随着全球气候的变化,全球平均温度在近130年(1880—2012年)升高了0.85(0.65~1.06)℃,地球表面大气温度预计在21 世纪中叶升高1.5~4.5 ℃[1-2]。全球气候变暖会对生态环境造成不可逆转的影响。在我国西部地区平均气温也呈逐年上升趋势,降水呈减少趋势,暖干化趋势明显增强[3]。研究高温与干旱对植物协同作用表明,高温和干旱协同作用对植物表现出不同的影响机制[4-5]。
灵武长枣(Ziziphus jujuba Mill.‘Lingwuchangzao’)是宁夏特色鲜食枣品种,其经济、生态及社会效益显著。随着人们生活水平和消费水平的不断提高,消费者对枣的保健价值关注更多,其红色喜庆的外观更容易得到消费者的喜爱。果实颜色的差异及深浅与花青苷等黄酮类物质有关,使枣着色的主要色素物质是花青苷与其他色素综合作用的产物[6-9]。花青苷不仅是红色、紫红色果实色泽的主要组成成分,也是具有营养价值的重要次生代谢产物之一,花青苷对果实的商品价值同样具有重要作用。目前,随着组学技术的发展,转录组和代谢组关联分析可在植物中用于筛选植物器官显示相关基因与代谢物[9],以较为全面地揭示调控机制。其中有关果皮色泽代谢调控研究表明,结构基因编码合成代谢相关酶UFGT、PAL、CHS、CHI、F3H、ANS 等,在花青苷合成中扮演着重要角色,尤其在苹果[10]、猕猴桃[11]、梨[12]等园艺植物中的研究报道较多。
目前,关于灵武长枣的研究内容,涉及嫁接和根蘖植株果实转录组测序,筛选到调控糖、有机酸代谢关键差异基因BAM1、PCO 和HAOX1[13]。宁夏地区气温升高与干旱环境下灵武长枣生长、光合产物分配、果实糖积累、花青苷合成相关酶活性及基因表达的研究已有报道[14-16]。关于花青苷合成代谢机制的研究已引起人们广泛关注,而在逆境胁迫下,灵武长枣果实色泽相关基因和代谢物方面的研究鲜见报道。因此,需要进一步对气温升高与干旱环境下灵武长枣果实色泽的差异及其内在机制进行探讨。笔者在本研究中通过整合转录组学和代谢组学分析,挖掘升温和干旱协同处理下调控花青苷合成的关键差异基因,提出代谢网络,阐明气温升高与干旱胁迫对灵武长枣果实着色的影响机制,揭示气候变化对枣果实品质形成的影响机制。
1 材料和方法
1.1 试验材料与处理
本试验在宁夏永宁县宁夏大学实验农场(106°04′00″E,38°47′07″N)进行,该地区属中温带干旱气候区,年均降水180~200 mm,灌淤土。试验材料为5年生灵武长枣嫁接植株。
采取大气温度、干旱(土壤水分含量)二因素裂区试验设计,大气温度为主处理,土壤含水量为副处理;大气温度设置2 个水平:自然大气温度T1,升高的大气温度T2(自然大气温度±2.0 ℃)(表1);土壤含水量设置3 个梯度:正常土壤供水条件D1(土壤含水量为70%~75%的田间最大持水量(maximum field capacity,MFC),中度干旱D2(50%~55%MFC)和重度干旱D3(30%~35% MFC),共6 个处理。采用开顶气室(OTC)控制系统模拟气温升高,土壤含水量采用土壤水分传感器、紫藤精准灌溉控制器(图1)。2个温度处理各设置3个气室,每个开顶气室种植9株枣树(株行距1 m×1 m),每3株1个土壤水分水平,共54 株枣树(各植株间根部用阳光板隔离)[17]。枣树萌芽期开始试验处理,分别在果实S1时期(白熟期)(图2-A)、S2时期(半红脆熟期)(图2-B)、S3 时期(全红脆熟期)(图2-C)于灵武长枣植株(每重复)各方位随机采集外表均一的果实样本10个。液氮速冻带回测定果皮花青苷含量,剩余样品储存于-80 ℃冰箱用于转录组测序。

图1 试验控制系统
Fig.1 Control system for experiment
A.精准灌溉器;B.多功能数据采集器。
A.Precision irrigator;B.Multifunctional data collector.

图2 果实成熟着色变化
Fig.2 Fruit ripening and coloration change
A.白熟期;B.半红脆熟期;C.全红脆熟期。
A.White mature period;B.Half red period;C.Full red period.
表1 试验处理
Table 1 Experimental treatment list
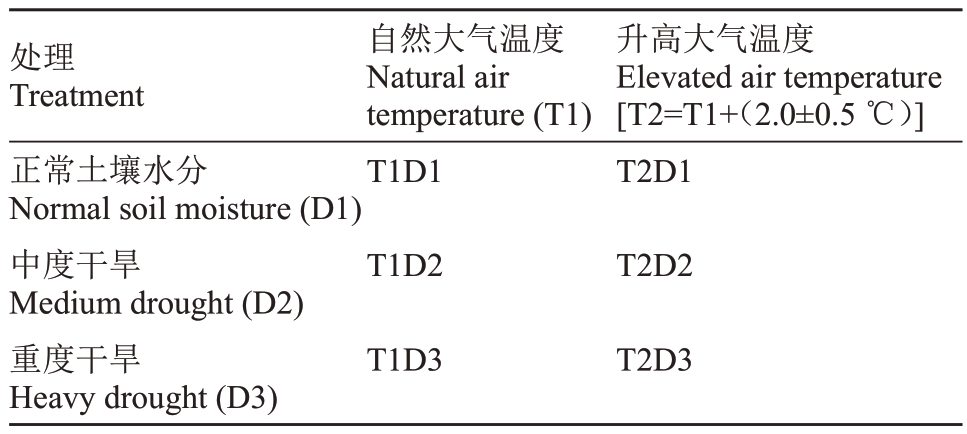
处理Treatment正常土壤水分Normal soil moisture(D1)中度干旱Medium drought(D2)重度干旱Heavy drought(D3)自然大气温度Natural air temperature(T1)T1D1 T1D2 T1D3升高大气温度Elevated air temperature[T2=T1+(2.0±0.5 ℃)]T2D1 T2D2 T2D3
1.2 果皮花青苷含量测定
参考周丹蓉等[18]的方法获得果皮花青苷提取液,并采用pH示差法[19]测定果皮花青苷含量。
1.3 果皮转录组测序分析
取灵武长枣果实两侧果皮,充分混合后用于总RNA提取。总RNA的提取参考全能型植物RNA提取试剂盒OmniPlant RNA Kit(DNase Ⅰ),2%琼脂糖凝胶电泳检测RNA 质量,利用Nanodrop 2000 分光光度计(Thermo Scientific,Waltham,MA,USA)检测RNA浓度和纯度,获得检测合格样品用于构建文库。第一链cDNA 合成使用TruseqTM RNA sample prep Kitt 试剂盒(Illumina,USA),将合格样品送到上海美吉生物医药科技有限公司在Illumina Novaseq 6000平台测序。测序完成后对每个样本获得的原始数据过滤,去除reads的接头序列、低质量adapter等,获得高质量的质控数据(clean data)。利用HISAT2软件将高质量clean data与冬枣参考基因组比对和定位分析(https://www.ncbi.nlm.nih.gov/genome/?term=Ziziphus%20jujuba%20Mill.var.spinosa)。
1.4 差异表达基因筛选及富集分析
利用表达定量软件RSEM对基因表达水平进行定量分析。此外,对果皮样品间的raw counts 标准化处理,然后利用DEGseq 软件进行组间差异表达分析(P<0.001&|log2FC|≥1)。采用GO和KEGG数据库对筛选的差异基因进行富集分析(padjust<0.05)。
1.5 PCR荧光定量
以Actin基因作为内参基因[20],候选基因的引物序列见表2,引物由美吉生物科技有限公司合成。实时荧光定量PCR(quantitative real-tirne PCR,qRTPCR)采用ABI 7300型荧光定量PCR仪(美国)PCR扩增,每次试验设3 次重复。反应体系参照ChamQ SYBR Color qPCR Master Mix(2X)(南京诺维赞生物科技有限公司),数据采用2-ΔΔCt方法计算[21]。
表2 实时荧光定量PCR 引物序列
Table 2 Nucleotide sequences of Primer in real-time PCR
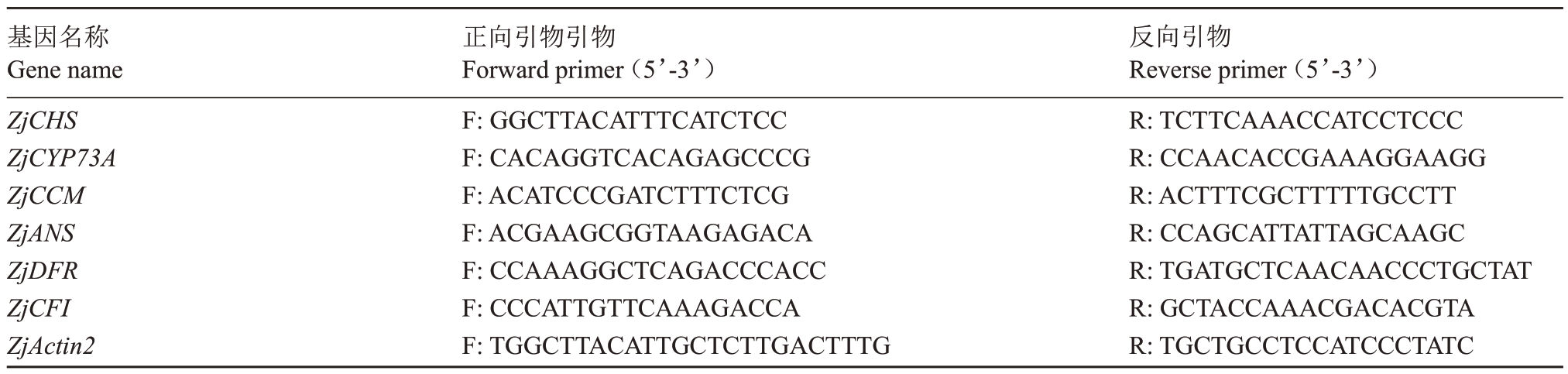
基因名称Gene name ZjCHS ZjCYP73A ZjCCM ZjANS ZjDFR ZjCFI ZjActin2正向引物引物Forward primer(5’-3’)F:GGCTTACATTTCATCTCC F:CACAGGTCACAGAGCCCG F:ACATCCCGATCTTTCTCG F:ACGAAGCGGTAAGAGACA F:CCAAAGGCTCAGACCCACC F:CCCATTGTTCAAAGACCA F:TGGCTTACATTGCTCTTGACTTTG反向引物Reverse primer(5’-3’)R:TCTTCAAACCATCCTCCC R:CCAACACCGAAAGGAAGG R:ACTTTCGCTTTTTGCCTT R:CCAGCATTATTAGCAAGC R:TGATGCTCAACAACCCTGCTAT R:GCTACCAAACGACACGTA R:TGCTGCCTCCATCCCTATC
1.6 果皮代谢组测序分析
利用超高效液相色谱串联傅里叶变换质谱UHPLC-Q Exactive HF-X 系统(赛默飞公司)进行LCMS 分析。色谱条件:色谱柱为ACQUITY UPLC HSS T3(100 mm×2.1 mm i.d. 1.8 µm; Waters,Milford,USA);流动相A为95%水+5%乙腈(含0.1%甲酸),流动相B 为47.5%乙腈+47.5%异丙醇+5%水(含0.1%甲酸),进样量为2 μL,柱温为40 ℃。流动相洗脱程序为:0~3.5 min,100%~75.5% A;3.5~5.0 min,75.5%~35% A;5.0~7.4 min,100% B;7.4~7.6 min,48.5%A;7.6~10.0 min,100%A。质谱条件:样品经电喷雾电离,分别采用正、负离子扫描模式采集质谱信号。将原始数据导入代谢组学处理软件ProgenesisQI(WatersCorporation,Milford,USA)处理,最终得到含保留时间、质荷比和峰强度等信息的数据矩阵。对样本进行主成分分析(PCA)预测模型的可靠性。以p <0.05、VIP>1 作为差异化合物筛选条件确定差异代谢物。对筛选的不同处理下差异代谢物KEGG 通路富集分析,确定关注的色泽相关差异代谢物。
2 结果与分析
2.1 气温升高与干旱对灵武长枣果皮花青苷含量的影响
从果实生长期S1到S3,灵武长枣果皮花青苷含量受气温升高和干旱交互作用的显著影响(表3)。随着果实的逐渐成熟,果皮花青苷含量总体呈上升趋势(图3)。在S1时期,3种干旱水平下,大气升温处理下果皮花青苷含量显著增加且增幅在15.68%~49.19%之间(p <0.05);同一气温环境下,与正常土壤水分相比,干旱处理对花青苷含量的影响均呈现极显著差异(p <0.01),其含量降低且降幅在16.93%~40.21%之间。S2 时期,大气升温处理增加了果皮花青苷含量,增幅在14.17%~52.16%之间;自然大气温度下,与正常土壤水分相比,中度干旱、重度干旱处理下花青苷含量分别极显著降低31.34%、35.81%;升高的大气温度下,2种干旱处理下果皮花青苷含量分别显著降低8.49%、17.00%。S3 时期果皮花青苷含量达到最大值,与自然大气温度相比,升高大气温度环境中正常土壤水分、中度干旱处理的花青苷含量分别显著上升12.93%、13.28%,重度干旱使果皮花青苷含量上升1.73%;自然大气温度环境下,与正常土壤水分相比,2种干旱处理的果皮花青苷含量分别显著降低12.58%、3.15%;升高的大气温度环境中,中度干旱、重度干旱处理的花青苷含量分别较正常土壤水分显著下降0.05%、12.75%。整个生育期T2D2、T2D3处理的花青苷含量较T1D1降低0.97%~10.79%。综上,不同水分水平下,气温升高会增加灵武长枣果皮花青苷含量,干旱的加剧降低了花青苷含量。气温升高与干旱协同处理使花青苷含量呈下降趋势,即气温升高缓解了干旱对灵武长枣果皮花青苷的影响。
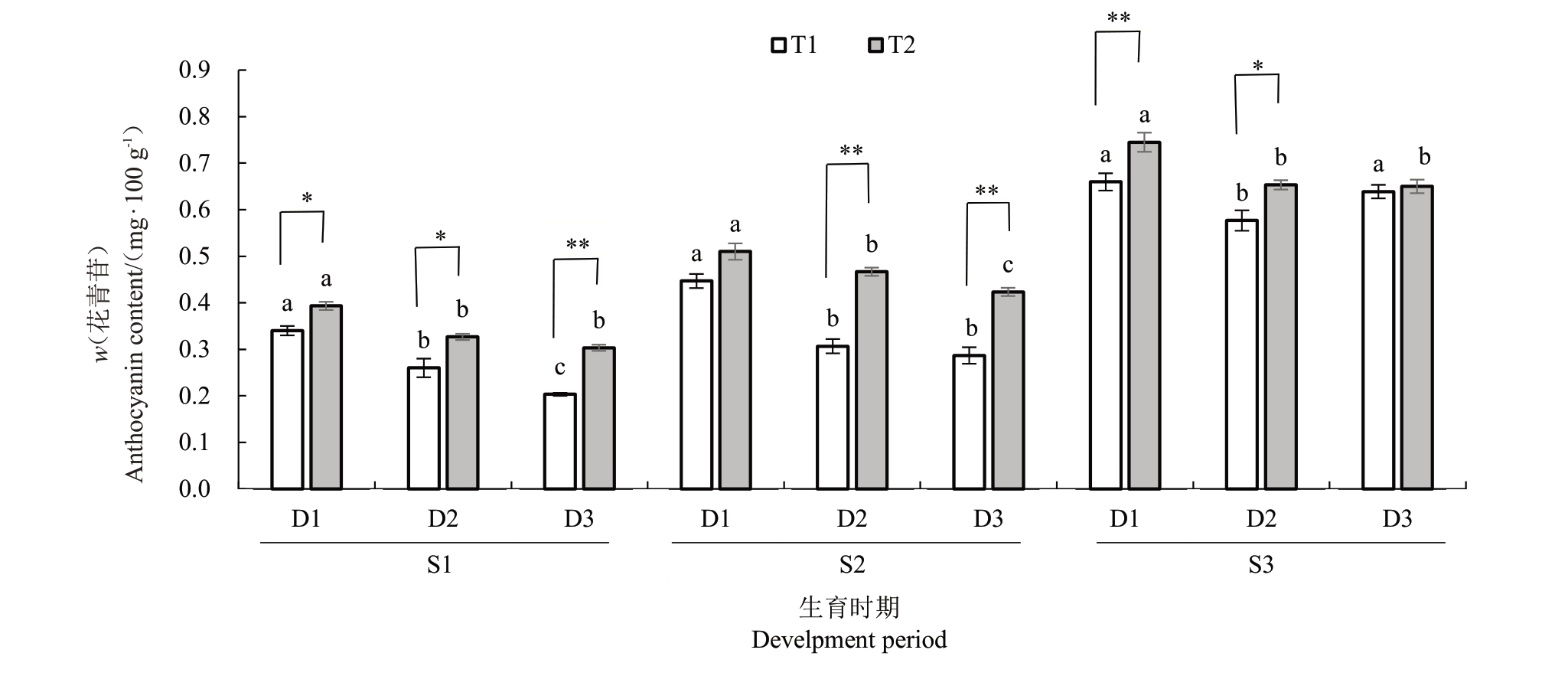
图3 气温升高与干旱对灵武长枣果皮花青苷含量的影响
Fig.3 Effects of elevated temperature and drought on anthocyanin content in fruit peel of Lingwuchangzao
不同小写字母表示同一时期同一温度处理下不同干旱间的Duncan’s 检验差异显著(p <0.05);*表示同一干旱水平下不同气温间的差异在0.05 水平显著;**表示差异在0.01 水平显著。
Different small letters indicated significant difference in Duncan’s test between different droughts under the same temperature treatment in the same period(p <0.05).*indicates that the difference between different temperatures at the same drought level is significant at 0.05 level;**represents a significant difference at 0.01 level.
表3 气温升高与干旱对灵武长枣果皮花青苷含量主效应和交互效应的方差分析
Table 3 P values of main effects and interaction on anthocyanin content in fruit peel of Lingwuchangzao under elevated temperature and drought
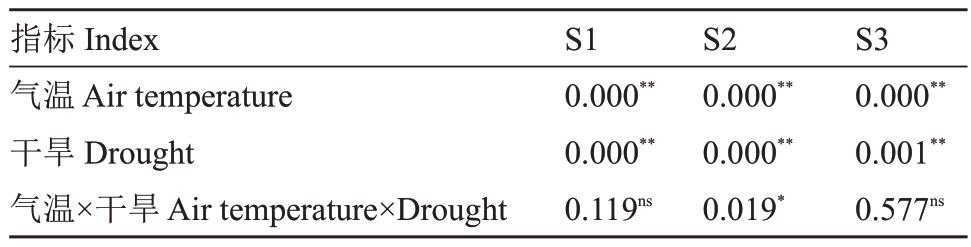
注:*:p <0.05,**:p <0.01,ns:p >0.05.
指标Index气温Air temperature干旱Drought气温×干旱Air temperature×Drought S1 0.000**0.000**0.119ns S2 0.000**0.000**0.019*S3 0.000**0.001**0.577ns
2.2 气温升高与干旱协同处理下灵武长枣果皮转录组测序分析
2.2.1 转录组测序 不同处理灵武长枣果皮样品转录组测序数据的组装分析见表4,共获得129.66 Gb Clean Data。各样本Clean reads 在42 696 576 和53 209 934 之间,Q20 均高于95%,且在96.3%和96.96%之间;Q30在89.75%和91.18%之间。分别将各样品Clean Reads与冬枣参考基因比对,比对效率为85.93%~89.92%,表明测序获得数据可靠,可用于后续分析。
表4 质控数据统计及比对分析
Table 4 Statistics and comparative analysis of quality control data
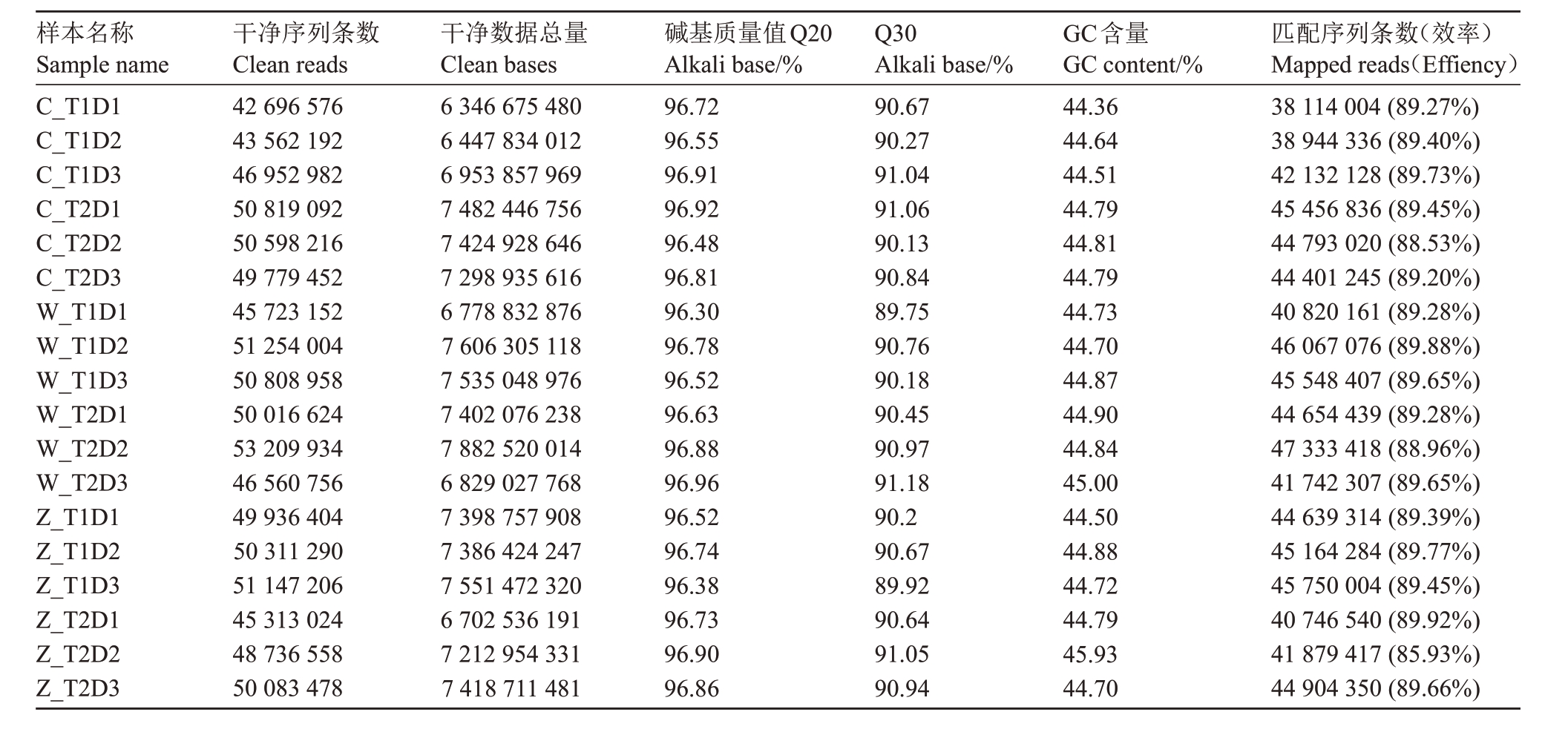
样本名称Sample name C_T1D1 C_T1D2 C_T1D3 C_T2D1 C_T2D2 C_T2D3 W_T1D1 W_T1D2 W_T1D3 W_T2D1 W_T2D2 W_T2D3 Z_T1D1 Z_T1D2 Z_T1D3 Z_T2D1 Z_T2D2 Z_T2D3干净序列条数Clean reads 42 696 576 43 562 192 46 952 982 50 819 092 50 598 216 49 779 452 45 723 152 51 254 004 50 808 958 50 016 624 53 209 934 46 560 756 49 936 404 50 311 290 51 147 206 45 313 024 48 736 558 50 083 478干净数据总量Clean bases 6 346 675 480 6 447 834 012 6 953 857 969 7 482 446 756 7 424 928 646 7 298 935 616 6 778 832 876 7 606 305 118 7 535 048 976 7 402 076 238 7 882 520 014 6 829 027 768 7 398 757 908 7 386 424 247 7 551 472 320 6 702 536 191 7 212 954 331 7 418 711 481碱基质量值Q20 Alkali base/%96.72 96.55 96.91 96.92 96.48 96.81 96.30 96.78 96.52 96.63 96.88 96.96 96.52 96.74 96.38 96.73 96.90 96.86 Q30 Alkali base/%90.67 90.27 91.04 91.06 90.13 90.84 89.75 90.76 90.18 90.45 90.97 91.18 90.2 90.67 89.92 90.64 91.05 90.94 GC含量GC content/%44.36 44.64 44.51 44.79 44.81 44.79 44.73 44.70 44.87 44.90 44.84 45.00 44.50 44.88 44.72 44.79 45.93 44.70匹配序列条数(效率)Mapped reads(Effiency)38 114 004(89.27%)38 944 336(89.40%)42 132 128(89.73%)45 456 836(89.45%)44 793 020(88.53%)44 401 245(89.20%)40 820 161(89.28%)46 067 076(89.88%)45 548 407(89.65%)44 654 439(89.28%)47 333 418(88.96%)41 742 307(89.65%)44 639 314(89.39%)45 164 284(89.77%)45 750 004(89.45%)40 746 540(89.92%)41 879 417(85.93%)44 904 350(89.66%)
2.2.2 基因表达及差异表达基因分析 共检测到表达基因29 610 个,其中已知基因27 290 个,新基因2320 个;表达转录本共60 498 个,其中已知转录本35 475 个,新转录本25 023 个。利用FPKM 值对应的基因表达丰度估算基因表达水平,18个果皮样品基因表达量主要集中在1.0~1.5 之间(图4-A)。以P <0.001、|log2FC|≥1 作为筛选标准进行差异基因分析,在S1 时期、S2 时期、S3 时期分别获得6473、8256、10 250个差异表达基因(图4-B)。分析获得差异基因数量最多的S3 时期,处理T1D1 vs T1D3 共获得1307 个差异基因,其中681 个上调,626 个下调;T1D1 vs T2D1 之间获得1148 个差异基因,其中605 个上调,543 个下调;T1D1 vs T2D3 之间6599 个差异基因,其中4966个上调,1633个下调。对S3时期3 个处理组合差异基因绘制韦恩图,其中共有的表达基因有257 个(图4-C)。对筛选获得的差异基因在4个处理样本中进行聚类分析(图4-D)。

图4 气温升高与干旱处理下灵武长枣果皮差异表达基因
Fig.4 Differential expression genes in fruit peel of Lingwuchangzao under elevated temperature and drought
2.2.3 差异基因KEGG 富集分析 S3 时期灵武长枣果皮转录组差异基因KEGG(Kyoto encyclopedia of genes and genomes)通路富集分析,在处理T1D1vs T1D3及T1D1 vs T2D3组中差异基因富集到与果色相关代谢通路,主要为类黄酮生物合成(Flavonoid biosynthesis)(图5)。其中,在T1D1 vs T1D3组中有13 个上调的差异基因富集到类黄酮合成,在T1D1 vs T2D3 中有3 个下调和10 个上调差异基因富集。此外,在T1D1 vs T1D3 组中,差异基因数量富集较多的代谢通路包括半乳糖代谢(Galactose metabolism)、糖酵解/糖异生(Glycolysis/Gluconeogenesis)、氨基酸糖和核苷酸糖代谢(Amino sugar and nucleotide sugar metabolism)、激素信号转导(Plant hormone signal transduction)、MAPK 信号通路-植物(MAPK signaling pathway-plant),分别富集12、13、14、17 和11 个基因(图5-A)。在T1D1 vs T2D3组中,差异基因富集数量较多的代谢通路主要有核糖体(Ribosome)167 个,内质网中的蛋白质加工(Protein processing in endoplasmic reticulum)74个,嘌呤代谢(Purine metabolism)38 个,光合作用(Photosynthesis)37个(图5-B)。
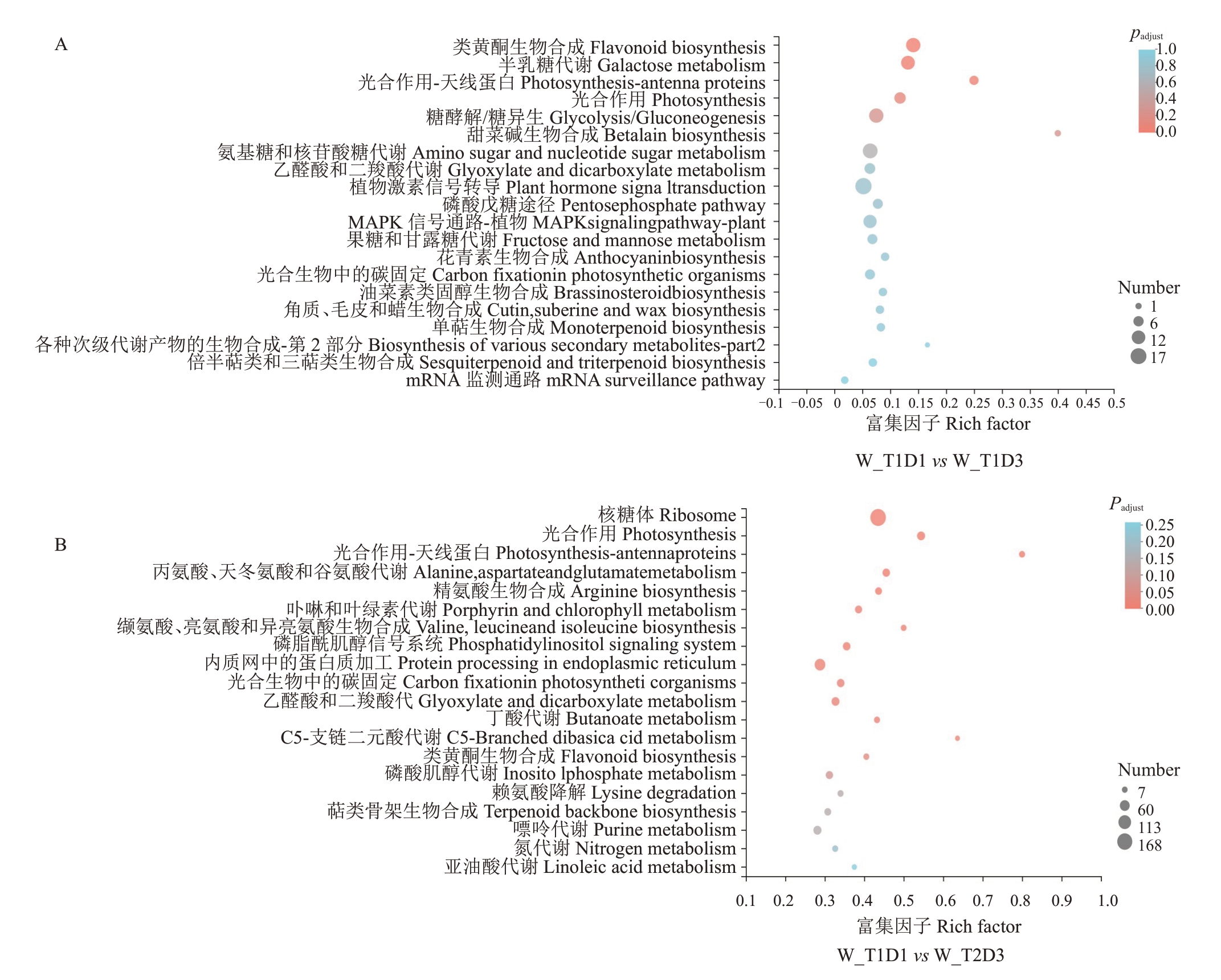
图5 灵武长枣全红脆熟期果皮差异基因KEGG富集分析
Fig.5 KEGG enrichment analysis of different genes in in fruit peel of Lingwuchangzao at full red period
2.3 气温升高与干旱条件对灵武长枣果皮代谢物的影响
2.3.1 差异代谢物分析 代谢组分析发现,所有灵武长枣果皮样本中共检测到745 种代谢物。以VIP >1用作鉴定标准共筛选615种差异代谢物。样品主成分分析(PCA)模型有效(R2X=0.536),主成分PC1 的贡献率为30.50%,主成分PC2 的贡献率为10.40%,6个处理组样品分布在不同的区域,组内重复性好,能够代表气温升高与干旱对灵武长枣果皮具有不同的代谢差异(图6-A)。结合转录组结果,主要分析S3时期关键差异代谢物变化,如图6-B所示,T1D2、T1D3、T2D1、T2D2、T2D3 与T1D1 相比,差异代谢物分别有293、259、279、280和289个,这些代谢物可以被认为是受气温升高与干旱影响的代表性差异代谢物。
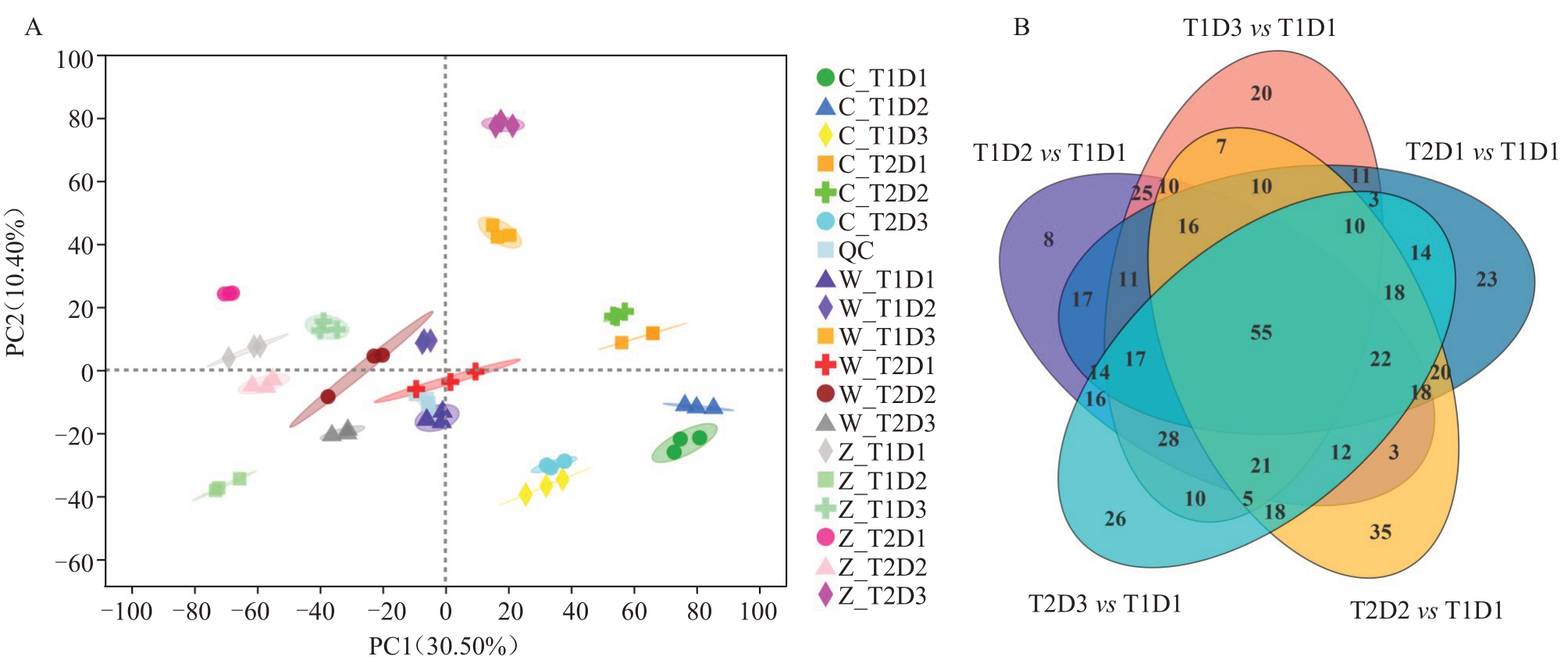
图6 灵武长枣果皮PCA 分析(A)及差异代谢物韦恩图(B)
Fig.6 PCA analysis(A)and differential metabolite Venn diagram(B)in fruit peel of Lingwuchangzao
2.3.2 差异代谢物富集分析 将T1D3 组和T2D3组筛选的差异代谢物进行KEGG 富集分析,富集最多通路是T2D3 处理,其中77 种代谢物主要富集到61 条代谢途径中。前20 条显著富集差异基因代谢通路如图7所示,其中在T2D3处理组中差异代谢物主要富集在氨酰基-tRNA的生物合成、ABC转运、精氨酸生物合成、嘌呤代谢、氰氨基酸代谢、类黄酮生物合成及丙氨酸、天冬氨酸和谷氨酸代谢通路中。分别有5种代谢物富集在氰氨基酸、甘氨酸,丝氨酸和苏氨酸代谢通路中,8 种代谢物富集在氨酰基tRNA的生物合成中,11种富集在嘌呤代谢途径中,7种代谢物富集在类黄酮合成通路中。类黄酮代谢在植物花青苷的合成转化中起关键作用,因此推断类黄酮生物合成途径中的代谢物可能是枣代谢过程中果皮颜色变化的关键代谢产物。该通路中筛选到儿茶素、槲皮素、飞燕草色素、圣草酚、天竺葵色素、表儿茶素、山柰酚7种代谢物均上调表达;飞燕草色素与天竺葵色素、圣草酚有较高的相关性(r=0.942 9)。

图7 差异代谢物KEGG 富集分析
Fig.7 KEGG enrichment analysis of differential metabolites
CP.细胞过程;EIP.环境信息处理;GIP.遗传信息处理;M.新陈代谢。
CP.Cellular process;EIP.Environmental information processing;GIP.Genetic information processing;M.Metabolism.
2.4 气温升高与干旱条件下影响灵武长枣果色的关键代谢通路的转录组和代谢组联合分析
类黄酮合成途径相关基因和代谢产物的变化,是枣果皮着色的基础。花青苷合成是类黄酮代谢的重要分支之一,从苯丙氨酸开始经一系列酶催化最终生成花青苷。对差异基因较多的T2D3 处理中6599 个差异基因进行MAPMAN 富集分析,6278 个差异基因注释到Overview Metabolism 的代谢通路中(图8-A)。其中,淀粉与蔗糖代谢(starch and sucrose)中有11 个基因,10 个上调,1 个下调;次级代谢中(Metabolism)有47 个差异基因,其中28 个上调,18个下调;氨基酸代谢中有15个基因,4个上调,11个下调。进一步对次级代谢通路(Secondary Metabolism)进行基因注释如图8-B 所示,类黄酮代谢(Flavonoids)中注释8个基因,3个上调,5个下调,其中3 个上调基因和3 个下调基因富集到花青苷通路中(Anthocyanins)。

图8 果实全红脆熟期T2D3 处理组差异基因富集MAPMAN 分析
Fig.8 MAPMAN enrichment analysis of differential genes in T2D3 treatment group at full red period
差异基因MAPMAN 富集分析表明,差异表达基因富集到多条基于枣全基因组注释Mapping的代谢通路中,其中类黄酮及花青苷相关关键代谢通路中注释富集有关键基因,进一步证实气温升高和干旱影响了枣果实的多个生理代谢途径,对类黄酮及花青苷关键代谢物合成具有显著影响,进而影响枣果实色泽。
基于MAPMAN 注释分析,结合筛选富集差异基因的关键类黄酮及花青苷代谢通路绘制关键基因调控网络如图9-A 所示。在类黄酮代谢通路中,从苯丙氨酸开始通过一系列酶催化,经其中一条支路生成最终产物天竺葵色素(pelargonidin)、表儿茶素(epicatechin)、飞燕草色素(delphindin)等物质参与花青苷代谢的合成及转化,在这一过程中有8 个关键差异基因在代谢物的合成及转化中显著上调(图7-B),检测到7个代谢物(图9黄亮部分)在不同处理代谢组中含量差异的变化。其中,无色花青素还原酶基因(leucoanthocyanidin reductase,LAR,EC:1.17.1.3)在代谢物无色花青素(leucocyanidin)转化为儿茶素(catechin)过程中,在3 个不同处理及对照组中均表现为显著上调,推测类黄酮代谢通路中该基因参与花青苷的转化调控,但受环境影响较弱。基因查尔酮合成酶基因(chalcone synthase,CHS,EC:2.3.1.74)、二氢黄酮醇-4-还原酶基因(bifunctional dihydroflavonol 4- reductase,DFR,EC:1.1.1.2191.1.1.234)、花青苷合成酶基因(anthocyanidin synthase,ANS,EC:1.14.20.4)及查尔酮-黄酮异构酶基因Ⅰ(chalcone-flavonone isomerase,CFI,EC:5.5.1.6)在处理T1D3 中表达量升高(图9-B),推测这4 个基因在响应重度干旱中起到关键调控作用。此外,代谢产物山柰酚(kaempferol)、槲皮素(quercetin)、飞燕草色素(delphindin)含量在T1D3 处理下与对照T1D1 相比有增加趋势,推测其在ZjCHS、ZjDFR、ZjANS、ZjCFI 基因调控作用下含量积累,有利于花青苷的合成。进一步分析表明,7 个关键代谢物在T2D1 和T2D3 处理下总体有增加趋势,其中T2D1处理比T2D3 处理趋势更为明显;结果表明高温处理有利于花青苷积累,而高温和干旱交互处理阻碍了关键代谢物的积累,即花青苷合成底物减少,进而影响了花青苷合成。气温升高和干旱处理下基因ZjCHS、ZjDFR、ZjANS、ZjCFI 在类黄酮和花青苷合成代谢中具有关键调控作用。

图9 灵武长枣果皮类黄酮合成通路
Fig.9 Flavonoid synthesis pathway in fruit peel of Lingwuchangzao
2.5 差异基因qRT-PCR表达分析
对筛选获得的类黄酮和花青苷代谢中关键调控基因ZjCHS、ZjDFR、ZjANS、ZjCYP73A、ZjCFI 及ZjCCM进行qRT-PCR表达,并与各基因在不同处理下转录本表达比较。如图10 所示,6 个基因在成熟期不同处理下表达量与转录本一致,且均上调表达(r=0.581*),初步表明6个基因是响应气温升高和干旱处理下的关键调控基因。
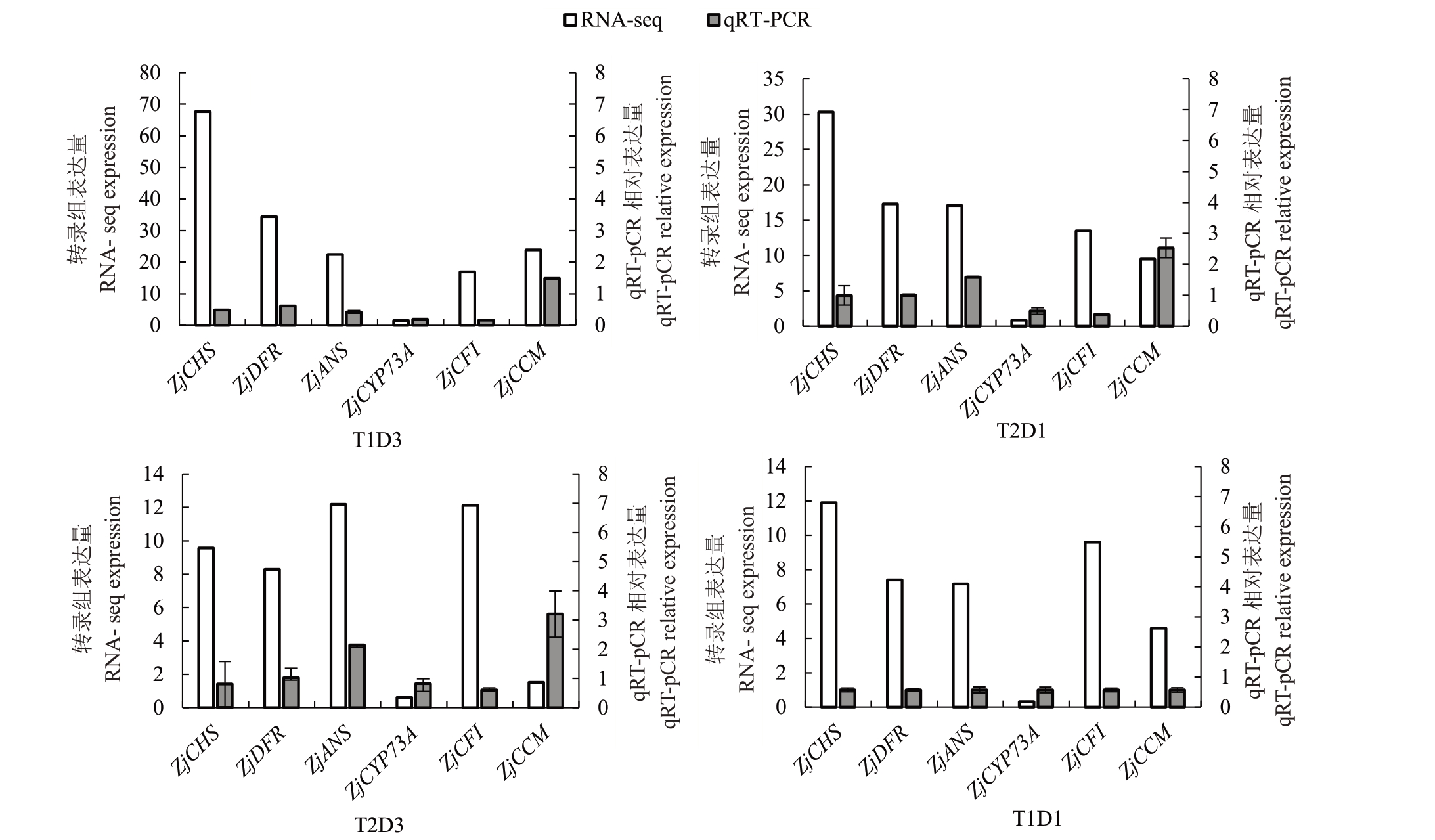
图10 花青苷合成关键基因qRT-PCR 验证
Fig.10 Validation of key genes in anthocyanin synthesis by qRT-PCR
3 讨 论
植物的物候及生长、光合特性、色泽都会不同程度地受到气候变化的影响。花青苷是决定灵武长枣果实颜色的重要因素之一,但易受环境的影响。其中温度对花青苷合成的影响是比较复杂的,高温处理草莓果实导致花青苷含量降低[22]。但也有研究表明高温对花青苷积累有积极作用[23]。前人研究多有报道干旱条件有利于葡萄、樱桃等果实着色[24-25]。笔者课题组前期研究表明,灵武长枣果实中糖、花青苷、类黄酮和类胡萝卜素含量及糖酸比在气温升高(比大气常温环境升高2 ℃左右)处理下显著增加,而干旱处理下(相同温度,田间最大持水量的30%~50%)显著降低。说明气温升高2 ℃左右有利于灵武长枣果实中糖分及花青苷积累,而干旱胁迫降低了花青苷含量[16]。花青素合成积累与糖分及类黄酮含量密切相关,参与类黄酮生物合成的基因表达模式与在花青苷中趋势基本一致[26]。花青苷合成代谢也受相关基因及转录组因子联合调控,例如MBW复合体、基因bHLH和MYB组合表达显著提高了低温处理下花青苷合成,MYB 和启动子ANS 组合表达显著提高苹果果皮中花青苷含量[27-28]。在高温(35 ℃)处理下李子果实中的花青苷调控关键基因PsPAL、PsCHS、PsDFR表达水平比在20 ℃温度下显著降低,基因PsANS和PsUFGT表达水平相似,同时这些基因编码的酶活性在35 ℃下显著增加,花青苷含量也在高温处理初期显著增高[29],这表明高温处理下花青苷含量取决于它的合成和降解平衡。
花青苷作为植物中重要调控物质可以有效保护植物免受各种环境胁迫,同时也可作为一种抗氧化剂清除氧化胁迫下植物中的ROS[30],在高温环境中,植物可通过呼吸和细胞膜流动增加线粒体中ROS,从而促进ROS向其他细胞器扩散,花青苷会大量清除ROS而抵御胁迫[31]。研究也发现在高温和强光照交互处理下梨果皮中花青苷含量明显增加,其主要是减弱高温的损害[32]。本研究中升温处理下灵武长枣果皮花青苷含量明显增加,而干旱处理下花青苷含量呈下降趋势,与前人研究存在相似的调控机制;但需在后期的研究中进一步探究高温处理下活性氧ROS、H2O2、SOD和APX等含量,及其与花青苷分解合成代谢相关酶、基因及非酶催化的降解速率等,进而明确高温处理对花青苷含量的积累机制。
查尔酮合成酶CHS 是花青苷等类黄酮生物合成途径中的第一个关键酶,已在多种植物中分离,如海棠CHS 基因在烟草中过表达可导致转基因烟草花瓣的花冠颜色更深[33]。类黄酮合成通路中二氢黄酮醇4-还原酶DFR是催化生成无色花青素的酶,该步骤是花青苷合成过程中的另一个关键步骤。2个不同颜色的海棠品种中,过表达DFR基因是海棠叶片和果皮呈红色的主要原因[34]。DFR等基因在枣果皮全红时期表达量最高,说明枣在发育过程中与花青苷合成相关的通路活跃[35]。本研究中,ZjDFR 基因在各处理中有较高表达,其表达模式与升温与干旱处理下灵武长枣果皮花青苷含量一致。花青素合成酶ANS催化无色花青素氧化形成有色花青素,是花青素合成后期的关键酶,其在红色品种白里肥桃和白色品种秋雪桃中表达模式上存在较大差别[36]。龙胆花瓣中ANS 基因突变导致花青苷无法积累,花瓣呈白色[37]。本研究中ZjANS基因受气温升高与干旱处理影响,与之密切相关的代谢物质天竺葵色素与飞燕草色素为主要的花青苷物质,基因的表达模式与灵武长枣果皮花青苷含量变化趋势一致,初步证明这些基因是枣果皮花青苷合成的关键基因,但需要进一步进行功能验证。
转录组与代谢组技术已成功运用到很多研究中,如筛选低温贮藏期间哈密瓜激素代谢中的关键基因与代谢物[38]、桃树型调控关键基因[39]、香菜营养药用相关的代谢产物和基因[40]等。笔者在本研究中经转录组和代谢组技术联合分析,可筛选到色泽相关的关键基因和代谢物,还可通过KEGG 富集将代谢物和基因与代谢通路联系起来,系统地揭示果皮花青苷合成的代谢机制。杜仲叶色的双组学分析中,F3’H、F3’5’H 及CHI 基因是华仲12 号与华仲11号杜仲叶色差异的关键基因,关键差异物质有二氢槲皮素、矢车菊素、矢车菊素3,5-二葡萄糖苷[41]。对3 个不同甜瓜果皮样品的转录组和代谢组分析,ANS 和UFGT 等2 个下调基因参与了花青苷的积累[42]。本研究将筛选到的关键基因与代谢物注释到通路中,在T1D3处理下筛选到基因ZjCHS、ZjDFR、ZjANS、ZjCFI在代谢通路中显著富集。代谢产物山柰酚、槲皮素、飞燕草色素含量在T1D3处理有增加趋势,推测其在基因调控作用下积累,进而参与了花青苷的合成与转化。7 个关键代谢物在T2D1 和T2D3 处理下总体有增加趋势,其中T2D1 处理中含量更高,表明气温升高有利于花青苷积累,而升温和干旱协同处理阻碍了关键代谢物的积累。类黄酮和花青苷合成受基因ZjCHS、ZjDFR、ZjANS、ZjCFI 调控,因此说明它们是花青苷合成的关键基因,4个基因对花青苷合成的调控机制及其功能与互作关系还需进一步研究。
4 结 论
气温升高有利于灵武长枣果皮花青苷积累,而干旱处理下花青苷含量降低,气温升高与干旱协同也降低了花青苷含量。转录组分析共获得129.66 Gb Clean Data,S3时期T2D3处理下获得的差异基因数量最多6599个。KEGG富集分析表明,类黄酮代谢为花青苷合成代谢关键通路,MAPMAN 注释分析有6278个差异基因注释到总的代谢通路中,其中次级代谢通路类黄酮代谢中注释8个基因。代谢组和转录组关联分析表明,ZjCHS、ZjDFR、ZjANS、和ZjCFI 是响应气温升高和干旱处理的关键调控基因,其在重度干旱T1D3处理下促进了山柰酚、槲皮素、飞燕草色素积累;在气温升高T2D1处理下促进了7个关键代谢物的积累,而在交互处理T2D3下降低了花青苷含量。综上表明,ZjCHS、ZjDFR、ZjANS和ZjCFI是灵武长枣响应气温升高与干旱处理下与花青苷合成相关的关键基因。
[1] 赵义海,柴琦.全球气候变化与草地生态系统[J].草业科学,2000,17(5):49-54.ZHAO Yihai,CHAI Qi. Global climate change and its impacts on rangeland ecosystem[J]. Pratacultural Science,2000,17(5):49-54.
[2] KRONER Y,WAY D A. Carbon fluxes acclimate more strongly to elevated growth temperatures than to elevated CO2 concentrations in a northern conifer[J]. Global Change Biology,2016,22(8):2913-2928.
[3] 张强,张存杰,白虎志,李林,孙兰东,刘德祥,王劲松,赵红岩.西北地区气候变化新动态及对干旱环境的影响:总体暖干化,局部出现暖湿迹象[J].干旱气象,2010,28(1):1-7.ZHANG Qiang,ZHANG Cunjie,BAI Huzhi,LI Lin,SUN Landong,LIU Dexiang,WANG Jinsong,ZHAO Hongyan. New development of climate change in northwest China and its impact on arid environment[J].Journal of Arid Meteorology,2010,28(1):1-7.
[4] BONADA M,JEFFRRY D W,PETRIE P R,MORAN M A,SADRAS V O.Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines[J].Australian Journal of Grape and Wine Research,2015,21(2):240-253.
[5] 孙欧文,杨倩倩,章毅,蔡建国.四个绣球品种对高温干旱复合胁迫的生理响应机制[J].植物生理学报,2019,55(10):1531-1544.SUN Ouwen,YANG Qianqian,ZHANG Yi,CAI Jianguo.Physiological response mechanism of four kinds of Hydrangea under high temperature and drought stress[J]. Plant Physiology Journal,2019,55(10):1531-1544.
[6] 张琼.枣着色过程中果皮结构及色素积累相关组分研究[D].保定:河北农业大学,2020.ZHANG Qiong.Analysis of peel structure and components related to pigment accumulation during jujube coloring[D].Baoding:Hebei Agricultural University,2020.
[7] 翟龙飞.枣中黄酮类化合物及枣皮红色素的初步研究[D].保定:河北农业大学,2014.ZHAI Longfei. Study on flavonoid contents and pigments of Chinese Jujube[D]. Baoding:Hebei Agricultural University,2014.
[8] 常世敏,生吉萍,申琳.冬枣果皮红色素提取及其性质的分析研究[J].保鲜与加工,2004,4(5):18-20.CHANG Shimin,SHENG Jiping,SHEN Lin. Extraction and analysis of pigments from Dongzao jujube[J]. Storage and Process,2004,4(5):18-20.
[9] KYOUNGWON C,KWANG-SOO C,HWANG-BAE S,JIN H I,SU Y H,HYERIM L,YOUNG-MI K,MYUNG H N. Network,analysis of the metabolome and transcriptome reveals novel regulation of potato pigmentation[J]. Journal of Experimental Botany,2016,67(5):1519-1533.
[10] HONDA C,KOTODA N,WADA M,KONDO S,KOBAYASHI S,SOEJIMA J,ZHANG Z L,TSUDA T,MORIGUCHI T.Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin[J].Plant Physiology and Biochemistry,2002,40(11):955-962.
[11] 张计育,莫正海,宣继萍,贾晓东,刘永芝,郭忠仁.猕猴桃果肉颜色相关色素代谢研究进展[J]. 中国农学通报,2013,29(13):77-85.ZHANG Jiyu,MO Zhenghai,XUAN Jiping,JIA Xiaodong,LIU Yongzhi,GUO Zhongren.Advance of research on flesh color related pigment metabolism in kiwifruit[J]. Chinese Agricultural Science Bulletin,2013,29(13):77-85.
[12] 俞波. 红色砂梨花青苷生物合成相关基因分离及表达研究[D].杭州:浙江大学,2012.YU Bo. Studies on isolation of anthocyanin biosynthesis related genes and their expression analysis in red Chinese sand pears[D].Hangzhou:Zhejiang University,2012.
[13] 马亚平,白琳云,徐伟荣,曹兵,赵思明,宋丽华,刘嘉佳.灵武长枣嫁接和根蘖繁殖植株果实转录组差异分析[J].分子植物育种,2021,19(16):5297-5306.MA Yaping,BAI Linyun,XU Weirong,CAO Bing,ZHAO Siming,SONG Lihua,LIU Jiajia. Transcriptome differences analysis of fruit of grafting and root tiller propagation of‘Lingwuchangzao’jujube[J]. Molecular Plant Breeding,2021,19(16):5297-5306.
[14] 陈丽华,杨喜盟,贾昊,宋丽华.气温升高与干旱对灵武长枣果实糖积累、蔗糖代谢关键酶及相关基因表达的影响[J].核农学报,2020,34(9):2112-2123.CHEN Lihua,YANG Ximeng,JIA Hao,SONG Lihua. Effects of elevated temperature and drought on sugar accumulation,key sucrose enzymes metabolism and related gene expression in fruit of jujube cultivar Lingwuchangzao[J]. Journal of Nuclear Agricultural Sciences,2020,34(9):2112-2123.
[15] 秦芳.气温升高与不同土壤水分条件对灵武长枣光合产物分配与果实品质的影响[D].银川:宁夏大学,2017.QIN Fang. Effect of elevated air temperature and different soil drought stress on photosynthate distribution and fruit quality of Lingwu long jujube[D].Yinchuan:Ningxia University,2017.
[16] JIANG W Q,LI N,ZHANG D P,MEINHARDT L,CAO B,LI Y J,SONG L H.Elevated temperature and drought stress significantly affect fruit quality and activity of anthocyanin-related enzymes in jujube (Ziziphus jujuba Mill. cv. Lingwuchangzao)[J].PLoS One,2020,15(11):e0241491.
[17] JIANG W Q,CHEN L H,HAN Y R,CAO B,SONG L H. Effects of elevated temperature and drought stress on fruit coloration in the jujube variety‘Lingwuchangzao’(Ziziphus jujuba cv. Lingwuchangzao)[J]. Scientia Horticulturae,2020,274(5):109-113.
[18] 周丹蓉,方智振,廖汝玉,叶新福,姜翠翠,潘少霖.李果皮花色素苷、类黄酮和类胡萝卜素含量及抗氧化性研究[J].营养学报,2013,35(6):571-576.ZHOU Danrong,FANG Zhizhen,LIAO Ruyu,YE Xinfu,JIANG Cuicui,PAN Shaolin. Contents of anthocyanin,flavonoids and carotenoids and antioxidant capacity of plum peels[J].Nutrimenta Sinica,2013,35(6):571-576.
[19] 刘奕琳,王振宇.蓝靛果中花色苷含量的测定及其体外抗氧化性[J].中国林副特产,2011(5):14-17.LIU Yilin,WANG Zhenyu. Determination the anthocyanin content in Lonicera edulis by pH-differential spectrophotometry and the antioxidance[J].Forest By-product and Speciality in China,2011(5):14-17.
[20] 卜娇迪.枣实时荧光定量PCR 中内参基因的筛选及应用[D].保定:河北农业大学,2016.BU Jiaodi. Screening and application of internal reference genes in jujube real-time fluorescent quantitative PCR[D]. Baoding:Hebei Agricultural University,2016.
[21] LIVARK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2- Δ Δ CT method[J].Methods,2001,25(4):402-408.
[22] CIVELLO P M,MARTÍNEZ G A,CHANES A R,AÑÓN M C.Heat treatments delay ripening and postharvest decay of strawberry fruit[J]. Journal of Agricultural and Food Chemistry,1997,45(12):4589-4594.
[23] 姜文倩,连亚妮,贾昊,宋丽华,曹兵.模拟气温升高对枣果实主要色素含量的影响[J]. 西北林学院学报,2019,34(6):115-119.JIANG Wenqian,LIAN Yanni,JIA Hao,SONG Lihua,CAO Bing. Effect of simulated temperature rising on the main pigment content of jujube fruit[J]. Journal of Northwest Forestry University,2019,34(6):115-119.
[24] 温鹏飞,袁晨茜,杨刘燕,杨运良,邢延富,牛兴艳,冀铮春.轻度土壤干旱对赤霞珠果实品质的影响[J]. 山西农业科学,2013,41(3):238-242.WEN Pengfei,YUAN Chenxi,YANG Liuyan,YANG Yunliang,XING Yanfu,NIU Xingyan,JI Zhengchun. Effect of light soil drought on the qualities of grape berry[J].Journal of Shanxi Agricultural Sciences,2013,41(3):238-242.
[25] 阳姝婷.干旱胁迫对甜樱桃生理及果实品质的影响[D].成都:四川农业大学,2016.YANG Shuting. Effect of drought stress on physiology and fruit quality of sweet cherry[D]. Chengdu:Sichuan Agricultural University,2016.
[26] SONG T T,LI K T,WU T,WANG Y,ZHANG X Z,XU X F,YAO Y C,HAN Z H. Identification of new regulators through transcriptome analysis that regulate anthocyanin biosynthesis in apple leaves at low temperatures[J]. PLoS One,2019,14(1):e0210672.
[27] CRIFO T,PUGLISI I,PETRONE G,RECUPERO G R,LO PIERO A R. Expression analysis in response to low temperature stress in blood oranges:Implication of the flavonoid biosynthetic pathway[J].Gene,2011,476(1/2):1-9.
[28] BAN Y,HONDA C,HATSUYAMA Y,IGARASHI M,BESSHO H,MORIGUCHI T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin[J]. Plant and Cell Physiology,2007,48(7):958-970.
[29] NIU J P,ZHANG G J,ZHANG W T,GOLTSEV V,SUN S,WANG J Z,LI P M,MA F W. Anthocyanin concentration depends on the counterbalance between its synthesis and degradation in plum fruit at high temperature[J]. Scientific Reports,2017,7(1):7684-7690.
[30] GOULD K S,MCKELVIE J,MARKHAM K R. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury[J].Plant,Cell and Environment,2002,25:1261-1269.
[31] MEDINA-SILVA R,BARROS M P,GALHARDO R S,NETTO L E S,COLEPICOLO P,MENCK C F M. Heat stress promotes mitochondrial instability and oxidative responses in yeast deficient in thiazole biosynthesis[J]. Research in Microbiology,2006,157(3):275-281.
[32] LI P M,CHENG L L. The elevated anthocyanin level in the shaded peel of‘Anjou’pear enhances its tolerance to high temperature under high light[J]. Plant Science,2009,177(5):418-426.
[33] TAI D Q,TIAN J,ZHANG J,SONG T T,YAO Y C.A Malus crabapple chalcone synthase gene,McCHS,regulates red petal color and flavonoid biosynthesis[J]. PLoS One,2014,9(10):e110570.
[34] TIAN J,CHEN M C,ZHANG J,LI K T,SONG T T,ZHNAG X,YAO Y C. Characteristics of dihydroflavonol 4-reductase gene promoters from different leaf colored Malus crabapple cultivars[J].Horticulture Research,2017,4(1):170-177.
[35] 李云飞.冬枣果皮红变分子机理的研究[D].天津:天津大学,2017.LI Yunfei. The molecular mechanism research on the red pigment development of Zizyphus jujuba Mill cv. Dongzao[D].Tianjin:Tianjin University,2017.
[36] 何平,李林光,王海波,常源升.基于转录组分析不同着色桃果皮花青苷表达模式与转录因子[J].植物生理学报,2019,55(3):310-318.HE Ping,LI Linguang,WANG Haibo,CHANG Yuansheng.Comparative analysis of anthocyanin expression patterns and transcription factors in different colored peach skins based on transcriptome data[J]. Plant Physiology Journal,2019,55(3):310-318.
[37] NAKATSUKA T,NISHIHARA M,MISHIBA K,YAMAMURA S. Two different mutations are involved in the formation of white-flowered gentian plants[J]. Plant Science,2005,169(5):949-958.
[38] 周发科,唐凤仙,张琴,宋文,宁明,蔡文超,单春会.哈密瓜低温贮藏期间激素代谢的代谢组和转录组分析[J/OL].食品科学.[2021-07-15].http://kns.cnki.net/kcms/detail/11.2206.TS.2021 0406.1053.002.html.ZHOU Fake,TANG Fengxian,ZHANG Qin,SONG Wen,NING Ming,CAI Wenchao,SHAN Chunhui. Metabolomic and transcriptome analysis of phytohormone metabolism in Hami Melon during low temperature storage[J/OL]. Food Science.[2021- 07- 15].http://kns.cnki.net/kcms/detail/11.2206.TS.20210406.1053.002.html.
[39] 谭彬,程钧,郑先波,王志强,冯建灿.桃树型及其调控关键基因研究进展[J].果树学报,2020,37(4):599-605.TAN Bin,CHENG Jun,ZHENG Xianbo,WANG Zhiqiang,FENG Jiancan. Progress on tree architecture and key genes of its regulation in peach[J]. Journal of Fruit Science,2020,37(4):599-605.
[40] WU T,FENG S Y,YANG Q H,BHETARIYA P J,GONG K,CUI C L,SONG J,PING X R,PEI Q Y,YU T,SONG X M.Integration of the metabolome and transcriptome reveals the metabolites and genes related to nutritional and medicinal value in Coriandrum sativum[J]. Journal of Integrative Agriculture,2021,20(7):1807-1818.
[41] 杨赟.‘华仲12 号’杜仲叶片呈红色的代谢组和转录组分析[D].郑州:河南农业大学,2019.YANG Yun. Metabolomes and transcriptomes analysis on leaves in red color of Eucommia ulmoides‘Huazhong No.12’[D].Zhengzhou:Henan Agricultural University,2019.
[42] ZHANG A A,ZHENG J,CHEN X M,SHI X Y,WANG H S,FU Q S. Comprehensive analysis of transcriptome and metabolom reveals the flavonoid metabolic pathway is associated with fruit peel coloration of melon[J].Molecules,2021,26(9):2830.