枣树(Ziziphus jujuba Mill.)是世界上栽培最古老的果树树种之一,主要分布在亚洲的中国、印度、韩国和日本。枣树在中国的栽培与利用具有7000多年的历史[1-2]。中国枣树的栽培面积占世界栽培总面积的98%,主要分布在山西、河南、河北和新疆。截至目前,新疆枣树的种植面积达345 000 hm2,其中阿克苏地区枣树的种植面积达136 000 hm2,并成为世界上最大的红枣生产基地[3]。在新疆,危害枣树的病虫害相对较少,然而随着枣树栽培面积的不断扩大,栽培模式的多样化,一些次要病害逐渐上升为主要病害,一些新病害也相继发生[4-6],但鲜见枣树病毒病的发生。2016年,枣树病毒病在新疆阿克苏地区枣园突然发生,造成大量果实畸形,严重地影响了红枣的产量和品质,给枣农造成了严重的经济损失,对新疆红枣产业构成严重威胁。自该病在新疆发生以来,新疆林科院林业有害生物科研团队联合疆内外专家针对枣树病毒病进行了高通量测序[7-8],并以高通量测序为指向,先后从阿克苏地区枣树样本中分离鉴定到不同种类的新病毒,初步推测枣树病毒病很可能是由不同种类的病毒复合侵染发生的一种枣树新病害。2019年刘达等[9]从枣树病害样本中分离鉴定到一种RNA病毒,该病毒为山梣环斑病毒属(Emaravirus)中的一个新种,暂命名为中国枣树花叶伴随病毒(Chinese date mosaic-associated virus,CDMaV)。通过对危害枣树昆虫介体进行病毒检测,在枣瘿螨体内检测到了CDMaV的存在,符合该属病毒由瘿螨科传播的特征[10-13]。2020年新疆林科院林业有害生物科研团队再次从发病叶片和果实样本中分离鉴定到1种DNA新病毒,该病毒为杆状病毒属,基因组结构具备杆状病毒属(Badnavirus)基因组的结构特征[14-17],是杆状病毒属中的一个新种,这是自2017 年Du 等[18]自北京地区表现枣树花叶症状的枣树叶片上分离鉴定到的一种DNA 病毒以来,再次在我国枣树上分离鉴定到的DNA 新病毒,其基因组碱基序列与北京地区报道的枣树DNA病毒在RT/RNase H的编码区存在20%的差异,且在新疆表现枣花叶和畸形果症状的样本中均分离鉴定到该病毒。杆状病毒属(Badnavirus)侵染的物种大多数是热带和亚热带作物,如香蕉[19]、柑橘[20]、黑胡椒[21]、甘蔗[22]、可可[23]、山药[24]和芋头[25]等。也可以侵染一些温带气候的植物,如红树莓[26]、无花果[27]和醋栗[28]等。这是首次报道在我国新疆阿克苏地区特殊气候条件下从果木中鉴定出杆状病毒。因此,笔者的研究结果表明:新疆枣树样本分离物为杆状病毒属中的一个新种,暂命名为枣树花叶伴随病毒A(jujube mosaic-associated virus A,JuMaVA)。JuMaVA主要危害枣树叶片和果实,造成叶片斑驳、卷曲,树势明显减弱,受害果实初期在果面上出现水浸状褪绿斑,随后病斑凹陷,果实畸形,后期逐渐萎缩脱落,造成整株产量损失在30%以上。该病具有传播速度快,危害严重,且前期隐症不易发现,一旦显症就已错过最佳防治时间等特点。常规的症状观察和病原的电镜扫描鉴定方法耗时长、程序繁琐,很难实现对病害发生动态的及时监测和有效控制病害的传播和流行。因此,建立枣树花叶伴随病毒A的快速、准确的分子检测技术,对于明确枣树花叶伴随病毒A的侵染时期,探索病害防控的关键点以及制定适时有效的防治措施具有重要的理论指导意义。笔者在本研究中在获得枣树花叶伴随病毒A 基因组全序列(GenBank Accession Number: MW892537.1)的基础上,以病毒基因组序列为靶标,利用Primer5.0 软件设计了灵敏度极高的特异性引物,通过PCR反应体系优化后的引物特异性扩增与灵敏度的检测,建立枣树花叶伴随病毒A的分子检测技术,并利用该技术在红枣整个生育期进行病害侵染动态的追踪检测与分析。通过病害田间侵染动态的追踪检测,明确枣树花叶伴随病毒A的田间侵染动态,探索病害防治的关键时间节点,为新疆枣树花叶病毒病的流行监测和早期防治提供了理论依据和技术支持。
1 材料和方法
1.1 材料及主要试剂和仪器
供试材料:枣树花叶伴随病毒A 阳性对照样本为本实验室收集于阿克苏地区阿克苏市和温宿县枣园具有典型花叶症状的叶片样本(图1),提取总DNA,并保存于-80 ℃低温冰箱。引物特异性验证材料是由新疆林科院植物病理实验室收集于新疆不同地区不同作物病毒病的叶片样本提取总DNA,并保存于-80 ℃低温冰箱的叶片DNA 样本,包括枣疯病(jujube witches broom)、丁香环斑病毒病(lilac ring mottle virus)、无花果病毒病(fig mosaic virus)、苹果花叶病(apple mosaic)、番茄黄化曲叶病毒病(tomato yellow leaf curl virus)、苜蓿病毒病(alfalfa mosaic virus)和西甜瓜病毒病(melon mosaic virus)等7 种作物病毒病总DNA 阳性样本。追踪检测材料是在枣树整个生育期内的不同时间点自不同监测点采集到的枣树病毒病叶片样本(360份)。

图1 携带枣树花叶伴随病毒A的枣树病叶和病果症状
Fig.1 Symptoms of diseased leaves and fruits of jujube tree with jujube mosaic-associated virus A
A.田间病叶症状;B.田间病果症状;C、E.健康叶和果;D、F.病叶和病果。
A.Field symptoms of diseased leaves;B.Field symptoms of diseased fruits;C,E.Healthy leaf and fruits;D,F.Diseased leaf and fruits.
供试引物:依据高通量测序后克隆得到的Ju-MaVA全基因组序列,利用Primer 5.0软件设计一对特异性引物JCD-F(5’-ATCCATCGAAGCACCAGCAG-3’)和JCD-R(5’-CGGAATCTGCTCCGTCTACT-3’),并在NCBI 数据库中进行了特异性比对分析,预计产物片段大小为263 bp,引物由上海生工生物工程有限公司合成。
主要试剂和仪器:DYY6C 电泳仪,北京六一生物科技有限公司;BIO-RAD T100 PCR,美国伯乐中国上海分公司;KCBIO2008 凝胶成像系统,北京原平皓生物技术有限公司;植物基因组DNA提取试剂盒和2×PCR 反应液等试剂购自上海生物工程有限公司。
1.2 方法
1.2.1 叶片样本总DNA提取 按照植物基因组提取试剂盒操作说明分别提取360 份采自3 个不同监测点的枣树花叶病毒病叶片样本的总DNA,置于-70 ℃超低温冰箱保存备用。
1.2.2 引物的特异性检测 取-70 ℃保存的枣树花叶伴随病毒A 病叶片总DNA 阳性样本作为阳性对照,以灭菌的双蒸水为阴性对照,以枣疯病、苹果花叶病毒、无花果病毒、西甜瓜病毒、苜蓿花叶病毒、番茄黄化曲叶病毒、丁香环斑病毒等7 种作物的叶片总DNA做为特异性检测的对照,利用引物(JCD-F)/(JCD-R)对枣树叶片样本总DNA 和来源于不同作物阳性病毒病叶片的DNA 样本进行PCR的特异性检测。PCR 反应体系(25 μL)为:10 μmol·L-1的上、下游引物各1 μL,2×PCR 反应液12.5 μL,模板DNA/cDNA 2 μL,dd H2O 补足至25 μL。PCR 反应条件:94 ℃,3 min;94 ℃,1 min,58 ℃,45 s,72 ℃,2 min,35个循环;72 ℃延伸10 min。
1.2.3 引物的灵敏性检测 将-70 ℃保存的枣树花叶伴随病毒A 叶片总DNA 阳性样本用紫外分光光度计测定浓度后,取阳性DNA 样本10 μL,按10 倍梯度稀释(107、106、105、104、103、102、10、1)8 个不同浓度,采用已建立的PCR 技术体系进行扩增,检测引物(JCD-F)/(JCD-R)的灵敏度。
1.2.4 枣树花叶伴随病毒A田间侵染动态的追踪监测 依据枣树花叶病毒病在阿克苏地区的发生与分布情况,选取枣树花叶病毒病发生较重的地块作为病害田间发生动态追踪检测的对象,包括阿克苏市依杆旗乡、阿克苏地区实验林场和阿克苏市托普鲁特乡红枣种植园,总面积约28 hm2。枣树品种均为灰枣,树龄分别为8、9 和9 a,种植模式为4 m×3 m。自2020年5月初枣树展叶期至8月下旬枣树花叶病毒病叶片显症高峰期,采用五点取样法分别于5月、6月、7月和8月采集疑似枣树病毒病叶片样本,每个枣园每个时间点各取30份,提取各样品总DNA,利用已建立的PCR 技术进行带毒率的分子检测和病害田间侵染动态分析。
2 结果与分析
2.1 引物的特异性检测
特异性引物(JCD-F)/(JCD-R)的扩增产物在JuMaVA 基因序列上的位点为698~960,通过在NCBI 数据库blastx 对比,其与JuMaVA 核酸序列有100%的匹配,与JuMaV序列有99.62%的匹配,与其他病毒无匹配,此特异性引物(JCD-F)/(JCD-R)扩增片段为JuMaVA的特异保守片段。引物的特异性验证结果表明,检测引物(JCD-F)/(JCD-R)对供试枣树花叶病毒病叶片样本总DNA 均能稳定地扩增出263 bp的单一目的条带,而其他不同作物的DNA样本和阴性对照均未扩增出目的条带,扩增稳定性好,特异性强(图2)。

图2 枣树花叶伴随病毒A 引物的特异性PCR 检测扩增结果
Fig.2 The sensibility amplification results of jujube mosaic-associated virus A from jujube by PCR of specific primers
M.2000 bp Marker;1~3.枣树花叶病毒病;4~6.枣疯病;7~9.无花果病毒;10~12.苹果花叶病毒;13~15.丁香环斑病毒;16~18.番茄黄化曲叶病毒;19~21.西甜瓜病毒;22~24.苜蓿花叶病毒;CK.阴性对照。
M.2000 bp Marker;1-3.Jujube mosaic-associated virus A;4-6.Jujube witches’broom;7-9.Fig mosaic virus;10-12.Apple mosaic virus;13-15.Lilac rings pot virus;16-18.Tomato yellow leaf curl virus;19-21.Melon mosaic virus;21-24.Alfalfa mosaic virus;CK.Negative control.
2.2 引物的灵敏度检测
枣树花叶伴随病毒A 叶片阳性样本总DNA的分光光度计测定质量浓度为4.168 ng·μL-1,将初始DNA梯度稀释至104倍液(4.168 pg·μL-1)时,仍能检测到目的条带(图3),说明(JCD-F)/(JCD-R)引物在优化的PCR反应体系条件下,对枣树花叶伴随病毒A 具有较高的检测灵敏度,可用于枣树花叶伴随病毒A田间侵染动态的追踪检测与验证。
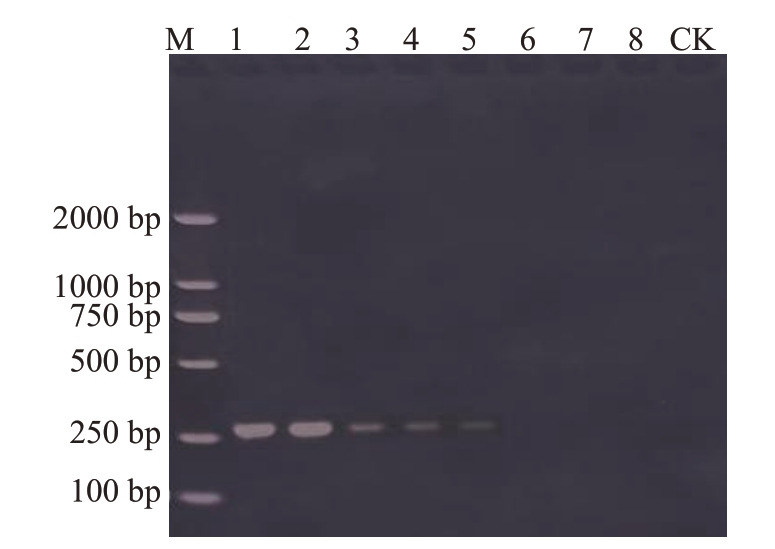
图3 枣树花叶伴随病毒A 引物的PCR 灵敏度检测
Fig.3 Sensibility detection of PCR primers of jujube mosaic-associated virus A
M.DNA Marker;1~8.总DNA 依次稀释梯度为1~107 倍液;CK.阴性对照。
M.DNA Marker;1-8.Serial dilution of total DNA(1-107);CK.Negative control.
2.3 枣树花叶伴随病毒A 田间侵染动态的追踪检测
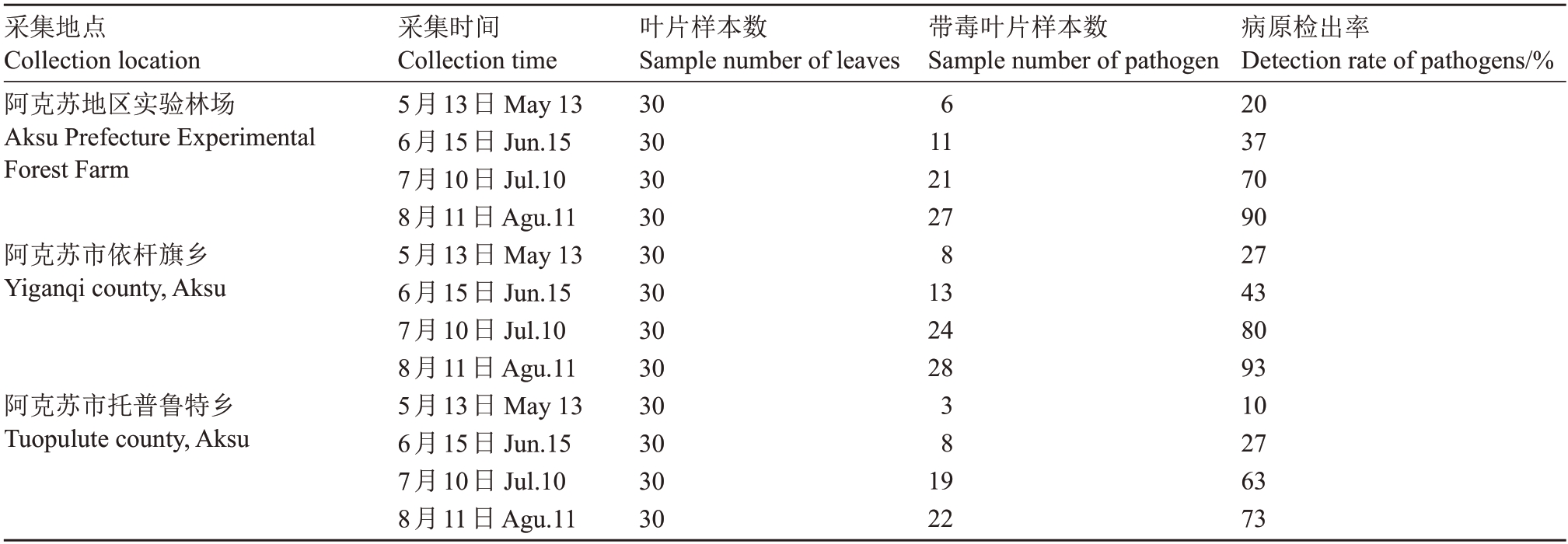
枣树花叶伴随病毒A田间侵染动态追踪检测结果显示,3 个枣园不同时期分别采集的30 份叶片样本中均有部分叶片样本可检测到枣树花叶伴随病毒A,且随着生育期内月份(5—8月)的增加,枣树花叶伴随病毒A的检出率呈逐渐升高的趋势。其中5月份采集的枣树叶片样本中枣树花叶伴随病毒A的检出率相对较低,最高检出率为27%,最低检出率10%,而在6 月份采集的叶片样本中枣树花叶伴随病毒A的检出率明显高于5月份的病害检出率,7—8月份达到高峰,最低检出率为63%,最高检出率达93%(表1)。
表1 样品材料及枣树花叶伴随病毒A 田间侵染动态的检测结果
Table 1 Specimen materials and the detection results of dynamic condition of jujube mosaic-associated virus A in field.
3 讨论
枣树病毒病是2016 年笔者研究团队在新疆阿克苏地区发现的一种国际新病害。2017 年Du 等[18]在北京地区表现花叶病的枣树上发现并报道了一种引起枣树花叶的DNA病毒(JuMaV),并对其基因组进行了测序分析,获取了该病毒的全基因组序列,该病毒侵染枣树,仅造成叶片黄化畸形,而笔者在新疆阿克苏地区枣树上发现的枣树花叶伴随病毒A(Ju-MaVA)来自于表现黄化卷曲的叶片,同时在对表现畸形症状的果实上也检测到该病毒的存在,但该杆状病毒(JuMaVA)是否是引起枣树花叶病毒病的主要病原,还需要后续的回接验证。枣树花叶病毒病的症状一般在每年7 月初,首先在当年生枣头枝上的幼嫩叶片显症,前期没有明显的发病症状,果农通常忽略前期对枣树病毒病的防治,仅在叶片或枣果显症期进行防治,致使防治效果很难达到预期的目标。因此,利用分子检测技术可以有效地检测出该DNA病毒的田间发生动态,同时也为后续开展病原的回接验证,以及探索病害防治的关键时间节点提供技术支持和理论依据。因此,笔者在枣树病毒病叶片样本的高通量测序分析的基础上,再次获得了1个DNA病毒(枣树花叶伴随病毒A)的全基因组序列,并以基因组序列为模板设计出一对高特异性引物,建立了针对该枣树花叶伴随病毒A的PCR分子检测技术,检测灵敏度达到4.168 pg·μL-1,引物(JCD-F)/(JCD-R)特异性强而且灵敏度高,具有实际可操作性,可用于枣树花叶伴随病毒A 田间侵染动态的追踪检测。
在枣树花叶伴随病毒A(JuMaVA)的田间追踪检测研究中,笔者发现3 个枣园不同时期采集的枣树花叶病毒病病害样本病原检出率随着月份的增加呈逐渐升高的趋势。其中5—6 月份检出率较低,7—8月份的检出率明显高于5—6月份,这与病害前期隐症、7 月份叶片显症、8 月份进入显症高峰期的田间调查结果相对应,同时也与上一年针对枣树花叶病毒病的田间追踪检测和调查结果相一致。因此,枣树花叶病毒病的发病症状具体是由单一病毒侵染,还是由多种病毒复合侵染的作物病毒病还需要进一步研究。在新疆,枣树花叶病毒病的潜伏期长,症状一旦出现便已错过了病害防治的关键时间节点。因此,明确枣树花叶病毒病主要病原种类及其分类地位、病毒与枣树花叶症状的关系,以及探索病害防控的关键点将是进一步深入研究的重点。
[1] 曲泽洲,王永惠.中国果树志·枣卷[M].北京:中国林业出版社,1993.QU Zezhou,WANG Yonghui.Chinese fruit trees record·Chinese jujube[M].Beijing:China Forestry Press,1993.
[2] 刘孟军,王玖瑞.新中国果树科学研究70 年:枣[J].果树学报,2019,36(10):1369-1381.LIU Mengjun,WANG Jiurui.Fruit scientific research in new China in the past 70 years:Chinese jujube[J].Journal of Fruit Science,2019,36(10):1369-1381.
[3] 新疆维吾尔族自治地方统计局.新疆统计年鉴[M].北京:中国统计出版社,2012.Xinjiang Uygur Autonomous Region Bureau of Statistics.Xinjiang statistical yearbook[M].Beijing:China Statistics Press,2012.
[4] BAI J Y,GUO Q Y,WANG X M,DUAN C X.First report of jujube leaf spot caused by Alternaria alternata in China[J].Plant Disease,2015,99(11):1624.
[5] BAI J Y,WANG X M,SHI Y J,DUAN C X.Occurrence and identification of Nothophoma quercina causing brown spot of jujube in China[J].Canadian Journal of Plant Pathology,2016,38(4):527-532.
[6] BAI J Y,GUO Q Y,SONG F J,LIU Z X,LIU Y,LEI C J.First report of Alternaria alternata causing brown spot of jujube in China[J].Journal of Plant Pathology,2015,97(2):391-403.
[7] BARBA M,CZOSNEK H,HADIDI A.Historical perspective,development and applications of next-generation sequencing in plant virology[J].Viruses,2014,6(1):106-136.
[8] ROOSSINCK M J,MARTIN D P,ROUMAGNAC P.Plant virus metagenomics:advances in virus discovery[J].Phytopathology,2015,105(6):716-727.
[9] 刘达,白剑宇,方荣祥,张莉莉.Emaravirus 属新病毒:中国枣树花叶伴随病毒全基因组测序[J].微生物学报,2020,60(2):397-405.LIU Da,BAI Jianyu,FANG Rongxiang,ZHANG Lili.Full genome sequence of Chinese date mosaic-associated virus,a new member of the genus Emaravirus[J].Acta Microbiologica Sinica,2020,60(2):397-405.
[10] ELBEAINO T,DIGIARO M,MARTELLI G P.Complete nucleotide sequence of four RNA segments of fig mosaic virus[J].Archives of Virology,2009,154(11):1719-1727.
[11] ELBEAINO T,DIGIARO M,UPPALA M,SUDINI H.Deep sequencing of dsRNAs recovered from mosaic-diseased pigeonpea reveals the presence of a novel emaravirus:pigeonpea sterility mosaic virus 2[J].Archives of Virology,2015,160(8):2019-2029.
[12] KUMAR P L,JONES A T,REDDY D V R.A novel mite-transmitted virus with a divided RNA genome closely associated with pigeonpea sterility mosaic disease[J].Phytopathology,2003,93(1):71-81.
[13] MCGAVIN W J,MITCHELL C,COCK P J A,WRIGHT K M,MACFARLANE S A.Raspberry leaf blotch virus,a putative new member of the genus Emaravirus,encodes a novel genomic RNA[J].Journal of General Virology,2012,93(2):430-437.
[14] BOUHIDA M,LOCKHART B E L,OLSZEWSKI N E.An analysis of the complete sequence of a sugarcane bacilliform virus genome infectious to banana and rice[J].Journal of General Virology,1993,74(1):15-22.
[15] XU D L,MOCK R,KINARD G,LI R H.Molecular analysis of the complete genomic sequences of four isolates of gooseberry vein banding associated virus[J].Virus Genes,2011,43(1):130-137.
[16] STAVOLONE L,HERZOG E,LECLERC D,HOHN T.Tetramerization is a conserved feature of the virion-associated protein in plant pararetroviruses[J].Journal of Virology,2001,75(16):7739-7743.
[17] HULL R.Caulimoviridae (Plant pararetroviruses) [M].Chichester,UK:John Wiley&Sons,Ltd.,2014.
[18] DU K T,LIU S J,CHEN Z R,FAN Z F,WANG H,TIAN G Z,ZHOU T.Full genome sequence of jujube mosaic-associated virus,a new member of the family Caulimoviridae[J].Archives of Virology,2017,162(10):3221-3224.
[19] JAMES A P,KIDANEMARIAM D B,HAMILL S D,DALE J L.HARDING R M.Infectivity of an infectious clone of banana streak CA virus in A-genome bananas(Musa acuminata ssp.)[J].Viruses,2021,13(6):1071.
[20] QI H,JOHN H.Cloning and sequence analysis of an infectious clone of Citrus yellow mosaic virus that can infect sweet orange via Agrobacterium-mediated inoculation[J].Journal of General Virology,2001,82(10):2549-2558.
[21] BHADRAMURTHY V,RETHEESH S T,BHAT A I,MADHU-BALA R,HAREESH P S,PANT P.Development of ELISAbased technique for the detection of a putative badnavirus infecting black pepper (Piper nigrum L.)[J].Indian Phytopathology,2012,58(3):314-318.
[22] MULLER E,DUPUY V,BLONDIN L,BAUFFE F,DAUGROIS J-H,NATHALIE L ISKRA-CARUANA M-L.High molecular variability of sugarcane bacilliform viruses in Guadeloupe implying the existence of at least three new species[J].Virus Research,2011,160(1/2):414-419.
[23] MULLER E,ULLAH I,DUNWELL J,DAYMOND A,RICHARDSON M,ALLAINGUILLAUME J,WETTEN A.Identification and distribution of novel badnaviral sequences integrated in the genome of cacao (Theobroma cacao)[J].Scientific Reports,2021,11(1):8270.
[24] MARAIS A,UMBER M,FILLOUX D,GOMEZ R-M,FAURE C,JULIAN C,ROUMAGNAC P,ACINA-MAMBOLE I,BONHEUR L,THEIL S,CONTRERAS S,CANDRESSE T,TEYCHENEY P-Y.Yam asymptomatic virus 1,a novel virus infecting yams(Dioscorea spp.)with significant prevalence in a germplasm collection[J].Archives of Virology,2020,165(11):2653-2657.
[25] KIDANEMARIAM D B,SUKAL A C,ABRAHAM A D,STOMEO F,HARDING R M.Identification and molecular characterization of taro bacilliform virus and taro bacilliform CH virus from East Africa[J].Plant Pathology,2018,67(9):1977-1986.
[26] DIAZ-LARA A,MOSIER N J,KELLER K E,MARTIN R.A variant of Rubus yellow net virus with altered genomic organization[J].Virus Genes,2015,50(1):104-110.
[27] LANEY A G,HASSAN M,TZANETAKIS I E.An integrated badnavirus is prevalent in fig germplasm[J].Phytopathology,2012,102(12):1182-1189.
[28] JONES A T,MCGAVIN W J,GEERING A D W,LOCKHRT B E L.A new badnavirus in Ribes species,its detection by PCR,and its close association with gooseberry vein banding disease[J].Plant Disease,2001,85(4):417-422.