甜瓜(Cucumis melo)是我国重要的葫芦科经济作物。近年来,设施甜瓜栽培模式的推广及连年重茬种植使甜瓜根部病害发生严重。2018 年海南三亚乐东大棚种植的20 hm2金香玉甜瓜在成熟期前约20 d出现整株急性萎蔫、枯死现象,植株根部及茎基部表现出褐色腐烂症状,且有大量的黑色小颗粒,感病植株的果实不能成熟,发病率达90%,根据发病症状初步判断是甜瓜炭腐病。
甜瓜炭腐病是由菜豆壳球孢(Macrophomina phaseolina(Tassi)Goid)引起的土传真菌病害[1]。M.phaseolina菌核呈球形至椭圆形,外面黑色、光滑,内部褐色或暗褐色,菌核大小为50~150 μm,分生孢子阶段很少被发现[2]。该病菌主要发生在温带和热带地区,寄主范围广,可侵染750 多种植物[3]。寄主被侵染后,组织表现出黑色症状被称为炭腐[4];植物的根及茎基部受害,地上部枯死,地下根部断续变黑、腐烂;受害部产生大量的小黑点,即病原菌的菌核,是病害的初侵染源[5]。我国最早在1981年报道该病菌可侵染哈密瓜,成熟前15~20 d 植株表现症状,初期萎蔫,随后茎基部坏死、腐烂,病株呈渐进性枯死或急性萎蔫枯死,果实不能正常成熟,严重影响哈密瓜的产量和品质,重病田块发病率为80%~90%[6]。目前,我国已报道该菌可侵染哈密瓜[6]、芝麻[7]、绿豆[8]及小豆[9]等。
笔者在本研究中为了分析引起海南大棚甜瓜急性萎蔫的病因及筛选防控该病害的适宜化学药剂,通过分离、鉴定及致病性测定明确该病菌,并采用生长速率法测定了8 种杀菌剂对该菌的室内毒力,旨在筛选出适宜防治甜瓜炭腐病的杀菌剂,为该病害的有效防治提供指导。
1 材料和方法
1.1 样本采集
每1 hm2内随机采集1个样品,共采集了10株表现萎蔫、死亡植株的根部及茎基部样本。
1.2 培养基制备
称取洗净去皮的马铃薯200 g,切成小块,加水1000 mL煮沸0.5 h,3层纱布过滤,加入葡萄糖20 g,搅拌均匀并溶解后,定容至1000 mL,分装于250 mL三角瓶。每100 mL培养基中加入1.8 g琼脂粉,混合均匀。121 ℃下0.1 MPa灭菌20 min,即为马铃薯葡萄糖琼脂(potato dextrose agar,PDA)培养基,备用。
1.3 病原菌分离
采用传统的组织分离法[10],从根部病健交界处切下约4 mm×4 mm的小块,用75%(φ,后同)乙醇消毒15 s,随后置于5%的次氯酸钠溶液中浸泡3 min,用无菌水清洗3遍后,放置于灭菌滤纸上干燥,最后放于PDA 培养基上,27 ℃培养,3 d 后挑取菌丝,纯化,并将纯化后的菌株保存在PDA 培养基斜面上,于4 ℃冰箱中保存备用。
1.4 致病性鉴定
采用菌饼接种法鉴定病原菌对金香玉的致病性[11-13]。当甜瓜幼苗生长至25 d时,取甜瓜茎部及茎基部,用75%乙醇进行表面消毒,用无菌接种针轻轻划微伤口,将直径5 mm的菌饼接种到伤口上。每个处理接种5个,3 次重复。以不接菌的PDA 培养基为阴性对照,用无菌脱脂棉进行保湿处理,置于27 ℃恒温箱培养。逐日观察并记录发病情况,取病健交界处组织再次分离并鉴定病原菌。并利用该方法接种生长15 d的南瓜品种思壮12(市售)。
1.5 病原菌鉴定
1.5.1 形态学鉴定 将获得菌株接种于PDA培养基平板上,27 ℃恒温黑暗培养5 d,期间不间断观察、记录菌落的形态,并用光学显微镜观察微菌核及菌丝。
1.5.2 分子鉴定 菌株培养4 d 后收集50~100 mg菌丝,采用改良的十六烷基三甲基溴化铵法(cetyltrimethylammonium bromide,CTAB)提取真菌DNA[14]。以内部转录间隔区1(internal transcribed spacer 1,ITS1)(5’-TCC GTA GGT GAA CCT GCG G-3’)和内部转录间隔区4(internal transcribed spacer 4,ITS4)(5’-TCC TCC GCT TAT TGA TAT GC-3’)为引物进行PCR 扩增[15]。为了进一步鉴定该病菌,依据ITS序列,选用特异引物MPKFI(5’-CCG CCA GAG GAC TAT CAA AC-3’)/MpKRI(5’-CGT CCG AAG CGA GGT GTA TT-3’)[16]进行扩增。20 μL PCR 反应体系:真菌DNA 模板1 μL(约100 ng)、上下游引物各1 μL、2×Taq Mixture 10 μL,补ddH2O 至20 μL。PCR反应程序为:94 ℃预变性3 min;94 ℃变性30 s,58 ℃退火30 s,72 ℃延伸30 s,循环33次;最后72 ℃延伸5 min,16 ℃保存。扩增产物用1.0%(w)的琼脂糖凝胶电泳检测,发现有目的片段后,送至生工生物工程(上海)股份有限公司进行测序。ClustalX 进行序列比对,采用MEGA X 最大相似自然法(maximum likelihood method)进行发育树分析[17]。
1.6 病菌的室内毒力测定
选用表1 中的8 种杀菌剂采用生长速率法测定室内毒力[18]。所有的杀菌剂均配置为1 mg·mL-1的母液,依据农药的推荐用量进行预实验,随后依据结果调整培养基药剂加入量,制成含有不同质量浓度药剂的含药PDA 培养基备用。将获得的病原菌在PDA 培养基上活化4 d,用直径为5 mm的打孔器从菌落边缘打取菌饼,并转接到含有8 种不同质量浓度的含药PDA培养基上。将培养基置27 ℃恒温培养箱暗培养,3次重复,以不含药剂空白PDA培养基为对照。
表1 供试药剂
Table 1 Tested fungicides
待不含药剂的PDA 对照平板的病原菌长满时(约4 d),采用十字交叉法,用游标卡尺测定菌落直径。计算每处理对病原菌菌丝增长的抑制率,菌丝抑制生长率/%=[(对照菌落生长直径-处理菌落生长直径)/对照菌落生长直径]×100。以设定的质量浓度的对数为横坐标(x),抑制率的概率值为纵坐标(y),求出线性回归方程y=a+bx、相关系数r 及各药剂对菌株的EC50值。用Microsoft Excel 2003、DPS数据处理工作平台对试验数据进行统计分析。
2 结果与分析
2.1 病害田间症状
甜瓜受到病原菌侵染后表现为植株萎蔫,随着病害的发展,根部出现大量小黑点,发病严重的地块植株萎蔫,直至枯萎死亡(图1)。

图1 甜瓜受到病原菌侵染后的田间症状
Fig.1 The field symptoms of melon infected with pathogen
2.2 病原菌致病力分析
2.2.1 病原菌接种甜瓜 经室内分离纯化,获得纯化的病原菌。将获得的病原菌进行回接,黑暗保湿72 h后,调查发现接种部位表现出明显的坏死(图2-A),接种PDA 空白对照的植株不表现任何症状(图2-B),从表现症状的部位重新分离到该病原菌,表明该病菌是引起甜瓜萎蔫的致病菌。

图2 人工接种接种甜瓜茎及茎基部72 h 后坏死斑症状
Fig.2 The necrotic symptoms of melon stems and basal stems at 72 h after artificial infection
A.人工接种甜瓜茎及茎基部;B.PDA 空白对照。
A.The melon plants were inoculated at the stems and basal stems;B.The blank PDA control.
2.2.2 病原菌接种南瓜 采用菌饼接种法将5 mm的病原菌菌饼接种至南瓜品种思壮12的茎部及茎基部,黑暗保湿72 h后发现人工接种南瓜茎部及茎基部不表现任何症状,接种时间延长至96 h后仍未表现任何症状(图3-A),同时PDA 空白对照的接种部位也未表现任何症状(图3-B)。说明该病原菌不侵染南瓜茎及茎基部,南瓜品种思壮12可作为嫁接砧木。

图3 人工接种南瓜茎及茎基部96 h 后未表现症状
Fig.3 The no symptoms of pumpkin stems and basal stems at 96 h after artificial infection
A.人工接种南瓜茎及茎基部;B.PDA空白对照;红色箭头为接种茎基部部位。
A.The pumpkin plants were inoculated at the stems and basal stems;B.The blank PDA control;Red arrow is the base of the basal stems of pumpkin.
2.3 形态学及分子生物学鉴定
2.3.1 形态学鉴定 病原菌的菌丝生长快,由接种菌饼处沿培养基表面向四周放射状生长,27 ℃恒温黑暗培养3 d的菌落直径可达80 mm,培养4 d 时可长满90 mm的PDA 培养基平板。菌丝初期24 h 呈白色薄绒状;随着培养时间的延长,48 h时菌落中间至周围颜色由白色向灰白色,出现椭圆形或圆形的微菌核;72 h 后随着微菌核增多,微菌核集结变黑(图4-A~B)。微菌核大小(80.3~120.0)μm×(65.6~120.0)μm(图4-C~D),在PDA培养基平板上不产生有性繁殖结构。

图4 病原菌在PDA 上的菌落及显微镜下的形态特征
Fig.4 The colony on PDA and its morphological characteristics of the pathogen
A.菌落正面;B.菌落背面;C.10 倍显微镜下的菌丝和微菌核;D.40 倍显微镜下的菌丝和微菌核。
A.Frontal colony;B.Backside colony;C.Hyphae and micro sclerotia at 10×microscope;D.Hyphae and micro sclerotia at 40×microscope.
2.3.2 分子生物学鉴定 ITS1/ITS4 扩增获得约600 bp的条带,测序分析表明该序列长度为583 bp,经BLAST 分析发现其与GenBank 中菜豆壳球孢M.phaseolina的序列一致性为99%(GenBank accession:FJ827625.1)。基于ITS 序列的系统进化树分析表明海南甜瓜分离物HN-melon 与M.phaseolina以99%的自展支持率聚为一个分支,Botryosphaeria fabicerciana为外群菌株处于外围(图5-A)。依据测序结果,利用特异引物MPKFI/MpKRI 扩增获得了350 bp的基因,BLAST 分析发现与菜豆壳球孢(GenBank accession:FJ960441)的序列一致性达99%。特异序列的进化树分析发现海南甜瓜分离物HN-melon 与M.phaseolina 聚为一类(图5-B)。该结果说明在甜瓜上分离到的病原菌是菜豆壳球孢。
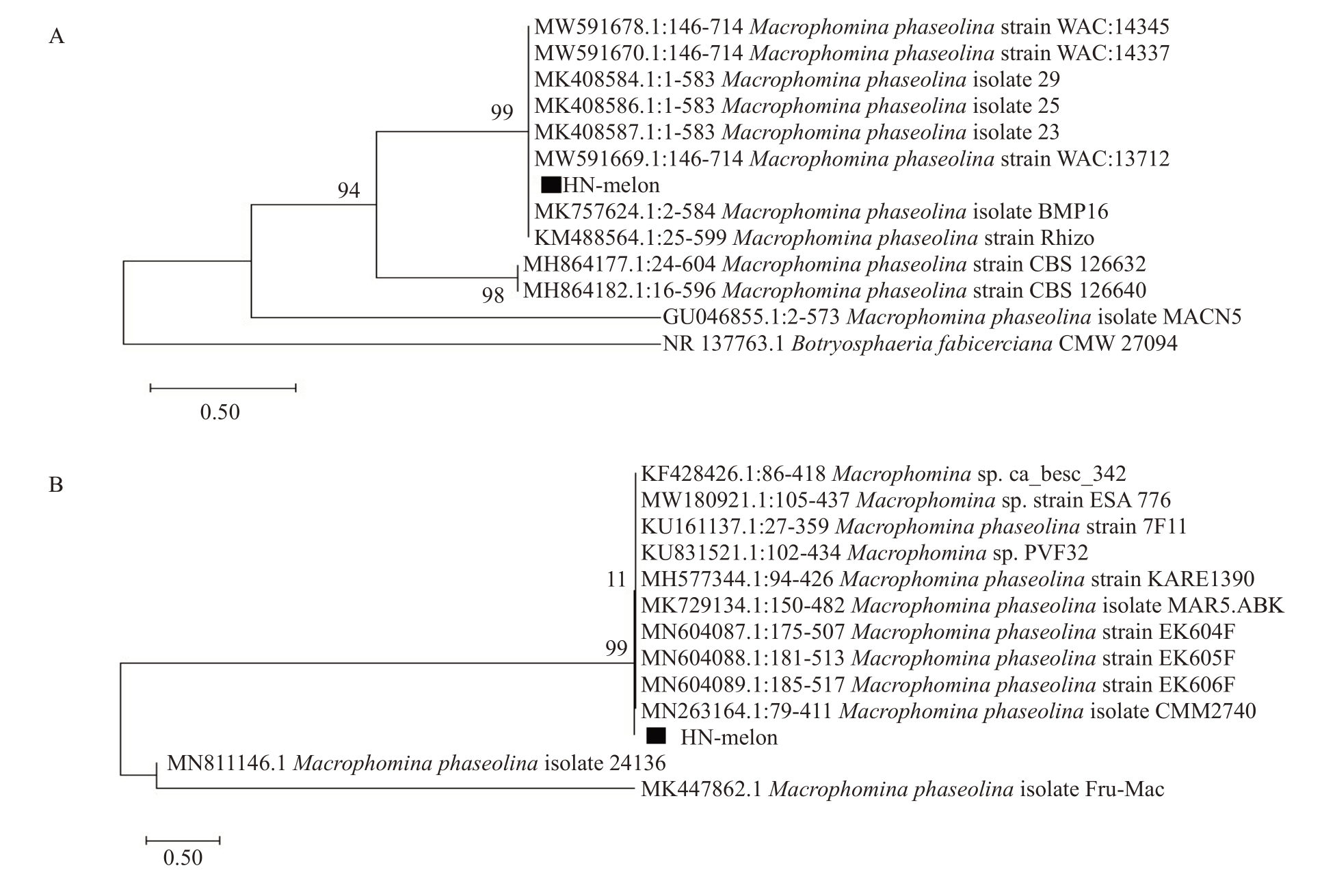
图5 基于ITS 及菜豆壳球孢特异序列的系统发育树
Fig.5 Phylogenetic tree of based on ITS and M.phaseolina specific sequences
A.基于ITS 序列的系统发育树;B.基于菜豆壳球孢特异序列的系统发育树。
A.Phylogenetic tree based on ITS sequence;B.Phylogenetic tree of M.phaseolina specific sequence.
通过病原菌、微菌核的生长状态观察及分子生物学鉴定,表明分离获得的病菌为菜豆壳球孢,这是首次在海南发现该病菌侵染甜瓜,引起甜瓜急性萎蔫。
2.4 8种药剂对病菌菌丝的抑制作用
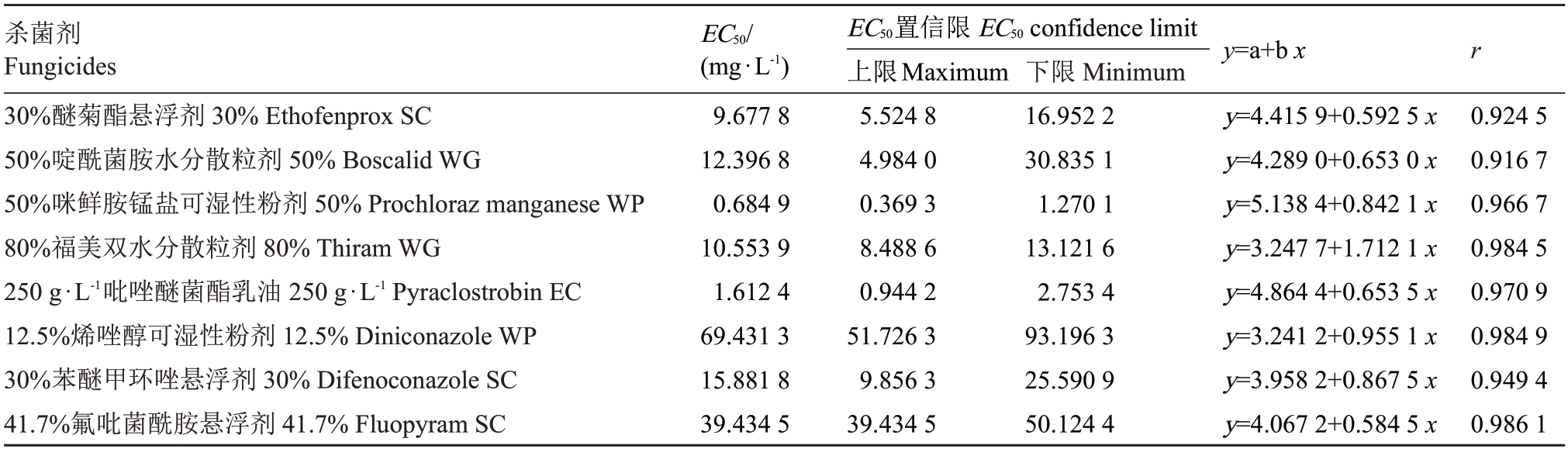
室内毒力测定结果(表2)可知,8种药剂的相关系数均在0.90 以上,表明药剂剂量与抑制作用呈较高的相关性,8 种杀菌剂对甜瓜炭腐病病原菌的毒力差异显著。50%咪鲜胺锰盐毒力最强,EC50 为0.684 9 mg·L-1;其次为250 g·L-1吡唑醚菌酯,EC50为1.612 4 mg·L-1,其他6种药剂的EC50值均很高,毒力较弱。
表2 8 种杀菌剂对菜豆壳球孢甜瓜分离物的抑制作用
Table 2 Inhibitory effects of eight fungicides on M.phaseolina melon isolates
3 讨论
M.phaseolina 是土传真菌,寄主范围广。我国首次报道该菌在新疆危害哈密瓜[6],之后一直未见该菌侵染甜瓜的报道。2018 年笔者团队在海南进行病害调查时发现,甜瓜成熟期前20 d 左右出现急性萎蔫,病害发生率高达90%,种植者急需认识并防控该病害。经室内分离、纯化及分子生物学分析表明侵染甜瓜的病原菌为M.phaseolina。分离到的M.phaseolina在人工培养条件下不产生有性繁殖结构,这与Zhao等[19]的结果一致。这是首次明确菜豆壳球孢是海南大棚甜瓜急性萎蔫的病原菌。
笔者采用菌饼接种法鉴定了M.phaseolina的致病性并从接种部位重新分离到该病原菌,说明M.phaseolina是甜瓜上的致病菌。该方法接种后黑暗保湿72 h后即可表现症状,与牙签接种法[3-4](一般需要10~15 d)相比,该方法结果稳定、可靠、用时短。研究表明嫁接可较好防控该病害,未嫁接甜瓜的发病率为70%~80%,而嫁接甜瓜枯萎现象很少发生[20]。因此,笔者也进行了M.phaseolina 对南瓜品种思壮12的致病性分析,结果表明南瓜茎及茎基部接种96 h 后不表现任何症状,说明该病菌不侵染南瓜。该结果为大范围的田间筛选南瓜砧木防控该病害提供了依据。
目前炭腐病难于防控,土壤消毒和暴晒防效甚微[21]。微菌核可在干燥土壤存活10个月以上,甚至超过2 a[22]。化学药剂仍是防控M.phaseolina的主要手段[7],一些杀菌剂对该菌有一定作用。室内毒力分析表明,三唑类杀菌剂及烯唑醇杀菌剂可显著抑制菜豆壳球孢菌丝发展[23],将40%五氯硝基苯粉剂药液淋于植株茎基部及周围土壤,14 d 后防效可达69.76%[24]。为了探索防治该病害的防治方案,为甜瓜产业持续健康发展提供保障,笔者在本研究中采用了8 种化学药剂进行了其室内毒力测定,结果发现咪鲜胺锰盐和吡唑醚菌酯的EC50 值分别为0.684 9 mg·L-1和1.612 4 mg·L-1,对菜豆壳球孢菌丝的抑制效果好,与吡唑醚菌酯对芝麻的M.phaseolina菌丝的抑制效果一致[25]。研究发现烯唑醇对不同菜豆壳球孢的抑制能力不同,对芝麻M.phaseolina的EC50值为0.06 μg·mL-1,皿内抑制效果很好[23],而笔者发现烯唑醇对甜瓜的M.phaseolina EC50值为69.431 3 mg·mL-1,抑制效果不好。对同一病原菌室内毒力的差异,推测是菌株的不同分离物对农药敏感性不同造成的。基于本研究结果,建议田间可采用咪鲜胺锰盐和吡唑醚菌酯交替灌根防控该病害。田间防治效果受到很多因素影响,室内杀菌剂毒力测定结果可为田间防治提供指导,真正应用于大田防治前还有待于进一步的田间试验验证。
4 结论
通过组织分离、纯化、致病性测定及系统发育树分析,首次明确了造成海南大棚甜瓜急性萎蔫的病原菌是菜豆壳球孢M.phaseolina。室内毒力分析表明50%咪鲜胺锰盐和250 mg·L-1吡唑醚菌酯对甜瓜的M.phaseolina 菌丝有明显的抑制作用,为大田防控该病害奠定了基础。
[1] MARQUEZ N,GIACHERO M L,GALLOU A,DEBAT H,CANENBROUCK S,DI RIENZO J,POZO M,DUCASSE D A,DECLERCK S.Transcriptional changes in mycorrhizal and non-mycorrhizal soybean plants upon infection with the fungal pathogen Macrophomina phaseolina[J].Molecular Plant-Microbe Interactions,2018,31(8):842-855.
[2] GUPTA G K,SHARMA S K,RAMTEKE R.Biology,epidemiology and management of the pathogenic fungus Macrophomina phaseolina(Tassi)Goid with special reference to charcoal rot of soybean (Glycine max (L.) Merrill)[J].Journal of Phytopathology,2012,160(4):167-180.
[3] MELO N J A,LIMA A G,NEGREIROS A M P,AMBRÓSIO M M Q,NASCIMENTO L V,SALES R.Pathogenicity of Macrophomina phaseolina in cultivars and accessions of Cucumis melo[J].Journal of Plant Pathology,2021,103(3):969-972.
[4] PRATT R G,MCLAUGHLIN M R,PEDERSON G A,ROWE D E.Pathogenicity of Macrophomina phaseolina to mature plant tissues of alfalfa and white clover[J].Plant Disease,1998,82(9):1033-1038.
[5] COHEN R,ELKABETZ M,EDELSTEIN M.Variation in the responses of melon and watermelon to Macrophomina phaseolina[J].Crop Protection,2016,85:46-51.
[6] 贾菊生.新疆哈密瓜碳腐病研究初报[J].新疆农业科学,1986,23(2):18-20.JIA Jusheng.A preliminary report on charcoal rot caused by Macrophomina phaseolina of Xinjiang Hami melon[J].Xinjiang Agricultural Sciences,1986,23(2):18-20.
[7] 孟祥锋,高新国,张春生.河南省芝麻茎点枯病发病规律及防治措施[J].河南农业科学,2003,32(10):69.MENG Xiangfeng,GAO Xinguo,ZHANG Chunsheng.Occurrence and control measures of sesame stem spot blight in Henan province[J].Journal of Henan Agricultural Sciences,2003,32(10):69.
[8] ZHANG J Q,ZHU Z D,DUAN C X,WANG X M,LI H J.First report of charcoal rot caused by Macrophomina phaseolina on mungbean in China[J].Plant Disease,2011,95(7):872.
[9] SUN S L,WANG X M,ZHU Z D,WANG B,WANG M.Occurrence of charcoal rot caused by Macrophomina phaseolina,an emerging disease of adzuki bean in China[J].Journal of Phytopathology,2016,164(3):212-216.
[10] 方中达.植病研究方法[M].3 版.北京:中国农业出版社,1998.FANG Zhongda.Research methods of plant disease[M].3rd.Beijing:China Agriculture Press,1998.
[11] 吴望,吴玉珠,胡军华,陈娜,张嘉,周彦.‘沃柑’果斑病病原菌鉴定及防治药剂筛选[J].果树学报,2021,38(3):385-393.WU Wang,WU Yuzhu,HU Junhua,CHEN Na,ZHANG Jia,ZHOU Yan.Pathogen identification and fungicide screening of‘Orah’fruit spot disease[J].Journal of Fruit Science,2021,38(3):385-393.
[12] 崔一平,彭埃天,宋晓兵,凌金锋,陈霞,程保平.番石榴枯萎病病原菌的分离及分子生物学鉴定[J].植物保护学报,2021,48(2):467-468.CUI Yiping,PENG Aitian,SONG Xiaobing,LING Jinfeng,CHEN Xia,CHENG Baoping.Isolation and molecular identification of the pathogen causing wilt in Psidium guajava[J].Journal of Plant Protection,2021,48(2):467-468.
[13] 周增强,侯珲,王丽,朱发亮.枝干苹果轮纹病人工接种方法与品种抗性评价[J].果树学报,2010,27(6):952-955.ZHOU Zengqiang,HOU Hui,WANG Li,ZHU Faliang.Trunk apple ring rot artificial inoculation method and the identification of cultivar resistance[J].Journal of Fruit Science,2010,27(6):952-955.
[14] 闫玖英,马长青,常博,范献光,李征,杨亚州,赵政阳.改良CTAB 法用于苹果果实基因组DNA的提取[J].分子植物育种,2017,15(9):3610-3615.YAN Jiuying,MA Changqing,CHANG Bo,FAN Xianguang,LI Zheng,YANG Yazhou,ZHAO Zhengyang.A modified CTAB method for genomic DNA extraction from apple fruit[J].Molecular Plant Breeding,2017,15(9):3610-3615.
[15] WHITE T J,BRUNS T D,LEE S B,TAYLOR F,WHITE T J,LEE S H,TAYLOR L,SHAWE-TAYLOR J.Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[J].PCR Protocols,A Guide to Methods and Application,1990,1:315-322.
[16] BABU B K,SAXENA A K,SRIVASTAVA A K,ARORA D K.Identification and detection of Macrophomina phaseolina by us-ing species-specific oligonucleotide primers and probe[J].Mycologia,2007,99(6):797-803.
[17] SUDHIR K,GLEN S,MICHAEL L,CHRISTINA K,KOICHIRO T.MEGA X:Molecular evolutionary genetics analysis across computing platforms[J].Molecular Biology&Evolution,2018,35(6):1547-1549.
[18] 宋化稳,高德良,徐娜娜,胡尊纪,庄治国,庄占兴.5 种甲氧基丙烯酸酯类杀菌剂对芦笋茎枯病菌的室内毒力及田间药效评价[J].农药,2019,58(7):532-534.SONG Huawen,GAO Deliang,XU Na’na,HU Zunji,ZHUANG Zhiguo,ZHUANG Zhanxing.Toxicity and field efficacy of five strobilurins fungicides against Phomopsis asparagi(Sacc.)Bubak[J].Agrochemicals,2019,58(7):532-534.
[19] ZHAO X,NI Y,LIU X,HUI Z,WANG J,CHEN Y,CHEN W,LIU H.A simple and effective technique for production of pycnidia and pycnidiospores by Macrophomina phaseolina[J].Plant disease,2020,104(4):1183-1187.
[20] RON C,NABIL O,ASAF P,MENAHEM E.Management of Macrophomina wilt in melons using grafting or fungicide soil application:Pathological,horticultural and economical aspectssciencedirect[J].Crop Protection,2012,35(1):58-63.
[21] HARTZ T K,CARTER W W,BRUTON B D.Failure of fumigation and solarization to control Macrophomina phaseolina and subsequent muskmelon vine decline[J].Crop Protection,1987,6(4):261-264.
[22] KHAN S. Macrophomina phaseolina as causal agent for charcoal rot of sunflower[J].Mycopath,2007,5:111-118.
[23] 倪云霞,王飞,刘玉霞,刘新涛,赵辉,刘红彦.芝麻茎点枯病菌的生物学特性及9 种杀菌剂对其抑制作用测定[J].河南农业科学,2016,45(6):72-76.NI Yunxia,WANG Fei,LIU Yuxia,LIU Xintao,ZHAO Hui,LIU Hongyan.Biological characteristics of the pathogen of sesame stem blight and inhibitory effects of nine fungicides[J].Journal of Henan Agricultural Sciences,2006,45(6):72-76.
[24] 宁运任,张济能,文洪波.40%五氯硝基苯粉剂防治菜豆炭腐病试验[J].广西植保,1999,12(1):21-22.NING Yunren,ZHANG Jineng,WEN Hongbo.Control of bean carbon rot test of 40% pentachloronitrobenzene powder[J].Guangxi Plant Protection,1999,12(1):21-22.
[25] 李梦姣,王振军,刘红彦.9 种杀菌剂对芝麻茎点枯病菌的室内毒力测定[J].中国园艺文摘,2013,29(11):49-50.LI Mengjiao,WANG Zhenjun,LIU Hongyan.Toxicity test of nine different fungicides to the pathogen causing sesame stem blight in laboratory[J].Chinese Horticulture Abstracts,2013,29(11):49-50.