柑橘是我国南方重要果树,在其果实成熟过程中对丰产树进行立杆拉枝,可以避免果实挤压和枝干折断,确保果实品质和树体健康。但是,立杆拉枝需要大量劳动投入。在当前从业劳动力不足和老龄化现象日渐严重的情况下,降低劳动投入对实现柑橘种植降本增效非常必要。促进枝干增粗、简化树体结构有助于立杆拉枝的逐步取消。但是,柑橘枝梢增粗机制及调控措施的相关研究严重滞后。
目前,植物增粗生长的研究主要集中在杨树[1-5]和拟南芥[6-13]等模式植物上。植物增粗生长主要受维管形成层的驱动。维管形成层在茎中呈闭环状态并且具有持续分裂的能力,通过平周分裂和切向分裂不断向内外产生次生木质部和次生韧皮部[9,14-16]。维管组织的不断积累是双子叶和裸子植物的茎不断增粗的重要原因[12,17-19]。此外,维管形成层的分子调控途径的相关研究亦有报道。拟南芥根部维管形成层的研究表明,形成层的活动受WOX4、WOX14、MYB87、KNAT1 和PTL 等基因的转录因子构建而成的网络调控[4,7,11,17,20];杨树茎部的转录组学分析表明,MYB家族和NAC家族包括MYB59、MYB46、NAC2、NAC3、NST1、AF1等基因,均参与杨树茎部次生生长的调控[1-2,13]。此外,植物激素是调控植物生长发育的重要因素,对维管形成层的活动起到了关键作用[3,5,10,21-23]。例如,生长素响应基因ARF5能够通过激活ATHB8 来诱导生长素转运载体基因PIN1 的表达,从而在木本植物次生生长中发挥作用[5,10];细胞分裂素能够引起萝卜根中形成层相关转录因子的差异表达,进而影响形成层细胞的增殖活性[22];ARR11和ARR12 是属于磷酸化介导的细胞分裂素信号途径中结合DNA的转录因子,其中ARR12能够影响生长素向内运输[23],而细胞分裂素可以通过阻断生长素的梯度重塑进而影响杨树形成层的形成[3]。以上报道表明植物增粗生长受到木质部、韧皮部、形成层以及激素代谢相关基因的表达调控,在柑橘枝梢增粗过程中是否如此,目前并不清楚。
笔者在本试验中以红心柚和南丰蜜橘为材料,从解剖和分子2个层面比较直立枝和斜生枝的增粗差异,以期明确柑橘直立枝梢比斜生枝梢增粗快的原因,为后续柑橘枝梢增粗的进一步研究、生产上轻简化树形培育奠定基础。
1 材料和方法
1.1 试验材料
2020年秋季(2020-09-08)随机选择枳砧红心柚(Citrus grandis‘Hongxinyou’)、枳砧南丰蜜橘(C.reticulata‘Kinokuni’)直立枝(垂直于地面水平线)和斜生枝(与地面水平线呈45°~60°)发育良好、老熟程度基本一致的秋梢各30根,分别用游标卡尺和卷尺测定枝梢(基部)的粗度和长度,然后各随机剪取10根枝梢,去掉叶片放入冰盒中带回实验室。
1.2 目的基因挖掘和引物设计
根据拟南芥和杨树中与次生生长相关的基因登录号[2,9],利用同源比对法分别在Phytozome(http://www.phytozome.net)甜橙、克里曼丁基因组数据库和华中农业大学甜橙基因组数据库[24](http://citrus.hzau.edu.cn/orange/)中筛选出柑橘中次生生长相关基因的序列。筛选的标准为有相同的注释名称和evalue≤e-30。将查找到的同一类基因利用BioEdit 软件进行序列比对分析[25],确定目的基因及其序列,采用Primer 5.0进行定量引物设计(表1)。
表1 柑橘增粗相关基因及其引物
Table 1 Secondary growth-related genes in citrus and their primer sequences

归类Category木质部中基因Genes in xylem韧皮部中基因Genes in phloem形成层中基因Genes in cambium激素相关基因Phytohormone-related genes基因名Gene name KNAT1 STM SND1 BOP1/2 APL BRX BRXL2 BRXL4 NAC45/86 NEN1/2-1 NEN1/2-2 NEN4 OPS BAM3 BES1 WOX4 WOX14 CLE8 HAM4 KNAT6 MYB46-1 MYB46-2 MYB59 PXY SD1 NAC2 AF1 NAC3 NST1 ARR11 ARR12 ARF5 ATHB8 PIN1序列ID Sequence ID Cs3g16460 Cs2g02590 Cs5g01350 Orange1.1t00760 Orange1.1t03247 Cs3g07250 Cs1g17720 Orange1.1t00156 Orange1.1t03242 Cs5g11010 Orange1.1t03999 Cs1g01750 Cs6g07730 Cs2g12040 Cs3g24470 Cs3g23280 Cs1g26550 Cs7g12750 Cs1g23790 Cs7g08990 Cs2g30230 Cs7g01800 Orange1.1t00911 Cs9g14980 Cs9g07450 Cs7g21830 Cs3g19890 Cs1g06760 Orange1.1t00561 Cs7g10020 Cs7g06180 Cs3g25860 Cs4g19310 orange1.1t00089引物序列Primer sequence(5’-3’)正向引物Forward primer AGCCAAGATCATCTCCCA TAATGGCTCATCCTCACT AATCGGATTGAGAAAGAC CTACTGCTTCCCGTATCAA GCTTACGCTTTACCACCT CGGCAAGCTCTTAACACT CCAATACACTGGCAGGTC AAAGATGGTGGGCTGAAA GGCACCTGTAGGATTACC CTCCAACCCTACTCCACTC GCTGTAACGGCATCACTC AGTTCGGAGCAATCGTAG GCAAATGGGAATAGCAAA TCCGCAGTCTGGTCTATC GAGGAAGACGGCACCACT GATAGGGATACTGGAGATGC ACTTACGCAACCTCCCTC ACCACCAGCAATACTATCC GTCGTGCCGTTGTTTAG CCGATGAGTTACTATCTGCT AGCCACGCAACAGCAAT GAAAGGCAATAACAACAAC CTCTTCGTCCTCTAACTACTC AACAGCATTGTGGATTGGGTG GGGACAGCCACATTAGCC TTGCCTCGGAGTTCCCTAC TGAGTTACAGTTACCACCAGG GTCTTGCGGGTCTTCATT GAGCTACTGCTGCTGGTT CATTGCCAGGACCACATC ACCACAAGTCAGGCAATC TGGCACATCTTCGAGTAA ATGAGGGATGGTCTATGTT GTGCCAGGTTGCTTATCT反向引物Reverse primer TCGTAATAAGCCTCCATAA GATCTTGACCGATACAGC CATTACTGAAGTGGAGGTT ACAACCTCGTTCACCACA TTGCATCCTAATCGCATC CGTATCTCGGACCACCTC TAGTAGGCGTTCCAATCA TTGGACGATACAAGTTACGAG CTGAACCCATTCGGATAT CTCGCACAATCAAACTCC CTTATTCTCGCACAATCAAA ATATTATGCCCTGCCCAC CGGCGTCAAGTAGAACCT GAACCGTTGAACTCGTTG CTCGGAAAGGATGAAGATAAAG CAATGGCTAAGACCAAGAC TCCACCCATCTTTCCTGT TCAATCTCCGCACTTCCA CCGCAGCAGATAGGTTTC CTCCTACCTTCTGGCAAT ATCCAGCGAAGGCGACAG AAAGGAAAGGAGGAAATG TTGCCATCCTGTTTCATA GCTTCGGCTTCGCTTCCT CATTTCTGCCCTCCTTGC ATTCTTGCCAACCGACCA CTTGAACCGTTTGGATACTT TGATTCCTAAGCCCACTC ATTTCATTGGGATTGTCG AAAGACGACCCAACTCCC TCCATCTCAAGTCCCACA ATCGCAGAGCATAGTTCA AGAAGTATTGCTGGAGGC ATCTCAGCGTCTGTTTCA
1.3 石蜡切片
随机剪取5根枝梢上部(距枝梢顶端5 cm)和下部(距枝梢基部5 cm)2~3 mm 的圆柱形茎段,迅速保存于福尔马林醋酸乙醇(formalin-acetic acid-alcohol,FAA)固定液中,采用微波快速石蜡切片法[26]进行切片和解剖结构观察。
1.4 总RNA提取和cDNA合成
随机选择直立枝和斜生枝各3 根,分别剪碎混匀后迅速用液氮处理。枝梢总RNA 提取采用艾德莱TRIpure Reagent(Aidlab,北京)提取剂和生工植物柱式RNA 提取试剂盒。参照TransScript Onestep gDNA Removal and cDNA Synthesis SuperMix试剂盒(北京全式金生物技术)中的方法,将1 μg高质量总RNA合成cDNA。
1.5 实时荧光定量PCR分析
实时荧光定量PCR(quantitative real-time PCR,qRT-PCR)分析采用Power SYBR Green PCR Master Mix试剂盒(翊圣公司),反应体系为:0.5 μL cDNA、5.0 μL Mix、0.2 μL正向引物、0.2 μL反向引物、4.1 μL ddH2O,每个样品进行4 次重复。采用QuantStudioTM 6Flex Real-time PCR System,程序反应为:准备阶段,50 ℃2 min,95 ℃5 min;扩增阶段,95 ℃15 s,60 ℃15 s,72 ℃30 s,40 个循环。参照2−ΔΔCt法[27]计算基因的相对表达量。
1.6 数据分析
试验数据利用SPSS V20、采用最小显著性差异法(least significant difference,LSD)进行差异性显著分析,p<0.05认定为处理之间差异显著。
2 结果与分析
2.1 生长比较
柑橘直立枝垂直地面,一般生长势较旺,而斜生枝与地面形成一定角度,一般缓慢向上生长(图1-A)。分别测定红心柚、南丰蜜橘的秋梢直立枝和斜生枝长度和粗度,发现红心柚、南丰蜜橘的直立枝的粗度均显著大于斜生枝,2 个品种的直立枝的长度略长于斜生枝,但是差异不显著(图1-B~C)。
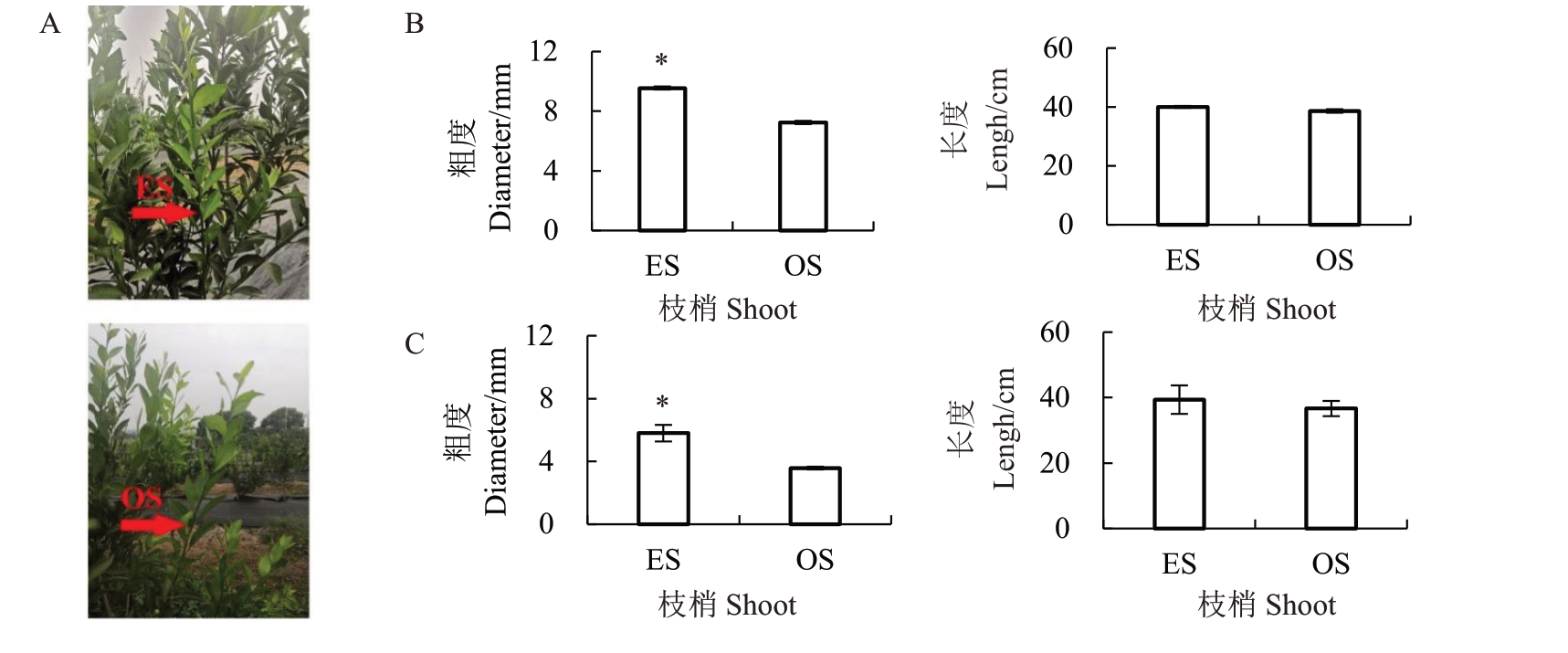
图1 红心柚、南丰蜜橘直立枝和斜生枝生长差异比较
Fig.1 Comparison of the shoot growth between the erect and the oblique shoots of red pomelo(C.grandis‘Hongxinyou’)and Nanfeng tangerine(C.reticulata‘Kinokuni’)
A.直立枝和斜生枝生长状态;B.红心柚秋梢直立枝和斜生枝粗度和长度;C.南丰蜜橘秋梢直立枝和斜生枝粗度和长度。ES、OS 分别代表直立枝、斜生枝。*表示直立枝和斜生枝粗度和长度的差异达到显著差异水平(p<0.05)。下同。
A.Growth status of erect and oblique shoots;B.The length of erect and oblique shoots of hongxinyou;C.The length of erect and oblique shoots of Nanfeng tangerine.ES and OS represent erect shoots and oblique shoots,respectively.*represents significant difference of the diameter and length between erect shoots and oblique shoots at p<0.05 by t-test.The same below.
2.2 解剖结构比较
进一步分析红心柚直立枝和斜生枝解剖结构,发现上部分茎中以髓部为主,髓细胞较大,未形成明显的髓射线,初生木质部初步形成,形成层细胞排列整齐;其中直立枝的初生韧皮部细胞要大于斜生枝、形成层宽度显著高于斜生枝、木质部宽度显著低于斜生枝(图2-A)。此外,枝梢下部茎中主要以次生木质部为主,髓射线明显凸现,导管细胞数量增加,形成层细胞呈扁平长方形、排列紧密,直立枝形成层和木质部的宽度均显著高于斜生枝(图2-B)。
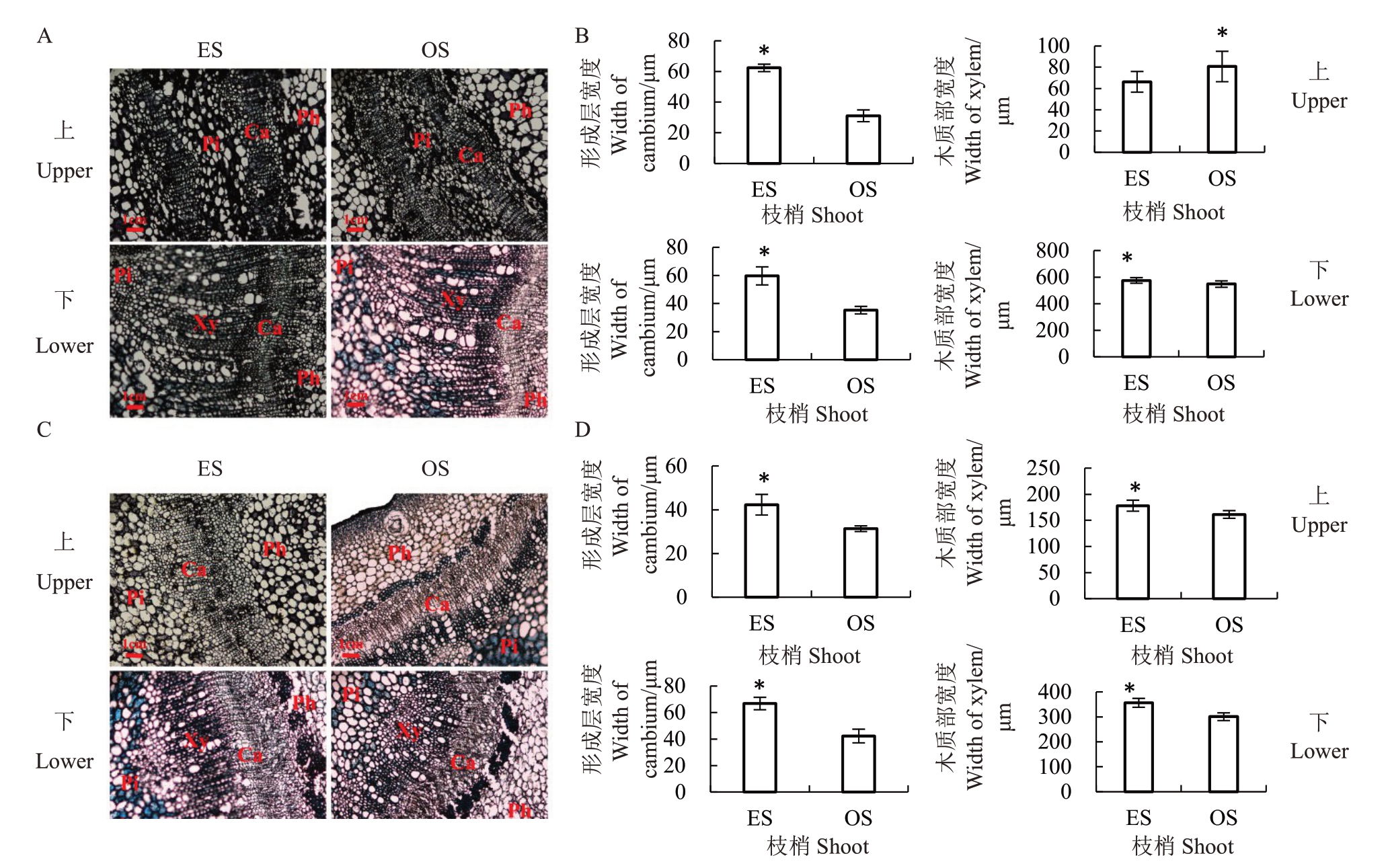
图2 红心柚、南丰蜜橘直立和斜生枝的横切结构
Fig.2 The upper and lower transverse sections of erect and oblique shoots of red pomelo(C.grandis‘Hongxinyou’)and Nanfeng tangerine(C.reticulata‘Kinokuni’)
A.红心柚枝梢上下部横切结构;B.红心柚枝梢上下部形成层和木质部的宽度;C.南丰蜜橘枝梢上下部横切结构;D.南丰蜜橘枝梢上下部形成层和木质部的宽度。Pi.髓;Xy.木质部;Ca.形成层;Ph.韧皮部。
A.The upper and lower transverse sections of Hongxinyou erect and oblique shoots;B.The width of cambium and xylem in upper and lower shoots of Hongxinyou;C.The upper and lower transverse sections of erect and oblique shoots of Nanfeng tangerine;D.The width of cambium and xylem in upper and lower shoots of Nanfeng tangerine.Pi,Xy,Ca,and Ph refer to pith,xylem,cambium,and phloem,respectively.
进一步分析南丰蜜橘直立枝和斜生枝解剖结构,发现枝梢上部的髓部及其细胞较大,形成层的细胞呈扁平状,排列比较规整;其中直立枝韧皮部的细胞数量多于斜生枝,但是暂未形成明显的次生木质部,而斜生枝则已经形成次生木质部,并出现导管细胞(图2-C)。此外,直立枝上部形成层和木质部宽度均显著高于斜生枝,枝梢下部均以木质部为主,髓射线明显形成;直立枝的导管细胞数量少但细胞较大,形成层细胞数量多且比斜生枝排列紧致;直立枝下部的形成层和木质部宽度均显著高于斜生枝(图2-D)。
2.3 木质部内增粗相关基因表达比较
通过分析4个木质部内增粗相关基因的表达发现(图3),红心柚中KNAT1 和STM 在直立枝中的相对表达量显著高于斜生枝,但是在南丰蜜橘中的相对表达量无显著差异;2个品种直立枝中SND1的相对表达量均显著高于斜生枝,而BOP1/2 的相对表达量在直立枝和斜生枝间无显著差异。
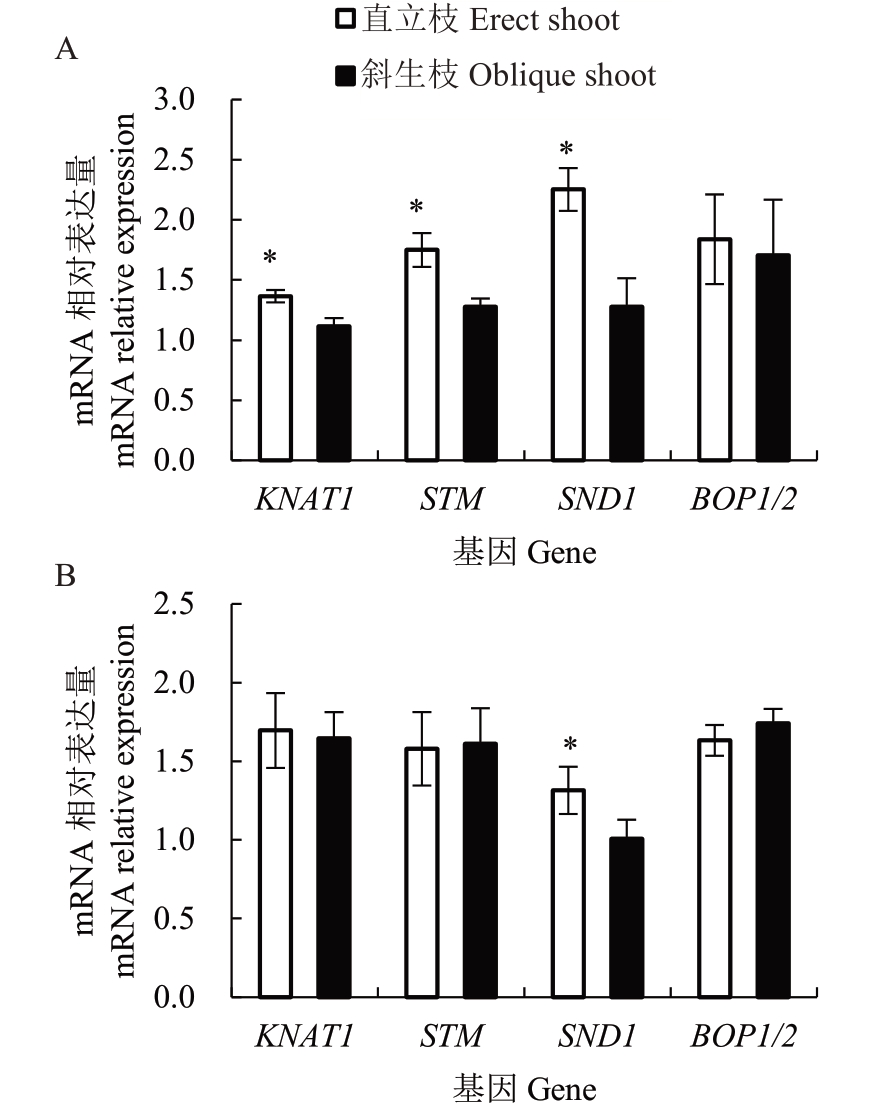
图3 红心柚、南丰蜜橘木质部中增粗相关基因的相对表达量比较分析
Fig.3 Comparative analysis of the expression of thickening-related genes in xylem of red pomelo(C.grandis‘Hongxinyou’)and Nanfeng tangerine(C.reticulata‘Kinokuni’)
A.红心柚;B.南丰蜜橘。数据表示4 个重复的平均值±标准误。下同。
A and B represent Honxinyou and Nanfeng tangerine, respectively.Values are means of four replications±SE.The same below.
2.4 韧皮部内增粗相关基因表达比较
通过分析10 个韧皮部内增粗相关基因的表达(图4),发现红心柚直立枝中APL、BRX、BRXL2、NEN1/2-1、NEN4和BAM3的相对表达量均显著低于斜生枝,而BRXL4 和NAC45/86 的相对表达量显著高于斜生枝,NEN1/2-2 和OPS 则无显著差异;在南丰蜜橘中,直立枝中的APL、BRXL2、BRXL4、NAC45/86 和NEN1/2-1 的相对表达量显著低于斜生枝,而NEN4、OPS 和BAM3 的相对表达量显著高于斜生枝,NEN1/2-2 和BRX 的相对表达量则无显著差异。综合来看,2 个品种直立枝中的APL 和BRXL2 的相对表达量均显著低于斜生枝。
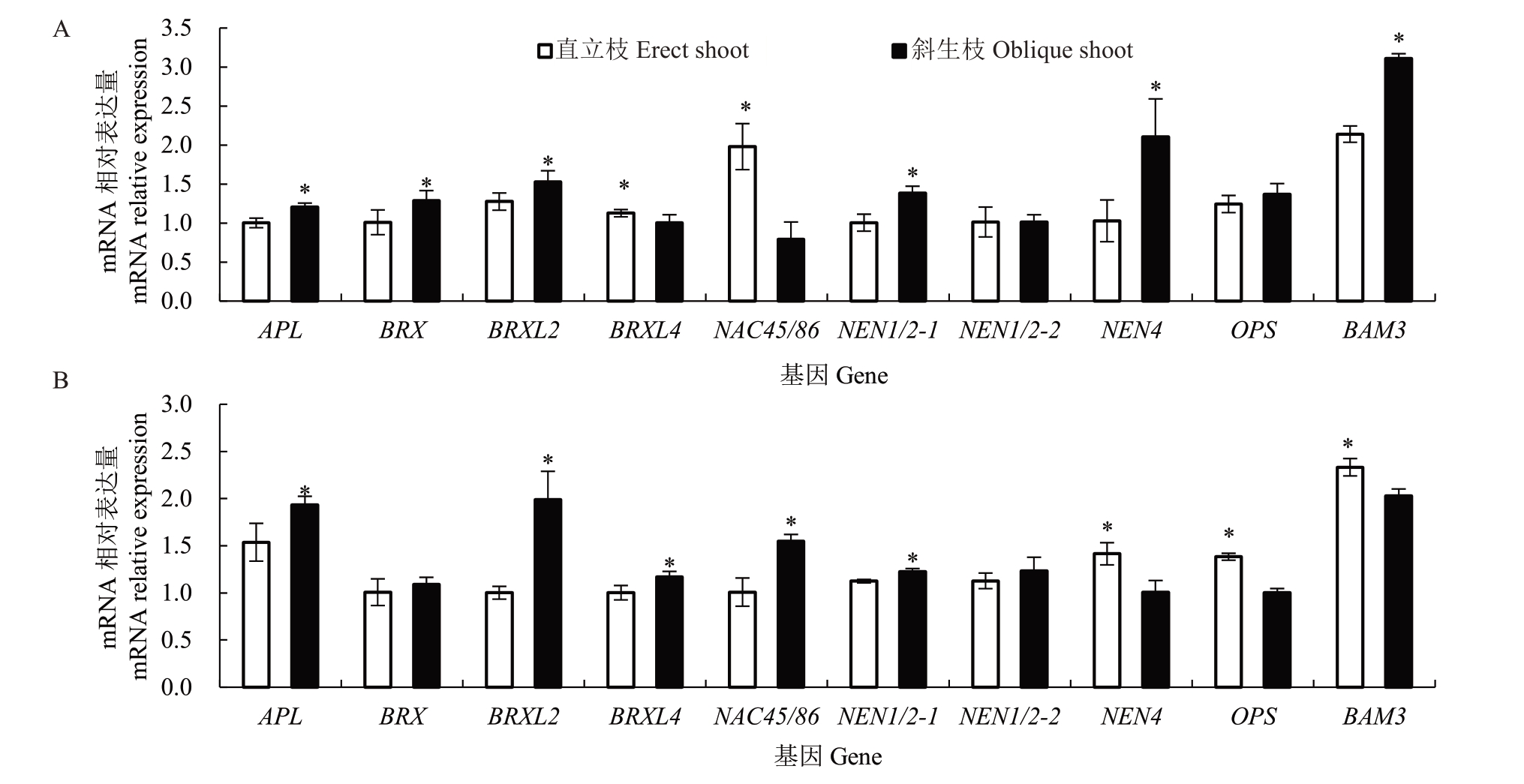
图4 红心柚、南丰蜜橘韧皮部中增粗相关基因的表达量比较分析
Fig.4 Comparative analysis of the expression of thickening-related genes in phloem of red pomelo(C.grandis‘Hongxinyou’)and Nanfeng tangerine(C.reticulata‘Kinokuni’)
2.5 形成层内增粗相关基因表达比较
通过分析15 个在形成层内增粗相关基因的相对表达量(图5),发现红心柚直立枝中的WOX4、WOX14、MYB46-1、MYB46-2、PXY、AF1和NST1的相对表达量显著高于斜生枝,而BES1、CLE8、HAM4和NAC3的相对表达量则显著低于斜生枝,KNAT6、MYB59、SD1 和NAC2 的表达量无显著差异;南丰蜜橘直立枝中的WOX4、CLE8、MYB46-1 和PXY 的相对表达量显著高于斜生枝,而BES1、WOX14、HAM4、KNAT6、MYB46-2、MYB59、SD1、NAC2、AF1和NAC3 的相对表达量则显著低于斜生枝,NST1 在直立枝和斜生枝间的相对表达量无显著差异。综合来看,2 个品种直立枝中WOX4、PXY 和MYB46-1 的相对表达量均显著高于斜生枝,而BSE1、HAM4 和NAC3的相对表达量均显著低于斜生枝。
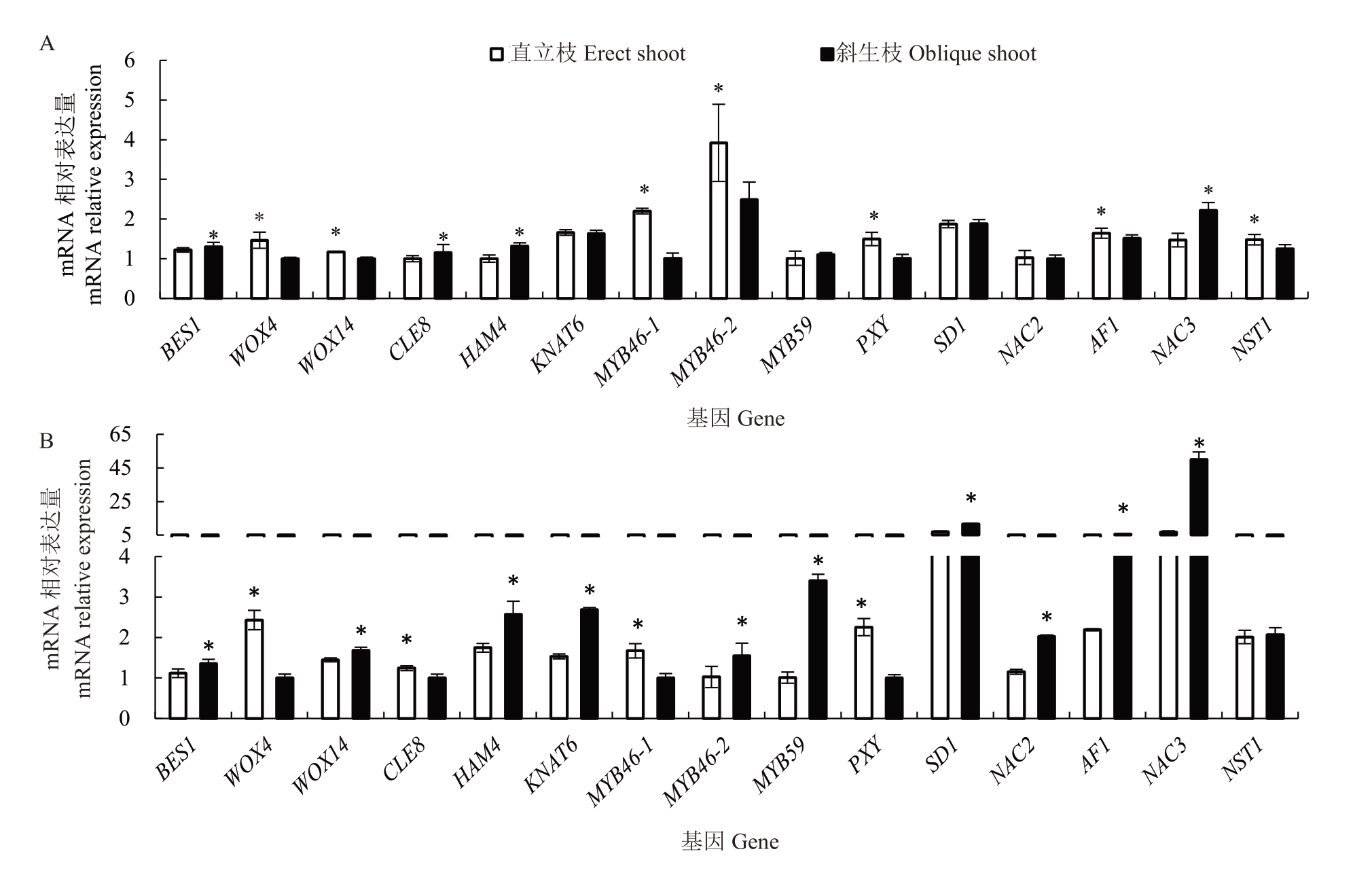
图5 红心柚、南丰蜜橘形成层中增粗相关基因的表达量比较分析
Fig.5 Comparative analysisn of the expression of thickening-related genes in cambium of red pomelo(C.grandis‘Hongxinyou’)and Nanfeng tangerine(C.reticulata‘Kinokuni’)
2.6 增粗相关的激素基因表达比较
通过比较细胞分裂素合成基因(ARR11 和ARR12)以及生长素响应运输相关基因(ARF5、ATHB8、PIN1)的相对表达量(图6),发现红心柚的直立枝和斜生枝中ARR11 和ARR12 的相对表达量无显著差异,ARF5在直立枝中的相对表达量显著高于斜生枝,而ATHB8 和PIN1 的相对表达量显著低于斜生枝;南丰蜜橘直立枝中ARR11 的相对表达量显著低于斜生枝,而ARR12的相对表达量则显著高于斜生枝,ARF5 和ATHB8 在直立枝中的相对表达量显著低于斜生枝,PIN1的相对表达量在直立枝和斜生枝间没有显著差异。
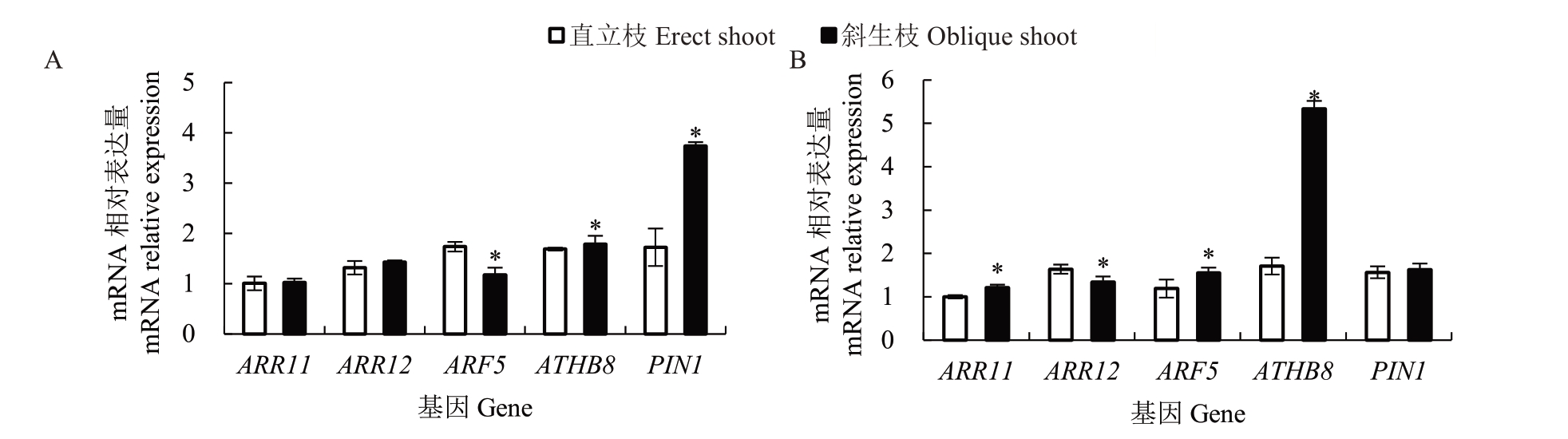
图6 红心柚、南丰蜜橘枝梢中细胞分裂素合成和生长素运输相关基因的表达量比较分析
Fig.6 Comparative analysis of the expression of cytokinin synthesis and auxin transport related genes in shoots of red pomelo(C.grandis‘Hongxinyou’)and Nanfeng tangerine(C.reticulata‘Kinokuni’)
3 讨 论
维管形成层是不断进行细胞分裂的侧分生组织[15-16]。维管形成层的活动能够影响植物茎的增粗生长[12,18-19],有效增加花序茎和下胚轴的径向直径[11-12]。前人研究发现,形成层组织可以不断向内外分化成木质部和韧皮部组织[6,15,18],并且杨树中木质部的产出速率明显高于韧皮部,从而木质部不断积累形成更多有用的木材[1,4]。本研究中,无论是红心柚还是南丰蜜橘的直立枝粗度均显著高于斜生枝,其形成层和木质部的横截面宽度也均显著高于斜生枝,说明柑橘枝梢增粗的快慢与形成层的分裂和木质部增粗快慢有密切关系。
维管形成层属于多向分化的次生分生组织[15-17],受激素、基因、激酶和多肽等因子的调控[5,8,21],其中以PXY-WOX4调控途径研究最为广泛。WOX4属于WOX 转录因子家族成员,是CLE41/44 相关信号通路的关键靶点,并且位于PXY 途径下游[7]。PXY 是导管分子分化的抑制因子(tracheary element differentiation inhibitory factors,TDIF)受体,属于富含亮氨酸的受体激酶,主要在形成层中表达,同时还参与了细胞分裂素的信号传导[28-29]。TDIF-PXY 调节下游WOX4 以维持原形成层和形成层细胞的分裂活性[8]。在杨树中,PtPXY和PtWOX4共同调节杨树茎的次生生长,TDIF-PXY-WOX4 形成固定保守的途径,调节着植物维管组织的活性[4]。同样在拟南芥中,经过RNA 干扰处理后,WOX4 的低表达强烈抑制形成层的细胞分裂[20]。在本研究中,红心柚和南丰蜜橘直立枝的粗度显著高于斜生枝,而且直立枝中WOX4 和PXY 的相对表达量显著高于斜生枝,说明PXY和WOX4的高表达是柑橘直立枝梢增粗生长高于斜生枝的重要原因。
另外,前人研究发现SND1 是参与木质部纤维次生壁合成的主要激活因子,在茎的束间纤维中特异性表达,其高表达是促进植物增粗生长过程中木质部纤维和导管细胞次生壁加厚的重要原因,而SND1 的低表达会导致纤维次生壁厚度明显下降[13]。本研究解剖发现,2个柑橘品种直立枝的木质部厚(宽)度显著高于斜生枝,而直立枝中SND1 的相对表达量也显著高于斜生枝,说明SND1 的高表达可能是直立枝的木质部纤维和导管细胞次生壁厚度显著高于斜生枝的重要原因。
植物增粗生长同样受到内源激素的调控,木本植物中的生长素-ARF5-ATHB8-PIN1 正反馈调节环在次生生长过程中起调控作用[5,10]。ARR11 和ARR12 在细胞分裂素转导途径中起正调控作用[3,22-23,29]。本研究中,生长素和细胞分裂素相关基因在红心柚和南丰蜜橘直立枝和斜生枝中的表达并没有表现出一致的规律,说明其可能不是决定柑橘直立枝和斜生枝粗度差异的重要因素。
4 结 论
柑橘直立枝粗度显著高于斜生枝,并且与直立枝的形成层层数增加和木质部增厚有关。其中,PXY、WOX4这2个基因的表达量高低可能是决定柑橘枝梢增粗生长快慢的关键因素,而SND1 高表达可能是柑橘直立枝比斜生枝木质部更厚(宽)的重要原因。相关基因的功能有待进一步验证。
[1]BOSSINGER G,SPOKEVICIUS A V.Sector analysis reveals patterns of cambium differentiation in poplar stems[J].Journal of Experimental Botany,2018,69(18):4339-4348.
[2]CHAO Q,GAO Z F,ZHANG D,ZHAO B G,DONG F Q,FU C X,LIU L J,WANG B C.The developmental dynamics of the Populus stem transcriptome[J].Plant Biotechnology Journal,2019,17(1):206-219.
[3]CHEN J J,WANG L Y,IMMANEN J,NIEMINEN K,SPICER R,HELARIUTTA Y,ZHANG J,HE X Q.Differential regulation of auxin and cytokinin during the secondary vascular tissue regeneration in Populus trees[J].New Phytologist,2019,224(1):188-201.
[4]KUCUKOGLU M,NILSSON J,ZHENG B,CHAABOUNI S,NILSSON O.WUSCHEL-RELATED HOMEOBOX4 (WOX4)-like genes regulate cambial cell division activity and secondary growth in Populus trees[J].New Phytologist,2017,215(2):642-657.
[5]ZHU Y Y,SONG D L,XU P,SUN J Y,LI L G.A HD-ZIP III gene,PtrHB4,is required for interfascicular cambium development in Populus[J].Plant Biotechnology Journal,2018,16(3):808-817.
[6]CHAFFEY N,CHOLEWA E,REGAN S,SUNDBERG B.Secondary xylem development in Arabidopsis:A model for wood formation[J].Physiologia Plantarum,2002,114(4):594-600.
[7]ETCHELLS J P,PROVOST C M,MISHRA L,TURNER S R.WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation[J].Development,2013,140(10):2224-2234.
[8]HIRAKAWA Y,KONDO Y,FUKUDA H.TDIF Peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis[J].Plant Cell,2010,22(8):2618-2629.
[9]LEHMANN F,HARDTKE C S.Secondary growth of the Arabidopsis hypocotyl-vascular development in 4 dimensions[J].Current Opinion in Plant Biology,2016,29:9-15.
[10]MÜLLER C J,VALDÉS A E,WANG G D,RAMACHANDRAN P,BESTE L,UDDENBERG D,CARLSBECKER A.PHABULOSA mediates an auxin signaling loop to regulate vascular patterning in Arabidopsis[J].Plant Physiology,2015,170(2):956-970.
[11]ZHANG J,ESWARAN G,SERRA J A,KUCUKOGLU M,XIANG J L,YANG W B,ELO A,NIEMINEN K,DAMÉN T,YUN Y J,LEE J H,RAGNI L,REUILLE P B,E.AHERT S,LEE J Y,MÄHÖNEN P,HELARIUTTA Y.Transcriptional regulatory framework for vascular cambium development in Arabidopsis roots[J].Nature Plants,2019,5(10):1033-1042.
[12]ZHAO C S,CRAIG J C,PETZOLD H E,DICKEMAN A W,BEERS E.The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl[J].Plant Physiology,2005,138(2):803-818.
[13]ZHONG R Q,DEMURA T,YE Z H.SND1,a NAC domain transcription factor,is a key regulator of secondary wall synthesis in fibers of Arabidopsis[J].Plant Cell,2006,18(11):3158-3170.
[14]潘国清.植物茎构造剖析[J].现代农业科技,2009(11):108-109.PAN Guoqing.Anatomy of plant stem structure[J].Modern Agricultural Sciences and Technology,2009(11):108-109.
[15]李正理.维管形成层的活动及其增生方式[J].生物学通报,1983,18(4):7-9.LI Zhengli.The activity of vascular cambium and its proliferation mode[J].Bulletin of Biology,1983,18(4):7-9.
[16]JOUANNET V,BRACKMANN K,GREB T.(Pro)cambium formation and proliferation:Two sides of the same coin?[J].Current Opinion in Plant Biology,2015,23:54-60.
[17]DE RYBEL B,MÄHÖNEN A P,HELARIUTTA Y,WEIJERS D.Plant vascular development:From early specification to differentiation[J].Nature Reviews Molecular Cell Biology,2016,17(1):30-40.
[18]SHI D,LEBOVKA I,LÓPEZ-SALMERÓN V,SANCHEZ P,GREB T.Bifacial cambium stem cells generate xylem and phloem during radial plant growth[J].Development,2019,146(1):171355.
[19]SPICER R,GROOVER A.Evolution of development of vascular cambia and secondary growth[J].New Phytologist,2010,186(3):577-592.
[20]JI J B,STRABLE J,SHIMIZU R,KOENIG D,SINHA N.WOX4 promotes procambial development[J].Plant Physiology,2009,152(3):1346-1356.
[21]SORCE C,GIOVANNELLI A,SEBASTIANI L,ANFODILLO T.Hormonal signals involved in the regulation of cambial activity,xylogenesis and vessel patterning in trees[J].Plant Cell Reports,2013,32(6):885-898.
[22]JANG G,LEE J H,RASTOGI K,PARK S,OH S H,LEE J Y.Cytokinin-dependent secondary growth determines root biomass in radish(Raphanus sativus L.)[J].Journal of Experimental Botany,2015,66(15):4607-4619.
[23]ZHANG W J,SWARUP R,BENNETT M,SCHALLER G E,KIEBER J J.Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem[J].Current Biology,2013,23(20):1979-1989.
[24]XU Q,CHEN L L,RUAN X A,CHEN D J,ZHU A D,CHEN C L,BERTRAND D,JIAO W B,HAO B H,LYON M P,CHEN J J,GAO S,XING F,LAN H,CHANG J W,GE X H,LEI Y,HU Q,MIAO Y,WANG L,XIAO S,BISWAS M K,ZENG W,GUO F,GAO H,YANG X,XU X W,CHENG Y J,XU J,LIU J H,LUO O J,TANG Z,GUO W W,KUANG H H,ZHANG H Y,ROOSE M L,NAGARAJAN N,DENG X X,RUAN Y.The draft genome of sweet orange(Citrus sinensis)[J].Nature Genetics,2013,45(1):59-66.
[25]THOMPSON J D,GIBSON T J,PLEWNIAK F,JEANMOUGIN F,HIGGINS D G.The CLUSTAL_X windows interface:Flexible strategies for multiple sequence alignment aided by quality analysis tools[J].Nucleic Acids Research,1997,25(24):4876-4882.
[26]魏欣悦,苏源,杨静,刘林,李成云.微波快速石蜡切片法观察水稻叶片组织[J].云南农业大学学报,2011,26(4):454-457.WEI Xinyue,SU Yuan,YANG Jing,LIU Lin,LI Chengyun.Microwave paraffin section in rice leaf tissue[J].Journal of Yunnan Agricultural University,2011,26(4):454-457.
[27]LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method[J].Methods,2001,25(4):402-408.
[28]HIRAKAWA Y,SHINOHARA H,KONDO Y,INOUE A,NAKANOMYO I,OGAWA M,SAWA S,OHASHI-ITO K,MASTUBAYASHI Y,FUKUDA H.Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system[J].Proceedings of the National Academy of Sciences of the United States of America,2008,105(39):15208-15213.
[29]HAN S,CHO H,NOH J,QI J Y,JUNG H J,NAM H,LEE S,HWANG D,GREB T,HWANG I.BIL1-mediated MP phosphorylation integrates PXY and cytokinin signalling in secondary growth[J].Nature Plants,2018,4(8):605-614.