枇杷(Eriobotrya japonica L.)是我国南方的特产果树,有“初夏特产的江南珍果”之称。尤其白沙枇杷品质优良,肉质甜美,深受消费者的喜爱。但白沙枇杷果皮薄,膨大期至成熟期遇到阴雨天气果皮吸水膨胀易引发裂果问题。据统计,白沙枇杷平均裂果率在20%左右,雨水多的年份可超过40%,严重威胁枇杷产业的发展[1]。
裂果是由于果实内部生长与外界环境不协调导致的一种生理性病害。果实开裂不仅影响外观品质,且易感染病虫害出现烂浆果,使果实失去商品价值[2]。在生产上许多果树易发生裂果现象,如葡萄、柑橘、油桃、樱桃等[3-6]。果实裂果受气候因子[7]、内源激素[8]、果皮组织结构[9]、细胞水势[10]等因素的影响。
钙是构建细胞壁组织力学性能的重要成分,能与细胞壁中果胶物质结合,形成果胶酸钙,增加原生质的弹性,增强细胞的耐压力和延展性[11]。Ca2+作为植物细胞中的第二信使,还参与许多生物与非生物胁迫,如干旱、盐碱、病虫害等,并对细胞壁、抗氧化系统及激素合成产生影响[12]。有研究表明,葡萄、甜樱桃、枣等果树的裂果率与果实含钙量呈负相关,裂果果皮中的钙含量显著低于正常果果皮中的钙含量[13-15]。外源喷钙可提高锦橙、壶瓶枣果实活性氧清除酶活性、降低果皮果胶酶和纤维素酶活性,减少裂果的发生[4,16]。但钙调控激素合成影响裂果的研究鲜有报道。枇杷裂果的研究多集中在裂果原因分析及防控栽培技术等方面[17]。在裂果生理方面,冯健君等[18]研究表明,果实水势与白沙枇杷裂果率呈正相关;王引等[1]研究了不同裂果易发性枇杷品种果实相关酶活性的差异,发现抗裂品种果皮SOD活性显著高于易裂品种。除此之外,有关枇杷裂果与钙素营养关系的研究尚未见报道,其调控的生理机制也不明了。笔者在本研究中以易发生裂果的软条白沙枇杷为试材,测定其裂果果皮、果肉中矿质元素、钙素营养、相关酶活性以及内源激素含量的变化,并研究了外源喷钙对裂果及相关生理指标的影响,旨在探讨钙调控枇杷裂果的生理机制,为防控白沙枇杷裂果提供理论依据。
1 材料和方法
1.1 供试材料与试验处理
试验地概况:供试果园位于浙江省台州市黄岩区浙江省柑橘研究所(E121.25°,N28.65°)。试验地2019 年1 月、2020 年1 月最低气温分别为5.6 ℃、5.1 ℃;3月20日—5月15日(果实膨大期—成熟期)总降雨量分别为476.5 mL、368.9 mL,土壤含水量为19.6%~31.4%。试验果园土壤肥力概况为:pH=5.8,全氮含量(w,后同)1.7 g·kg-1,有机质含量16.9 g·kg-1。
于2019 年4 月20 日—5 月14 日对试验果园裂果状况和裂果规律进行调查和采样分析。调查品种为8年生软条白沙(Eriobotrya japonica L.‘Ruantiaobaisha’),随机选取生长势基本一致的枇杷树40株,调查每株树的结果量、裂果数、裂果类型,计算裂果率(裂果率%=裂果数/总果数×100)。5月15日在裂果较轻的果树上(裂果率<5%)采集正常果样品,在裂果严重的果树上(裂果率>20%)采集裂果样品,取样部位为果树外围东、南、西、北及内膛中部5个方位大小一致的果实,每棵树选取不同裂果程度的5个果,4棵树的20个果分别测定单果质量、纵横径、种子数,然后将20个果混合为1个样品,正常果处理和裂果处理各设3 个重复。并随机选取20 株树,每株树采集5个不同程度的裂果果实混合为1个样品,20 株树共计20 个果实样品,用于相关性分析。另外,分别于幼果期的2 月1 日、膨大期的3 月25日、转色期的4月15日和成熟期的5月15日采集正常果果实,用于测定果实不同发育时期Ca2+含量。
根据裂果调查结果,选择裂果严重的果树,于2019年11月25日(头性花花后30 d)、12月25日(头性花花后60 d)、2020年1月25日(头性花花后90 d)进行喷钙试验,试验设2个处理,CK(喷施蒸馏水),钙处理(喷施0.5%CaCl2 溶液),每处理3 株树,3 次重复,共18 株树。喷施方法为沿植株至下而上喷施,直至叶片、花、果充分湿润并有水滴滴下。于2020年5月调查裂果率,并于15日在喷钙树上摘取正常果,在对照树上摘取裂果,用于相关生理指标测定。
1.2 测定方法
1.2.1 果实样品处理 将果皮、果肉分离,一部分经液氮冷冻后放入-80 ℃超低温冰箱保存,用于测定Ca2+含量,超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)、多酚氧化酶(PPO)、果胶酶(PG)和纤维素酶(CL)活性,GA3、IAA、ABA含量。另一部分在105 ℃下杀青20 min,然后60 ℃烘干研磨成粉,用于全氮、全磷、全钾、全钙、镁含量的测定。
1.2.2 枇杷果皮、果肉中矿质元素与Ca2+含量的测定 测定N、P、K、Ca、Mg含量。全N:凯氏定氮法;全P:紫外分光光度法;K、Ca、Mg:原子吸收法,具体方法见土壤农化分析[19]。Ca2+含量采用甲基百里香酚蓝比色法测定,委托南京建成生物科技有限公司完成。
1.2.3 酶活性测定 SOD活性:氮蓝四唑光化还原法;蛋白含量:考马斯亮蓝法[20];POD活性:愈创木酚法;PPO 活性:邻苯二酚法;CAT 活性:分光光度法[21];PG、CL活性采用试剂盒检测,委托南京建成生物科技有限公司测定。
1.2.4 激素含量测定 GA3、IAA、ABA含量采用高效液相色谱法测定[22]。
1.3 数据分析
使用Excel 2010 和SAS9.0进行相关性分析、差异显著性分析和作图,各试验处理组数据均与对照组进行差异显著性分析。
2 结果与分析
2.1 软条白沙枇杷裂果发生规律
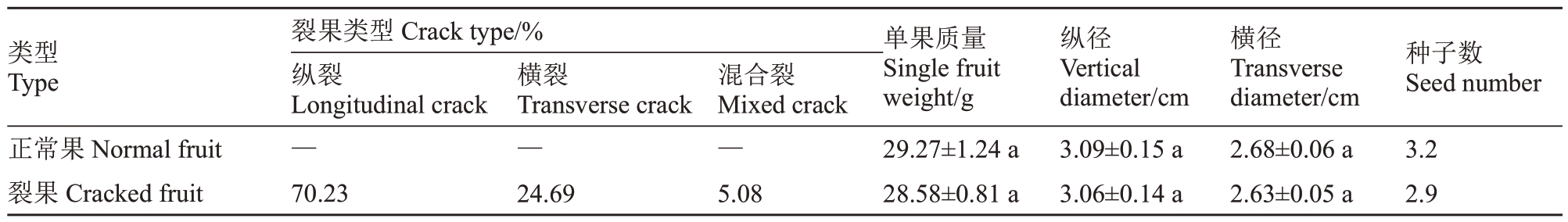
由表1可知,软条白沙主要裂果方式为纵裂,占全部裂果的70.21%,其次是横裂,为24.69%,混合裂最少,仅占5.08%。裂果单果质量、纵横径较正常果稍小,但两者之间无显著差异性。裂果与正常果种子均以3颗为主,裂果平均种子数(2.9颗)小于正常果(3.2颗)。
表1 软条白沙裂果类型及正常果与裂果果实性状比较
Table 1 fruit crack type of white flesh loquat(Ruantiaobaisha)and comparison of fruit characters between normal fruits and cracked fruits
注:相同小写字母表示在p <0.05 无显著差异。
Note:The same small letters indicate no significant difference at p <0.05.
类型Type正常果Normal fruit裂果Cracked fruit裂果类型Crack type/%纵裂Longitudinal crack—70.23横裂Transverse crack—24.69混合裂Mixed crack—5.08单果质量Single fruit weight/g 29.27±1.24 a 28.58±0.81 a纵径Vertical diameter/cm 3.09±0.15 a 3.06±0.14 a横径Transverse diameter/cm 2.68±0.06 a 2.63±0.05 a种子数Seed number 3.2 2.9
台州地区2019 年4 月下旬至5 月上旬雨量充沛,4 月20 日之前枇杷裂果已经少量发生,在7%左右,20—24 日之间裂果率缓慢上升,随着时间的推移,裂果率迅速升高,4 月28 日达到10.32%,4 月28日至5月6日进入裂果高峰期,裂果率直线上升,由10.32%上升至21.25%,随后裂果率趋于平稳,到5月14日裂果率几乎不再增加(图1)。
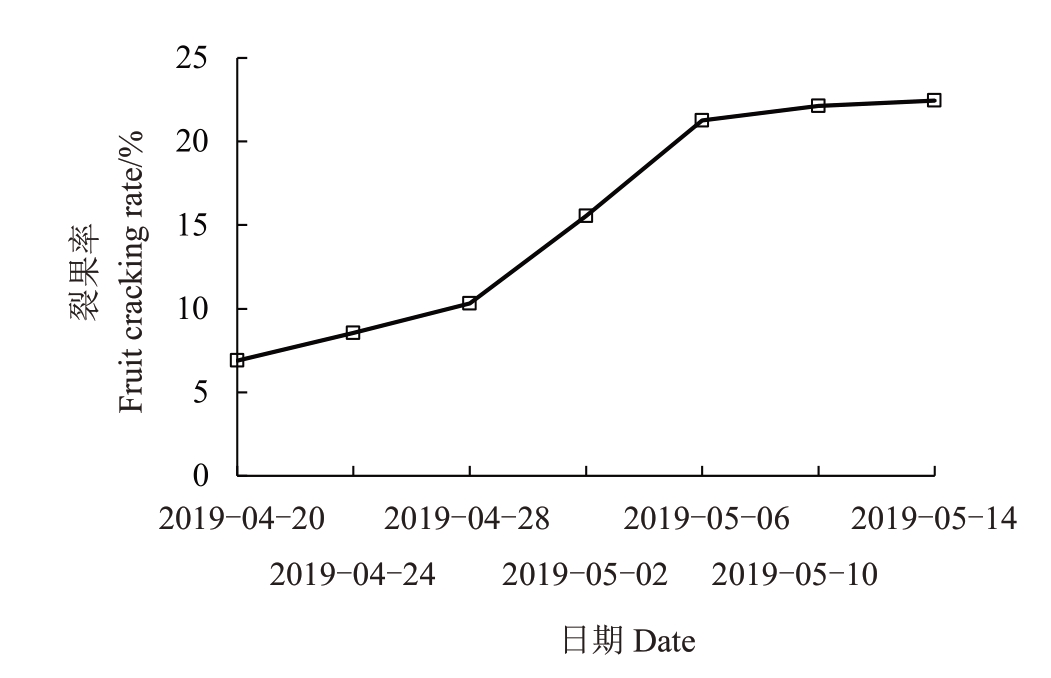
图1 软条白沙枇杷裂果发生规律
Fig.1 Regularity of fruit cracking of white flesh loquat(Ruantiaobaisha)
2.2 白沙枇杷裂果与正常果中矿质元素含量
对调查采集的正常果和裂果样品分析(表2)表明,正常果果皮和果肉中氮含量略低于裂果果皮和果肉,但差异不显著。正常果果皮中的磷含量(w,后同)为7.06 g·kg-1,显著高于裂果果实(4.54 g·kg-1),果肉中磷含量无显著差异。正常果果皮、果肉中钾、镁含量与裂果果皮、果肉中钾、镁含量均无显著差异。
表2 裂果与正常果中矿质元素含量差异
Table 2 Difference of mineral element concentrations between cracked and normal fruits (g·kg-1)

注:表中数据为平均值±标准差。同列中同一果实部位的不同小写字母表示在p <0.05 差异显著。下同。
Note: Data in table above are mean±SD. Different small letters in the same column and same fruit organ represent significant difference at p <0.05.The same below.
样品Samples果皮Peel果肉Pulp类型Type正常果Normal fruit裂果Cracked fruit正常果Normal fruit裂果Cracked fruit w(N)0.52±0.05 a 0.61±0.04 a 0.44±0.07 a 0.53±0.05 a w(P)7.06±0.65 a 4.54±0.34 b 2.09±0.09 a 2.28±0.11 a w(K)74.74±6.32 a 76.54±5.63 a 31.10±3.35 a 27.74±2.12 a w(Ca)7.25±0.45 a 5.82±0.38 b 2.06±0.05 a 1.31±0.28 b w(Mg)7.05±0.43 a 6.93±0.35 a 6.04±0.04 a 6.16±0.55 a
由表2 还可以看出,果实钙含量在果皮和果肉中表现出一致的规律,钙在裂果果皮、果肉中的含量均低于相应部位正常果中的含量,正常果果皮、果肉中钙含量分别为7.25、2.06 g·kg-1,而裂果同一部位钙含量分别比裂果中相应部位的钙含量低19.72%和36.41%,正常果与裂果果皮、果肉中的钙含量均具有显著差异。相关性分析表明,裂果率与果皮钙含量呈极显著负相关(相关系数R2=0.866 5)(图2)。
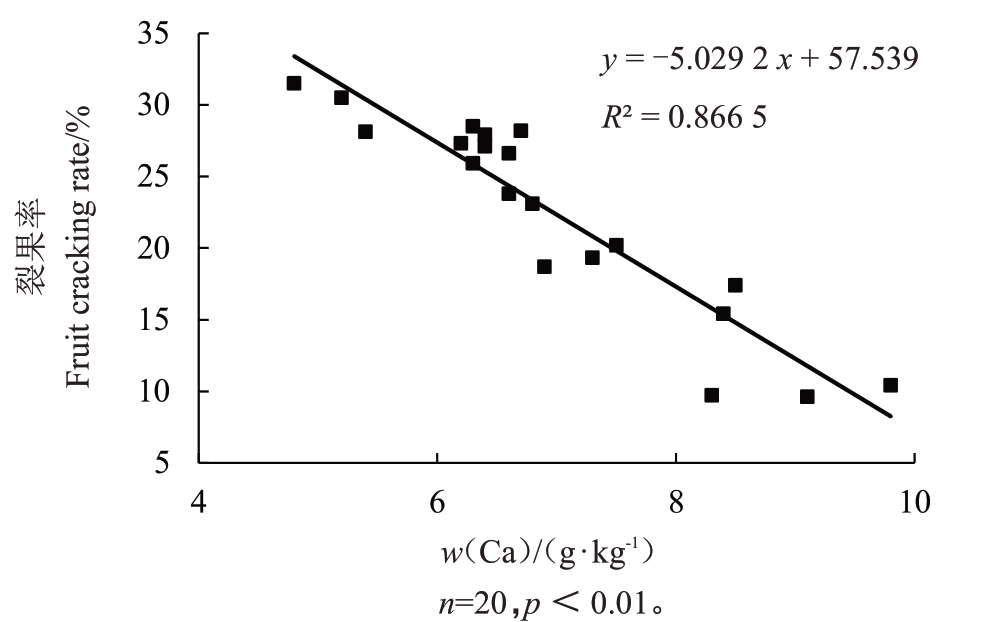
图2 裂果率与果皮钙含量的相关关系
Fig.2 The correlation of fruit cracking rate and Ca concentration in loquat peel
2.3 白沙枇杷裂果与正常果中活性氧清除酶与氧化酶活性差异
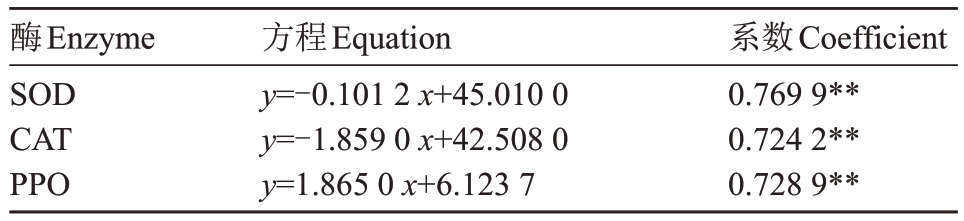
如表3所示,SOD 和CAT 在果皮中呈现出一致的规律,正常果活性显著高于裂果活性。正常果果皮SOD活性为292.31 U·g-1,高于裂果17.81%,正常果果肉SOD 活性为207.23 U·g-1,高于裂果果肉40.58%。CAT活性裂果果皮、果肉分别高于裂果果皮果肉17.78%和7.87%。相关性分析结果表明,果实的裂果率与果皮中的SOD活性、CAT活性呈显著负相关(相关系数分别为R2=0.769 9 和R2=0.724 2)(表4)。
表3 裂果与正常果中SOD、POD、CAT 和PPO 活性的差异
Table 3 Difference in SOD,POD,CAT and PPO activities between cracked and normal fruits(U·g-1)
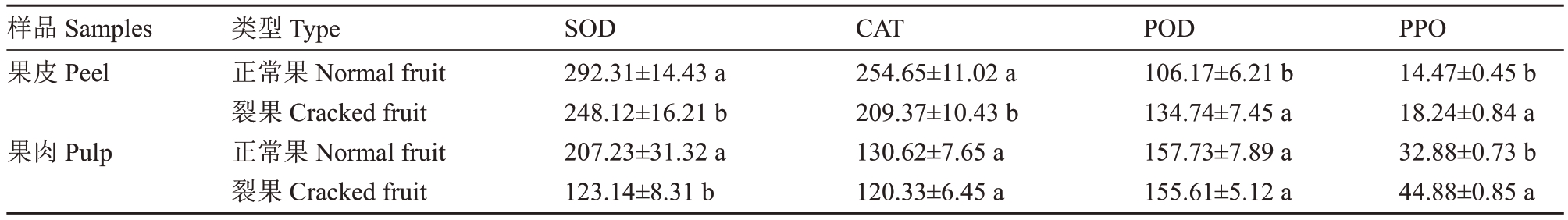
样品Samples果皮Peel果肉Pulp类型Type正常果Normal fruit裂果Cracked fruit正常果Normal fruit裂果Cracked fruit SOD 292.31±14.43 a 248.12±16.21 b 207.23±31.32 a 123.14±8.31 b CAT 254.65±11.02 a 209.37±10.43 b 130.62±7.65 a 120.33±6.45 a POD 106.17±6.21 b 134.74±7.45 a 157.73±7.89 a 155.61±5.12 a PPO 14.47±0.45 b 18.24±0.84 a 32.88±0.73 b 44.88±0.85 a
表4 裂果率与果皮SOD、CAT、PPO 活性的相关性
Table 4 The correlation of fruit cracking rate and SOD,CAT,PPO activities in loquat peel
注:**p <0.01。下同。
Note:**p <0.01.The same below.
酶Enzyme SOD CAT PPO方程Equation y=-0.101 2 x+45.010 0 y=-1.859 0 x+42.508 0 y=1.865 0 x+6.123 7系数Coefficient 0.769 9**0.724 2**0.728 9**
从表3 中还可以发现POD、PPO 活性与SOD、CAT活性呈现相反的结果,果皮中POD和PPO活性正常果均低于裂果。其中POD 活性在正常果果皮中为106.17 U·g-1,比裂果处理低21.20%,果肉中POD 活性两者无显著差异,正常果果皮、果肉中PPO 活性分别为14.47、32.88 U·g-1,显著低于裂果26.01%和26.73%。相关性分析表明,果皮PPO活性与裂果率呈显著正相关(表4)。
2.4 白沙枇杷裂果与正常果中细胞壁水解酶活性差异
分析(图3-A)表明,裂果果皮中PG 和CL 活性显著高于正常果果皮,其中裂果果皮PG 活性为263.18 U·g-1,高于正常果皮28.82%,CL活性高于正常果皮54.85%。果肉中2种酶活性与果皮酶活性呈现一致的规律,裂果果肉PG 活性高于正常果肉17.43%,CL活性高于正常果肉16.71%(图3-B)。果皮PG、CL活性与裂果率呈显著正相关,相关系数分别为0.775 4和0.733 2(表5)。
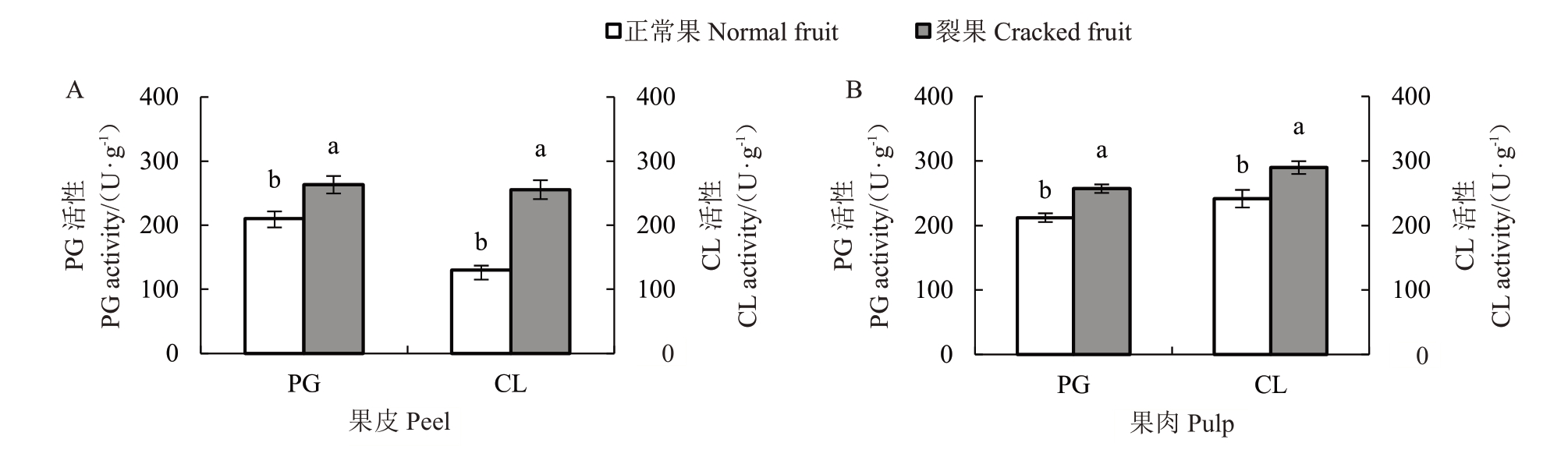
图3 裂果与正常果中细胞壁水解酶活性
Fig.3 PG and CL activities in normal and cracked fruits
表5 果皮中裂果率与细胞壁水解酶活性的相关性
Table 5 The correlation of fruit cracking rate and PG,CL activities in loquat peel
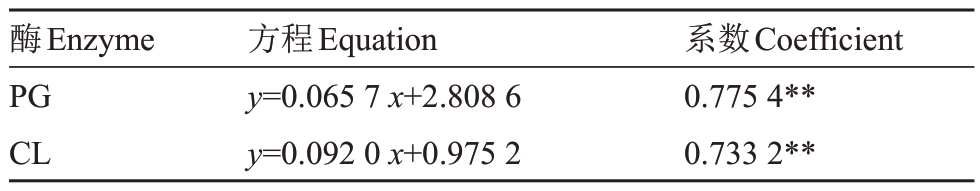
酶Enzyme PG CL方程Equation y=0.065 7 x+2.808 6 y=0.092 0 x+0.975 2系数Coefficient 0.775 4**0.733 2**
2.5 白沙枇杷裂果与正常果中激素水平差异
由表6 可知,裂果与正常果果皮中GA3与IAA含量无显著差异,而裂果果肉中GA3含量显著高于正常果31.75%,IAA含量两者差异不大。裂果果皮中ABA 含量为155.75 ng·g-1,显著高于正常果果皮21.56%,果肉中ABA含量无显著差异。
表6 裂果与正常果中激素水平差异
Table 6 Differences in hormone levels between cracked and normal fruits(ng· g-1)
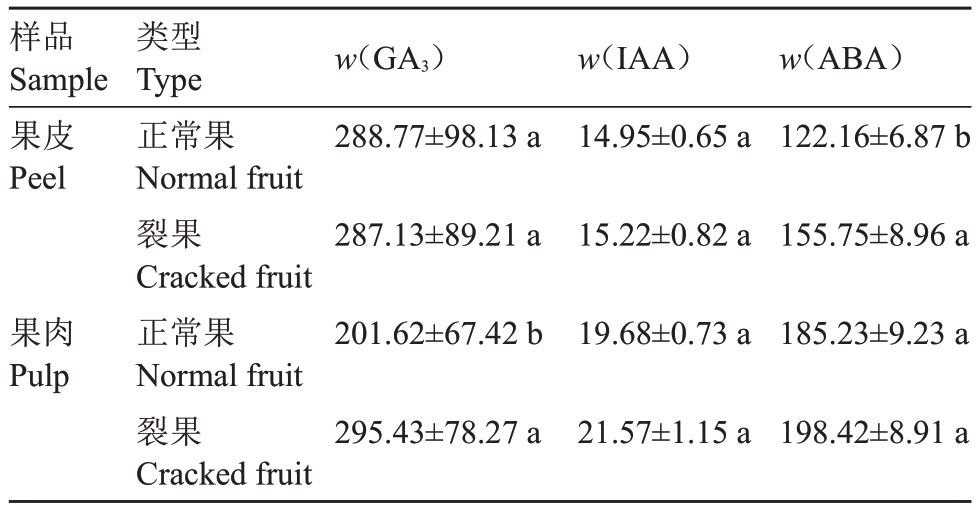
样品Sample果皮Peel果肉Pulp类型Type正常果Normal fruit裂果Cracked fruit正常果Normal fruit裂果Cracked fruit w(GA3)288.77±98.13 a 287.13±89.21 a 201.62±67.42 b 295.43±78.27 a w(IAA)14.95±0.65 a 15.22±0.82 a 19.68±0.73 a 21.57±1.15 a w(ABA)122.16±6.87 b 155.75±8.96 a 185.23±9.23 a 198.42±8.91 a
2.6 白沙枇杷果实发育期间Ca2+浓度的变化
由图4 可以看出,枇杷果皮、果肉中Ca2+含量的变化趋势表现出相同的规律,且果肉中的Ca2+含量显著低于果皮。幼果期果皮、果肉Ca2+含量最高(分别为12.31、9.10 μmol·g-1),到膨大期时急剧下降了41.6%和43.5%,膨大期到转色期果皮中Ca2+含量变化不大,果肉中有所上升,成熟期时果皮、果肉Ca2+含量降到最低值(4.54、2.52 μmol·g-1),且果肉中Ca2+含量显著低于果皮。
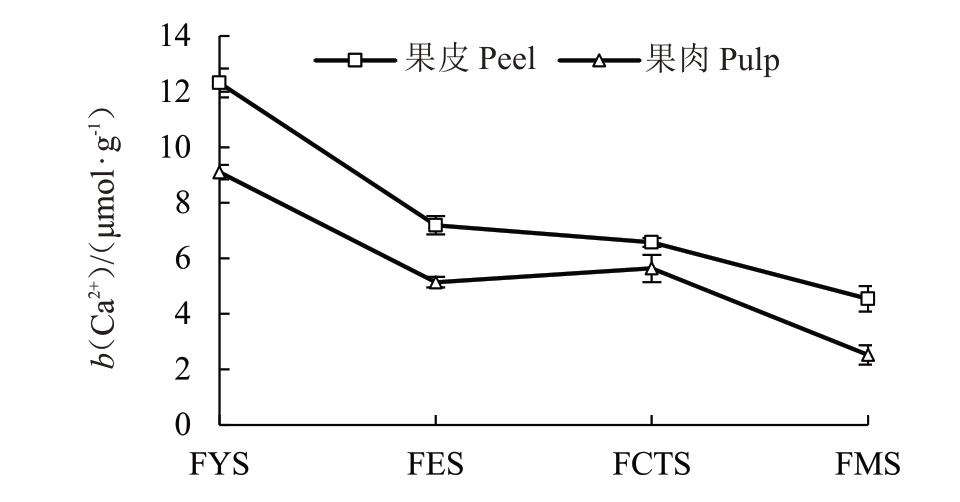
图4 枇杷果实不同发育时期果皮、果肉Ca2+动态变化
Fig.4 Dynamic changes of Ca2+in peel and pulp of loquat fruit at different developmental stages
FYS、FES、FCTS、FMS 分别表示幼果期、膨大期、转色期和成熟期。
FYS,FES,FCTS,FMS stand for young fruit stage, fruit expanding stage,fruit color turning stage and fruit mature stage,respectively.
2.7 不同裂果程度果实果皮钙含量与各生理指标的相关性分析
由表7 可知,不同裂果程度成熟期果实果皮钙含量与果皮SOD活性呈显著正相关,与果皮PPO活性、PG 活性、CL 活性、ABA 含量呈显著负相关,而与果皮CAT、POD 活性,果肉GA3含量的相关性不显著。其中Ca 与PG、CL 活性相关性最显著,相关系数分别为-0.841、-0.810。SOD 活性与PPO、PG、CL 活性、果肉GA3含量呈显著负相关。PPO、PG 与CL 活性呈显著正相关关系。果肉GA3含量与果皮ABA含量呈显著正相关。CAT、POD活性与其他指标之间均无显著相关性。
表7 果皮钙含量与酶活性、内源激素含量相关性
Table 7 Correlation analysis of calcium in peel with enzyme activity and endogenous hormone content
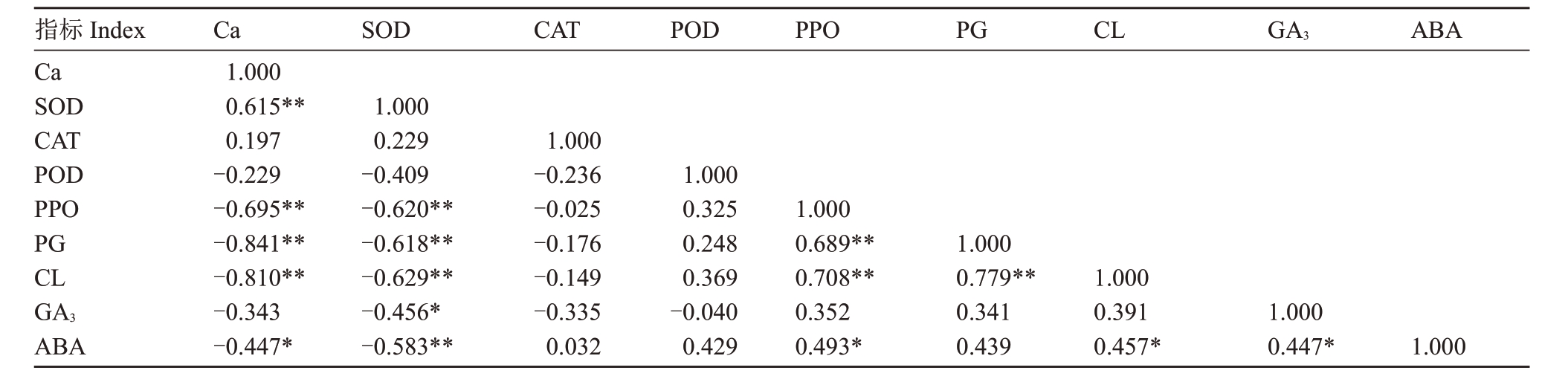
注:**表示在0.01 水平上相关性极显著,*表示在0.05 水平上相关性显著;表中数据除GA3 含量为果肉含量,其余均为果皮含量,n=20。
Note:** indicates extremely significant difference at 0.01 level, * indicates significant difference at 0.05 level; the data in the table all indicates content in peel except for GA3 content(in pulp),n=20.
指标Index Ca SOD CAT POD PPO PG CL GA3 ABA Ca 1.000 0.615**0.197-0.229-0.695**-0.841**-0.810**-0.343-0.447*SOD 1.000 0.229-0.409-0.620**-0.618**-0.629**-0.456*-0.583**CAT 1.000-0.236-0.025-0.176-0.149-0.335 0.032 POD 1.000 0.325 0.248 0.369-0.040 0.429 PPO 1.000 0.689**0.708**0.352 0.493*PG 1.000 0.779**0.341 0.439 CL 1.000 0.391 0.457*GA3 1.000 0.447*ABA 1.000
2.8 外源喷钙对裂果率与果实钙含量的影响
由图5-A可知,外源喷施0.5%CaCl2溶液,软条白沙枇杷裂果率由27.1%降低到14.4%。喷钙后果皮钙、果肉钙含量为8.34、4.95 g·kg-1,分别是对照的1.68 倍和1.69 倍(图5-B)。喷钙可显著提高果实钙含量,降低裂果率。
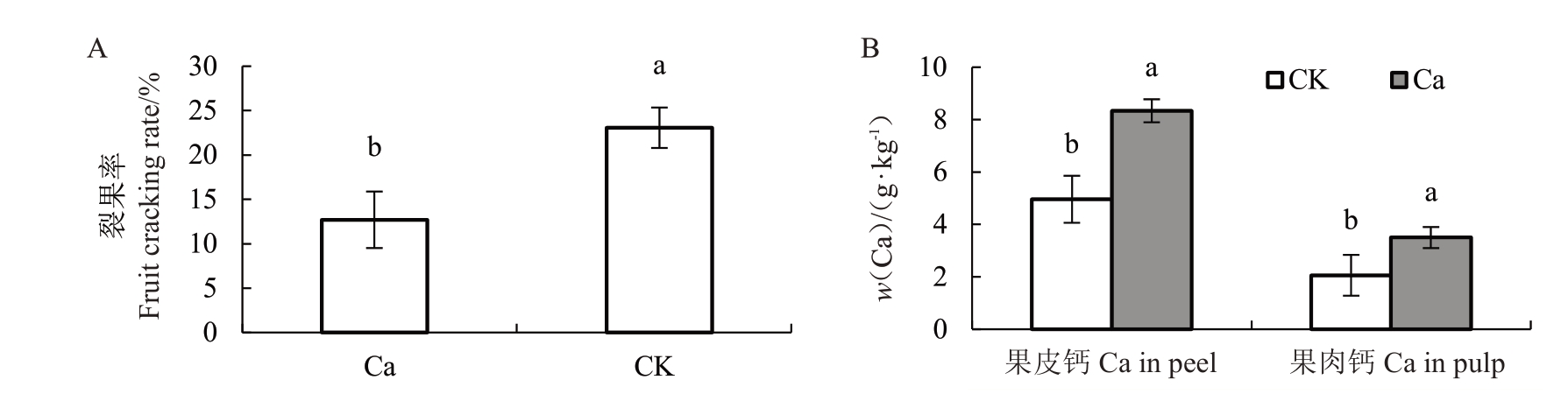
图5 外源CaCl2处理对白沙枇杷裂果率及果实钙含量的影响
Fig.5 Effect of CaCl2treatment on fruit cracking rate and fruit Ca content on white flesh loquat
2.9 外源喷钙对相关酶活性及内源激素含量的影响
如图6 所示,喷钙可显著提高果皮SOD 活性、CAT活性。喷钙后果皮SOD活性为256.71 U·g-1,较对照提高17.76%;CAT 活性为303.63 U·g-1,较对照提高12.08%。果肉SOD 活性在钙处理后虽有所升高,却不具显著差异性。果肉CAT活性为129.99 U·g-1,较对照显著提高了18.5%。
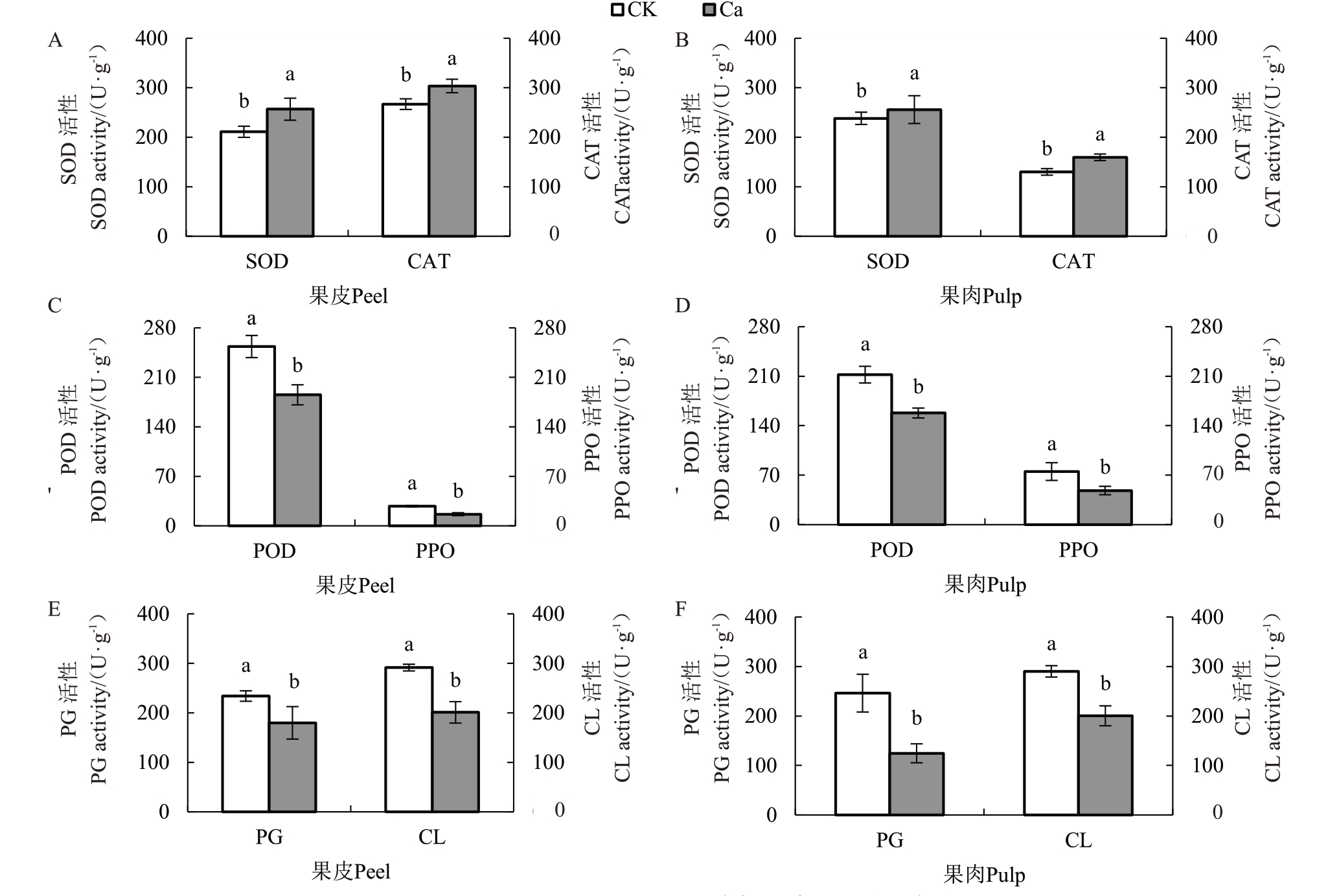
图6 外源CaCl2处理对果实相关酶活性的影响
Fig.6 Effect of CaCl2treatment on fruit related enzyme activity
喷钙后POD、PPO 活性与SOD、CAT 活性变化趋势相反,外源Ca2+降低了这2种酶的活性。如图6-C~D 所示,果皮POD、PPO 活性分别较对照降低了26.93%和41.02%,果肉POD、PPO 活性分别较对照降低了25.68%、35.68%,且处理前后均具有显著差异性。
外源喷钙显著降低了果皮、果肉PG、CL 活性。由图6-E~F可知,果皮PG、CL活性分别较对照降低了23.17%和30.05%,果肉PG、CL 活性分别较对照降低了97.5%和28.12%。
图7 显示了外源CaCl2处理后果皮、果肉ABA与GA3的变化情况。喷钙可显著降低果皮生长类激素ABA含量。喷钙后果皮ABA含量较对照降低了19.41%,钙处理同时降低了果肉促生长类激素GA3水平,处理后GA3含量为208.03 ng·g-1,较对照降低了28.18% 。果皮GA3含量、果肉ABA 含量处理前后无显著差异。
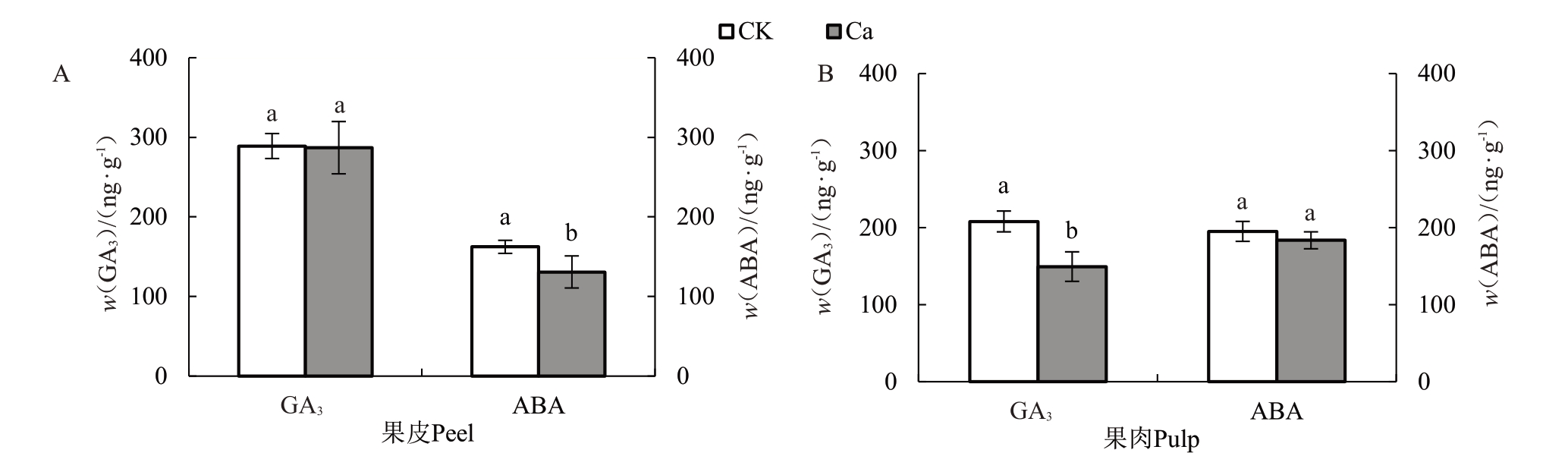
图7 外源CaCl2处理对果实内源激素的影响
Fig.7 Effect of CaCl2treatment on fruit endogenous hormones
3 讨 论
裂果是果实发育过程中的一种生理性病害。试验期间,浙东南地区枇杷裂果始于果实膨大前期,4月底、5 月初进入裂果高峰期,裂果类型以横裂为主。果实迅速膨大期需要大量营养元素供给,前人研究结果表明,与裂果相关的矿质元素有K、N、Ca、Mg、B,其中Ca 与裂果的关系尤为密切[23]。本研究中,果实中N、K、Mg 含量与裂果相关性关系不显著,而较高的果皮P含量可以减少裂果的发生,这与温明霞等[4]的研究结果不一致,究其原因,可能跟P能够增强枇杷抗逆性有关[24]。钙是植物生长过程中必需的矿质元素,并作为第二信使参与了生物和非生物胁迫的各种反应,在果实生长发育尤其是果皮细胞壁结构形成过程中起着重要的作用。对果皮、果肉中总Ca 含量的测定结果表明,正常果果皮、果肉中的Ca含量均显著高于裂果,说明钙素营养不足是造成白沙枇杷裂果的主要原因之一。高钙减少裂果的直接原因在于:一方面,钙与果胶酸结合形成果胶酸钙,对增加细胞间的连接有重要作用;另一方面,Ca2+可降低细胞膜透性,防止果皮水分过量吸收[25]。本研究中,枇杷果实幼果期Ca2+含量最高,膨大期Ca2+显著下降,推测果实对钙的吸收主要集中在幼果期到膨大期。这是因为果实发育早期细胞分裂比较迅速,果实需要吸收大量的Ca2+用于细胞壁的形态建成,曹一博[26]也有类似的研究结果。由此推断,幼果期钙素营养的缺乏造成果皮细胞壁形态建成受阻,导致后期裂果的发生。
SOD和CAT是活性氧清除剂,参与抗寒、抗旱、抗盐碱等多种逆境胁迫,能够在逆境胁迫或衰老过程中清除体内过量的活性氧,减少细胞毒害、保护细胞膜结构[27]。本研究中,正常果SOD、CAT活性显著高于裂果,且裂果率与果皮中的SOD 活性、CAT 活性呈显著负相关,推测在枇杷果实遭遇裂果胁迫时,果实中产生了大量的活性氧,而果皮、果肉中低SOD、CAT 活性不足以清除有毒害作用的活性氧而引发裂果,这与温明霞等[4]在锦橙裂果中的研究结果一致。POD是植物体内常见的氧化还原酶,通过参与细胞壁成分的酚基交联降低果皮细胞壁的延伸性,PPO可促使果实发生褐化[28-29]。曹一博[26]研究表明,易裂枣品种成熟果实中这2 种酶的活性显著高于抗裂枣果实。本研究中,裂果果实中POD、PPO活性均显著升高,可能在于高POD活性在降低果皮延伸性的同时,高PPO 活性进一步导致果实褐化,从而加速了裂果发生。果胶酶(PG)和纤维素酶(CL)分别是水解细胞结构物质(果胶和纤维素)的酶,其活性高低与果胶和纤维素的水解程度有关。Ca2+可抑制细胞壁可溶性果胶水解,低钙果实中的果胶酶和纤维素酶升高,引起构建果皮细胞壁重要组分的果胶和纤维素水解,造成果皮强度降低,从而引发裂果[30]。
内源激素的变化是引起裂果的一个重要原因,尤其是在果实发育的中后期。裂果多的树生长促进类物质(IAA、CTK、GA3)含量较高,特别在果肉中表现尤为明显[31]。本研究中,裂果果肉中GA3含量显著高于正常果,而裂果果皮中ABA含量显著高于正常果。原因可能在于果皮中生长抑制类激素ABA的增加会导致果皮衰老加速,与此同时果肉中促进生长类激素的积累导致果肉膨胀过快,果皮与果肉生长速度不平衡引起果实开裂。
花期至幼果期对软条白沙进行喷钙处理可显著提高果实钙含量,降低裂果率,说明在幼果及前期补钙可促进细胞壁形态建成,减轻裂果。本研究中,外源喷施0.5%CaCl2可显著提高果实SOD、CAT活性,原因可能在于钙可调控活性氧清除途径,降低活性氧积累,缓解氧化胁迫[27]。Yu等[32]研究发现,钙通过调节细胞壁代谢相关酶的转录和活性,提高了原果胶含量,抑制了水溶性果胶含量的增加。本研究虽未测定原果胶与可溶性果胶的含量,但从果胶酶和纤维素酶的活性变化可以推断、验证这个观点。喷钙可显著降低果肉GA3含量、果皮ABA 含量,原因在于Ca2+信号可通过参与调控植物激素的合成响应非生物胁迫,维持激素平衡[33]。
果实的裂果是一个复杂的过程,钙影响裂果的各种生理过程并不是独立的[11]。本研究中,果皮钙含量与果皮SOD活性呈显著正相关,与果皮PPO活性、PG 活性、CL 活性、ABA 含量呈显著负相关,说明钙信号可直接影响上述酶与激素合成。同时,研究结果还表明,酶与酶之间、酶与激素之间、激素与激素之间同样存在显著的相关关系,说明酶系统与激素合成系统之间通过相互影响与相互作用,形成了一个复杂的裂果调控网络,其具体的调控机制还有待进一步研究。
4 结 论
白沙枇杷钙素营养的吸收主要发生在幼果期到膨大期,果皮中钙素营养不足是导致裂果发生的主要原因之一。幼果期钙素营养缺乏影响果皮细胞壁建成,花期及幼果期喷钙可显著提高果实钙含量,缓解膜脂过氧化毒害,降低细胞壁水解程度,维持内源激素平衡,降低裂果率。
[1] 王引,陈方永,倪海枝,颜帮国.裂果易发性不同的枇杷品种果实相关酶活性比较[J].农业科技通讯,2019(7):195-197.WANG Yin,CHEN Fangyong,NI Haizhi,YAN Bangguo. Comparison of fruit related enzyme activities in loquat cultivars with different cracking susceptibility[J]. Bulletin of Agricultural Science and Technology,2019(7):195-197.
[2] GINE-BORDONABA J,ECHEVERRIA G,UBACH D,AGUILO-AGUAYO I,LUISA L M,LARRIGAUDIERE C.Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance[J].Plant Physiology and Biochemistry,2016,111:216-225.
[3] 王旭旭,樊秀彩,李傲,张超博,房经贵,刘崇怀,上官凌飞.葡萄品种资源裂果性状调查与分析[J].园艺学报,2016,43(11):2099-2108.WANG Xuxu,FAN Xiucai,LI Ao,ZHANG Chaobo,FANG Jinggui,LIU Chonghuai,SHANGGUAN Lingfei. Investigation and analysis on cracking trait in grape berry[J]. Acta Horticulturae Sinica,2016,43(11):2099-2108.
[4] 温明霞,石孝均. 锦橙裂果的钙素营养生理及施钙效果研究[J].中国农业科学,2012,45(6):1127-1134.WEN Mingxia,SHI Xiaojun.Influence of calcium on fruit cracking of Jincheng orange and its physiological mechanism[J]. Scientia Agricultura Sinica,2012,45(6):1127-1134.
[5] 陈银朝.不同品种油桃裂果比较及防治措施研究[J].西北农业学报,2007,16(2):165-168.CHEN Yinchao. The comparison of dehiscent fruit of different nectarines and controlling measures[J].Acta Agriculturae Boreali-Occidentalis Sinica,2007,16(2):165-168.
[6] 张琪静,谷大军. 甜樱桃果实裂果机理研究进展[J]. 果树学报,2014,31(4):704-709.ZHANG Qijing,GU Dajun.A review of the mechanisms of fruit cracking in sweet cherries[J]. Journal of Fruit Science,2014,31(4):704-709.
[7] 冯美利,李杰,曾鹏,孙程旭,陈思婷.香水椰子裂果率与气候因子的通径分析[J].热带作物学报,2010,31(11):1922-1926.FENG Meili,LI Jie,ZENG Peng,SUN Chengxu,CHEN Siting.Path analysis between climatic factors and fruit cracking of aromatic coconut[J]. Chinese Journal of Tropical Crops,2010,31(11):1922-1926.
[8] 曹一博,李长江,孙帆,张凌云.抗裂与易裂枣内源激素含量和细胞壁代谢相关酶活性比较[J]. 园艺学报,2014,41(1):139-148.CAO Yibo,LI Changjiang,SUN Fan,ZHANG Lingyun. Comparison of the endogenous hormones content and the activities of enzymes related to cell-wall metabolism between Jujube cultivars susceptible and resistant to fruit cracking[J].Acta Horticulturae Sinica,2014,41(1):139-148.
[9] 曹一博,孙帆,刘亚静,张凌云.枣果实组织结构及果皮中矿质元素含量对裂果的影响[J].果树学报,2013,30(4):621-626.CAO Yibo,SUN Fan,LIU Yajing,ZHANG Lingyun. Effects of anatomical structure and mineral contents in pericarp on fruit cracking in jujube fruit[J].Journal of Fruit Science,2013,30(4):621-626.
[10] 王保明,丁改秀,王小原,付春宝,秦国杰,杨俊强,仓国营,温鹏飞.枣果实裂果的组织结构及水势变化的原因[J].中国农业科学,2013,46(21):4558-4568.WANG Baoming,DING Gaixiu,WANG Xiaoyuan,FU Chunbao,QIN Guojie,YANG Junqiang,CANG Guoying,WEN Pengfei. Changes of histological structure and water potential of Huping jujube fruit cracking[J]. Scientia Agricultura Sinica,2013,46(21):4558-4568.
[11] 马雯彦,庞晓明,续九如,李颖岳.果实裂果影响因子研究进展[J].华中农业大学学报,2010,29(6):798-804.MA Wenyan,PANG Xiaoming,XU Jiuru,LI Yingyue.Advances in research on the factors influencing fruit cracking[J]. Journal of Huazhong Agricultural University,2010,29(6):798-804.
[12] 章文华,陈亚华,刘友良.钙在植物细胞盐胁迫信号转导中的作用[J].植物生理学通讯,2000,36(2):146-153.ZHANG Wenhua,CHEN Yahua,LIU Youliang. Calcium action in signal transduction in plant cells under salt stress[J]. Plant Physiology Communications,2000,36(2):146-153.
[13] 赵玉华.钙在葡萄中的积累规律及钙对果实品质和裂果的影响[D].长沙:湖南农业大学,2017.ZHAO Yuhua. The Calcium accumulation rule of the grape and the effect of Ca to the fruit quality and dehiscent of grape[D].Changsha:Hunan Agricultural University,2017.
[14] 张阁,朱国英,刘成连,原永兵,李志军,王永章.甜樱桃果实果肉Ca2+质量浓度变化规律及其与裂果的关系[J].果树学报,2008,25(5):646-649.ZHANG Ge,ZHU Guoying,LIU Chenglian,YUAN Yongbing,LI Zhijun,WANG Yongzhang. Change of Ca2+ concentration in sweet cherry (Prunus avium L.) fruit and its relationship with fruit cracking[J].Journal of Fruit Science,2008,25(5):646-649.
[15] 李克志,高中山.枣裂果机理的初步研究[J].果树科学,1990,7(4):221-226.LI Kezhi,GAO Zhongshan. Preliminary study on the mechanism of fruit cracking in Chinese jujube[J]. Journal of Fruit Science,1990,7(4):221-226.
[16] 郭红彦,白晋华,段风琴,郗鑫,李涛,郭晋平.钙处理对“壶瓶枣”裂果细胞壁降解酶活性及组织结构的影响[J].园艺学报,2019,46(8):1486-1494.GUO Hongyan,BAI Jinhua,DUAN Fengqin,XI Xin,LI Tao,GUO Jinping. Effect of CaCl2 treatment on cell wall degrading enzymes activities and microstructure of fruit cracking of Ziziphus jujuba‘Huping Zao’[J]. Acta Horticulturae Sinica,2019,46(8):1486-1494.
[17] 潘丹红. 枇杷裂果原因及防止措施[J]. 浙江柑橘,2021,38(1):41-43.PAN Danhong. Causes and prevention measures of loquat fruit cracking[J].Zhejiang Citrus,2021,38(1):41-43.
[18] 冯健君,周慧芬,胡美君,郑洁,郭延平.白沙枇杷果实发育、果实水势及与裂果关系研究[J].热带亚热带植物学报,2010,18(5):536-540.FENG Jianjun,ZHOU Huifen,HU Meijun,ZHENG Jie,GUO Yanping. Relationship between fruit cracking and fruit development,water potential of white flesh loquat[J]. Journal of Tropical and Subtropical Botany,2010,18(5):536-540.
[19] 鲍士旦.土壤农化分析[M].北京:中国农业出版社,2008.BAO Shidan. Soil agro-chemical analysis[M]. Beijing:China Agricultural Press,2008.
[20] 曲敏,刘羽佳,范宏臣,朱姝,王金凤.两种检测SOD 酶活性方法的比较[J].食品安全质量检测学报,2014,5(10):3318-3323.QU Min,LIU Yujia,FAN Hongchen,ZHU Shu,WANG Jinfeng.Comparison of two methods for detecting SOD activity[J].Food SafetyandQualityDetectionTechnology,2014,5(10):3318-3323.
[21] WANG Y,FU X Z,LIU J H,HONG N. Differential structure and physiological response to canker challenge between‘Meiwa’kumquat and‘Newhall’navel orange with contrasting resistance[J].Scientia Horticulturae,2011,128(2):115-123.
[22] 祁业凤,刘孟军.两个胚败育率不同的枣品种果实生育期内源激素的变化[J].园艺学报,2004,31(6):800-802.QI Yefeng,LIU Mengjun. Change of endogenous hormone in cultivars of Chinese jujube with different type of embryo abortion[J].Acta Horticulturae Sinica,2004,31(6):800-802.
[23] 林兰稳. 矿质营养对荔枝裂果率的影响[J]. 土壤与环境,2001,10(1):55-56.LIN Lanwen. Effects of mineral nutrition on fruit cracking rate of Litchi chinensis[J]. Soil and Environmental Sciences,2001,10(1):55-56.
[24] 蔡礼鸿.枇杷学[M].北京:中国农业出版社,2012.CAI Lihong.Loquat[M].Beijing:China Agriculture Press,2012.
[25] 崔守尧,吴震,吕海萌,薛灵姿,蒋芳玲.外源CaCl2 缓解番茄裂果的生理机制[J].南京农业大学学报,2019,42(1):59-65.CUI Shouyao,WU Zhen,LÜ Haimeng,XUE Lingzi,JIANG Fangling. The physiological mechanism of exogenous CaCl2 relieving tomato fruit cracking[J]. Journal of Nanjing Agricultural University,2019,42(1):59-65.
[26] 曹一博.枣果实发育过程中生理代谢与裂果的关系[D].北京:北京林业大学,2013.CAO Yibo. The relationship between the metabolism and fruit cracking during the development stages of Zizyphus jujuba Mill.[D].Beijing:Beijing Forestry University,2013.
[27] 宋姗姗,杨方圆,杨莎,万书波,李新国.钙离子对盐胁迫下花生根系发育的调控[J].植物生理学报,2021,57(7):1547-1558.SONG Shanshan,YANG Fangyuan,YANG Sha,WAN Shubo,LI Xinguo. Regulation of calcium ion on root development of peanut under salt stress[J]. Plant Physiology Journal,2021,57(7):1547-1558.
[28] 刘欢. 枣果皮结构及细胞壁代谢酶活性与抗裂果关系的研究[D].阿拉尔:塔里木大学,2019.LIU Huan. Study on the relationship between jujube peel structure,cell wall metabolic enzyme activity and resistance to cracking[D].Alaer:Tarim University,2019.
[29] 高美玲,于长宝,李佳益. 小型西瓜果皮酶系与裂果性的关系[J].江苏农业科学,2016,44(10):228-230.GAO Meiling,YU Changbao,LI Jiayi.Relationship between enzyme system and fruit cracking of small watermelon peel[J]. Jiangsu Agricultural Sciences,2016,44(10):228-230.
[30] 栗现芳,姚瑞,赵瑞华,陈国梁,陈宗礼.枣果纤维素酶对裂果发生的影响[J].安徽农业科学,2016,44(27):65-68.LI Xianfang,YAO Rui,ZHAO Ruihua,CHEN Guoliang,CHEN Zongli. Effect of cellulase on jujube fruit cracking[J].Journal of Anhui Agriculture Sciences,2016,44(27):65-68.
[31] 邱燕萍,陈洁珍,欧良喜,王碧青,袁沛元.糯米糍荔枝裂果与内源激素变化的关系[J].果树学报,1999,16(4):276-279.QIU Yanping,CHEN Jiezhen,OU Liangxi,WANG Biqing,YUAN Peiyuan.Relationship between fruit cracking and endogenous hormones in‘Nuomici’litchi variety[J]. Journal of Fruit Science,1999,16(4):276-279.
[32] YU J,ZHU M T,BAI M,XU Y S,FAN S G,YANG G S.Effect of calcium on relieving berry cracking in grape (Vitis vinifera L.)‘Xiangfei’[J].PeerJ,2020,8(12):9896.
[33] HE Y D,LI R M,WEI C B,SUN G M.Effect of exogenous calcium on root growth and endogenous hormone contents in pineapple seedlings[J]. Advanced Materials Research,2013,864/867:106-110.