葡萄是在世界上广受欢迎的水果,因其品种繁多,不仅可以鲜食、制干,还可以酿酒、酿醋、酿汁,世界各地均有大面积栽培[1]。截至2019 年,我国葡萄总产量为1 419.5万t,居世界产量首位[2]。但我国葡萄主要栽培区土壤大多为盐碱土[3],对葡萄生长发育及品质产生巨大影响,制约着葡萄产业的发展[4]。大量研究也已证明,在葡萄耐盐碱栽培生产上,一般使用优良的抗性砧木来提高植株的耐盐碱性,增产增质[5-6],其通过砧穗相互作用改变植株内源物质,影响葡萄植株内部生理生化的变化、调节生长,从而提高葡萄抗性[7],葡萄栽培上抗性砧木的应用会成为葡萄耐盐碱高效生产的必然趋势[8-10]。
光合作用是植物能量的重要来源,是作物高产高质的基础,盐碱胁迫下,植物叶片的PSⅡ反应中心受到损伤,植株的光合作用受到抑制[11-12]。叶绿素荧光与光合作用之间有着密切联系,可以快速检测植株对环境变化的响应。近年来,随着对叶绿素荧光技术研究的深入,人们认识到叶绿素荧光诱导动力学曲线能提供大量的生理信息,鉴定植物对环境胁迫的敏感性,为植物的耐盐碱栽培提供一定的理论依据[13-15]。
目前,我国葡萄砧木的耐盐机制研究中大多集中于单盐胁迫方面,而在抗盐碱混合胁迫方面鲜见报道。笔者在本研究中选取常用的几种砧木进行盐碱混合胁迫,探讨混合盐碱胁迫对不同葡萄砧木光合及叶绿素荧光特性的影响,揭示相关机制,并利用主成分分析来评价不同砧木的耐盐碱性,从中筛选出耐盐碱性较好的品种,以期为葡萄砧木的抗性研究以及耐盐碱栽培提供参考依据。
1 材料和方法
1.1 材料
于2020 年11 月在石河子大学葡萄园内采集3309M、5BB、1103P、420A、5C、SO4等6种抗性砧木枝条,置于聚乙烯塑料袋中、封口,进行沙藏处理。2021年1月取出沙藏枝条,于大棚内催根催芽,20 d后扦插至营养钵中,大棚内常规管理培养幼苗。待幼苗长至8~11枚叶片时移栽于桶中进行水培,每桶2 株苗,每桶加改良式Hoagland 营养液6 L。缓苗10 d 后开始处理,CK:改良式Hoagland 营养液;盐碱胁迫处理:改良式Hoagland 营养液+50 mmol·L-1(NaCl + NaHCO3)(按物质的质量1∶1 混合,pH=8.3)。试验期间营养液用充气泵连续通气,每天补充水培盆中散失的水分至6 L 刻度线,每5 d 更换1次营养液。
1.2 试验指标测定
1.2.1 新梢生长量测定 分别于处理后0、15 d用卷尺测定植株地上部分长度,3 次重复,取平均值,精确至0.1 cm。
1.2.2 叶绿素含量测定 于处理后15 d采集新鲜叶片,擦净后去掉叶脉剪碎,称取0.2 g于试管中,加入10 mL 95%乙醇,避光浸提至叶片变白。用分光光度计分别测定在波长470、649、665 nm下吸光度值,并应用Arnon[16]的公式计算叶绿素含量。
1.2.3 光合指标的测定 于处理后15 d使用配置红蓝光源(光量子通量密度为1000 μmol·m-2·s-1)的LI-6400XT 便携式光合仪(美国LI-COR 公司生产)测量叶片各光合指标(上午9:00—11:00)。
1.2.4 叶绿素荧光诱导动力学曲线的测定与JIPtest 参数计算 于处理后15 d 使用植物效率仪(MPEA,Hansatech,UK)测定叶片的快速叶绿素荧光诱导动力学曲线(OJIP 曲线)。测定前暗处理30 min,共记录2 s(红光诱导)。OJIP 曲线上的O、J、I、P 相对应的时间分别为0.000 02、0.002、0.03、0.4~1 s。JIP-test 参数的计算参考李红杰等[17]、胡文海等[18]的方法,相关参数及其含义见表1。
表1 JIP 测定分析所用的参数和公式
Table 1 Parameters and formulae used in JIP-test analysis

参数和公式Parameters and formulas Fo Fj Fi Fm Fv=Ft-Fo Fv/Fm=(Fm-Fo)/Fm Fv/Fo=(Fm-Fo)/Fo Vj=(Fj-Fo)/(Fm-Fo)Vi=(Fi-Fo)/(Fm-Fo)Mo=4(F300μS-Fo)/(Fm-Fo)ψo=ETo/TRo=(1-Vj)φEo=ETo/ABS=[1-(Fo/Fm)]×ψo φPo=TRo/ABS=1-(Fo/Fm)ABS/CSm≈Fm TRo/CSm=φPo(ABS/CSm)ETo/CSm=φEo(ABS/CSm)DIo/CSm=(ABS/CSm)-(TRo/CSm)RC/CSm=φPo(Vj/Mo)(ABS/CSm)ABS/RC=Mo(1/Vj)(1/φPo)TRo/RC=Mo(1/Vj)ETo/RC=Mo(1/Vj)ψo PIabs=(RC/ABS)[φPo/(1-φPo][ψo(1-ψo)]参数含义Explanation of the parameters暗适应后的最小荧光强度Minimum fluorescence intensity after dark adaptation J点处(2 ms)的荧光强度Fluorescence intensity at J point(2 ms)I点处(30 ms)的荧光强度Fluorescence intensity at point I(30 ms)暗适应后的最大荧光强度Maximum fluorescence intensity after dark adaptation在t时可变荧光强度Variable fluorescence intensity at t最大光化学效率Maximum photochemical efficiency of PSⅡ潜在光化学效率Potential photochemical efficiency of PSⅡJ点时的相对可变荧光强度Fluorescence intensity at the J step I点时的相对可变荧光强度Fluorescence intensity at the I step 0JIP荧光诱导曲线的初始斜率Initial slope of the 0JIP fluorescence induction curve反应中心捕获的激子中用来推动电子传递到电子传递链中超过QA的其他电子受体的激子占用来推动QA还原激子的比率The ratio of excitons captured in the reaction center used to push electrons to other electron receptors in the electron transport chain that exceed QA's occupation to push the QA reduced excitons用于电子传递的量子产额Quantum yield for electron transport初级光化学反应最大量子产额Primary photochemical reaction of the largest quantum yield单位面积吸收的光能Light energy absorbed per unit area单位面积捕获的光能Light energy captured per unit area单位面积电子传递的量子产额Quantum yield of electron transport per unit area单位面积的热耗散Heat dissipation per unit area单位面积内反应中心的数量The number of reaction centers per unit area单位RC吸收的光能Light energy absorbed per unit RC单位RC捕获的光能Light energy captured per unit RC单位RC电子传递的量子产额Quantum yield of electron transport per unit RC以吸收光能为基础的性能指数Performance index based on light energy absorption
1.3 数据分析
采用Microsoft Excel 和SPSS 20.0 进行数据统计、分析处理及主成分分析。
2 结果与分析
2.1 盐碱胁迫对不同葡萄砧木生长发育的影响
由图1 可知,盐碱胁迫处理后,6 个葡萄砧木植株新梢生长量相较于对照大幅度降低,其中新梢生长量最高的砧木为1103P,5BB 次之,品种420A 的新梢生长量显著低于其他砧木。与对照相比,胁迫15 d 后1103P、3309M、420A、5BB、5C、SO4 的新梢生长量降幅分别为75.68%、83.81%、39.74%、75%、78.87%、52.09%。
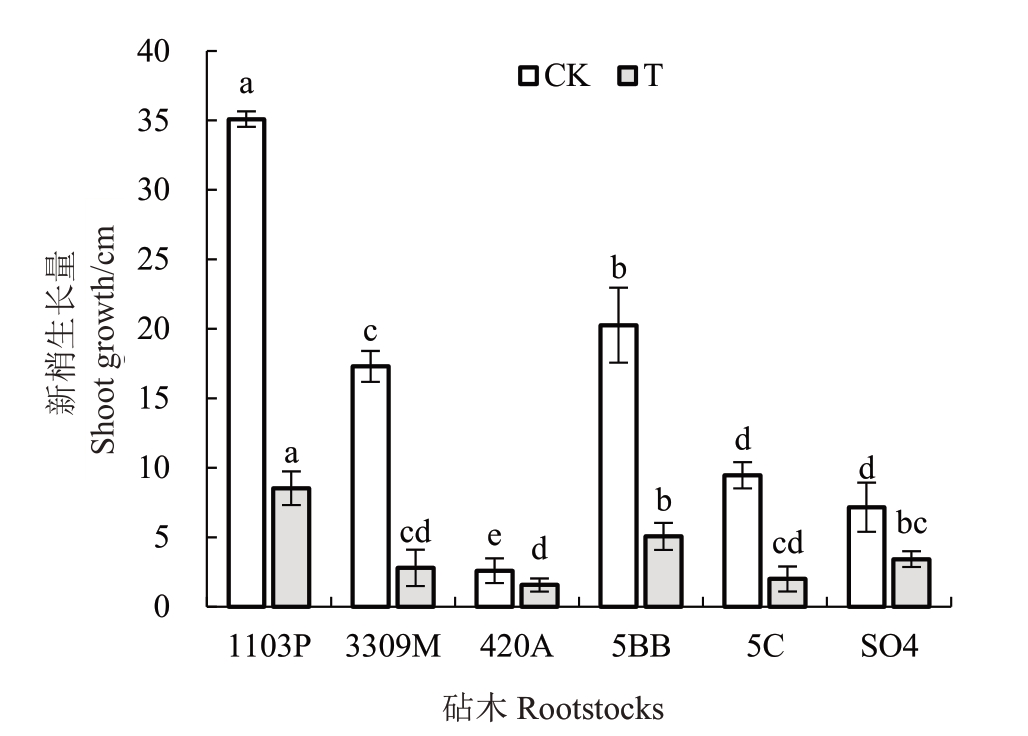
图1 不同砧木新梢生长量
Fig.1 Shoot growth of different rootstocks
不同小写字母表示相同处理不同砧木间差异显著(p <0.05)。下同。
Different lowercase letters indicated the same treatment days, and there were significant differences among different rootstocks(p <0.05).The same below.
2.2 盐碱胁迫对不同砧木叶片叶绿素含量的影响
盐碱胁迫影响植物叶片光合色素的合成。由图2 可以看出,不同砧木叶片的叶绿素a(Chl a)含量、叶绿素b(Chl b)含量、总叶绿素(Chl a+b)含量、叶绿素a/b(Chl a/b)在处理后均呈下降趋势。由图2-A~C可知,盐碱胁迫15 d后,Chl a、Chl b、Chl a+b含量最高的均为5BB,最低的均为3309M;与对照相比,Chl a、Chl b、Chl a+b含量下降最少的是420A,降幅分别为8.72%、7.03%、8.39%;5BB次之,降幅分别为17.64%、7.06%、15.41%;降幅最多的均为3309M,降幅为35.58%、31.26%与34.65%。如图2-D 所示,胁迫15 d 后,不同砧木的Chl a/b 值有显著差异,由高到低分别为420A、SO4、5BB、3309M、1103P、5C,其中下降最少的为420A,降幅为2.08%,下降最多的为5C,降幅为26.31%。
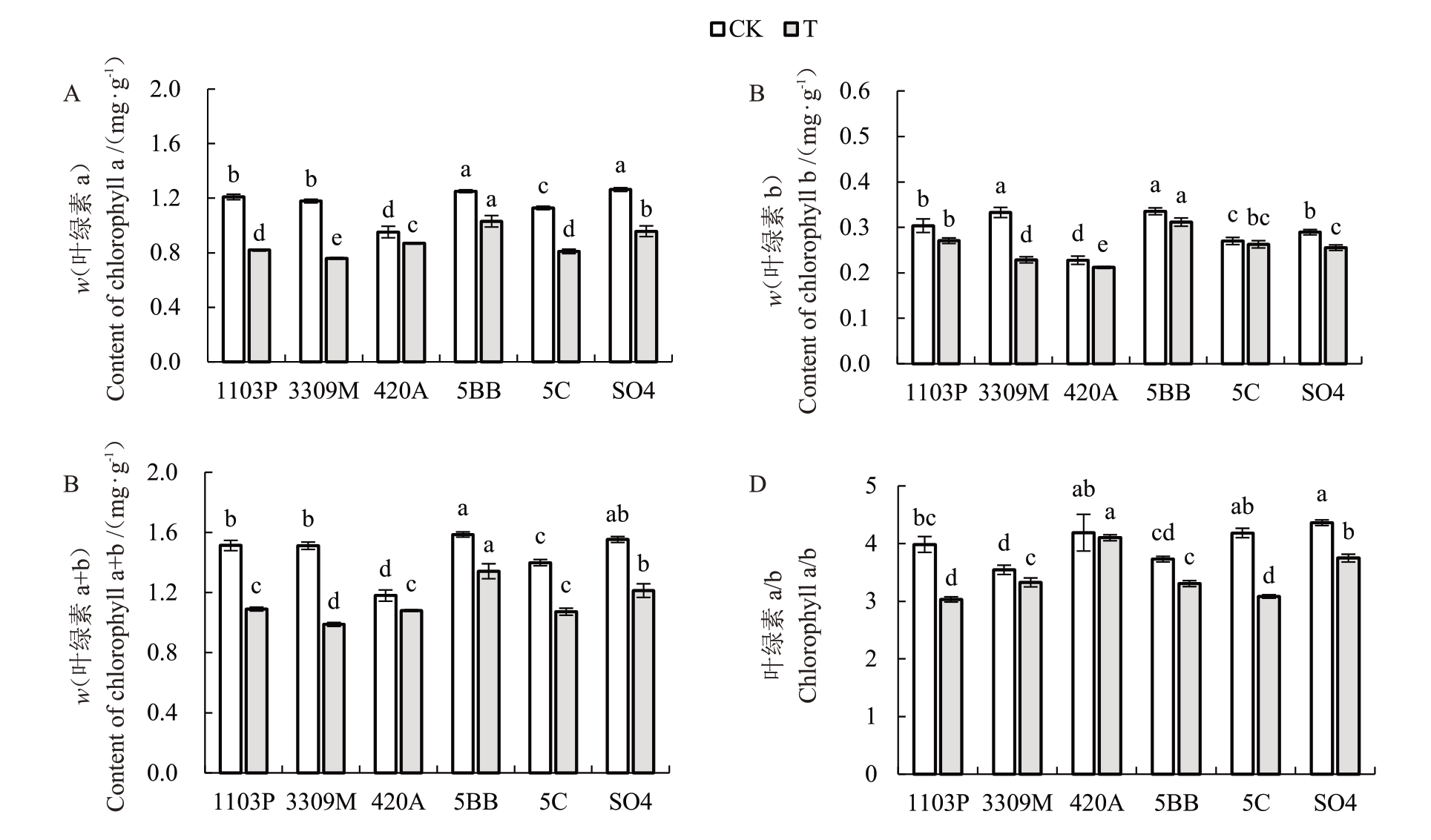
图2 盐碱胁迫对不同砧木叶绿素含量的影响
Fig.2 Effects of saline-alkali stress on Chlorophyll content of different rootstocks
2.3 盐碱胁迫对不同砧木叶片光合参数的影响
盐碱胁迫对不同葡萄砧木叶片光合参数均有所影响。经盐碱胁迫后,各砧木叶片的净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)、蒸腾速率(Tr)均下降。由图3-A 可知,与对照相比,Pn下降最少的为5BB,降幅为36.78%,下降最多的为3309M,降幅为79.32%;在6 个葡萄砧木中,Pn 最高的为5BB,最低的是3309M。由图3-B 可知,盐碱胁迫对各砧木叶片的Gs影响较大,与对照相比,5C、3309M 2种砧木降幅高达94.55%、94.66%,下降较少的砧木为1103P、420A,降幅也分别高达79.48%、80.83%;在6 个葡萄砧木中,1103P 的Gs显著高于其他砧木,5BB次之。如图3-C所示,盐碱胁迫处理后,Ci最高的为5C,与对照相比,降幅为28.09%;SO4 次之,相比于对照降幅为26.21%;Ci最低的为5BB,其与对照相比降幅也最大,为51.60%。由图3-D所示,盐碱胁迫处理后,5BB 的Tr显著高于其他砧木,1103P、420A、SO4 次之,最低的为5C;Tr下降较多的为5C,下降幅度为97.69%,下降较少的是5BB 与420A,降幅为70.11%与70.26%。

图3 盐碱胁迫对不同砧木光合参数的影响
Fig.3 Effects of saline-alkali stress on photosynthetic parameters of different rootstocks
2.4 盐碱胁迫对不同砧木叶片OJIP曲线的影响
如图4-A~B 所示,不同葡萄砧木O 点均无明显差异。由图4-A可知,各砧木OJIP曲线形状整体相似,较陡峭,各曲线均在P点达到最大值,其中J、I、P的荧光强度最高的为SO4,最低的为3309M。在盐碱胁迫处理15 d 后(图4-B),各砧木OJIP 曲线形状有较大差异,总体较为平缓,且荧光强度较对照均有所下降,其中下降较多的且荧光强度最低的均为5C;SO4 曲线各点J、I、P 的荧光强度均高于其他砧木,1103P次之,其他砧木无明显差异。

图4 盐碱胁迫对不同砧木OJIP 曲线的影响
Fig.4 Effects of saline-alkali stress on OJIP curve of different rootstocks
A、C. 对照;B、D. 处理;A、B. 不同砧木OJIP 曲线;C,D. 不同砧木O-P 标准化OJIP 曲线。
A,C.Control;B,D.Treatment;A,B.OJIP curve of different rootstocks;C,D.O-P standardized OJIP curve of different rootstocks.
将不同葡萄砧木OJIP 曲线按O-P 标准化处理后如图4-C、图4-D所示,盐碱胁迫处理后,相较于对照,各砧木I-P 段趋于平缓,J 相、I 相整体呈上升趋势,其中5C、420A受胁迫影响更大,J相、I相增长幅度较明显,SO4变化较小。
2.5 盐碱胁迫对不同砧木叶片叶绿素荧光参数的影响
盐碱胁迫下不同砧木叶绿素荧光参数如表2所示。由对照可知,砧木5C 的性能指数(PIabs)、PSⅡ的最大光化学效率(Fv/Fm)高于其他砧木;SO4 具有较高的光能的吸收(ABS/CSm)、捕获(TRo/CSm)能力与电子传递产额(ETo/CSm),以及单位面积内反应中心数量(RC/CSm);5BB 具有较高热耗散(DIo/CSm)。盐碱胁迫15 d后,与对照相比,各砧木J点相对可变荧光强度(Vj)、I 点相对可变荧光强度(Vi)、单位RC吸收的光能(ABS/RC)、单位RC 捕获的光能(TRo/RC)整体呈上升趋势,DIo/CSm变化不一,其他荧光参数整体呈下降趋势。其中Vj、Vi、ABS/RC、TRo/RC 增幅最大的为5C,增幅为30.74%、5.60%、31.55%、21.62%,且5C的Vj、Vi值显著高于其他砧木;不同砧木的ABS/RC、TRo/RC、DIo/CSm值无显著差异;PIabs、Fv/Fm、TRo/CSm、ETo/RC 降幅最小的均为SO4;ABS/CSm、ETo/CSm、RC/CSm降幅最小的均为3309M。
表2 盐碱胁迫对不同砧木叶绿素荧光参数的影响
Table 2 Effects of saline-alkali stress on Chlorophyll fluorescence parameters of different rootstocks

砧木Rootstocks 1103P 3309MCK 420A 5BB 5C SO4处理Treatment CK T T CK T CK T CK T CK T以吸收光能为基础的性能指数PIabs 1.75±0.42 bc 1.03±0.25 a 2.50±0.45 ab 1.54±0.17 a 2.07±0.70 bc 1.20±0.43 a 1.49±0.09 c 1.54±0.20 a 3.18±0.25 a 0.51±0.14 b 1.96±0.48 bc 1.38±0.33 a PSⅡ的最大光化学效率Fv/Fm 0.814±0.005 ab 0.781±0.020 ab 0.817±0.015 ab 0.786±0.021 ab 0.813±0.014 ab 0.764±0.009 b 0.801±0.000 b 0.781±0.019 ab 0.830±0.009 a 0.724±0.001 c 0.817±0.006 ab 0.808±0.004 a J点处(2 ms)的荧光强度Vj 0.553±0.04 a 0.584±0.03 a 0.486±0.03 bc 0.570±0.09 a 0.516±0.04 ab 0.536±0.08 a 0.513±0.02 ab 0.527±0.11 a 0.452±0.02 c 0.653±0.01 a 0.537±0.03 ab 0.582±0.04 a I点处(30 ms)的荧光强度Vi 0.865±0.02 a 0.893±0.02 ab 0.850±0.03 a 0.848±0.00 b 0.851±0.01 a 0.823±0.04 c 0.843±0.00 a 0.829±0.06 c 0.867±0.01 a 0.918±0.01 a 0.874±0.01 a 0.904±0.01 a单位面积吸收的光能ABS/CSm 48 261.67±3 088.98 a 42 417.00±7 578.46 ab 41 499.00±4 858.51 b 39 284.00±2 360.79 b 46 651.33±1 503.07 ab 39 301.67±701.23 b 48 125.33±495.94 a 39 621.00±2 176.45 b 49 863.00±1 258.87 a 30 307.33±644.55 c 51 679.00±3 790.46 a 47 931.00±5 431.71 a单位面积捕获的光能TRo/CSm 39 260.00±2 441.73 a 33 197.67±6 624.92 ab 33 948.00±4 566.62 b 30 894.33±2 706.04 b 37 914.33±780.05 ab 30 030.00±212.78 b 38 556.67±418.58 ab 30 953.33±2 027.99 b 41 376.33±1 461.01 a 21 940.00±687.21 c 42 243.67±3 296.34 a 38 731.00±4 579.54 a单位面积电子传递的量子产额ETo/CSm 44 252.00±3 146.94 a 38 592.00±7 101.86 ab 37 618.33±4 888.15 b 35 695.33±1 758.61 b 42 432.67±1 410.15 ab 35 011.00±1 015.95 b 43 470.33±611.99 a 35 577.33±2 327.19 b 45 211.67±1 516.43 a 27 403.00±529.05 c 47 315.00±3 568.12 a 44 065.67±4 733.12 a单位面积的热耗散DIo/CSm 9 001.67±697.47 ab 9 219.33±1 152.31 a 7 551.00±291.89 c 8 389.67±345.26 a 8 737.00±873.16 ab 9 271.67±503.99 a 9 568.67±77.37 a 8 667.67±757.02 a 8 486.67±223.13 bc 8 367.33±84.55 a 9 435.33±556.52 ab 9 200.00±852.17 a单位面积的活性反应中心数量RC/CSm 2.41±0.16 a 2.09±0.17 b 2.18±0.33 a 2.08±0.09 b 2.25±0.25 a 1.74±0.24 c 2.07±0.07 a 1.87±0.25 bc 2.21±0.21 a 1.71±0.11 c 2.40±0.10 a 2.44±0.09 a单位RC吸收的光能ABS/RC 2.065±0.16 b 2.516±0.15 a 1.933±0.33 b 2.083±0.19 a 2.073±0.36 b 2.450±0.37 a 2.573±0.12 a 2.560±0.67 a 1.869±0.18 b 2.731±0.14 a 2.021±0.18 b 2.220±0.12 a单位RC捕获的光能TRo/RC 1.68±0.12 b 1.96±0.12 a 1.58±0.23 b 1.63±0.11 a 1.68±0.27 b 1.87±0.27 a 2.06±0.04 a 1.99±0.47 a 1.55±0.13 b 1.98±0.08 a 1.65±0.14 b 1.79±0.09 a单位RC电子传递的量子产额ETo/RC 0.75±0.02 b 0.82±0.06 ab 0.81±0.16 b 0.70±0.10 b 0.81±0.14 b 0.87±0.20 ab 1.00±0.03 a 0.91±0.09 a 0.85±0.10 ab 0.69±0.02 b 0.76±0.03 b 0.75±0.02 ab
2.6 不同砧木耐盐碱能力主成分分析
如表3、表4所示,对盐碱胁迫下不同葡萄砧木的18个指标标准化后进行主成分分析,提取特征值>1的4 个主成分,特征值分别为8.542、4.194、2.879、1.414,累计方差贡献率为94.61%,具有较强信息代表性,达到分析要求。第1 主成分在Tr、PIabs、Fv/Fm、Vj、ABS/CSm、TRo/CSm、ETo/CSm上有较高的荷载量,第2 主成分在Ci、RC/CSm、ABS/RC、ETo/RC 上有较高的荷载量,第3 主成分在Vi、DIo/CSm、TRo/RC 上有较高的荷载量,第4主成分在新梢生长量、Chla+b、Pn、Gs上有较高的荷载量。综合得分(F)是每个主成分得分与对应贡献率乘积之和,并根据F进行排名,各砧木在盐碱胁迫下的排名为SO4、1103P、5BB、420A、3309M、5C。
表3 主成分分析成分荷载矩阵
Table 3 Principal component analysis load matrix

注:*表示某指标在各因子中的最大绝对值。
Note:*indicates the biggest absolute value of each index in all factors.
指标Index新梢生长量Shoot growth Chl a+b Pn Gs Ci Tr PIabs Fv/Fm Vj Vi ABS/CSm TRo/CSm ETo/CSm DIo/CSm RC/CSm ABS/RC TRo/RC ETo/RC特征值Eigen values方差贡献率Proportion of variance/%累计贡献率Cumulative variance/%第1主成分The first principal component 0.147 0.199 0.206 0.234-0.137 0.320*0.288*0.318*-0.264*-0.137 0.311*0.312*0.304*0.204 0.215-0.177-0.068 0.193 8.542 47.460 47.460第2主成分The second principal component-0.065-0.225-0.175-0.106 0.357*-0.162 0.0005 0.169 0.217 0.270 0.177 0.186 0.201 0.008 0.351*-0.323*-0.343-0.388*4.194 23.300 70.760第3主成分The third principal componen 0.352 0.129 0.169 0.270 0.271-0.017-0.290-0.033 0.252 0.418*0.092 0.078 0.107 0.232*0.136 0.316 0.401*0.034 2.879 15.900 86.750第4主成分The fourth principal componen-0.503*0.517*0.505*-0.399*0.170-0.093 0.034-0.005 0.040 0.093 0.038 0.044 0.043-0.043 0.069 0.053 0.048-0.019 1.414 7.860 94.610
表4 不同砧木盐碱胁迫后综合得分及其排名
Table 4 Comprehensive scores and ranking of different rootstocks after saline alkali stress
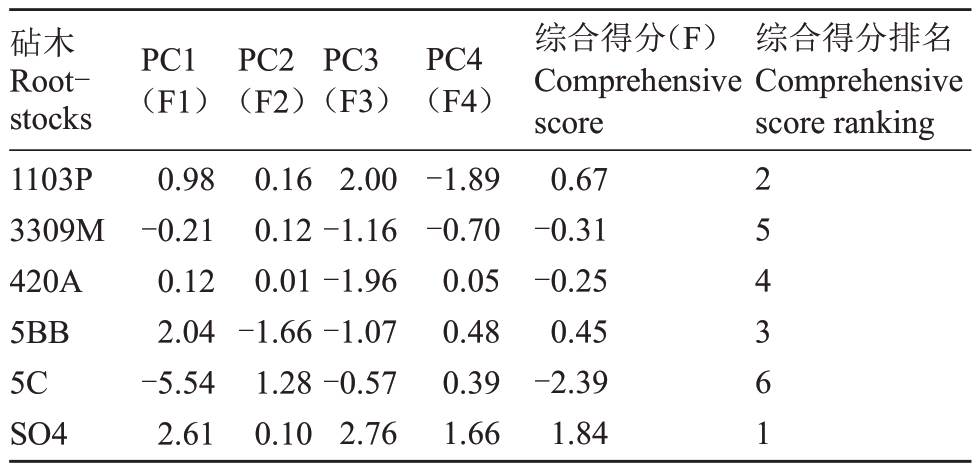
砧木Rootstocks 1103P 3309M 420A 5BB 5C SO4 PC1(F1)0.98-0.21 0.12 2.04-5.54 2.61 PC2(F2)0.16 0.12 0.01-1.66 1.28 0.10 PC3(F3)2.00-1.16-1.96-1.07-0.57 2.76 PC4(F4)-1.89-0.70 0.05 0.48 0.39 1.66综合得分(F)Comprehensive score 0.67-0.31-0.25 0.45-2.39 1.84综合得分排名Comprehensive score ranking 2 5 4 3 6 1
3 讨 论
经过盐碱胁迫,植株最直接的表现在于生长发育受到限制[19-20]。于昕等[21]用复合盐碱处理葡萄植株后,葡萄的叶片枯黄萎蔫,生长量显著降低。仪泽会等[22]研究发现,复合盐碱胁迫会显著抑制青椒幼苗的生长,且随着复合盐碱浓度的升高,幼苗的株高逐渐降低。本试验中,不同葡萄砧木在遭受胁迫后,新梢生长量均明显下降,但胁迫后新梢生长量与对照相比,降幅最小的砧木为420A 与SO4,与新梢生长量较高的砧木1103P、5BB不一致,这可能是由于砧木自身差异所造成的。
叶绿素是植物主要的光合色素,密切影响植物光合效率,其含量在一定程度上衡量植株的抗逆性[23-24]。本试验中,不同砧木在盐碱胁迫下,叶片中Chl a、Chl b 以及Chl a+b 含量较对照均呈下降趋势。植株遭受盐碱胁迫后叶绿素含量降低,可能是因为胁迫后pH 升高,植株胞内离子平衡被打破,导致叶片吸收与叶绿素合成相关的Mg2+能力下降,从而叶绿素合成受阻,含量降低[25]。或者是盐碱胁迫破坏了植物叶片叶绿体内部结构,使叶绿素的合成与降解失衡,导致叶绿素合成受阻[26]。本试验中,在盐碱胁迫15 d 后,Chl b 含量较对照下降幅度小于Chl a。这与刘兵等[27]在盐碱胁迫下对垂丝海棠的研究结果相一致,经胁迫后的植株Chl a+b 含量下降,且Chl b含量对胁迫的反应更敏感,在处理20 d时显著降低,早于Chl a含量于40 d时显著降低。这可能是因为叶绿素酶对Chl b的降解大于Chl a[28]。
盐碱胁迫可通过叶片气孔限制与非气孔限制2种方式影响植物的光合作用,随着盐碱胁迫浓度的增加以及胁迫时间的延长,影响方式会从气孔性限制转变为非气孔性限制[29-31]。在盐碱胁迫下,植株内部离子平衡被打破,导致叶片气孔关闭,Gs下降,从而限制外界CO2进入叶片,使Ci下降。所以当Ci与Gs同时降低时,说明植株光合作用受气孔因素影响[32]。张瑞等[33]发现烟富六号苹果在不同砧木上的光合荧光系统在盐碱胁迫下会受到一定程度的损坏,Pn、Gs、Tr和qP呈下降趋势。本研究中,不同葡萄砧木遭受盐碱胁迫15 d 后,叶片的Pn、Gs、Ci、Tr均显著低于CK,且Ci与Gs同时处于下降趋势,说明各砧木植株光合作用受到抑制是由气孔因素导致的,且叶片Pn与Chl a+b含量变化相同,说明各砧木叶绿素的降低也是植株光合作用下降的原因之一。与尹勇刚等[34]、王振兴等[35]研究结果相一致。
当植物所处环境变化时,叶绿素荧光会发生相应的变化来响应环境对植物的影响[36]。OJIP曲线可直观地反映一段时间内植物PSⅡ反应中心原初光化学反应信息,以及电子传递信息变化动态[37]。研究表明,胁迫会导致植株J、I、P相荧光值下降,使曲线趋于平缓,使O-P标准化后的曲线J、I相升高[38-40]。若受到胁迫,O点荧光强度显著上升,则表明植株有一定程度的可逆反应[39,41]。本试验中,不同葡萄砧木在遭受盐碱胁迫后,曲线特征位点改变,O、J、I、P相整体呈下降趋势,OJIP曲线趋于平缓,说明盐碱胁迫降低了砧木PSⅡ反应中心的活度,影响植株叶片叶绿素荧光强度;O-P标准化后的曲线中,J相呈上升趋势,I-P段趋于水平,说明,反应中心电子传递受阻;其中,不同砧木中,受影响最严重的为5C。此外,O点荧光强度下降的有3309M、420A以及5BB,表明其反应中心遭到不可逆失活。
由叶绿素荧光动力学参数可清晰地从植物对光能的吸收、转化、热耗散、电子传递状态等方面综合反映植株对光能的利用率[27]。由于不同葡萄砧木的PSⅡ荧光参数对盐碱胁迫的响应有所不同,所以各砧木对光能利用效率有所差异。前人研究中,一般使用Fv/Fm或者PIabs来反映胁迫对植物光合结构的影响,并评估植株受胁迫的程度以及植株对光能的利用率[42]。植物处于正常生长发育状态时,会有较高的光能吸收(ABS/CSm)及光能捕获(TRo/CSm)能力,且Vj与Vi处于较低的状态,使QA(初级醌受体)更通畅地向QB(次级醌受体)进行电子传递,有较高的电子传递产额(ETo/CSm)以及反应中心数量(RC/CSm),此外,保持较低的热耗散(DIo/CSm),所以植株有较高的Fv/Fm与PIabs值[43-45]。Fv/Fm与PIabs的降低,表明植物叶片PSⅡ反应中心受到了破坏,电子传递速率和光化学量子效率遭到光抑制,植株对光能的利用率降低[46]。本研究中,盐碱胁迫后,不同葡萄砧木的RC/CSm、ABS/CSm、TRo/CSm、ETo/CSm、ETo/RC 值整体呈下降趋势,ABS/RC、TRo/RC、Vj、Vi、DIo/CSm整体升高,表明植株光合反应中心失活,叶片单位面积内吸收光能、捕获光能的能力低,单位反应中心吸收光能、捕获的光能增加,导致PSⅡ反应中心受体侧QA大量积累,电子在受体侧的传递受阻,电子传递能力下降[47-48],加上胁迫后的砧木均有较高的DIo/CSm,所以导致Fv/Fm与PIabs降低。在盐碱胁迫下,5C与420A 的单位面积反应中心的数量(RC/CSm)显著低于其他砧木,而SO4具有显著高于其他砧木的RC/CSm;且ABS/CSm、TRo/CSm、ETo/CSm值最高、降幅较少的砧木均为SO4,但SO4 的PIabs与Fv/Fm低于5BB、3309M,可能是为了不损伤其光系统,SO4把吸收的光能大部分以热耗散的形式消耗,较少部分用于电子传递,从而使同化受到影响,导致光合能力下降[41]。
植物耐盐碱机制复杂,单一指标不足以说明问题,需要从多方面综合来阐述。笔者以盐碱胁迫对不同葡萄砧木生理影响的18 个指标为依据进行主成分分析,结果表明,第1 主成分中,Tr、PIabs、Fv/Fm、Vj、ABS/CSm、TRo/CSm、ETo/CSm等是评价各砧木受盐碱胁迫后损伤的主要指标,其中,Tr是调节光合作用的指标,PIabs、Fv/Fm 是评价荧光能力的指标,ABS/CSm、TRo/CSm、Vj、ETo/CSm是控制光能利用率的重要指标,说明盐碱胁迫主要通过改变植株叶片的气孔、蒸腾能力,以及叶片对光能的吸收、传递、转换、利用能力等方面来影响植株。此外,从主成分分析结果可得出,各砧木耐盐碱胁迫强弱的排名为:SO4、1103P、5BB、420A、3309M、5C。
4 结 论
综上所述,在盐碱胁迫下,不同葡萄砧木新梢生长受到抑制,植株光系统的结构和功能被破坏,使叶绿素合成受阻,加上气孔因素限制,光合作用强度降低,叶绿素荧光特性进而受到影响。利用主成分分析得到耐盐碱性最强的是SO4,1103P、5BB 次之,420A、3309M、5C耐盐碱性较差。
[1] 田野,陈冠铭,李家芬,向雄鹰,刘扬,李宏杨.世界葡萄产业发展现状[J].热带农业科学,2018,38(6):96-101.TIAN Ye,CHEN Guanming,LI Jiafen,XIANG Xiongying,LIU Yang,LI Hongyang. Present development of grape industry in the world[J]. Chinese Journal of Tropical Agriculture,2018,38(6):96-101.
[2] 刘爱华,叶植材.中国统计年鉴[M].北京:中国统计出版社,2020.LIU Aihua,YE Zhicai. China statistical yearbook[M]. Beijing:China Statistics Press,2020.
[3] 刘凤之.中国葡萄栽培现状与发展趋势[J].落叶果树,2017,49(1):1-4.LIU Fengzhi. Current situation and development trend of grape cultivation in China[J].Deciduous Fruits,2017,49(1):1-4.
[4] ZOHREH E B,MASOUD H,MICHELLE D,LYLE C,FILIPPO M,MOHAMMAD R M.Effect of salinity stress on the physiological characteristics,phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak[J].Industrial Crops&Products,2019,135:311-320.
[5] 李超,白世践,赵荣华,陈光,蔡军社.葡萄砧木及其应用的研究进展[J].农学学报,2016,6(5):53-59.LI Chao,BAI Shijian,ZHAO Ronghua,CHEN Guang,CAI Junshe. Progresses in research and application of grapevine rootstocks[J].Journal of Agriculture,2016,6(5):53-59.
[6] LI R L,SHI F C,FUKUDA K.Interactive effects of various salt and alkali stresses on growth,organic solutes,and cation accumulation in a halophyte Spartina alterniflora(Poaceae)[J].Environmental&Experimental Botany,2010,68(1):66-74.
[7] 丁璇,袁坤,曹建华,徐智娟,何哲,林位夫.嫁接树砧穗互作研究进展[J].热带农业科学,2010,30(5):68-71.DING Xuan,YUAN Kun,CAO Jianhua,XU Zhijuan,HE Zhe,LIN Weifu. Research progress on interaction between rootstock and scion[J]. Chinese Journal of Tropical Agriculture,2010,30(5):68-71.
[8] 马振强,臧兴隆,孙鲁龙,翟衡.几种葡萄砧木及其与赤霞珠嫁接组合磷效率比较[J].园艺学报,2014,41(7):1289-1296.MA Zhenqiang,ZANG Xinglong,SUN Lulong,ZHAI Heng.Comparison of phosphorus efficiency on different rootstocks and their grafted seedlings with cabernet sauvignon[J].Acta Horticulturae Sinica,2014,41(7):1289-1296.
[9] 金仲鑫.不同砧木对葡萄果实品质的影响及机理初探[D].泰安:山东农业大学,2017.JIN Zhongxin. Modifications of grape berry quality as affected by the rootstocand preliminary exploration on the underlying mechanism[D].Tai’an:Shandong Agricultural University,2017.
[10] 赵艳侠,张久慧,亓桂梅. 葡萄砧木的应用研究现状及展望[J].北方园艺,2019(11):136-142.ZHAO Yanxia,ZHANG Jiuhui,QI Guimei. Research status and prospects of application of grape rootstocks[J]. Northern Horticulture,2019(11):136-142.
[11] MASSONNET C,REGNARD J L,PÉ LAURI,COSTES E,SINOQUET H. Contributions of foliage distribution and leaf functions to light interception,transpiration and photosynthetic capacities in two apple cultivars at branch and tree scales[J].Tree Physiology,2008,28(5):665-678.
[12] KALAJI H M,JAJO A,OUKARROUM A,BRESTIC M,ZIVCAK M,SAMBORSKA I A,CETNER M D,LUKASIK,IZABELA,GOLTSEV V,LADLE R J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stressconditions[J].ActaPhysiologiaePlantarum,2016,38(4):102.
[13] 李秋利,高登涛,魏志峰,王志强,刘军伟.桃幼苗叶片叶绿素荧光特性和抗氧化酶活性对二氧化硫胁迫的响应[J].果树学报,2018,35(11):1374-1384.LI Qiuli,GAO Dengtao,WEI Zhifeng,WANG Zhiqiang,LIU Junwei. Response of chlorophyll fluorescence characteristics and antioxidant enzyme activities in peach leaves to sulfur dioxide stress[J].Journal of Fruit Science,2018,35(11):1374-1384.
[14] MATHUR S,JAJOO A,MEHTA P,BHARTI S.Analysis of elevated temperature-induced inhibition of photosystem II using chlorophylII a fluorescence induction kinetics in wheat leaves(Triticum aestivum)[J].Plant Biology,2011,13(1):1-6.
[15] 王延秀,李飞鸿,武云龙,郭爱霞,胡亚,朱燕芳,贾旭梅.盐碱胁迫下红地球葡萄对生化改良的光合与生理响应[J].干旱地区农业研究,2018,36(4):214-222.WANG Yanxiu,LI Feihong,WU Yunlong,GUO Aixia,HU Ya,ZHU Yanfang,JIA Xumei. Photosynthetic and physiological response to biochemical improvement of Red Globe grape under saline-alkali stress[J]. Agricultural Research in the Arid Areas,2018,36(4):214-222.
[16] ARNON D I.Copper enzymes in isolated chloroplasts:polyphenoxidase in Beta vulgaris[J].Plant Physiology,1949,24(1):1-15.
[17] 李红杰,刘佳,赵成凤,张宏军,张仁和.干旱胁迫及复水处理对玉米苗期叶片光化学活性的影响[J].植物生理学报,2020,56(5):997-1005.LI Hongjie,LIU Jia,ZHAO Chengfeng,ZHANG Hongjun,ZHANG Renhe. Effects of drought stress and rehydration on photochemical activity in maize seedling leaves[J]. Plant Physiology Journal,2020,56(5):997-1005.
[18] 胡文海,闫小红,李晓红,曹灶桂.24-表油菜素内酯对干旱胁迫下辣椒叶片快速叶绿素荧光诱导动力学曲线的影响[J].植物研究,2021,41(1):53-59.HU Wenhai,YAN Xiaohong,LI Xiaohong,CAO Zaogui. Effects of 24-epibrassinolide on the chlorophyll fluorescence transient in leaves of pepper under drought stress[J].Bulletin of Botanical Research,2021,41(1):53-59.
[19] 于昕,赵玉花,相广庆,杜远鹏,姚玉新. 盐碱处理下砧木SA15 和SA17 对美乐葡萄生长和果实品质的影响[J].南京农业大学学报,2019,42(6):1022-1029.YU Xin,ZHAO Yuhua,XIANG Guangqing,DU Yuanpeng,YAO Yuxin. Effects of SA15 and SA17 rootstocks on growth and berry quality of Merlot grape under salt and alkali treatment[J]. Journal of Nanjing Agricultural University,2019,42(6):1022-1029.
[20] 胡爱双,张小栋,王文成,李凯超,孙宇.盐胁迫对不同耐盐性八棱海棠株系生理特性的影响[J]. 果树学报,2021,38(3):335-343.HU Aishuang,ZHANG Xiaodong,WANG Wencheng,LI Kaichao,SUN Yu. Effects of sait sres on pysiogial caceristis of two srains of Malus micromalus Mak. with different salt tolerance[J].Journal of Fruit Science,2021,38(3):335-343.
[21] 于昕,岳倩宇,于梦,杜远鹏,姚玉新.复合盐碱处理下葡萄砧木SA15、SA17 和1103P 的生理响应分析[J].植物生理学报,2020,56(1):57-65.YU Xin,YUE Qianyu,YU Meng,DU Yuanpeng,YAO Yuxin.Physiological responses of grape rootstocks SA15,SA17 and 1103P to salt-alkali stress[J].Plant Physiology Journal,2020,56(1):57-65.
[22] 仪泽会,毛丽萍,赵婧. 嫁接对复合盐碱胁迫下青椒幼苗生长、抗氧化能力及渗透调节能力的影响[J]. 植物生理学报,2020,56(9):1943-1954.YI Zehui,MAO Liping,ZHAO Jing. Effects of grafting on growth,antioxidant capacity and osmotic adjustment capacity of green pepper seedlings under mixed salt-alkali stress[J]. Plant Physiology Journal,2020,56(9):1943-1954.
[23] YANG J Y,ZHENG W,TIAN Y,WU Y,ZHOU D W. Effects of various mixed salt-alkaline stresses on growth,photosynthesis,and photosynthetic pigment concentrations of Medicago ruthenica seedlings[J].Photosynthetica,2011,49(2):275-284.
[24] 贾旭梅,朱燕芳,王海,吴玉霞,赵通,程丽,朱祖雷,王延秀.垂丝海棠应对盐碱复合胁迫的生理响应[J].生态学报,2019,39(17):6349-6361.JIA Xumei,ZHU Yanfang,WANG Hai,WU Yuxia,ZHAO Tong,CHENG Li,ZHU Zulei,WANG Yanxiu.Physiological response of Begonia verticillata to salt alkali compound stress[J].Acta Ecologica Sinica,2019,39(17):6349-6361.
[25] GUO R,YANG Z Z,LI F,YAN C R,ZHONG X L,LIU Q,Xia X,LI H R,ZHAO L. Comparative metabolic responses and adaptive strategies of wheat(Triticum aestivum)to salt and alkali stress[J].BMC Plant Biology,2015,15(1):170.
[26] SUN J,JIA Y X,GUO S R,LI J,SHU S. Resistance of spinach plants to seawater stress is correlated with higher activity of xanthophyll cycle and better maintenance of chlorophyll metabolism[J].Photosynthetica,2010,48(4):567-579.
[27] 刘兵,贾旭梅,朱祖雷,张瑞,赵通,王延秀.盐碱胁迫对垂丝海棠光合作用及渗透调节物质的影响[J]. 西北植物学报,2019,39(9):1618-1626.LIU Bing,JIA Xumei,ZHU Zulei,ZHANG Rui,ZHAO Tong,WANG Yanxiu. Effects of saline-alkali stress on photosynthesis and osmoregulation substances of Begonia verticillata[J]. Acta Botanica Boreali-Occidentalia Sinica,2019,39(9):1618-1626.
[28] 卢艳,王飞,韩明玉,赵亮明,李涛.NaCl 胁迫对4 种砧穗组合苹果的生长及光合特性的影响[J]. 西北农业学报,2011,20(8):106-110.LU Yan,WANG Fei,HAN Mingyu,ZHAO Liangming,LI Tao.Effect of NaCl stress on growth and photosynthetic characteristics of apple on four different rootstock-scion combinations[J].Acta Agriculturae Boreali-Occidentalis Sinica,2011,20(8):106-110.
[29] 王晚霞,高立杨,张瑞,赵婷,张仲兴,王双成,王延秀.2,4-表油菜素内酯对盐碱胁迫下垂丝海棠光合及生理特性的影响[J].果树学报,2021,38(9):1479-1490.WANG Wanxia,GAO Liyang,ZHANG Rui,ZHAO Ting,ZHANG Zhongxing,WANG Shuangcheng,WANG Yanxiu. Effects of 2,4 epbrassinolide on photosynthetic and physiological characteristics of Malusa halliana under saline-alkali stress[J].Journal of Fruit Science,2021,38(9):1479-1490.
[30] 高光林,姜卫兵,俞开锦,汪良驹.盐胁迫对果树光合生理的影响[J].果树学报,2003,20(6):493-497.GAO Guanglin,JIANG Weibing,YU Kaijin,WANG Liangju.A review of studies on effect of salt stress on photosynthesis in fruitcrops[J].Journal of Fruit Science,2003,20(6):493-497.
[31] 邢庆振,郁松林,牛雅萍,于坤,宋曼曼.盐胁迫对葡萄幼苗光合及叶绿素荧光特性的影响[J].干旱地区农业研究,2011,29(3):96-100.XING Qingzhen,YU Songlin,NIU Yaping,YU Kun,SONG Manman.Effects of salt stress on photosynthetic physiology and chlorophyll fluorescence characteristics of grape (Red Globe)seedlings[J]. Agricultural Research in the Arid Areas,2011,29(3):96-100.
[32] FARQUHAR G D,SHARKEY T D . Stomatal conductance and photosynthesis[J].Annual Review of Plant Physiology,1982,33(1):317-345.
[33] 张瑞,贾旭梅,朱祖雷,张夏燚,赵通,郭爱霞,刘兵,高立杨,王延秀.‘烟富六号’苹果在不同砧木上响应盐碱胁迫的光合及生理特性[J].果树学报,2019,36(6):718-728.ZHANG Rui,JIA Xumei,ZHU Zulei,ZHANG Xiayi,ZHAO Tong,GUO Aixia,LIU Bing,GAO Liyang,WANG Yanxiu.Photosynthesis and physiological characteristics of‘Yanfu 6’apple under saline-alkali stress on different rootstocks[J]. Journal of Fruit Science,2019,36(6):718-728.
[34] 尹勇刚,袁军伟,刘长江,韩斌,李敏敏,孙艳,贾楠,郭紫娟,赵胜建.NaCl 胁迫对葡萄砧木光合特性与叶绿素荧光参数的影响[J].中国农业科技导报,2020,22(8):49-55.YIN Yonggang,YUAN Junwei,LIU Changjiang,HAN Bin,LI Minmin,SUN Yan,JIA Nan,GUO Zijuan,ZHAO Shengjian.Effects of NaCl stress on leaf photosynthesis and chlorophyll fluorescence parameters of Vitis sp.rootstocks[J].Journal of Agricultural Science and Technology,2020,22(8):49-55.
[35] 王振兴,吕海燕,秦红艳,赵滢,刘迎雪,艾军,曹建冉,杨义明,沈育杰.盐碱胁迫对山葡萄光合特性及生长发育的影响[J].西北植物学报,2017,37(2):339-345.WANG Zhenxing,LÜ Haiyan,QIN Hongyan,ZHAO Ying,LIU Yingxue,AI Jun,CAO Jianran,YANG Yiming,SHEN Yujie.Photosynthetic characteristics and growth development of amur grape (Vitis amurensis Rupr. ) under saline-alkali stress[J].Acta Botanica Boreali-Occidentalia Sinica,2017,37(2):339-345.
[36] 陈丽,王振兴,艾军,许培磊,秦红艳,赵滢,刘迎雪,焦竹青,沈育杰.干旱胁迫对山葡萄左山二幼苗叶片光系统Ⅱ活性的影响[J].果树学报,2011,28(6):977-983.CHEN Li,WANG Zhenxing,AI Jun,XU Peilei,QIN Hongyan,ZHAO Ying,LIU Yingxue,JIAO Zhuqing,SHEN Yujie.Effects of drought stress on activities of photosystems Ⅱin leaves of young plants of amur grape Zuoshan 2[J]. Journal of Fruit Science,2011,28(6):977-983.
[37] WASSINK E C. Chlorophyll fluorescence and photosynthesis[M]// NORD F F.Advances in Enzymology and Related Subjects of Biochemistry. New York:Interscience Publisher Inc.,1951.
[38] 全绍文,曹福军,牛建新.新疆早实核桃和晚实核桃叶绿素荧光特征差异比较[J].果树学报,2016,33(7):832-840.QUAN Shaowen,CAO Fujun,NIU Jianxin. A comparative study of chlorophyll fluorescence characteristics between early fruiting and late fruiting walnut cultivars in Xinjiang[J]. Journal of Fruit Science,2016,33(7):832-840.
[39] STRASSER R J,TSIMILLI M M,SHENG Q,GOLTSEV V.Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis[J].Biochimica et Biophysica Acta (BBA)-Bioenergetic,2010,1797(6/7):1313-1326.
[40] 李红杰,刘佳,赵成凤,张宏军,张仁和.干旱胁迫及复水处理对玉米苗期叶片光化学活性的影响[J].植物生理学报,2020,56(5):997-1005.LI Hongjie,LIU Jia,ZHAO Chengfeng,ZHANG Hongjun,ZHANG Renhe. Effects of drought stress and rehydration on photochemical activity in maize seedling leaves[J]. Plant Physiology Journal,2020,56(5):997-1005.
[41] 刘明秀,王泓丁,张宇娜,张帮燕,刘松,李韵佳,党江波,何桥,梁国鲁,郭启高.三倍体枇杷与其四倍体和二倍体亲本光系统活性差异[J].园艺学报,2021,48(1):37-48.LIU Mingxiu,WANG Hongding,ZHANG Yuna,ZHANG Bangyan,LIU Song,LI Yunjia,DANG Jiangbo,HE Qiao,LIANG Guolu,GUO Qigao. Comparative analysis of the photosystem activity of triploid loquats and their tetraploid,diploid parents[J].Acta Horticulturae Sinica,2021,48(1):37-48.
[42] 吴晓丽,汤永禄,李朝苏,吴春,黄钢.不同生育时期渍水对冬小麦旗叶叶绿素荧光及籽粒灌浆特性的影响[J].中国生态农业学报,2015,23(3):309-318.WU Xiaoli,TANG Yonglu,LI Chaosu,WU Chun,HUANG Gang. Effect of waterlogging at different growth stages on flag leaf Chlorophyll fluorescence and grain-filling properties of winter wheat[J]. Chinese Journal of Eco-Agriculture,2015,23 (3):309-318.
[43] KATE M,JOHNSON G N. Chlorophyll fluorescence-a practical guide[J].Journal of Experimental Botany,2000,51(345):659.
[44] 孔芬,王卫成,贺欢,汤玲,杨馥霞.NaCl 处理对芍药光合特性与快速叶绿素荧光特征的影响[J]. 分子植物育种,2019,17(22):7531-7537.KONG Fen,WANG Weicheng,HE Huan,TANG Ling,YANG Fuxia.Effects of NaCl treatment on photosynthetic and fast chlorophyll fluorescence characteristics in herbaceous peony[J].Molecular Plant Breeding,2019,17(22):7531-7537.
[45] 李辛,赵文智.荒漠区植物雾冰藜光合特性对混合盐碱胁迫的响应[J].生态学报,2018,38(4):1183-1193.LI Xin,ZHAO Wenzhi. Response of various salt-alkaline mixed stresses on the photosynthetic characteristics of Bassia dasyphylla in a desert region[J]. Acta Ecologica Sinica,2018,38(4):1183-1193.
[46] 周丹丹,刘德玺,李存华,陈苗苗,刘桂民,杨庆山,李永涛.盐胁迫对朴树和速生白榆幼苗光合特性及叶绿素荧光参数的影响[J].西北植物学报,2016,36(5):1004-1011.ZHOU Dandan,LIU Dexi,LI Cunhua,CHEN Miaomiao,LIU Guimin,YANG Qingshan,LI Yongtao.Photosynthetic characteristics and chlorophyll fluorescence parameters of Celtis sinensis and Ulmus pumila L.seedling under salt stress[J].Acta Botanica Boreali-Occidentalia Sinica,2016,36(5):1004-1011.
[47] 李旭新,刘炳响,郭智涛,常越霞,贺磊,陈芳,路丙社.NaCl 胁迫下黄连木叶片光合特性及快速叶绿素荧光诱导动力学曲线的变化[J].应用生态学报,2013,24(9):2479-2484.LI Xuxin,LIU Bingxiang,GUO Zhitao,CHANG Yuexia,HE Lei,CHEN Fang,LU Bingshe. Effects of NaCl stress on photosynthesis characteristics and fast chlorophyll fluorescence induction dynamics of Pistacia chinensis leaves[J]. Chinese Journal of Applied Ecology,2013,24(9):2479-2484.
[48] 苏晓琼,王美月,束胜,孙锦,郭世荣.外源亚精胺对高温胁迫下番茄幼苗快速叶绿素荧光诱导动力学特性的影响[J].园艺学报,2013,40(12):2409-2418.SU Xiaoqiong,WANG Meiyue,SHU Sheng,SUN Jin,GUO Shirong. Effects of exogenous Spd on the fast chlorophyll fluorescence induction dynamics in tomato seedlings under high temperature stress[J].Acta Horti culturae Si nica,2013,40(12):2409-2418.