葡萄是葡萄科葡萄属多年生藤本植物,因其用途广泛、商业价值可观,为世界广泛种植的经济类果树作物之一。在我国葡萄栽培历史悠久,其营养丰富,是果树栽培中经济效益最高的物种之一[1]。
由于北方地区干旱少雨、部分地块土壤盐渍化严重,难以种植葡萄等园艺作物,而南方地区葡萄生产季降雨量充沛,根系土壤含水量较高,病害严重,致使所产葡萄果实糖度低、着色差、风味淡,因此,亟需找到一种既可解决北方盐渍化严重的土壤不能种植葡萄,造成土壤资源浪费的问题,也可同时满足南方地区由于降雨量较大,地下水位高,所种植的葡萄果实品质差等一系列问题的技术措施。糖和花青素是葡萄果实内在品质和外观品质中最重要的组成部分,它们对植物和人体健康有着积极的作用[2]。
根域限制是利用物理或生态方式将根系控制在一定容积内来调控营养生长和生殖生长平衡的一种新型栽培方式。根域限制可以显著提高葡萄果实品质(糖、香味成分和花色苷的积累),并在各地大面积推广应用中得到充分验证。同时,已有大量研究致力于根域限制促进葡萄果实品质提升。早期的研究显示,根域限制栽培葡萄树体的碳素营养水平提高、而氮素营养含量降低[3],但根域限制栽培葡萄树单株的生物学产量(周年积累量)仅有对照的1/2左右,但经济学产量却接近对照的2倍,表明光合产物更多地分配给了果实[4]。进一步采用“浆果杯技术体系”研究发现,与对照相比,根域限制栽培促进了韧皮部质外体糖卸载[5],其可能与输导组织的结构变化及转化酶等关键酶的活性改善有关[6]。对花色苷的研究结果表明,根域限制栽培条件下巨峰葡萄果皮中的酰化和甲基化花色苷比例增加,花色苷合成关键基因表达显著上调[7]。植物生长发育过程中一直伴随着各种激素协同发挥调控功能。虽已有研究证明,根域限制可提高葡萄果实中糖和花色苷含量。根域限制栽培葡萄后,除休眠期与萌芽期以外的各个发育阶段,根、茎、叶、花和果穗等几乎所有组织、器官的脱落酸含量都大幅提高,转色期树液的脱落酸含量也增加到对照的近2倍。但仍缺乏系统的研究。鉴于此,笔者旨在通过探究根域限制对葡萄树体营养生长,果实糖、有机酸、花色苷和ABA等指标的影响,明确根域限制的表型特征变化及其与植物激素间存在的关联,为进一步探索根域限制条件下植物激素ABA 调控糖和花色苷积累的机制奠定基础。
1 材料和方法
1.1 材料
供试品种为3 年生玫瑰香葡萄(Vitis vinifera L.‘Muscat Hamburge’),于2017 年3 月定植于上海交通大学现代工程训练中心的玻璃温室(31°11’N,121°29’E)。温室长32 m,宽16 m,肩高4.3 m,顶高5 m。试验分2 组:根域限制和地栽(对照)处理,每一处理定植36 株葡萄树。根域限制的葡萄树定植于沙、土、有机肥(体积比1∶1∶1)为基质的直径40 cm的120 L 控根器中,对照葡萄树定植于基质完全相同的露地中作为对照。东西行向,株行距2 m×1 m,单株留10 个新梢,Y 字形整形,每个新梢留1 穗果,每一果穗留果40~50粒。采用霍格兰营养液进行滴灌,营养液浓度以120 mg·kg-1含N为准,每周1次。
1.2 试剂与仪器
1.2.1 试剂 甲醇(分析纯,CWN);已腈(色谱级,CWN);甲酸(色谱级,CWN);NaOH;氨水;葡萄糖和果糖(色谱纯,国药集团有限公司)。
1.2.2 仪器 台式高速冷冻离心机(湘仪,H2100R),超声波清洗机(KQ-500DE 型),手持糖度计,HPLC(LC3000型)配备示差折光检测器及紫外检测器,超纯水纯化系统(默克密理博,Milli-Q Advantag),旋转蒸发仪(RE-2000B,上海亚荣)配备SHE-Ⅲ型循环水真空泵,QTRAP5500 四级杆线性离子阱质谱仪。
1.3 方法
1.3.1 树体生长指标的测定 玫瑰香葡萄定梢后,处理和对照分别选择生长均匀一致植株10株,每株选2个长势一致且发育良好的新梢挂牌标记。新梢长到约20 cm时,每5 d及重要物候期(盛花期、果实膨大期、转色期)采用软尺测量新梢长度,用游标卡尺测定新梢基部向上5 cm 处的新梢粗度,每5 株树为1个生物学重复,设置3个生物学重复。
1.3.2 果实品质指标的测定 于花后20 d(20 DAA),从处理和对照组中随机选取10穗果,每穗果选取不同部位5 粒果粒(上、中、下、阴面、阳面),共50粒果做标记,每5 d采用游标卡尺测定1次果粒横径、纵径,直至成熟采收期。另外,每5 株葡萄树上随机选取30粒果实,为1个生物学重复,试验设置3个生物学重复(共15株葡萄树),带回实验室用万分之一天平测定单果质量,随后,挤压取葡萄汁,用于测定可溶性固形物含量。
(1)可溶性固形物含量的测定。待测完单果质量后,将葡萄果实挤压取果汁于50 mL 离心管中,8000 r·min-1离心10 min,取上清液用电子数显糖度计(DR103)测定其可溶性固形物含量,3次重复。
(2)单糖、有机酸含量的测定。将榨取的果汁在10 000 r·min-1的条件下离心15 min。吸取1 mL 上清液,加入离心管中,然后加入9 mL 水进行稀释。将稀释后的果汁用0.22 μm的针式滤头过滤,加入进样瓶,利用LC3000型高效液相色谱仪测定[8]。
糖测定色谱条件为,示差检测器,ZORBAX NH2色谱柱(250 mm×4.6 mm,5µm),流动相:乙腈-水(体积比75∶25,加入少量浓氨水,如750∶250∶2),流速:0.75 mL·min-1,柱温:25 ℃。每一样品重复进样3次,带入所得标准曲线计算对应有机酸或糖分含量,取平均值并计算标准差。
有机酸测定色谱条件为,紫外检测器,C18 色谱柱(250 mm× 4.6 mm,5 µm),流动相:V甲醇∶V0.01mol·L-1KH2PO4=3∶97,pH=2.5,流速:0.4 mL·min-1,柱温:45 ℃,检测波长为210 nm。
(3)总花色苷及单体花色苷含量的测定。花色苷提取参考Wang等[9]的方法有所改动:挑选大小均匀、成熟度一致、无机械损伤、无病虫害的果实,冷冻状态下剥皮,液氮研磨成粉末,1 g果皮加入5 mL含1%甲酸的甲醇溶液中,25 ℃下避光摇床萃取30 min,然后8000×g 低温离心10 min,收集上清液,残渣重复提取4次,合并上清液,30 ℃旋转蒸发除去甲酸和甲醇,然后用甲酸乙醇水溶液(V甲酸∶V乙醇∶V水=2∶10∶88)溶解复溶,定容至5 mL,该提取液用于总花色苷和单体花色苷测定。用pH 示差法测定总花色苷的含量(以矢车菊素-3-葡萄糖苷当量计算),每个处理3 次重复,花色苷含量(mg·g-1)X=∆A×F×M/ε×m,其中∆A为吸光值;F为稀释倍数;M为花青素-3-葡萄糖苷的相对分子质量449.2,ε为矢车菊素-3-葡萄糖苷的摩尔消光系数26 900;m为样品质量。
HPLC-MS(高效液相色谱-质谱联用技术)测定单体花色苷:G1315A 二极管阵列检测器,电喷雾离子源和离子阱质谱检测器,Kromasil C18 色谱柱(250 mm×4.6 mm,5 µm),流动相A:2%甲酸水溶液;流动相B:2.0%甲酸乙腈水溶液;流速1.0 mL·min-1,柱温:25 ℃,检测波长:525 nm,进样量:30µL。质谱采用电喷雾离子源(ESI),离子扫描范围:100~1000 m·z-1,雾化气压力:241.36 kPa;干燥器流速:10 L·min-1,干燥器温度:350 ℃。每一样品重复进样3次,利用标准曲线计算对应单体花色苷含量,取平均值并计算标准差[9]。
(4)类黄酮及原花青素含量的测定。挑选大小均匀、成熟度一致、无机械伤、无病虫害的果实,冷冻研磨成粉末后按照料液比1∶30(m/v)加入70%的乙醇提取液(含1%甲酸),超声辅助提取30 min,提取液在离心机上4000 r·min-1离心10 min。重复提取3次,合并上清液,利用高效液相色谱-质谱联用技术进行原花青素及类黄酮含量的测定。
色谱条件。色谱柱:ACQUITY HSS T3 column(100 mm×2.1 mm,1.8 μm,Waters);流动相:A相为0.1%甲酸水溶液,B 相为0.1%甲酸乙腈溶液;洗脱梯度:0~2 min,A 相:98%,B 相为2%;2~7 min,A 相80%,B 相:20%;7~12 min,A 相:50%,B 相:50%;12~14 min,A相:10%,B相:90%;柱温为30 ℃;进样量:2 μL,流速0.4 μL·min-1,检测波长为200~600 nm。
质谱条件:仪器采用负离子接口模式,多反应监测离子扫描模式(MRM)检测;气帘气(CUR)流速:35 L·min-1;雾化气(GS1)流速:55 L·min-1;辅助气(GS2)流速:55 L·min-1;离子化温度为550 ℃;喷雾电压(IS):负离子模式下-4500 V。通过LC-MS 相应峰值读出数据[10]。
(5)植物激素ABA含量的测定。准确称取0.1 g液氮速冻的葡萄果实样品至2 mL 离心管中,加入1 mL 提取液,置于-20 ℃浸提过夜;4 ℃,13 000×g离心20 min,收集上清液至新的2 mL 离心管,加入0.5 mL 提取液涡旋振荡,重复提取1次,4 ℃,13 000×g离心20 min,合并上清液,过C18 SPE Cartridge 柱子后旋转蒸发,甲酸溶解残渣,过已MCX SPE Cartridge 柱子,弃废液,继续用5 mL 1 mol·L-1的甲酸溶液冲洗Poly-Sery MCX SPE Cartridge 柱子,弃废液;5 mL甲醇洗脱至蒸馏瓶中,40 ℃旋转蒸发,1 mL的提取液2溶解残渣,过0.22 μm的有机相滤膜,上机检测。色谱柱:C18(250 mm × 4.6 mm,5 μm),流动相A:0.1%甲酸甲醇溶液,流动相B:0.1%甲酸水溶液,洗脱梯度:0~4 min,20%流动相A,4~8 min,50%流动相A,8~20 min,80%流动相A,20~22 min,80%流动相A,22~22.2 min,20%流动相A,22.2~30 min,20%流动相A。流速0.8 mL·min-1,柱温40 ℃,检测波长525 nm,进样量20 μL[11]。
1.4 数据分析
数据统计采用SPSS17.0 进行单因素方差分析和独立样本T检验法评估样本间差异的显著性,“*”和“**”分别表示样品间的显著性(p <0.05)和极显著性差异(p <0.01)。用Origin16.0 和Photoshop CS3完成图片绘制。
2 结果与分析
2.1 根域限制对玫瑰香葡萄生长发育的影响
根域限制对玫瑰香葡萄营养生长指标的影响如图1 所示。根域限制(RR)和地栽(NR)的模型图如图1-A所示。不同发育阶段根域限制和地栽条件下葡萄果实发育的表型如图1-B所示,其中40 DAA为膨大期,60 DAA 为转色期,80 DAA 为转色末期,110 DAA 为成熟采收期。花后20 d 至花后35 d,根域限制和对照间新梢长度无显著差异,同时,从花后40 d 至成熟采收期,根域限制条件下新梢长度均显著低于对照(图1-C)。而从花后20 d 至花后105 d,根域限制条件下新梢粗度几乎均显著低于对照(图1-D)。而对于果实横径和纵径,均呈双“S”生长曲线,从花后35 d至花后55 d,根域限制和地栽条件下果实横纵径呈线性上升,且根域限制条件下果实横纵径均高于对照;花后55 d至花后65 d,葡萄果实生长发育几乎处于停滞期,横纵径无明显的变化,说明该阶段葡萄果实几乎处于生长停滞状态;从花后65 d至花后110 d,葡萄果实又进入第2次快速生长发育期,其果实横纵径再次快速增大,尤其以果实横径变化最明显,在成熟采收期,根域限制条件下葡萄果实纵径、横径均无显著性差异(图1-E,图1-F)。2种栽培模式下单果质量几乎无差异(图1-G)。可溶性固形物含量是表征果实糖分含量的基本指标,可溶性固形物含量随葡萄果实的发育呈现整体升高的趋势,且根域限制条件下,除花后50 d、花后55 d 和花后60 d外,可溶性固形物含量差异不显著外,其余时期显著高于对照(图1-H)。
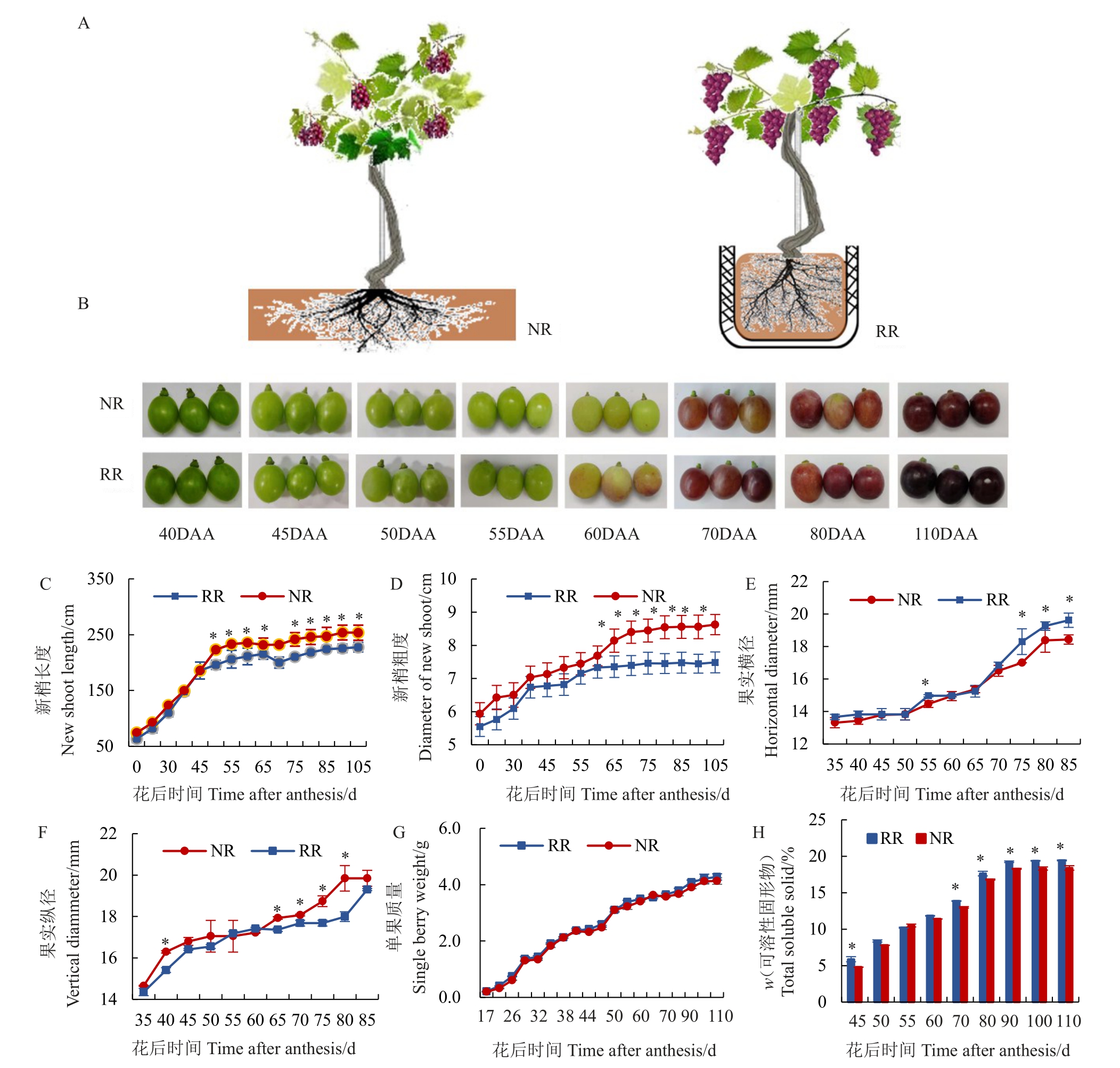
图1 根域限制对玫瑰香葡萄果实生长发育的影响
Fig.1 Effects of root restriction on the growth and development of grapevine
A.根域限制(RR)和传统地栽(NR)模型图;B.2 种栽培模式下葡萄果实不同生长发育过程的表型图;C.新梢长度;D.新梢直径;E.果实横径;F.果实纵径;G.单果质量;H.可溶性固形物含量。
A.The model of root restriction (RR) and no root restriction (NR);B.The process of grape berry development under root restriction cultivation and no restriction cultivation (control);C.New shoot length;D.New shoot diameter;E.Horizontal diameter of grape berry;F.Vertical diameter of grape berry;G.Single berry weight;H.Total soluble solid.
2.2 根域限制提高玫瑰香葡萄果实糖含量
根域限制对玫瑰香葡萄果实单糖含量的影响如图2 所示。利用HPLC 检测根域限制和对照条件下不同发育时期玫瑰香葡萄果实葡萄糖、果糖和蔗糖含量发现,葡萄果实中均未检测到蔗糖,果实中单糖主要为葡萄糖和果糖。随着葡萄果实的发育,葡萄糖和果糖含量均呈现出上升趋势,且根域限制条件下葡萄糖和果糖含量均高于对照。花后55 d时,根域限制条件下既可检测到葡萄糖和果糖,而此时地栽条件下则未检测到2 种单糖,于花后60 d(转色期),2种栽培模式下葡萄糖和果糖含量均大幅度上升,且根域限制条件下葡萄糖和果糖含量均高于地栽,尤以果糖含量提高较为显著。在成熟采收期,根域限制条件下葡萄糖和果糖含量(w,后同)分别为10.92 mg·g-1,9.84 mg·g-1,分别比对照(7.69 mg·g-1,7.55 mg·g-1)显著提高了42.0%和30.03%。
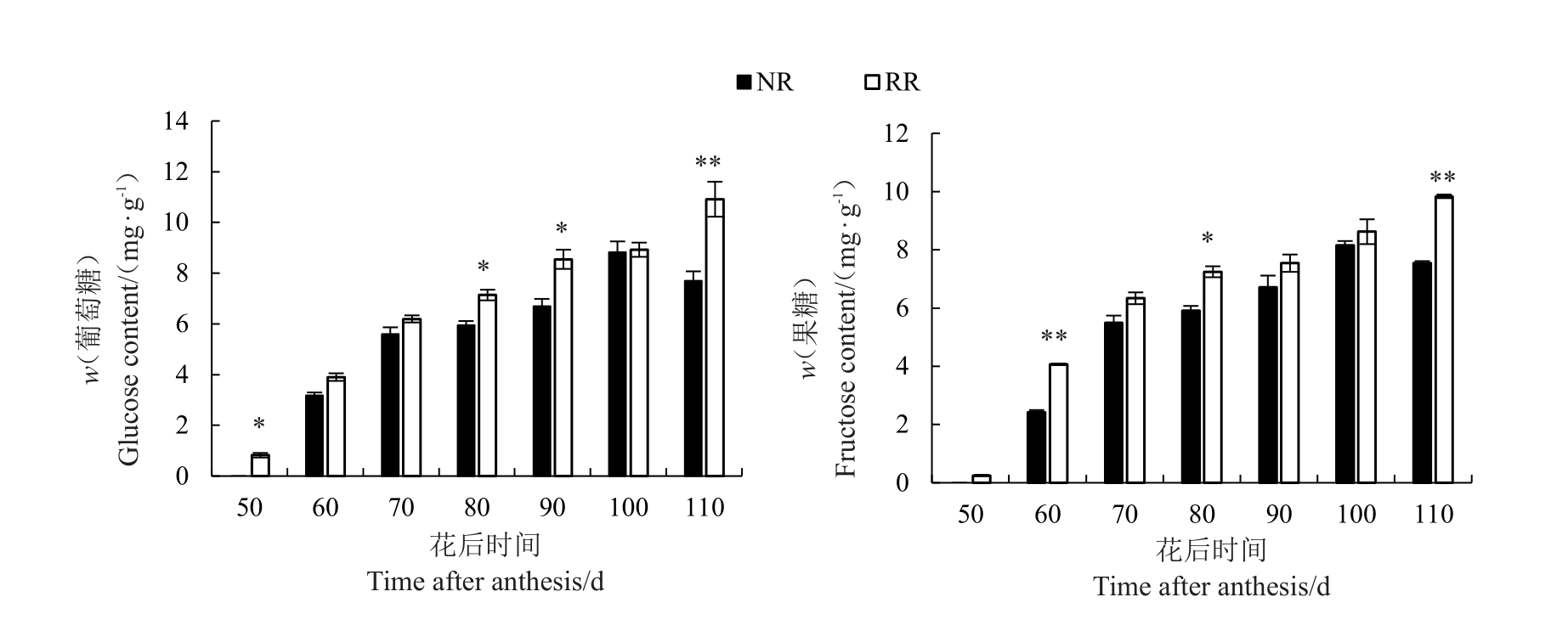
图2 根域限制对玫瑰香葡萄果实单糖组分含量的影响
Fig.2 Effects of root restriction on the individual sugar content in grape berry
2.3 根域限制降低玫瑰香葡萄果实有机酸含量
根域限制对玫瑰香葡萄果实有机酸酸含量的影响如图3 所示。2 种栽培模式下玫瑰香葡萄果实中均可检测到11 种有机酸。葡萄果实中有机酸含量从转色期(S2)至成熟采收期(S4)整体呈下降趋势,膨大期(S1)果实中苹果酸(D-苹果酸和L-苹果酸)、酒石酸和柠檬酸这3种主要有机酸在根域限制条件下分别为21.58、17.13、7.77、0.20 mg·g-1,而地栽分别为18.03、16.25、8.78、0.23 mg·g-1,根域限制栽培下苹果酸和酒石酸含量均高于对照;而在转色期(S2),根域限制条件下它们的含量分别为10.97、8.57、5.98 mg·g-1,地栽为16.04、12.41、6.94 mg·g-1,根域限制栽培的这3 种有机酸含量均低于对照,与膨大期(S1)根域限制栽培下它们的含量均高于对照完全相反;转色末期(S3),根域限制条件下它们的含量分别为4.63、4.53、5.80 mg·g-1,地栽为5.89、6.63、8.48 mg·g-1,由此可以看出,随着葡萄果实发育,有机酸含量逐渐下降,转色期是果实有机酸含量急剧下降的关键时期,根域限制栽培降低果实有机酸含量也主要在膨大期(S1)至转色期(S2)这一阶段发挥作用。
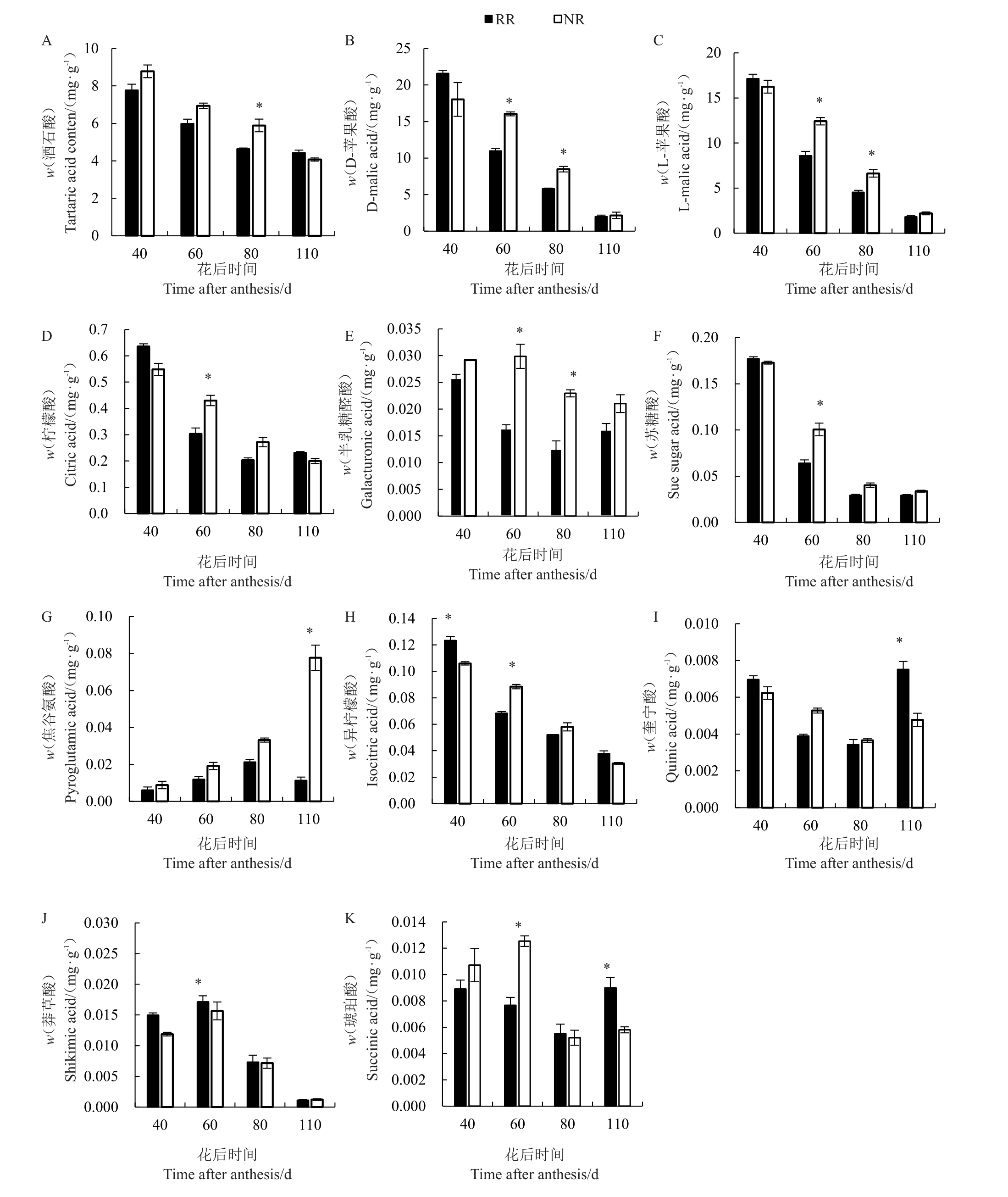
图3 根域限制对玫瑰香葡萄果实有机酸含量的影响
Fig.3 Effects of root restriction on organic acid content
A.酒石酸;B.D-苹果酸;C.L-苹果酸;D.柠檬酸;E.半乳糖醛酸;F.苏糖酸;G.焦谷氨酸;H.异柠檬酸;I.奎宁酸;J.莽草酸;K.琥珀酸。
A.Tartaric acid;B.D-malic acid;C.L-malic acid;D.Citric acid;E.Galacturonic acid;F.Sue sugar acid;G.Pyroglutamic acid;H.Isocitric acid;I.Quinic acid;J.Shikimic acid;K.Succinic acid.
2.4 根域限制提高玫瑰香葡萄果实花色苷含量
由图1-B的表型结果可知,根域限制条件下葡萄果实于花后60 d进入转色期,而地栽仍为绿色,2种处理果实发育进程存在一定差异,为此,笔者分别检测了两处理葡萄果实中总花色苷和单体花色苷含量。首先是总花色苷含量变化,发现花后40 d 和花后50 d时,2 种处理条件下的葡萄果实中几乎检测不到总花色苷,而在花后60 d,根域限制和地栽条件下的葡萄果实中均可检测到花色苷,且根域限制下花色苷含量显著高于地栽,从花后60 d至花后110 d,葡萄果实中花色苷含量呈现上升趋势,且根域限制的均显著高于地栽(图4-A和4-B),说明根域限制可提高果实中总花色苷含量。为探究根域限制和对照对葡萄不同生长发育期果实单体花色苷组分和含量的影响,分别在膨大期(花后40 d)、转色期(花后60 d)、转色末期(花后80 d)和成熟期(花后110 d)4个阶段检测葡萄果实中单体花色苷组分和含量变化,检测结果如图4-C所示。结果表明,2种栽培模式中可检测到13种单体花色苷。在成熟期,除花翠素-3-乙酰化葡萄糖苷和花翠素外,根域限制条件下葡萄果实中其他单体花色苷含量均显著或极显著高于对照,最高为对照的7倍。根域限制条件下二甲花翠素及其衍生物含量在成熟采收期显著高于对照。综上所述,根域限制可显著提高葡萄果实单体花色苷含量,但未改变花色苷组分。
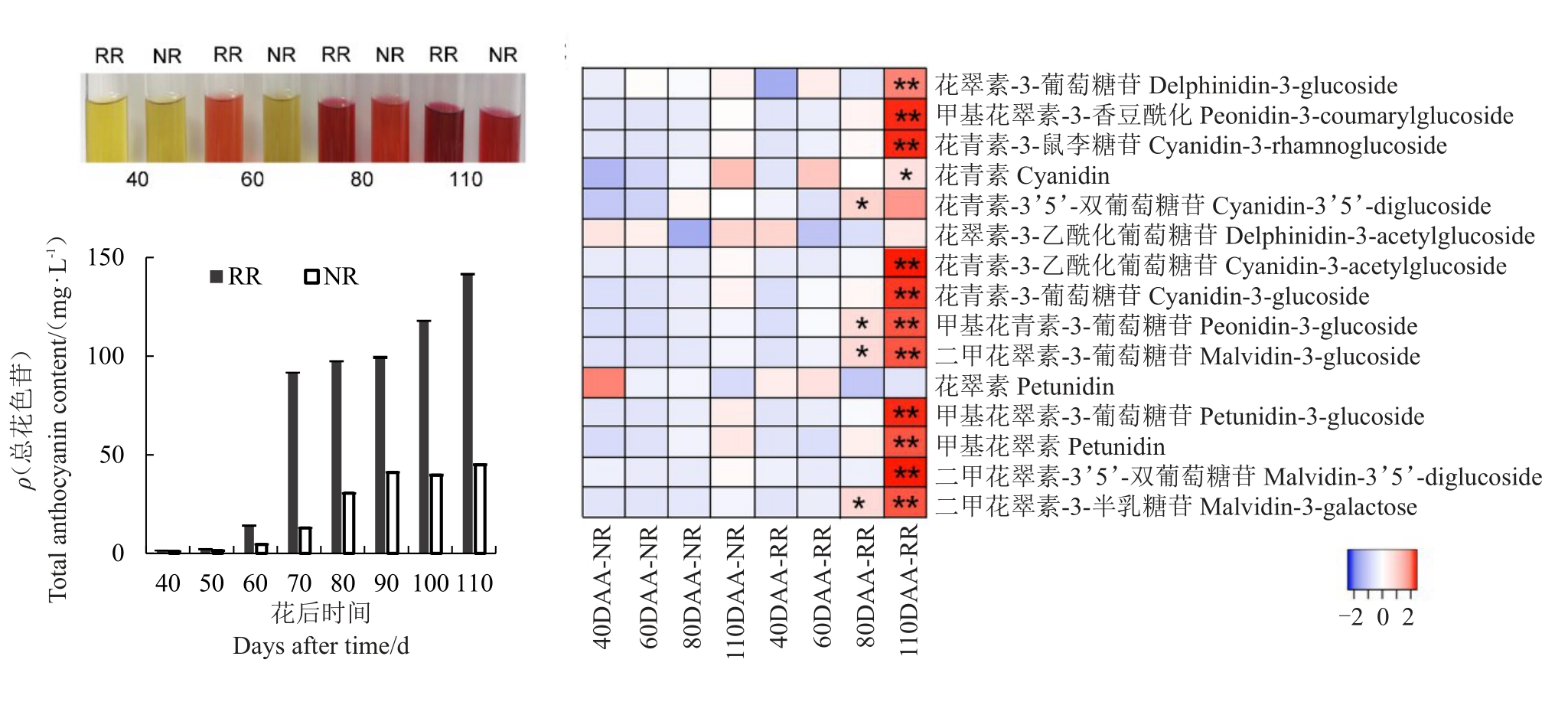
图4 根域限制对玫瑰香葡萄花色苷含量的影响
Fig.4 Effect of root restriction on anthocyanin content in grape
A 和B.根域限制条件下玫瑰香葡萄总花色苷的变化,C.根域限制条件下玫瑰香葡萄单体花色苷含量的变化热图。*代表与对照相比,差异显著,**代表与对照相比,差异极显著。数据用Log2 数值和Z-score 进行标准化。
A and B.Change of total anthocyanin content of grape berry under root restriction.C.Heatmap of individual anthocyanin content in Muscat Hamburg grape under root restriction.*indicated significant differences(p ≤0.05),**indicated extremely significant differences(p ≤0.01).Values were normalized with Log2(values)and converted to Z-scores.
2.5 根域限制促进玫瑰香葡萄果实非花色苷酚类物质的积累
在根域限制和对照条件下玫瑰香葡萄果实中均可检测到19 种酚酸(图5-A)。根域限制显著提高了果实中二羟基苯甲酸、没食子酸、3,4-二羟基苯甲酸和鞣花酸含量。根域限制和对照条件下玫瑰香葡萄果实中均可检测到12 种黄烷醇(图5-B)。2 种处理条件下原花青素含量整体呈现下降趋势,且在成熟采收期,根域限制条件下原花青素含量整体低于对照,但差异不显著。根域限制和对照条件下玫瑰香葡萄果实中均可检测到21种黄酮醇(图5-C)。且在成熟采收期,除山奈酚-3-葡萄糖苷、山奈酚、木樨草素、柚皮苷和根皮苷含量在两种处理间无显著性差异外,其他均以根域限制处理显著高于对照,为对照的1.7~5.9 倍。检测到的黄酮醇大多以黄酮醇苷形式存在,山奈酚和柚皮素含量下降,而糖苷结合态含量极显著上升,可能是山奈酚和柚皮素衍生化形成黄酮醇苷类物质以响应根域限制这一非生物胁迫的影响。3 种槲皮素苷类物质含量显著上升,可能是由于其竞争底物柚皮素的能力增强,进一步转化为糖苷类。根域限制和对照条件下玫瑰香葡萄果实中均可检测到4种芪类物质,分别为云杉新苷、紫檀芪、反式白藜芦醇和葡萄素(图5-D)。除紫檀芪外,其他3 种芪类物质随果实发育呈上升趋势,且根域限制条件下云杉新苷和葡萄素含量显著高于对照,而反式白藜芦醇含量呈相反趋势。

图5 根域限制条件下玫瑰香葡萄果实非花色苷酚类物质含量分析
Fig.5 Analysis of different phenolic substances(except for anthocyanin)in Muscat Hamburg grape under root restriction
A.酚酸;B.黄烷醇;C.黄酮醇;D.芪类。*代表与对照相比,差异显著,**代表与对照相比,差异极显著。数据用Log2 数值和Z-score 进行标准化。
A.Phenolic acid;B.Flavanols,C.Flavonols;D.Stilbenes.“*”indicated significant differences (p ≤0.05),“**”indicated extremely significant differences(p ≤0.01).Values were normalized with Log2(values)and converted to Z-scores.
2.6 根域限制条件下植物激素ABA含量的变化
根域限制栽培下不同发育阶段玫瑰香葡萄果实内源激素ABA 含量变化如图6 所示。随着葡萄果实的生长发育,果实内源ABA含量呈先上升后下降的趋势,在转色期ABA 含量最高。且在花后40 d(膨大期)、花后60 d(转色期)和花后80 d(转色末期),根域限制条件下果实内源ABA 含量均显著高于对照,分别为对照的2.50、1.95、1.93倍,而在成熟采收期,2 种处理中内源ABA 含量(ρ)分别为27.23 ng·mL-1和26.67 ng·mL-1,无显著差异。
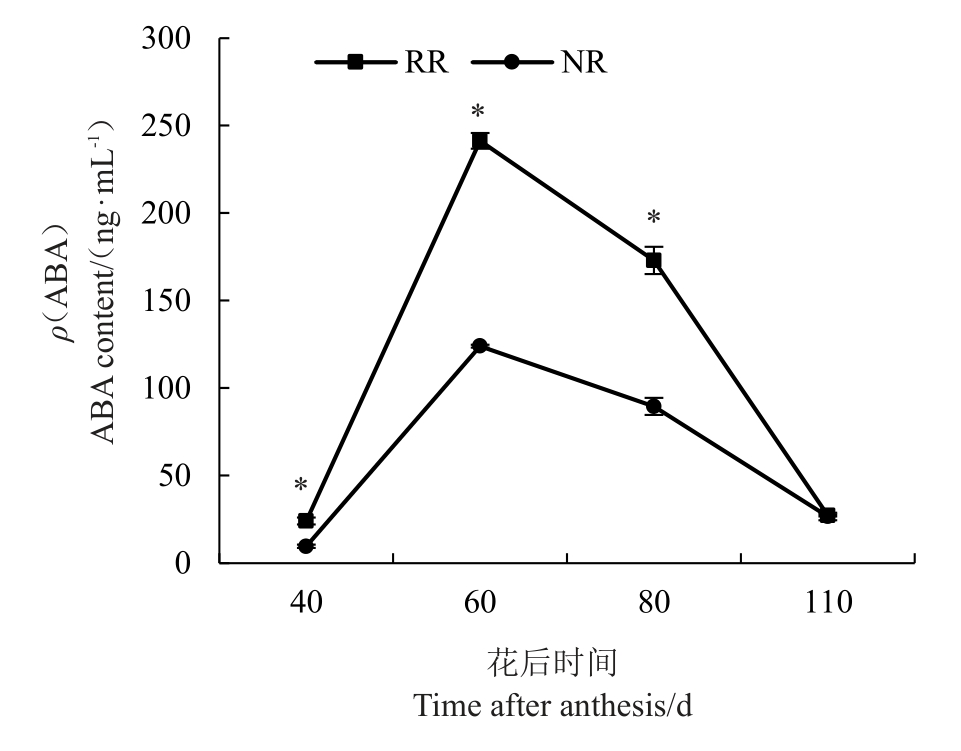
图6 根域限制栽培促进玫瑰香葡萄果实内源激素ABA的积累
Fig.6 Root restriction cultivation promoted the accumulation of endogenous ABA in grape berries befereand after veraison
NR 表示传统栽培(对照),RR 表示根域限制栽培。误差线表示3个生物学重复的平均值±标准误,采用单因素ANOVA 和Tukeys 事后检验分析差异显著性,“*”表示显著性差异(p ≤0.05)。
NR represents control,RR indicates root restriction.The data are presented as the mean values of three biological replicates,and the error bars indicate SD.Significances were tested by one-way ANOVA and Tukeys post-hoc test,“*”indicates significant difference (p ≤0.05).
3 讨论
3.1 根域限制影响葡萄地上部分的营养生长
葡萄是世界广泛栽植的经济类果树作物之一,在生长发育过程中果实经历复杂的生理变化[11]。根域限制栽培技术使葡萄植株处于胁迫状态,对树体的营养生长与生殖生长间的平衡具有一定的调控作用[12]。目前,根域限制栽培技术已被成功应用于葡萄[13]、桃[14]、苹果[15]、柑橘[16]和甜樱桃[17]等多种果树作物。在根域限制条件下,葡萄植株和果实生长发育代谢规律均发生显著变化,本课题组前期研究结果表明,根域限制条件下葡萄植株的营养生长(新梢生长量等)、叶片光合速率和干物质积累量均低于传统地栽,但果实中积累的光合同化产物量反而显著高于对照[18]。虽然根域限制抑制玫瑰香葡萄新梢生长,却促进了不定根的发育,尤其是毛细根的数量极显著高于对照。根系作为植物从土壤中吸收水分和营养的唯一器官,可为地上部分的生长发育提供充足的营养和矿质元素基础。根域限制下葡萄植株年生物积累量低于对照,但根域限制下果实生物量积累占整株的比例却显著升高[6],本研究与谢兆森等[6]研究结果相一致,说明根域限制通过促进地下部分从土壤中汲取水分和矿质营养元素运输至地上部分,为地上部分的生长提供有效营养保证,从而提升葡萄果实品质。
3.2 根域限制栽培提升葡萄果实品质
在葡萄果实整个发育期,果实呈现纵横径逐渐增长,果实软化及色泽变深等一系列外观特征变化。研究结果表明,根域限制栽培可有效促进葡萄果实转色,有利于果实色素积累,色泽明显优于对照。葡萄果实外部表观特征的变化是由果实内部一系列生理生化变化所引起的。葡萄果实糖酸是评价果实成熟度和口感质量的重要指标。笔者在本研究中发现,在膨大期葡萄果实以积累有机酸为主,而在转色期之后,葡萄果实开始积累糖分和次生代谢物,有机酸开始降解。结果表明,转色期,葡萄果实中葡萄糖和果糖迅速积累,且根域限制条件下两者含量均显著高于对照。同时,有机酸在呼吸作用下被逐渐降解。在柑橘果实发育前期,果肉中有机酸含量迅速增加,当体积为成熟体积1/2时,有机酸含量最高,随后逐渐下降,而在果实发育后期,有机酸作为呼吸作用和糖异生过程的底物或基质参与相应的生理过程,伴随着其含量的逐渐下降,糖分含量的逐渐升高[19],这与本章的研究结果相一致。笔者在本研究中发现,在果实转色期前(40 DAA),有机酸(主要为苹果酸和酒石酸)含量最高,且以根域限制栽培高于对照,而随着果实的发育,两处理果实中有机酸含量均呈降低趋势,且根域限制条件下有机酸含量下降速度高于对照,至成熟采收期,根域限制栽培葡萄果实有机酸含量明显低于对照,提高果实糖酸比,改善果实品质和口感。可溶性固形物含量作为一项检测快速、表征果实含糖量的指标,不能完全表征果实糖分含量变化。为此,为了进一步揭示根域限制对果实糖分含量的影响,笔者在本研究中利用HPLC检测了根域限制和对照条件下单糖组分的变化,在两种栽培模式下均可检测到葡萄糖和果糖两种单糖成分,但未检测到蔗糖成分,且葡萄糖和果糖的比例接近1∶1。另外,根域限制栽培可显著提高葡萄果实中葡萄糖和果糖含量,这与Wang等[9]的研究结果完全一致。进一步证实根域限制栽培方式下的葡萄果实生理代谢活动旺盛,库强增加,促进光合产物在库器官的积累。前人研究发现,适度干旱胁迫能够显著提高葡萄果实可溶性固形物、花色苷和总酚等物质含量,这与本研究结果相一致,可能是根域限制对葡萄果实初生和次生代谢物的影响与中度水分胁迫具有相似的作用效果,均有利于各种次生代谢物的积累。果皮颜色是葡萄果实重要的品质指标之一,它主要取决于花色苷的组分和含量[20]。前人研究认为,中度水分胁迫不仅有助于增加果皮中总花色苷和单体花色苷含量,而且还可以提高花色苷衍生物含量比例,如甲基化修饰和乙酰化修饰程度,而甲基化和酰化修饰的花色苷物质有助于提高花色苷的稳定性[20],在成熟采收期,花色苷的糖基化和酰基化衍生物含量均极显著高于对照,表明其不仅有助于果实着色,同时可提高葡萄果实的稳定性。这一研究结果再次证明,根域限制与水分胁迫具有类似的作用效果。
类黄酮是葡萄果实中重要的酚类化合物,它不仅在植物生长发育中发挥着重要作用,也在植物响应逆境环境胁迫中起着重要作用。槲皮素3-O-葡萄糖苷和槲皮素3-O-葡糖苷酸被认为是葡萄浆果中的主要黄酮醇[21],而本试验中仅检测到槲皮素3-O-葡萄糖苷的存在,且随着生育期的推进,其含量整体呈上升趋势。在成熟采收期,根域限制条件下其含量极显著高于对照,这与前人在水分胁迫条件下观察到的结果完全一致。另外,水分胁迫处理对槲皮素和山奈酚及其衍生物的影响较小[21],而笔者在本研究中发现,根域限制条件下槲皮素和山奈酚及其衍生物比例显著提高,说明根域限制与水分胁迫属于两种不同的逆境胁迫,对果实发育的影响机制不完全一致。水分胁迫可提高苯丙氨酸代谢通路中柚皮素及其代谢物含量,而笔者在本研究中发现,根域限制提高了葡萄果实中柚皮素7-O-葡萄糖苷含量,而柚皮素自身与对照间无差异,可能是由于大量的柚皮素被修饰形成稳定的衍生物,最终有助于提高葡萄果实的稳定性。以上研究结果均表明本次处理具备了根域限制葡萄树的典型特征。ABA 通过下游信号分子间的相互作用来调控甜樱桃果实发育、成熟及品质形成等过程[22-23]。同样地,ABA 也加速进蓝莓果实的着色及软化[24]。研究结果与其相一致,笔者发现根域限制条件下葡萄果实中ABA 含量显著提高,果实提前着色,糖含量显著提高。
4 结论
根域限制栽培显著抑制玫瑰香葡萄植株的营养生长,显著促进玫瑰香葡萄果实葡萄糖、果糖,总花色苷和酚类化合物积累,且甲基化和酰基化修饰花色苷衍生物含量居多。且植物激素ABA 与果实糖分、花色苷积累呈显著正相关。
[1] YANG B H,HE S,LIU Y,LIU B C,JU Y L,KANG D Z,SUN X Y,FANG Y L.Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries[J].Food Chemistry,2020,314:126170.
[2] LI T S,YAMANE H,TAO R.Preharvest long-term exposure to UV-B radiation promotes fruit ripening and modifies stage-specific anthocyanin metabolism in highbush blueberry[J].Horticulture Research,2021,8(1):1-2.
[3] WANG S P,OKAMOTO G,KEN H.Effect of root restriction on vine nutrition of Kyoho grape from dormancy to anthesis[J].Journal of the Japanese Society for Horticultural Science,1997,66(11):86-87.
[4] WANG S,OKAMOTO G,HIRANO K,LU J,ZHANG C X.Effects of restricted rooting volume on vine growth and berry development of Kyoho grapevines[J].American Journal of Enology and Viticulture,2001,52(3):248-253.
[5] 娄玉穗,杨天仪,刘晓清,李洪艳,赵丽萍,许文平,张才喜,王世平.根域限制对‘峰后’葡萄果实韧皮部糖卸载的影响[J].园艺学报,2013,40(5):817-27.LOU Yusui,YANG Tianyi,LIU Xiaoqing,LI Hongyan,ZHAO Liping,XU Wenping,ZHANG Caixi,WANG Shiping.Effects of root restriction on berry sugar phloem unloading of‘Fenghou’grape[J].Acta Horticulturae Sinica,2013,40(5):817-827.
[6] 谢兆森.根域限制对葡萄果实发育、源库器官及其输导组织结构的影响[D].上海:上海交通大学,2010.XIE Zhaosen.The effect of root restriction on grape berry development,the structure of source and sink organs,and their conducting tissue of grapevines[D].Shanghai:Shanghai Jiaotong University,2010.
[7] WANG B,HE J J,BAI Y,YU X M,LI J F,ZHANG C X,XU W P,BAI X J,CAO X J,WANG S P.Root restriction affected anthocyanin composition and up-regulated the transcription of their biosynthetic genes during berry development in‘Summer Black’grape[J].Acta Physiologiae Plantarum,2013,35(7):2205-2217.
[8] 段书延.钙胁迫与根域限制对葡萄生长发育和代谢产物的影响[D].上海:上海交通大学,2019.DUAN Shuyan.Impact of calcium stress and root restriction on development and metabolites of grape (Vitis vinifera L.)[D].Shanghai:Shanghai Jiaotong University,2019.
[9] WANG B,HE J J,DUAN C Q,YU X M,ZHU L N,XIE Z S,ZHANG C X,XU W P,WANG S P.Root restriction affects anthocyanin accumulation and composition in berry skin of‘Kyoho’grape(Vitis vinifera L.×Vitis labrusca L.)during ripening[J].Scientia Horticulturae,2012,137:20-28.
[10] 张钥,王呈阳,周嘉玲,李有梅,谢兆森,冷锋.不同水分调亏处理对葡萄果皮酚类物质的影响[J].果树学报,2021,38(8):1296-1307.ZHANG Yue,WANG Chengyang,ZHOU Jialing,LI Youmei,XIE Zhaosen,LENG Feng.Effects of different water regulated deficit treatments on phenols in grape fruits[J].Journal of Fruit Science,2021,38(8):1296-1307.
[11] JIA H F,CHAI Y M,LI C L,LU D,LUO J J,QIN L,SHEN Y Y.Abscisic acid plays an important role in the regulation of strawberry fruit ripening[J].Plant Physiology,2011,157(1):188-199.
[12] XIE Z S,LI B,FORNEY C F,XU W P,WANG S P.Changes in sugar content and relative enzyme activity in grape berry in response to root restriction[J].Scientia Horticulturae,2009,123(1):39-45.
[13] WANG S P,OKAMOTO G,HIRANO K,LU J,ZHANG C X.Effects of restricted rooting volume on vine growth and berry development of Kyoho grapevines[J].American Journal of Enology and Viticulture,2001,52(3):248-253.
[14] BOLAND A M,JERIE P H,MITCHELL P D,GOODWIN I.Long-term effects of restricted root volume and regulated deficit irrigation on peach:I.Growth and mineral nutrition[J].American Society for Horticultural Science,2000,125(1):135-142.
[15] ATKINSON C J,WEBSTER A D,VAUGHAN S P,TAYLOR L,KINGSWELL G.Interactions between root restriction,irrigation and rootstock treatments on‘Queen Cox’apple trees:Effects of soil and plant water relations[J].The Journal of Horticultural Science&Biotechnology,2000,75(4):376-382.
[16] MATAA M,TOMINAGA S.Effects of root restriction on tree development in Ponkan mandarin (Citrus reticulata Blanco)[J].Journal of the American Society for Horticultural Science,1998,123(4):651-655.
[17] WHITE M D,TUSTIN D S,FOOTE K F,CAMPBELL J M.Growth of young sweet cherry trees in response to root restriction using root control bags[C].2th International Symposium onOrchard and Plantation Systems,2001:391-397.
[18] XIE Z S,FORNEY C F,XU W P,WANG S P.Effects of root restriction on ultrastructure of phloem tissues in grape berry[J].American Society for Horticultural Science,2009,44(5):1334-1339.
[19] CALZARANO F,CICHELLI A,DEL CARLO M,SEGHETTI L.Effect of esca on the quality of berries,musts and wines[J].Phytopathologia Mediterranea,2004,43(1):125-135.
[20] ZHAI R,WANG Z M,ZHANG S W,MENG G,SONG L Y,WANG Z G,LI P M,MA F W,XU L F.Two MYB transcription factors regulate flavonoid biosynthesis in pear fruit (Pyrus bretschneideri Rehd.)[J].Journal of Experimental Botany,2016,67(5):1275-1284.
[21] VILLANGO S,SZEKERES A,BENCSIK O,LAPOSI R,PALFI Z,ZSOFI Z.The effect of postveraison water deficit on the phenolic composition and concentration of the Kékfrankos(Vitis vinifera L.)berry[J].Scientia Horticulturae,2016,209:113-116.
[22] TIJERO V,TERIBIA N,MUNOZ P,MUNNE-MOSCH S.Implication of abscisic acid on ripening and quality in sweet cherries:Differential effects during pre-and post-harvest[J].Frontiers in Plant Science,2016,7:602.
[23] SHEN X J,GUO X,ZHAO D,ZHANG Q,JIANG Y Z,WANG Y T,PENG X,WEI Y,ZHAI Z F,ZHAO W,LI T H.Cloning and expression profiling of the PacSnRK2 and PacPP2C gene families during fruit development,ABA treatment,and dehydration stress in sweet cherry[J].Plant Physiology and Biochemistry,2017,119:275-285.
[24] CHUNG S W,YU D J,OH H D,AN J H,HUH J H,HEE J L.Transcriptional regulation of abscisic acid biosynthesis and signal transduction,and anthocyanin biosynthesis in‘Bluecrop’highbush blueberry fruit during ripening[J].PLoS One,2019,14(7):e0220015.