甜樱桃(Prunus avium L.)为蔷薇科李属植物,在温带落叶果树中属于成熟较早的树种。甜樱桃营养丰富,含有多种营养元素和生物活性成分,例如,葡萄糖、维生素C和花色苷等[1-2],具有较高的营养和经济价值。
近年来,全球甜樱桃的种植面积和产量迅速增加,并呈现持续增长态势,甜樱桃产业发展潜力巨大。但其生长周期跨度大且比较复杂,花芽生长易受夏季高温和冬季低温影响,形成畸形果或不能结实,导致产量下降,品质参差不齐,经济价值急剧下滑。因此,探究甜樱桃花芽在不同温度胁迫下的生长发育情况有助于其产量和品质的提升。
高温胁迫对植物的光合作用、呼吸作用、细胞组织稳定性、渗透系统等造成一定程度的伤害,影响植物的正常生长[3],且夏季高温影响甜樱桃花芽分化,容易导致畸形果的形成[4-5],对产量和品质提升产生不利影响,经济效益下降。有学者研究,在面对高温胁迫时,野生型拟南芥体内出现茉莉酸积累;且外源应用茉莉酸有助于维持高温下植物的细胞活力[6]。花芽分化后,为了抵御冬季低温危害,花芽进入休眠状态,需要经过一定时间的低温积累才能解除休眠,促进花芽的进一步生殖生长。有研究表明,茉莉酸处理可以显著提高冷藏水稻幼苗的存活率[7],并且在拟南芥冷驯化诱导过程中提高冷冻耐受性方面具有积极作用[8]。
茉莉酸能够响应高温、低温等多种非生物胁迫[9],MYC2转录因子在茉莉酸信号转导途径中起着核心调控作用,增强植物对逆境的抵抗能力,有利于植物正常生长发育。目前,MYC2 转录因子在甜樱桃中的研究几乎处于空白状态。因此,研究MYC2基因如何调控甜樱桃花芽响应温度胁迫的机制有着重要意义。
基于此,依据樱桃基因组数据库信息,笔者成功克隆了茉莉酸信号途径的响应因子PavMYC2基因,并利用实时荧光定量测定了其在不同发育阶段的表达模式,探索PavMYC2 与PavJAZs 蛋白互作关系,为深入探究甜樱桃花芽发育阶段MYC2基因在温度胁迫中发挥的作用机制奠定基础。
1 材料和方法
1.1 植物材料
试验材料为栽植于上海交通大学甜樱桃栽培基地(121.48°E,31.25°N),2009—2010 年定植的10 年生甜樱桃树种,所选品种分别为低需冷量的罗亚理(Royal Lee)和高需冷量的红灯(Hongdeng),砧木为大青叶(P. pseudocerasus‘Daqingye’)。株行距为4 m×6 m,采用主干疏层形整枝,在避雨条件下栽培,其余均按常规管理。基地实时温度用HOBO UX100-003(HOBO,USA)记录。
于2019年7月15日、8月15日、9月15日、10月15 日、11 月15 日、12 月15 日和2020 年1 月15 日、2月5 日、3 月5 日、3 月10 日、3 月15 日采集樱桃花芽样品并用液氮速冻,然后保存于-80 ℃冰箱备用。
1.2 甜樱桃MYC2基因克隆
采用天根公司植物总RNA提取试剂盒,按照说明书进行甜樱桃花芽中总RNA 的提取。1 μg 罗亚理和红灯RNA 使用PrimeScriptTM Ⅱ1st Strand cDNA Synthesis Kit(TaKaRa)进行反转录成cDNA。使用Prime 5软件,依据甜樱桃参考基因组(http://cherry.kazusa.or.jp/)进行引物设计,克隆PavMYC2,使用PrimeSTAR Max Premix(TaKaRa)按照说明书进行PCR 反应。25 μL 体系,12.5 μL PrimeSTAR Max Premix,上下游引物各0.5 μL,模板1 μL,加水补齐25 μL,所用程序为:98 ℃变性5 min;98 ℃5 s,55 ℃5 s,72 ℃30 s,35 个循环;72 ℃延伸5 min。通过1%的琼脂糖凝胶电泳分离PCR 产物,并用SanPrep柱式DNA 胶回收试剂盒(B518131,生工生物)按照标准操作步骤进行纯化,PCR纯化产物连接pEASYBlunt Cloning Vector(TransGen),并送擎科公司测序。
1.3 序列分析、系统进化树构建及蛋白互作预测
应用DNAMAN 进行多序列的对比分析,分析其序列的一致度和相似性;采用MEGA 6 中的邻接法,进行系统进化分析,从NCBI 里GenBank DNA database(http://www.ncbi.nlm.nih.gov/genbank/)获得的同源蛋白构建系统进化树,步长值为1000。在STRING 网站(https://string-db.org/cgi/input?sessionId=xo2a1k8QkhcK&input_page_active_form=single_identifier)进行蛋白互作预测。
1.4 PavMYC2亚细胞定位实验
将PavMYC2 的cDNAs 与具有35S 强启动子和GFP 荧光蛋白的PHB 载体相连接。同源重组法构建PavMYC2-PHB 载体,然后转入GV3101 感受态,每10 μL 感受态加1 μL 连接好的阳性重组质粒,轻轻拍打管底混匀,依次于冰上静置5 min、液氮5 min、37 ℃水浴5 min、冰浴5 min。在超净工作台上加入500 μL 无抗生素的LB 液体培养基,在28 ℃下振荡培养2~3 h。涂布在含有卡那霉素(50 mg·L-1)、利福平(20 mg·L-1)抗性的LB平板上,2~3 d后挑斑,验证阳性克隆后,摇菌至OD600值在1.0 左右,使用10 mmol·L-1 MES-KOH(pH=5.2)、10 mmol·L-1 MgCl2、100 μmol·L-1 acetosyringone 配置的悬浮液进行悬浮,室温静置2~5 h 后,注射进入生长约35 d的烟草叶片,3~7 d 后在激光共聚焦(Zeiss LSM510/ConfoCor2)下扫描,并以GFP-PHB 空载作为对照。
1.5 PavMYC2荧光定量分析
以甜樱桃PavActin为内参。根据荧光定量PCR引物设计原则,使用Primer 5 设计引物。Bio-Rad System(Bio-Rad,CA,USA)用于荧光定量实验,程序为95 ℃30 s,95 ℃5 s,60 ℃30 s,40 个循环。3次生物学重复。荧光值变化曲线和熔解曲线用于分析结果的准确性。数据采用2-ΔΔCT法进行相对定量分析,利用Excel等软件进行统计学分析。
1.6 PavMYC2 与PavJAZs 双分子荧光互补实验(BiFC)
使用pXY104和pXY106载体,同源重组法构建BiFC实验的PavMYC2-pXY104、PavJAZ1-pXY106、PavJAZ2- pXY106、PavJAZ3- pXY106、PavJAZ4-pXY106、PavJAZ5-pXY106 和PavJAZ6-pXY106 载体。将载体分别转入GV3101 感受态,挑斑验证阳性克隆后,摇菌至OD600值为0.8~1.0。每一对互作载体菌液混合,室温放置2~5 h 后,注射入生长35 d左右的烟草叶片中,黑暗中培养1 d,正常光照培养2 d,然后在激光共聚焦显微镜下观察荧光信号。以pXY104和pXY106空载体为对照。
2 结果与分析
2.1 PavMYC2基因鉴定与进化分析
对甜樱桃中的MYC2基因依据GenBank登录号XP_021803466 进行基因克隆与序列鉴定。Pav-MYC2 开放阅读框长2070 bp,编码689 个氨基酸。PavMYC2 与桃和拟南芥的MYC2 基因编码的氨基酸序列进行比对发现,它们都具有bHLH-Zip保守结构域,表明PavMYC2 为bHLH 家族转录因子(图1-A)。
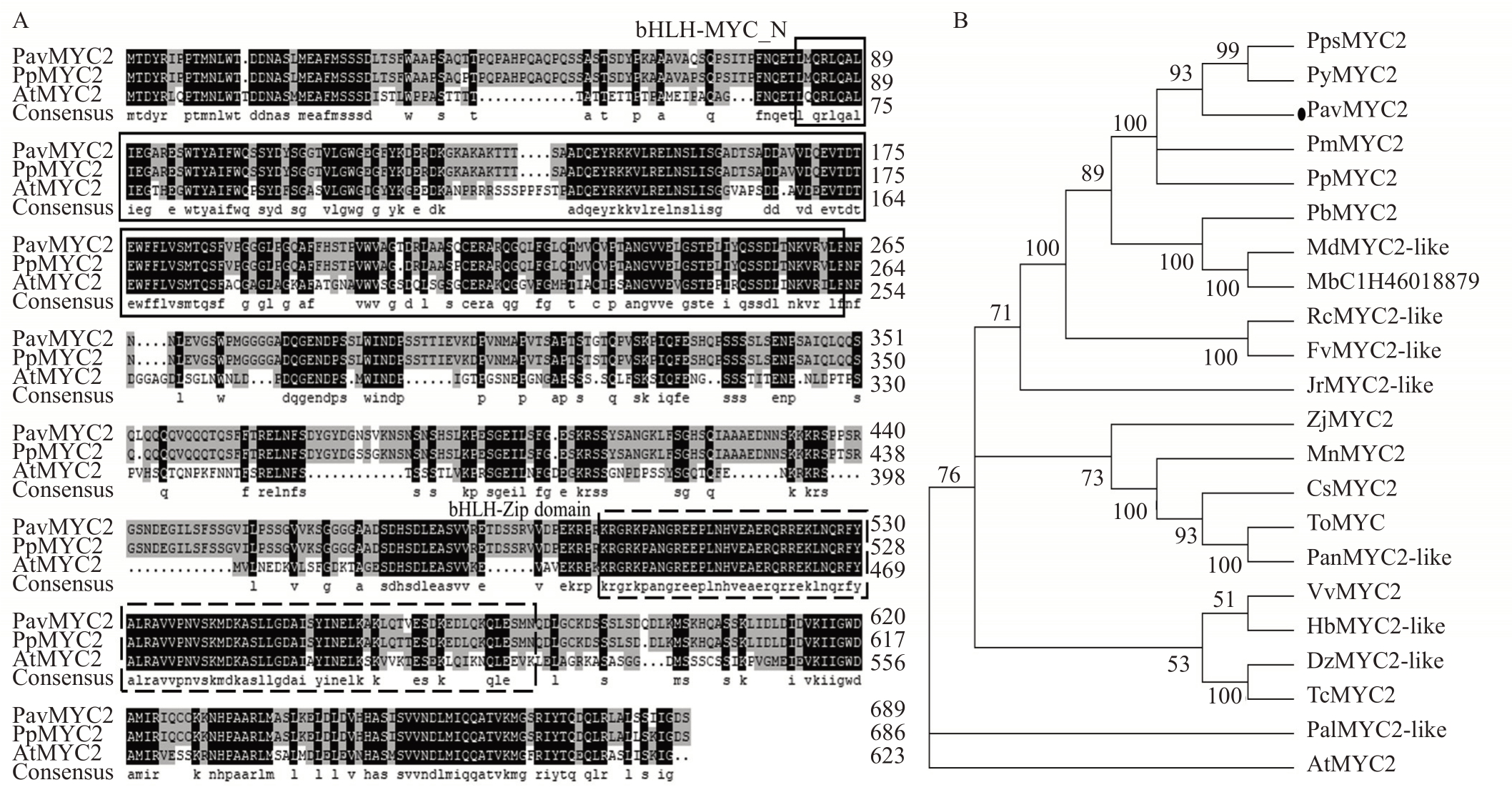
图1 PavMYC2 序列和进化分析
Fig.1 Sequence and phylogenetic analysis of PavMYC2
A.甜樱桃的MYC2 和桃、拟南芥的MYC2 的序列比对,bHLH-MYC_N 和bHLH-Zip 结构域分别用实线和虚线框在图中表明;B.进化树基于甜樱桃和其他物种MYC2 及其同源蛋白的氨基酸序列构建,甜樱桃的MYC2 蛋白在图中已用黑点表明,这些蛋白在Genebank 中的登录号为:AtMYC2. 拟南芥(NP_174541.1);PpsMYC2. 中国樱桃(ALN42127.1);PyMYC2. 樱花(PQQ11938.1);PmMYC2. 梅(XP_008238247.1);PpMYC2. 桃(XP_020419289.1);PbMYC2. 梨(XP_018500941.1);MdMYC2-like. 苹果(XP_008341963.2);MbC1H46. 山荆子(TQD95523.1);RcMYC2-like. 月季(XP_024169176.1);FvMYC2-like. 草莓(XP_004300239.1);JrMYC2-like. 核桃(XP_018811463.1);Zj-MYC2.枣(XP_015896923.1);MnMYC2.川桑(XP_010104300.1);CsMYC2.大麻(XP_030501877.1);ToMYC.异色山黄麻(PON87401.1);Pan-MYC2-like.糙叶山黄麻(PON45335.1);VvMYC2.葡萄(XP_002280253.2);HbMYC2-like.橡胶树(XP_021664832.1);DzMYC2-like.榴莲(XP_022737560.1);TcMYC2.可可(XP_017973469.1);PalMYC2-like.白牧豆树(XP_028802884.1)。
A.Alignment of the sequence of MYC2 from sweet cherry,peach,and Arabidopsis.bHLH-MYC_N and bHLH-Zip domains are indicated by solid and dotted boxes;B.Phylogenetic tree based on the amino acid alignment of PavMYC2 protein in sweet cherry marked with dots and other plant species.Accession numbers are as follows:AtMYC2.Arabidopsis(NP_174541.1);PpsMYC2.Prunus pseudocerasus(ALN42127.1);PyMYC2.Prunus yedoensis (PQQ11938.1); PmMYC2. Prunus mume (XP_008238247.1); PpMYC2. Prunus persica (XP_020419289.1); PbMYC2. Pyrus x bretschneideri (XP_018500941.1); MdMYC2-like. Malus domestica (XP_008341963.2); MbC1H46. Malus baccata (TQD95523.1); RcMYC2-like. Rosa chinensis (XP_024169176.1); FvMYC2-like. Fragaria vesca subsp. vesca (XP_004300239.1); JrMYC2-like. Juglans regia (XP_018811463.1); Zj-MYC2. Ziziphus jujuba (XP_015896923.1); MnMYC2. Morus notabilis (XP_010104300.1); CsMYC2. Cannabis sativa (XP_030501877.1); To-MYC. Trema orientalis (PON87401.1); PanMYC2-like. Parasponia andersonii (PON45335.1); VvMYC2. Vitis vinifera (XP_002280253.2); Hb-MYC2- like. Hevea brasiliensis (XP_021664832.1); DzMYC2- like. Durio zibethinus (XP_022737560.1); TcMYC2. Theobroma cacao (XP_017973469.1);PalMYC2-like.Prosopis alba(XP_028802884.1).
为了确定PavMYC2 的系统进化关系,从NCBI数据库中检索其他物种中与PavMYC2 蛋白同源性较高的氨基酸序列,利用MEGA 6.0 软件构建系统进化树(图1-B)。结果表明,甜樱桃与中国樱桃(Prunus pseudocerasus)、樱花(Prunus yedoensis)的MYC2 的遗传距离最近,而与拟南芥(Arabidopsis thaliana)之间的遗传距离较远。
2.2 PavMYC2亚细胞定位和基因表达模式分析
为了探究甜樱桃PavMYC2蛋白的亚细胞定位,用PavMYC2 的cDNA 序列构建具有强启动子CaMV 35S 和荧光蛋白标签的PHB-GFP 载体,空载作为对照(图2-A)。在烟草叶片中瞬时表达Pav-MYC2 的荧光蛋白,和对照相比,PavMYC2 只在细胞核中表达(图2-B)。
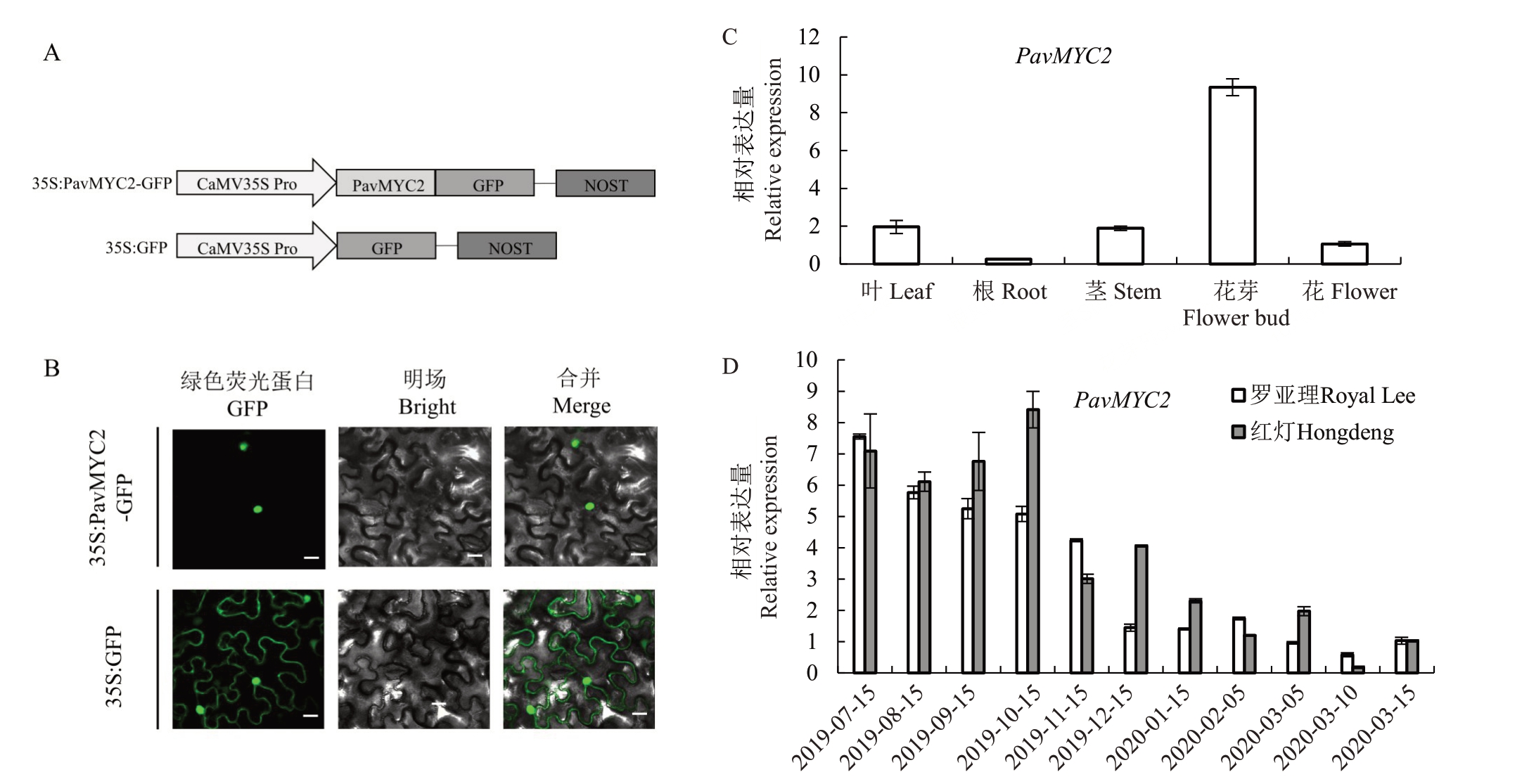
图2 PavMYC2 基因表达模式和亚细胞定位分析
Fig.2 Expression patterns analysis and subcellular localization of PavMYC2
A.35S:PavMYC2-GFP 和35S:GFP 载体构建示意图;B.在烟草叶片上表达35S:PavMYC2-GFP 和35S:GFP 载体蛋白,激光共聚焦采集荧光信号,观察亚细胞定位,比例尺为20 μm;C.PavMYC2 在甜樱桃不同部位的表达量分析,包括叶片、根、茎干、花芽和花;D.2019 年7 月15日到2020 年3 月15 日罗亚理和红灯两个品种花芽中PavMYC2 季节性基因表达分析。
A.Schematic representation of the 35S:PavMYC2-GFP fusion construct and the 35S:GFP construct;B.Leaves of Nicotiana benthamiana plants expressing 35S:PavMYC2-GFP and 35S:GFP.Scale bar=20 μm;C.Spatial expression of PavMYC2 was detected in different sweet cherry tissues,including leaves, roots, stems, flower buds, and flowers; D. Seasonal expression of PavMYC2 was detected in flower buds of the two cultivars Royal Lee and Hongdeng from 15 July 2019 to 15 March 2020.
进一步测定了PavMYC2 在甜樱桃各个组织中的表达量,以探究其在花芽生长发育过程中的功能。PavMYC2在叶子、根、茎、花芽和花中已检测到其表达,在根中的表达量最低,在花芽中表达量最高且花芽中的表达量依次比叶子、茎、花和根中的高4.8、4.9、8.8和37.4倍(图2-C)。
另外,为了进一步确定PavMYC2在樱桃花芽发育各个生长时期发挥的作用,对其季节性表达趋势进行分析。从夏季至次年春季依次取样,包含了花芽发育的重要阶段。PavMYC2 在夏秋季表达量最高,然后开始下降,春季表达量最低(图2-D)。
2.3 PavMYC2启动子顺式作用元件分析
PavMYC2 启动子顺式作用元件主要可以分为三种类型:光响应元件、激素响应元件和胁迫相关元件(表1)。光响应元件中,以G-Box 光响应元件的数量最多达7 个,另外还包括Box4、GATA-motif、Ibox、TCT-motif 等光响应元件;激素响应元件中,脱落酸响应元件ABRE 的数量高达10 个,因此,Pav-MYC2 可能在ABA 信号转导途径中起着一定的调控作用。其他还包括茉莉酸甲酯响应元件CGTCAmotif 和TGACG-motif,生长素响应元件TGA-box;胁迫相关元件中主要有低温诱导元件LTR 和厌氧诱导元件ARE,表明PavMYC2在植物生长发育过程和响应外界环境变化中发挥一定作用。
表1 PavMYC2 基因启动子顺式作用元件
Table 1 PavMYC2 gene promoter cis-acting elements
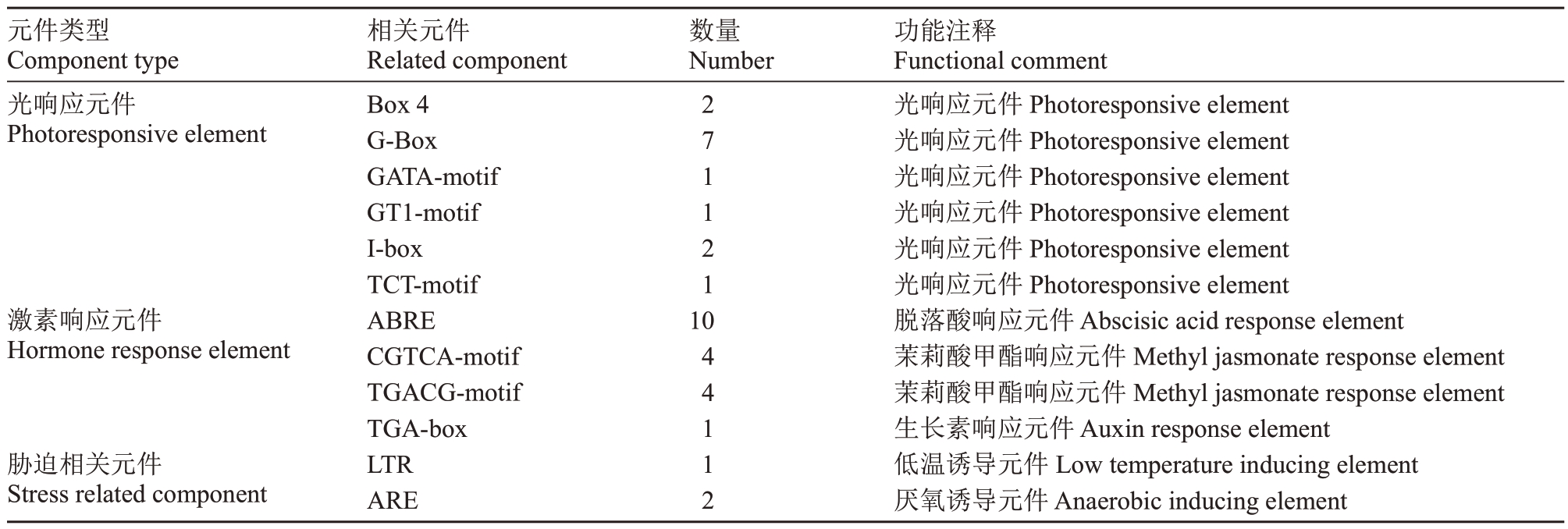
元件类型Component type光响应元件Photoresponsive element激素响应元件Hormone response element胁迫相关元件Stress related component相关元件Related component Box 4 G-Box GATA-motif GT1-motif I-box TCT-motif ABRE CGTCA-motif TGACG-motif TGA-box LTR ARE数量Number 2 7 1 1 2 1 10 4 4 1 1 2功能注释Functional comment光响应元件Photoresponsive element光响应元件Photoresponsive element光响应元件Photoresponsive element光响应元件Photoresponsive element光响应元件Photoresponsive element光响应元件Photoresponsive element脱落酸响应元件Abscisic acid response element茉莉酸甲酯响应元件Methyl jasmonate response element茉莉酸甲酯响应元件Methyl jasmonate response element生长素响应元件Auxin response element低温诱导元件Low temperature inducing element厌氧诱导元件Anaerobic inducing element
2.4 PavMYC2互作蛋白预测分析
互作蛋白的预测结果显示,得分在0.7 以上与MYC2互作的蛋白有13个(表2、图3)。其中大多是JAZ 蛋白,JAZ 类蛋白在JA 信号转导途径中起着抑制子的作用[10-11]。此外,互作蛋白还包括NINJA 家族蛋白、COI 家族蛋白和PPR 家族蛋白。利用MEME 软件,对PavJAZ 基因家族蛋白的保守基序进行分析,结果显示PavJAZ1-PavJAZ6 共有2 个保守基序,LOGO显示的即为保守基序Tify和jas的序列结构(图4),Tify基序的长度为21个氨基酸,jas基序的长度为27个氨基酸,其保守度相对较高。尽管基序所处的位置各有不同,但PavJAZs 全都具有相同的保守基序,且Tify 基序都位于5’端,jas 基序都位于3’端。
表2 PavMYC2 互作蛋白预测
Table 2 The prediction of PavMYC2 interacting proteins
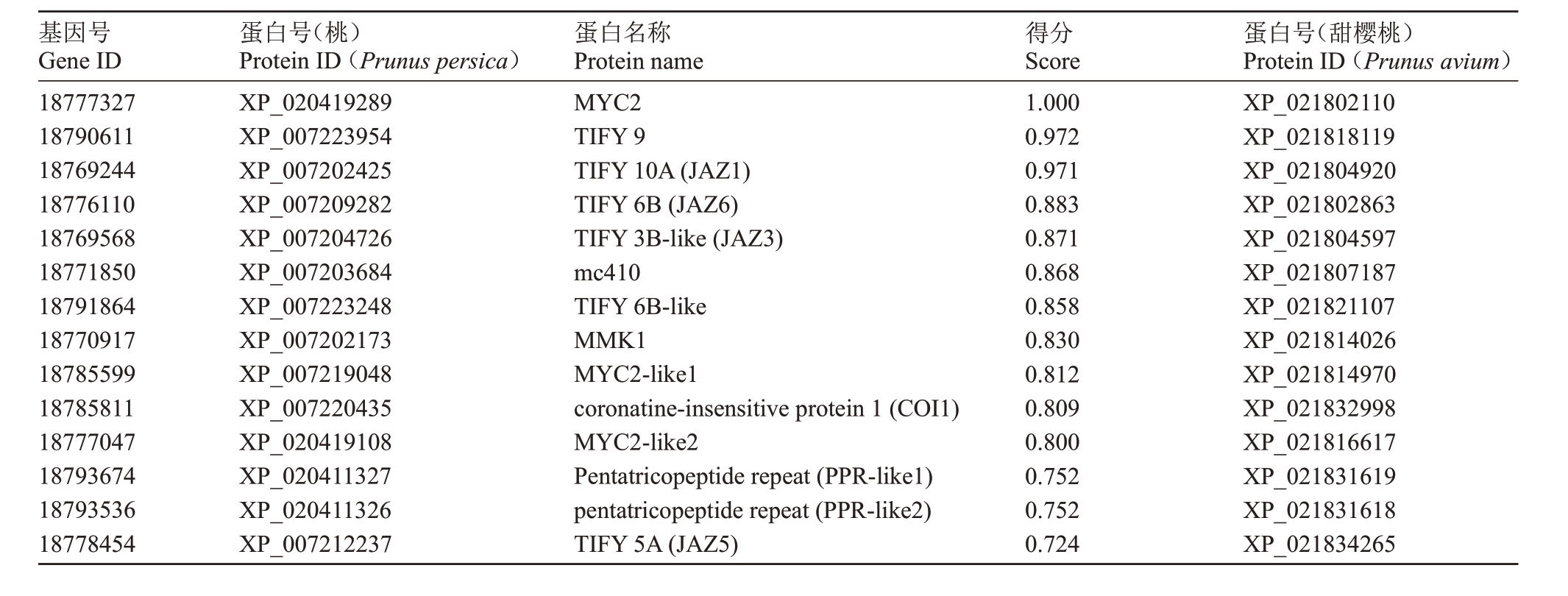
基因号Gene ID 18777327 18790611 18769244 18776110 18769568 18771850 18791864 18770917 18785599 18785811 18777047 18793674 18793536 18778454蛋白号(桃)Protein ID(Prunus persica)XP_020419289 XP_007223954 XP_007202425 XP_007209282 XP_007204726 XP_007203684 XP_007223248 XP_007202173 XP_007219048 XP_007220435 XP_020419108 XP_020411327 XP_020411326 XP_007212237蛋白名称Protein name MYC2 TIFY 9 TIFY 10A(JAZ1)TIFY 6B(JAZ6)TIFY 3B-like(JAZ3)mc410 TIFY 6B-like MMK1 MYC2-like1 coronatine-insensitive protein 1(COI1)MYC2-like2 Pentatricopeptide repeat(PPR-like1)pentatricopeptide repeat(PPR-like2)TIFY 5A(JAZ5)得分Score 1.000 0.972 0.971 0.883 0.871 0.868 0.858 0.830 0.812 0.809 0.800 0.752 0.752 0.724蛋白号(甜樱桃)Protein ID(Prunus avium)XP_021802110 XP_021818119 XP_021804920 XP_021802863 XP_021804597 XP_021807187 XP_021821107 XP_021814026 XP_021814970 XP_021832998 XP_021816617 XP_021831619 XP_021831618 XP_021834265
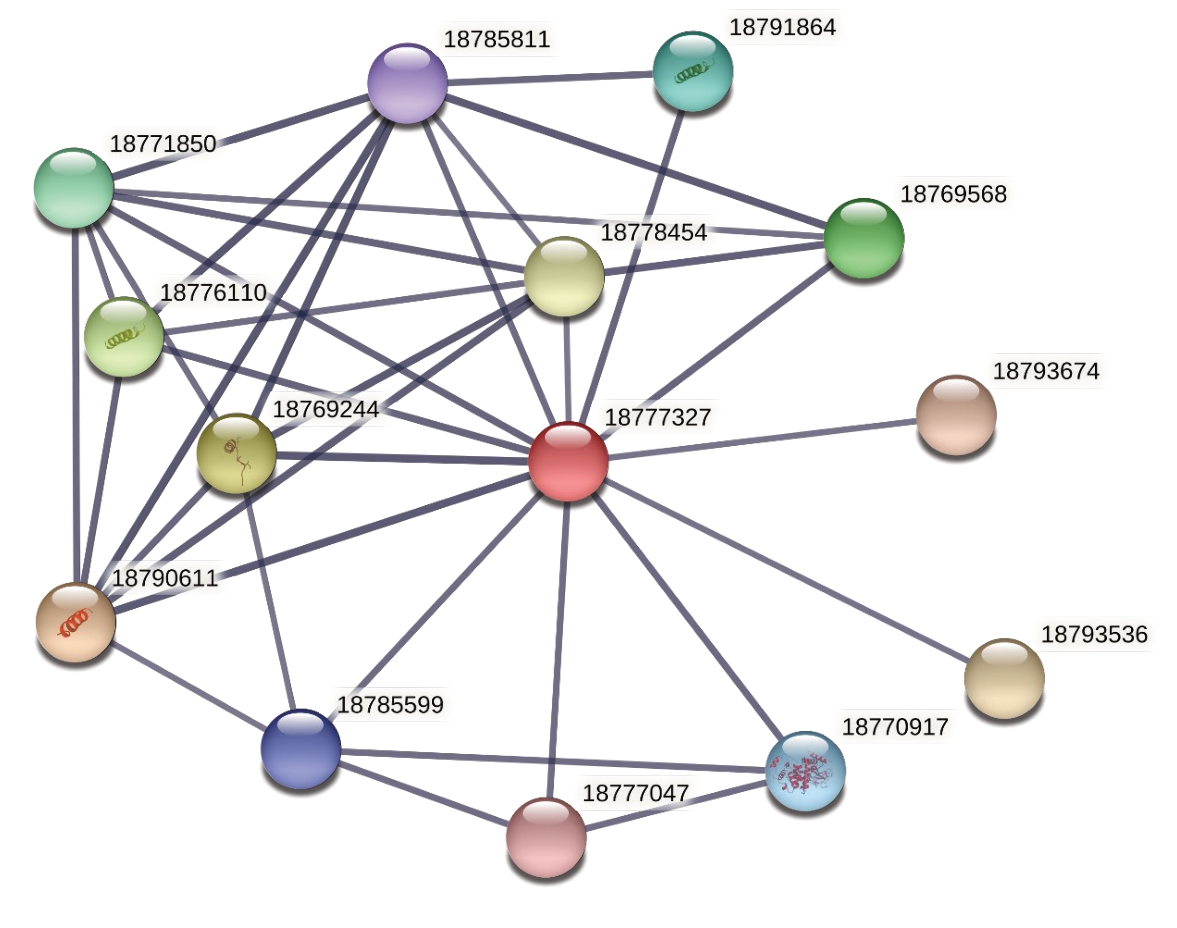
图3 与PavMYC2 互作的蛋白预测
Fig.3 Predicted proteins interacting with PavMYC2
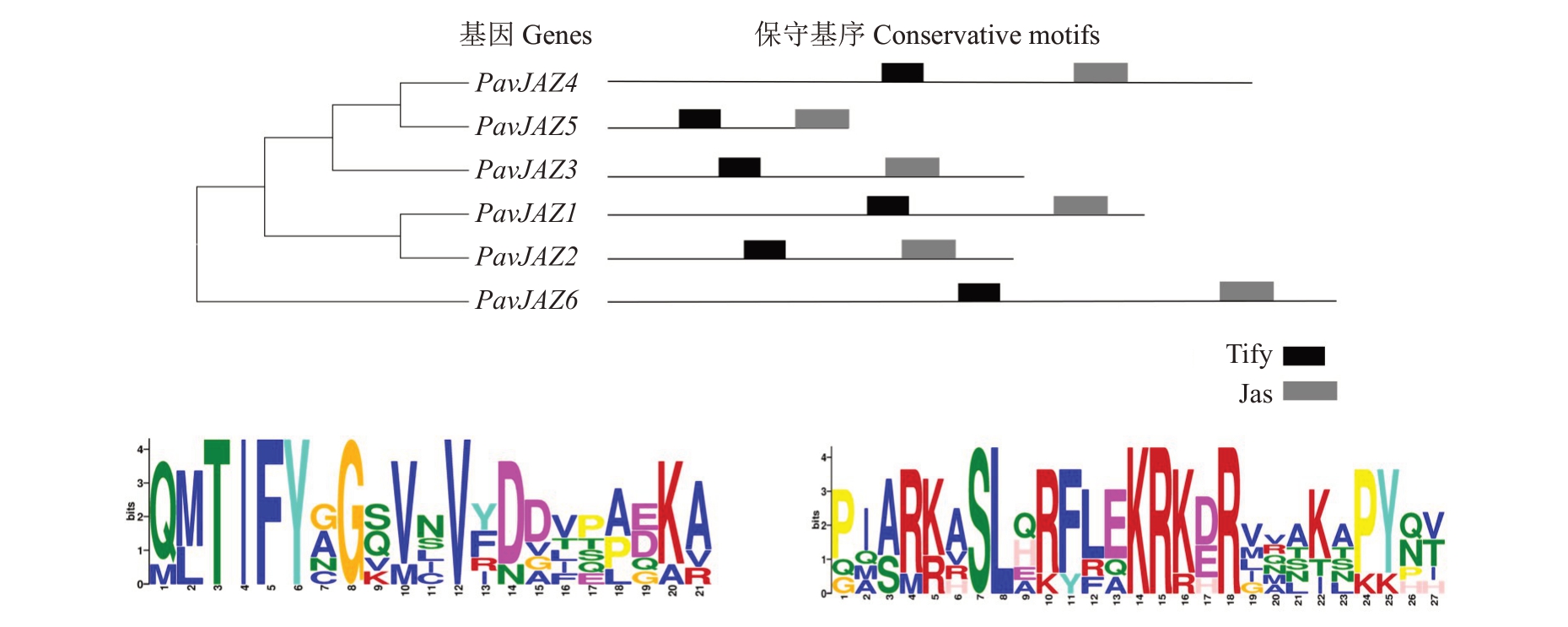
图4 PavJAZ1-6 蛋白保守基序分析
Fig.4 Amino acid motifs analysis of PavJAZ1-6 proteins
2.5 PavJAZs基因季节性表达趋势分析
通过INRA(https://bwenden.shinyapps.io/Dor-Patterns/)对PavJAZs在各个时期的相对表达量进行分析(图5),结果显示,PavJAZ1、2、3、5 的总体表达量呈现先上升后下降的趋势,并且在8 月份时表达量达到高峰。PavJAZ1、PavJAZ2、PavJAZ5 在高峰期之后表达量迅速下降,与PavMYC2的表达量变化趋势相似。然而,PavJAZ4 和PavJAZ6 的表达量不规则变化,推测二者与PavMYC2无直接的相关性。

图5 甜樱桃花芽中PavJAZ1-6 基因的季节性表达趋势
Fig.5 Seasonal expression levels of PavJAZ1-6 in sweet cherry flower buds
2.6 双分子荧光互补验证PavMYC2 与PavJAZs之间的互作
对PavMYC2 与PavJAZ1-PavJAZ6 分别进行双分子荧光互补实验,将构建好的PavMYC2-pXY104与PavJAZs-pXY106 的菌液充分混匀,注入烟草叶片中,3 d 后在激光共聚焦下观察荧光信号(图6)。结果显示,PavMYC2-pXY104+PavJAZ1-pXY106、PavMYC2-pXY104+PavJAZ2-pXY106和PavMYC2-pXY104+PavJAZ3-pXY106 组合可以观察到黄色的荧光信号,而且黄色荧光主要集中在细胞核上,而PavMYC2-pXY104+pXY106、pXY104+PavJAZ1-pXY106、pXY104+PavJAZ2-pXY106 和pXY104+PavJAZ3-pXY106 的组合中并未出现荧光信号,表明PavMYC2 与PavJAZ1、PavJAZ2 和PavJAZ3 之间存在蛋白互作,与PavJAZ4、PavJAZ5 和PavJAZ6 之间不存在蛋白互作。
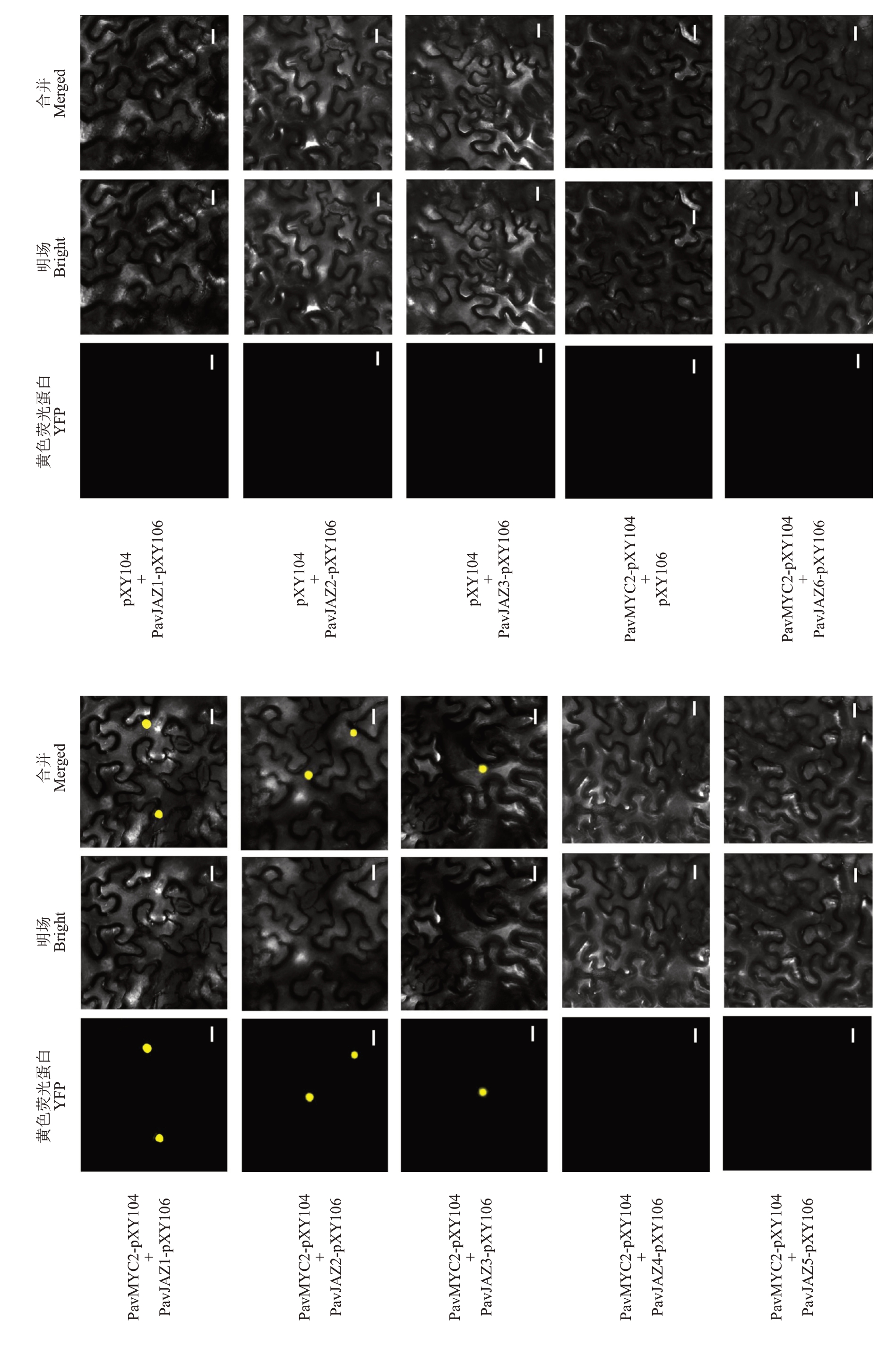
图6 PavMYC2 与PavJAZ1-6 蛋白互作分析
Fig. 6 Protein interactions between PavMYC2 and PavJAZ1-6
3 讨 论
花是植物重要的繁殖器官,在温带果树中,花发育的好坏决定了果实的数量和质量。夏季高温影响花芽分化,冬季低温会影响甜樱桃花芽的休眠进程,早春低温冻害会破坏甜樱桃的花蕾[12],所以如何响应温度胁迫对甜樱桃的生殖生长至关重要。
MYC2转录因子是bHLH转录因子家族的重要成员之一,在茉莉酸信号转导途径中起着核心调控作用,并且也参与了植物不同组织和器官应对逆境的过程[13-14]。本研究通过甜樱桃参考基因组设计引物,成功克隆了PavMYC2基因,其具有bHLH-Zip保守结构域,属于bHLH 家族。根据bHLH 特点和作用方式可以分为不同的亚族,有学者对拟南芥、水稻、藻类等bHLH 转录因子的各个方面进行综合分析,将其分为32 个亚族[15]。甜樱桃MYC2 基因的表达模式分析发现,其在花芽中的表达含量最高,说明PavMYC2基因主要在花芽中起作用,并且PavMYC2基因的表达趋势随季节变化,在夏秋季表达量最高,然后逐渐下降,在冬季维持一定的表达水平,春季开花时表达量最低,表明PavMYC2基因可能参与甜樱桃花芽响应高低温胁迫且和开花早晚有关。
茉莉酸可以提高植物可溶性蛋白含量,减轻高温造成的氧化伤害,维持植物正常生长[16-17]。MYC2转录因子激活茉莉酸途径下游基因表达,调控植物对温度胁迫的应答反应[18]。烟草中,高温诱导Nt-MYC2a 表达,促进茉莉酸的生物合成[19]。在研究植物耐冷性时,有学者发现,在热带果树香蕉中Ma-MYC2s 可能与MaICE1 之间存在相互作用,并且对CBF 低温应答途径相关基因的表达产生一定影响,在一定程度上提高了香蕉果实的耐寒性[20]。在外源MeJA 处理后,冬小麦TaMYC2 表达水平明显升高,而且脯氨酸和可溶性糖含量增加,耐寒性增强[21]。
植物开花主要受到光照和温度两个方面影响[22-23],光周期和光质对植物发育至关重要。光周期可以帮助植物预测每个季节到来的时刻[24],光质信号可以与温度信号相互作用调节植物开花[25]。研究表明MYC2 可以与FT(FLOWERING LOCUS T)基因的染色质区域特异性结合,并且通过MYC突变体的筛选证明MYC2/3/4 与FT 相互作用调节开花[26]。PavMYC2具有光启动子元件以及低温响应元件,可能通过外界的光和温度等环境条件影响PavMYC2基因表达,进而影响甜樱桃开花进程。
Zhai 等[27]研究表明JA 受体COI1 和JAZ 蛋白参与调控开花。JAZ蛋白是茉莉酸信号转导途径中的抑制子,JAZ1 蛋白抑制茉莉酸酯响应基因的转录[28]。感知激素信号后,JAZ 阻遏物可以通过释放MYC2 的蛋白酶体降解并且激活JA 的反应。在茉莉酸信号通路中,MdJAZ1/4 可以结合MdMYC2 共同影响苹果愈伤组织对低温的抵抗能力[29]。MYC2与大部分JAZ 蛋白相互作用[30],本研究发现甜樱桃MYC2 与JAZ1、JAZ2、JAZ3 之间存在互作,而与JAZ4、JAZ5、JAZ6 之间不存在相互作用,因此表明PavMYC2和JAZs在甜樱桃花芽中共同响应温度胁迫并协同调控开花。
MYC2基因在调控植物抵御高低温胁迫和开花过程中起着至关重要的作用,随着研究进一步深入,PavMYC2基因调控机制将逐渐明确,有助于阐明甜樱桃在不同温度胁迫下的生长发育机制,进一步提高其产量和品质。
4 结 论
笔者在研究中成功克隆PavMYC2基因,并且分别从生物信息和分子层面分析其结构,验证其功能。该基因在夏秋季花芽中高表达,随后逐渐下降,在冬季维持一定的表达水平,春季开花时最低。同时,PavMYC2与PavJAZ1/2/3互作,协同响应高低温胁迫。该研究为进一步探究甜樱桃花芽中MYC2在响应温度胁迫和调控开花进程中的作用奠定了基础。
[1] GAO L,MAZZA G. Characterization,quantitation,and distribution of anthocyanins and colorless phenolics in sweet cherries[J].Journal ofAgricultural and Food Chemistry,1995,43(2):343-346.
[2] QUERO-GARCÍA J,IEZZONI A,PULAWSKA J,LANG G.樱桃:科学与生产[M].张才喜. 译. 上海:上海交通大学出版社,2021:576-588.QUERO-GARCÍA J, IEZZONI A, PULAWSKA J, LANG G.Cherries:Botany,production and uses[M]. ZHANG Caixi.Trans. Shanghai:Shanghai Jiao Tong University Press,2021:576-588.
[3] 解玉玲.高温胁迫对植物生理影响的研究进展[J].吉林农业,2019(8):107-108.XIE Yuling. Research progress on the effects of high temperature stress on plant physiology[J]. Jilin Agriculture,2019(8):107-108.
[4] WANG J Y,LIU J X,JIU S T,LI Y T,MATTHEW W,SHE W J,WANG L,MA C,XU W P,WANG S P,ZHANG C X. The MADS-box genes PaMADS3/4/5 co-regulate multi-pistil formation induced by high temperature in Prunus avium L. [J]. Scientia Horticulturae,2019,256:108593.
[5] LIU J X,WANG J Y,SHE W J,WANG L,LUO M,CHEN Y J,LI Y T,WANG S P,ZHANG C X. MADS-Box genes are involved in cultivar- and temperature- dependent formation of multi-pistil and polycarpy in Prunus avium L.[J]. Journal of Plant Growth Regulation,2019,38(3):1017-1027.
[6] CLARKE S M,CRISTESCU S M,MIERSCH O,HARREN F J,WASTERNACK C,MUR L A. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana[J].New Phytologist,2009,182(1):175-187.
[7] LEE T M,LUR H S,CHU C. Role of abscisic acid in chilling tolerance of rice (Oryza sativa L.) seedlings:II. Modulation of free polyamine levels[J].Plant Science,1997,126(1):1-10.
[8] HU Y R,JIANG L Q,WANG F,YU D Q. Jasmonate regulates the inducer of CBF expression-C-repeat binding factor/DRE binding factor1 cascade and freezingtolerance in Arabidopsis[J].Plant Cell,2013,25(8):2907-2924.
[9] WASTERNACK C. Jasmonates:an update on biosynthesis,signal transduction and action in plant stress response,growth and development[J].Annals of Botany,2007,100(4):681-697.
[10] CHINI A,FONSECA S,FERNÁNDEZ G,ADIE B,CHICO J M,LORENZO O,GARCÍA-CASADO G,LÓPEZ-VIDRIERO I,LOZANO F M,PONCE M R,MICOL J L,SOLANO R.The JAZ family of repressors is the missing link in jasmonate signalling[J].Nature,2007,448(7154):666-671.
[11] SHEARD L B,TAN X,MAO H B,WITHERS J,BEN-NISSAN G,HINDS T R,KOBAYASHI Y,HSU F,SHARON M,BROWSE J,HE S Y,RIZO J,HOWE G A,ZHENG N. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ coreceptor[J].Nature,2010,468(7322):400-405.
[12] LIU J X,WANG J Y,SHE W J,WANG L,LUO M,CHEN Y J,LI Y T,WANG S P,ZHANG C X. MADS-box genes are involved in cultivar- and temperature- dependent formation of multi-pistil and polycarpy in Prunus avium L. [J]. Journal of Plant Growth Regulation,2019,38(3):1017-1027.
[13] 李罡,李文龙,许雪梅,李成浩.MYC2 转录因子参与植物发育调控的研究进展[J].植物生理学报,2019,55(2):125-132.LI Gang,LI Wenlong,XU Xuemei,LI Chenghao. Research progress of MYC2 transcription factors participating in plant development and regulation[J]. Plant Physiology Journal,2019,55(2):125-132.
[14] 沈乾,陆续,张凌,高尔娣,唐克轩.植物中MYC2 转录因子功能研究进展[J].上海交通大学学报,2012,30(6):51-56.SHEN Qian,LU Xu,ZHANG Ling,GAO Erdi,TANG Kexuan.Advances in the studies of MYC2 transcription factor in plants[J].Journal of Shanghai Jiao Tong University,2012,30(6):51-56.
[15] CARRETERO P L,GALSTYAN A,ROIG V I,MARTÍNEZGARCÍA J F,BILBAO-CASTRO J R,ROBERTSON D L. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis,poplar,rice,moss,and algae1[J].Plant Physiology,2010,153(3):1398-1412.
[16] 陈培琴,郁松林,詹妍妮,康喜亮.茉莉酸对葡萄幼苗耐热性的影响[J].石河子大学学报(自然科学版),2006,24(1):87-91.CHEN Peiqin,YU Songlin,ZHAN Yanni,KANG Xiliang. Effect of jasmonate acid on thermotolerance of grape seedlings[J].JournalofShiheziUniversity(NaturalScience),2006,24(1):87-91.
[17] 曹冬梅,王云山,康黎芳,张超,刘琛彬.高温对红掌幼苗叶片茉莉酸浓度及抗氧化系统的影响[J]. 中国生态农业学报,2007,15(5):102-104.CAO Dongmei,WANG Yunshan,KANG Lifang,ZHANG Chao,LIU Chenbin.Effect of high temperature on jasmonic acid(JA) concentration and antioxidation of Anthurium andraeanum seedling leaf[J]. Chinese Journal of Ecological Agriculture,2007,15(5):102-104.
[18] 丁永强. 茉莉酸和光照调控烟草叶片黄化的作用机制分析[D].重庆:西南大学,2017.DING Yongqiang.Regulation of leaf chlorosis by jasmonate and light signals in tobaco (Nicotiana tabacum L.)[D]. Chongqing:Southwest University,2017.
[19] YANG L M,LI J Y,JI J H,LI P,YU L L,ABD-ALLAH E F,LUO Y M,HU L W,HU X Y.High temperature induces expression of tobacco transcription factor NtMYC2a to regulate nicotine and JA biosynthesis[J].Frontiers in Physiology,2016,7:465.
[20] ZHAO M L,WANG J N,SHAN W,FAN J G,KUANG J F,WU K Q,LI X P,CHEN W X,HE F Y,CHEN J Y,LU W J.Induction of jasmonate signalling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit[J]. Plant Cell & Environment,2013,36(1):30-51.
[21] 赵虎.MeJA 对冬小麦抗寒生理及寒胁迫下TaCOI1/TaMYC2基因表达量的影响[D].哈尔滨:东北农业大学,2018.ZHAO Hu. Effect of MeJA on cold-resistance and cold stress of TaCoI1/TaMYC2 gene in winter wheat[D]. Haerbin:Northeast Agricultural University,2018.
[22] LEE J H,LEE J S,AHN J H.Ambient temperature signaling in plants:An emerging field in the regulation of flowering time[J].Journal of Plant Biology,2008,51:321-326.
[23] SANCHEZ S E,CAGNOLA J I,CREPY M,YANOVSKY M J,CASAL J J. Balancing forces in the photoperiodic control of flowering[J]. Photochemical & Photobiological Sciences,2011,10(4):451-460.
[24] IÑIGO S,ALVAREZ M J,STRASSER B,CALIFANO A,CERDÁN P D. PFT1,the MED25 subunit of the plant mediator complex,promotes flowering through CONSTANS dependent and independent mechanisms in Arabidopsis[J]. The Plant Journal,2012,69(4):601-612.
[25] FOREMAN J,JOHANSSON H,HORNITSCHEK P,JOSSE E M,FANKHAUSER C,HALLIDAY K J.Light receptor action is critical for maintaining plant biomass at warm ambient temperatures[J].The Plant Journal,2011,65(3):441-452.
[26] WANG H P,LI Y,PAN J J,LOU D J,HU Y R,YU D Q. The bHLH transcription factors MYC2,MYC3,and MYC4 are required for Jasmonate-Mediated inhibition of flowering in Arabidopsis[J].Molecular Plant,2017,10(11):1461-1464.
[27] ZHAI Q Z,ZHANG X,WU F M,FENG H L,DENG L,XU L,ZHANG M,WANG Q M,LI C Y.Transcriptional mechanism of jasmonate receptor COI1-Mediated delay of flowering time in Arabidopsis[J].The Plant Cell,2015,27(10):2814-2828.
[28] THINES B,KATSIR L,MELOTTO M,NIU Y J,MANDAOKAR A,LIU G H,NOMURA K,HE S Y,HOWE G A,BROWSE J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signaling[J]. Nature,2007,448(7154),661-665.
[29] WANG Y C,XU H F,LIU W J,WANG N,QU C Z,JIANG S H,FANG H C,ZHANG Z Y,CHEN X S.Methyl jasmonate enhances apple cold tolerance through the JAZ-MYC2 pathway[J].Plant Cell,Tissue and Organ Culture,2019,136:75-84.
[30] FERNÁNDEZ-CALVO P,CHINI A,FERNÁNDEZ-BARBERO G,CHICO J M,GIMENEZ-IBANEZ S,GEERINCK J,EECKHOUT D,SCHWEIZER F,GODOY M,FRANCO-ZORRILLA J M,PAUWELS L,WITTERS E,PUGA M I,PAZARES J,GOOSSENS A,REYMOND P,JAEGER G D,SOLANO R. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses[J]. The Plant Cell,2011,23(2):701-715.