物群落也发生改变,土壤生态破坏,所以棉隆化学熏蒸需要配合施用微生物菌剂来改良土壤生态环境,重塑健康的微生物群落[15-20]。研究发现,哈茨木霉+芽孢杆菌能防治土壤根结线虫、孢囊线虫等多种致病线虫,减轻由致病线虫引起的根腐病和枯萎病,诱导植株对致病线虫产生抗性[21-26]。
本试验研究结果表明,几个组合处理后土壤螺旋属致病线虫数量大致表现为不消毒(CK)>棉隆菌棒联合消毒(MJ)>菌棒熏蒸消毒(J)、棉隆消毒(M),不施菌剂(O)>芽孢杆菌(Y)、哈茨木霉(H)>哈茨木霉+芽孢杆菌(HY);而垫刃属表现为菌棒熏蒸(J)>不消毒处理(CK)>棉隆菌棒联合消毒(MJ)>棉隆消毒(M),不施用菌剂(O)>芽孢杆菌(Y)>哈茨木霉(H)>哈茨木霉+芽孢杆菌(HY)。相比不消毒组合,棉隆处理后致病线虫数量下降了77.97%~98.39%,而棉隆菌棒联合处理后致病线虫数量下降了30.28%~94.70%;菌剂使用中,哈茨木霉+芽孢杆菌处理相比对照致病线虫数量降低了81.46%~97.10%,在几个处理组合中最显著。可见单独使用棉隆、棉隆菌棒联合消毒和哈茨木霉+芽孢杆菌施用3种方法均对土壤致病线虫防治有良好效果,但棉隆消毒效果比棉隆菌棒联合消毒更好。相比之下,棉隆加哈茨木霉、棉隆加芽孢杆菌和棉隆加哈茨木霉+芽孢杆菌都具有显著降低致病线虫的效果,且处理间差异并不大,但是棉隆加哈茨木霉+芽孢杆菌显著提升了自由生活线虫的数量,其可以更大程度上降低致病线虫比例、提升土壤生物活性。因此,生产上建议在桃老果园改造过程中,宜采用定植前进行棉隆熏蒸、定植后施用哈茨木霉+芽孢杆菌的土壤改良技术。
另有研究表明,芸薹属植物残体、绿肥、秸秆、辣椒残体和禽畜有机肥等方式可以作为一种化学熏蒸剂的替代品,对植物寄生性线虫有良好的防治效果[27-31]。但本试验结果显示,单独使用蘑菇菌棒还原法的土壤消毒方法,尽管能提高土壤有机质和透气性,但对致病线虫防治并无显著效果,甚至还增加了垫刃属线虫的数量。在《植物线虫志》[9]中,垫刃科中的瓣膜茎线虫、食菌茎线虫等可以寄生在真菌菌丝中,同时可以以桃树作为寄主,单独菌棒处理致病线虫数量较多的原因可能是菌棒没有再次腐熟杀死原有致病线虫,导致线虫迁移寄主,在桃树果园土壤中生长繁殖。因此在使用有机物料、植物残体进行物理熏蒸时应该充分腐熟,并做好杀虫和除菌等相关工作。
4 结 论
土壤线虫以自由生活线虫为主,且主要分布在根际周围10~<30 cm深的浅层土壤中,土壤改良可重构线虫群落;主要致病线虫为螺旋属和垫刃属,利用分子鉴定表明,双宫螺旋线虫是主要的致病线虫;定植前使用棉隆熏蒸、定植后施用哈茨木霉+芽孢杆菌能有效地防治致病线虫。
[1] 李远想,王尚堃.果树再植病研究进展[J].北方园艺,2019(4):149-154.LI Yuanxiang,WANG Shangkun.Research progress on fruit tree replanting diseases[J].Northern Horticulture,2019(4):149-154.
[2] 董晓民,高晓兰,刘伟,李桂祥,李淼,张安宁.桃连作障碍中自毒作用的研究进展[J].黑龙江农业科学,2021(2):123-127.DONG Xiaomin,GAO Xiaolan,LIU Wei,LI Guixiang,LI Miao,ZHANG Anning. Research progress on autotoxicity in peach continuous cropping disorder[J]. Heilongjiang Agricultural Sciences,2021(2):123-127.
[3] 陈虹,周俗,刘刚,王钰,康晓慧.黑麦草品种对灰梨孢灰斑病的室内抗性评价[J].草原与草坪,2021,41(4):96-101.CHEN Hong,ZHOU Su,LIU Gang,WANG Yu,KANG Xiaohui. Indoor resistance evaluation of ryegrass varieties to gray pear spore gray spot disease[J]. Grassland and Turf,2021,41(4):96-101.
[4] MARIN B M,GRAYSTON S J,FORGE T.Isolation and characterization of Streptomycetes and Pseudomonad strains with antagonistic activity against the plant parasitic nematode Pratylenchus penetrans and fungi associated with replant disease[J].Biological Control,2021,158:104599.
[5] 齐永志,孙雅如,王冰,郭邯菲,马可,甄文超.草莓根系分泌物和腐解物化感作用研究进展[J].园艺学报,2021,48(4):778-790.QI Yongzhi,SUN Yaru,WANG Bing,GUO Hanfei,MA Ke,ZHEN Wenchao. Research progress on allelopathy of root exudates and decompositions of strawberry[J].Acta Horticulture Sinica,2021,48(4):778-790.
[6] 高飞,赵贺,周峰,倪玮,李辉信,焦加国,孙兴祥.江苏省不同种植年限的西/甜瓜农田土壤线虫群落特征[J].生态学杂志,2020,39(1):155-163.GAO Fei,ZHAO He,ZHOU Feng,NI Wei,LI Huixin,JIAO Jiaguo,SUN Xingxiang. Communitiy characteristics of nematodes in agricultural soil of watermelon and melon with different cultivation years in Jiangsu province[J].Chinese Journal of Ecology,2020,39(1):155-163.
[7] 邓艳凤. 球根植物线虫种类及风险评估[J]. 安徽农业科学,2021,49(8):145-148.DENG Yanfeng. Pest risk analysis of nematodes of bulbous plant[J]. Journal of Anhui Agricultural Sciences,2021,49(8):145-148.
[8] 李欢欢,金惺惺,于焦,何晋,徐春玲,谢辉.西藏作物根际垫刃科线虫3 个新记录种[J].高原农业,2020,4(2):131-136.LI Huanhuan,JIN Xingxing,YU Jiao,HE Jin,XU Chunling,XIE Hui. Three newly recorded species of family Tylenchidae from crop rhizosphere in Tibet[J]. Journal of Plateau Agriculture,2020,4(2):131-136.
[9] 刘维志.植物线虫志[M].北京:中国农业出版社,2004.LIU Weizhi.Plant nematode history[M].Beijing:China Agriculture Press,2004.
[10] 刘维志.植物病原线虫学[M].北京:中国农业出版社,2000.LIU Weizhi. Plant pathogen nematology[M]. Beijing:China Agriculture Press,2000.
[11] GOODFRIEND W L,OLSEN M W,FRYE R J.Soil microfloral and microfaunal response to Salicornia bigelovii planting density and soil residue amendment[J]. Plant and Soil,2000,223(1/2) :23-32.
[12] 赵雷,郑炜,梅圆圆,郑经武.基于rDNA 的PCR-RFLP 及序列分析对四种螺旋线虫的分子鉴定[J].农业生物技术学报,2010,18(5):981-984.ZHAO Lei,ZHENG Wei,MEI Yuanyuan,ZHENG Jingwu.Molecular identification of four Helicotylenchus species based on PCR-RFLP and sequencing rDNA[J]. Journal of Agricultural Biotechnology,2010,18(5):981-984.
[13] 颜秀娟,李明姝,李楠,孙星邈.连作大豆田土壤线虫的种群结构和垂直分布[J].湖北农业科学,2009,48(2):335-337.YAN Xiujuan,LI Mingshu,LI Nan,SUN Xingmiao. Preliminary report on population structure and vertical distribution of soil nematodes under continuous cropping soybean field[J]. Hubei Agricultural Sciences,2009,48(2):335-337.
[14] 潘凤娟,许艳丽,李春杰,赵丹.大豆不同轮作体系根围土壤线虫空间分布特征[J]. 农业系统科学与综合研究,2009,25(1):33-38.PAN Fengjuan,XU Yanli,LI Chunjie,ZHAO Dan.Spatial distribution of soil nematodes in soybean rhizosphere under different rotation systems[J]. Systems Sciences and Comprehensive Studies in Agriculture,2009,25(1):33-38.
[15] 乔康.1,3-D 防治蔬菜根结线虫病及其对土壤细菌多样性的影响[D].泰安:山东农业大学,2013.QIAO Kang. Control efficacy of 1,3-D to vegetable root-knot nematode and its effect on soil bacterial diversity [D]. Tai’an:Shandong Agricultural University,2013.
[16] 朱晓蕾,韩文风,任秀梅,牛文静,刘矿,张卫华.不同土壤处理对辣椒生长和根结线虫发生的影响[J].山东农业工程学院学报,2021,38(3):18-21.ZHU Xiaolei,HAN Wenfeng,REN Xiumei,NIU Wenjing,LIU Kuang,ZHANG Weihua. Effects of different application of soil fumigants on growing of pepper and controlling of root-knot nematode[J].Journal of Shandong Institute of Agricultural Engineering,2021,38(3):18-21.
[17] 徐晓琳,郭守鹏,门洪文,王文军.98%棉隆对防治设施蔬果土传病害的应用效果[J].世界热带农业信息,2020(8):29-30.XU Xiaolin,GUO Shoupeng,MEN Hongwen,WANG Wenjun.The application effect of 98% Mianlong on the prevention and control of plant-borne vegetable,fruit and soil-borne diseases[J].World Tropical Agriculture Information,2020(8):29-30.
[18] 曹坳程,刘晓漫,郭美霞,王秋霞,李园,欧阳灿彬,颜冬冬.作物土传病害的危害及防治技术[J]. 植物保护,2017,43(2):6-16.CAO Aocheng,LIU Xiaoman,GUO Meixia,WANG Qiuxia,LI Yuan,OUYANG Canbin,YAN Dongdong. Incidences of soilborne diseases and control measures[J]. Plant Protection,2017,43(2):6-16.
[19] 刘春艳,王万立,霍建飞,郝永娟,王勇.98%棉隆微粒剂防治黄瓜根结线虫田间药效试验[J].北方园艺,2011(23):128-130.LIU Chunyan,WANG Wanli,HUO Jianfei,HAO Yongjuan,WANG Yong. Field efficacy test of 98% Mianlong microparticles for controlling cucumber root-knot nematodes[J]. Northern Horticulture,2011(23):128-130.
[20] BIN H,QIAN W,MEI G,WEN S F,XIAO N W,QIU X W,DONG D Y,CAN B O,YUAN L,AO C C.The synergistic advantage of combining chloropicrin or dazomet with fosthiazate nematicide to control root-knot nematode in cucumber production[J]. Journal of Integrative Agriculture,2019,18(9):2093-2106.
[21] POCURULL M,FULLANA A M,FERRO M. Commercial formulates of trichoderma induce systemic plant resistance to meloidogyne incognita in tomato and the effect is additive to that of the Mi-1.2 resistance gene[J].Frontiers in Microbiology,2019,10:3042.
[22] 张雅静,宋美燕,张怡静,房庆,杨俊,彭德良,黄文坤,彭焕,朱英波,孔令安.兼防黄瓜根腐病和根结线虫病的淡紫拟青霉和哈茨木霉的筛选[J].生物技术通报,2021,37(2):40-50.ZHANG Yajing,SONG Meiyan,ZHANG Yijing,FANG Qing,YANG Jun,PENG Deliang,HUANG Wenkun,PENG Huan,ZHU Yingbo,KONG Lingan. Identification of Purpureocillium lilacinum and Trichoderma harzianum strains for simultaneously controlling cucumber root rot and root-knot nematode diseases[J].Biotechnology Bulletin,2021,37(2):40-50.
[23] POCRULL M,FULLANA A M,FERRO M,VALERO P,ESCUDERO N,SAUS E,GABALDO T,SORRIBAS F J. Commercial formulates of Trichoderma induce systemic plant resistance to Meloidogyne incognita in tomato and the effect is additive to that of the Mi-1.2 resistance gene[J].Frontiers in Microbiology,2019,10:3042.
[24] NEVIN A,ALAA E O.Efficiency of Trichoderma harzianum as a biocontrol agent in combination with humate and chitosan against Meloidogyne incognita on tomato[J].Archives of Phytopathology and Plant Protection,2018,51(11/12):673-683.
[25] HUANG M,BULUT A,SHRESTHA B,MATERA C,GRUNDLER F M W,SCHLEKER A S S. Bacillus firmus I-1582 promotes plant growth and impairs infection and development of the cyst nematode Heterodera schachtii over two generations[J].Scientific Reports,2021,11(1):1-15.
[26] ZÁRATE P A,YANG W,ANDRESEN B. The effects of nested miRNAs and their host genes on immune defense against Bacillus thuringiensis infection in Caenorhabditis elegans[J]. Developmental&Comparative Immunology,2021,123:104144.
[27] OKA Y. Mechanisms of nematode suppression by organic soil amendments:A review[J]. Applied Soil Ecology,2009,44(2):101-115.
[28] 肖同建,李蕊,王小慧,高超,朱震,马雪莲,沈其荣,冉炜.施用生防菌菌剂与日光消毒和生物熏蒸相结合防治番茄根结线虫的研究[J].中国生物防治学报,2012,28(2):262-268.XIAO Tongjian,LI Rui,WANG Xiaohui,GAO Chao,ZHU Zhen,MA Xuelian,SHEN Qirong,RAN Wei.Effect of bio-control agent coupled with solarization and soil biofumigation on root-knot nematode of tomato[J]. China Journal of Biological Control,2012,28(2):262-268.
[29] PIEDRA B A,GARCÍA-ÁLVAREZ A,DÍEZ-ROJO M A. Use of pepper crop residues for the control of root-knot nematodes[J].Bioresource Technology,2007,98(15):2846-2851.
[30] 张超,何立月,张桂娟,许小荣,林雪.生物土壤熏蒸防治根结线虫病技术应用效果[J].北京农业,2011(S2):24-25.ZHANG Chao,HE Liyue,ZHANG Guijuan,XU Xiaorong,LIN Xue. Biological prevention and control of soil fumigation root knot nematode technology application effect[J].Beijing Agriculture,2011(S2):24-25.
[31] D’ADDABBO T,MIGUNOVA V D,RENČO M,SASANELLI N.Suppressiveness of soil amendments with pelleted plant materials on the root-knot nematode Meloidogyne incognita[J]. Helminthologia,2020,57(4):376.