柑橘大实蝇(Bactrocera minax)属双翅目实蝇科,是一种严重危害柑橘类果实的寡食性害虫[1-2]。该虫广泛分布于亚洲的柑橘生产带上,严重影响了柑橘果实的品质和产量[1,3-5]。柑橘大实蝇主要以幼虫在果实中钻蛀取食进行危害,不易防控,而利用引诱剂诱捕成虫是一种高效、绿色环保的防治方法。因此,深入研究柑橘大实蝇的嗅觉识别机制,既可以加深对柑橘大实蝇嗅觉通路的认知,也为研制高效引诱剂或驱避剂提供重要的理论依据。
昆虫嗅觉在其识别配偶、寻找产卵位置和定位寄主植物等生命活动中起着至关重要的作用[6-8]。触角作为昆虫的主要嗅觉器官,其上分布着数量和种类众多的嗅觉感受器,而环境中的脂溶性气味分子从感受器上的微孔进入之后,需要穿过亲水性的淋巴液才能够到达并激活位于感觉神经元树突膜上的气味受体,这一过程被称为昆虫外周嗅觉信号转导过程。昆虫外周嗅觉信号转导是通过几种重要的功能蛋白介导的,主要包括气味结合蛋白(odorant binding proteins,OBPs)、化学感受蛋白(chemosensory proteins,CSPs)、感觉神经元膜蛋白(sensory neuron membrane proteins,SNMPs)、气味降解酶(odorant degrading enzymes,ODEs)、离子型受体(ionotropic receptors,IRs)、味觉受体(gustatory receptors,GRs)和气味受体(odorant receptors,ORs)等[9]。OBPs 和CSPs 具有选择性识别并运输疏水性气味分子穿过亲水性的感受器淋巴液的功能,在昆虫嗅觉识别的过程中发挥着极其重要的作用,是昆虫嗅觉识别的第一步生化反应[10]。
CSPs 是一类低分子水溶性蛋白,一般含有约120 个氨基酸残基,蛋白分子质量10~15 ku,具有4个保守的半胱氨酸残基形成的2 个二硫键[11]。与OBPs 不同的是,CSPs 不仅在触角这种化学感受组织中表达,同时也在非化学感觉组织中普遍表达。如信息素分泌腺体[12]、中肠[13]、腹部[14]、生殖器官[15]、卵[16]和足[17],暗示了其具有非嗅觉作用。已有研究表明CSPs 具有参与昆虫的再生和发育[16,18-19]、参与昆虫抗药性的形成[20-21]、参与昆虫的离居型和散居型的生理型态转换调节[22]、参与到昆虫取食过程中并行使表面活性剂的作用[23],而在昆虫触角中高表达的CSPs 往往参与了昆虫的嗅觉识别过程[24]。已有研究发现,在触角中高表达的CSPs与寄主植物挥发物具有较高的结合活性,如大螟(Sesamia inferens)的CSP19(SinfCSP19)在雄蛾触角中高表达,重组表达的SinfCSP19 不仅能有效结合6 种寄主挥发物组分(Ki=2.12~8.75 μmol·L-1),而且对3 种性信息素组分也表达出超强结合能力(Ki=0.49~1.78 μmol·L-1)[25];在触角中显著上调表达的苜蓿盲蝽(Adelphocoris lineolatus)CSP1-3,重组表达的AlinCSP1-3 能够有效结合叶醇、正戊醛和水杨酸甲酯等气味配体(Ki=5.42~16.30 μmol·L-1)[26]。因此,推测BminCSP3也具有同以上几种CSPs类似的功能。
CSPs蛋白首次在黑腹果蝇(Drosophila melanogaster)中鉴定出来,命名为OS-D(Olfactory specific protein D)[27],至今已鉴定了包括鳞翅目在内的十多个目昆虫的CSPs[28]。近期,笔者通过分析柑橘大实蝇头部转录组,鉴定了4 种CSPs 基因(BminCSP1-4),其中BminCSP3 在柑橘大实蝇成虫触角中显著上调表达[29],暗示该基因可能具有嗅觉功能。为了证明BminCSP3的嗅觉功能,笔者在本研究中通过RT-PCR 克隆BminCSP3 基因,并对其进行生物信息学分析;以原核表达系统体外表达纯化得到BminCSP3;利用荧光竞争结合实验检测BminCSP3 与14种气味配体(其中大部分气味能够引起柑橘大实蝇成虫行为反应)[30-31]的结合特性,以期为明确柑橘大实蝇化学感受机制及研发其行为调节剂奠定基础。
1 材料和方法
1.1 供试昆虫
柑橘大实蝇试虫均来自长江大学农学院昆虫化学生态实验室[30]。收集1~3 日龄雌雄成虫的触角,每组样本取自30头成虫(不同日龄各10头),收集好的样品于-80 ℃保存。
1.2 主要试剂及仪器
MiniBEST Universal RNA Extraction Kit、Prime-ScriptTM RT reagent Kit with gDNA Eraser、Premix TaTM(LA TaqTM Version 2.0)、DL2000 DNA Marker、TaKaRa MiniBEST Plasmid Purification Kit Ver.4.0、限制性内切酶EcoRⅠ和Hind Ⅲ、DNA Ligation Kit均购自宝生物工程(大连)有限公司;Ni-NTA His·Bind Resin 纯化树脂,上海七海复泰生物科技有限公司;肠激酶(Enterokinase),上海近岸科技有限公司;普通琼脂糖凝胶DNA 回收试剂盒,天根生化科技(北京)有限公司;BCA 蛋白浓度测定试剂盒,北京索莱宝科技有限公司;pGEM-T easy vector,Promega;F-DH5α 感受态细胞,唯地生物。Gel Doc XR+凝胶成像系统,T100TM PCR 仪(BIO-RAD);荧光分光光度计F-7000(日本日立公司)。
1.3 总RNA提取以及第一链cDNA合成
柑橘大实蝇组织总RNA 按照MiniBEST Universal RNA Extraction Kit说明书提取;第一链cDNA参照PrimeScriptTM RT reagent Kit with gDNA Eraser说明书合成。-20 ℃保存,备用。
1.4 柑橘大实蝇BminCSP3基因克隆鉴定
根据本实验室柑橘大实蝇成虫转录组数据中筛选到的BminCSP3基因序列。设计出带有酶切位点的引物用于扩增编码成熟BminCSP3蛋白的基因序列(表1,酶切位点用下划线标示),以雄成虫触角组织cDNA 为模板,对BminCSP3 进行克隆,PCR 反应体系(25 μL):cDNA 模板2 μL、Premix Taq(LA Taq Version 2.0)试剂12.5 μL、上下游引物各1 μL(10 μmol·L-1)、ddH2O 8.5 μL。反应条件:95 ℃预变性3 min;95 ℃16 s,58 ℃25 s,72 ℃30 s,29个循环;72 ℃延伸5 min。PCR产物用1%(w)的琼脂糖凝胶电泳检测正确后,使用琼脂糖凝胶DNA回收试剂盒回收目的片段。将回收的目的片段连接到pGEM-T easy vector,并转化至DH5α 感受态细胞中进行培养,挑取单克隆进行菌液PCR 检测,使用TaKaRa MiniBEST Plasmid Purification Kit Ver 4.0 提取检测正确的质粒送样测序。序列测定及引物合成均由北京六合华大基因科技有限公司完成。
表1 本研究所用引物序列
Table 1 Primer used in this study
1.5 柑橘大实蝇BminCSP3 基因的生物信息学分析
柑橘大实蝇BminCSP3 核苷酸序列利用ORF Finder(https://www.ncbi.nlm.nih.gov/orffinder/)查找开放阅读框,通过DNAMAN 7.0软件推导氨基酸序列;利用Expasy的ProtParam(https://web.expasy.org/protparam/)分析蛋白质的理化性质;信号肽采用SignalP 5.0(http://www.cbs.dtu.dk/services/SignalP-5.0/)服务器进行预测,同时运用TMHMM Server v 2.0(http://www.cbs.dtu.dk/services/TMHMM/)对 其跨膜结构域进行预测。
1.6 柑橘大实蝇BminCSP3 基因的原核表达载体构建及表达纯化
将测序正确的克隆质粒pGEM-T/BminCSP3 和表达载体pET-32a(+)用限制性内切酶EcoRⅠ和Hind Ⅲ双酶切,酶切产物按照DNA Ligation Kit 说明书进行连接,转化到BL21(DE3)表达菌株中,培养过夜,挑选单克隆菌落,摇菌并提取BminCSP3/pET-32a重组质粒测序验证。
将测序正确的表达菌株以1∶100加入液体培养基中,在37 ℃振荡培养到OD600 达到0.6,加入终浓度为0.5 mmol·L-1的IPTG,28 ℃,200 r·min-1诱导5 h。在4 ℃,8000 r·min-1离心10 min,收集菌体,并以SDS-PAGE 电泳检测表达量,用裂解缓冲液(50 mmol·L-1 Tris-HCI,pH 8.0,50 mmol·L-1 NaCl,0.5%TritonX-100,2 mg·mL-1溶菌酶)悬浮,超声破碎(超声时间4 s、间隔时间4 s,总时间25 min),在4 ℃,12 000 r·min-1离心10 min,分别收集上清液和沉淀,经处理后用SDS-PAGE 电泳检测目的蛋白表达形式。结果表明,目的蛋白以可溶性形式存在于上清液中,使用Ni-NTA His·Bind Resin 纯化树脂进行纯化,纯化后利用肠激酶切除His-Tag,获得不含His-Tag的目的蛋白BminCSP3。通过BCA 蛋白浓度测定试剂盒检测BminCSP3蛋白的浓度。
1.7 柑橘大实蝇BminCSP3荧光竞争结合实验
试验在荧光分光光度计F-7000上进行,石英荧光比色皿为四通光,激发光波长为337 nm,狭缝宽度为10 nm,发射光谱范围为370~500 nm。本实验中所用的荧光探针1-NPN和气味标样(表2)均用色谱级甲醇配置成终浓度为1 mmol·L-1的工作液,所选用的气味标样大部分能够引起柑橘大实蝇成虫行为反应。
表2 BminCSP3 与气味化合物的结合数据
Table 2 The binding data of BminCSP3 and odor chemical
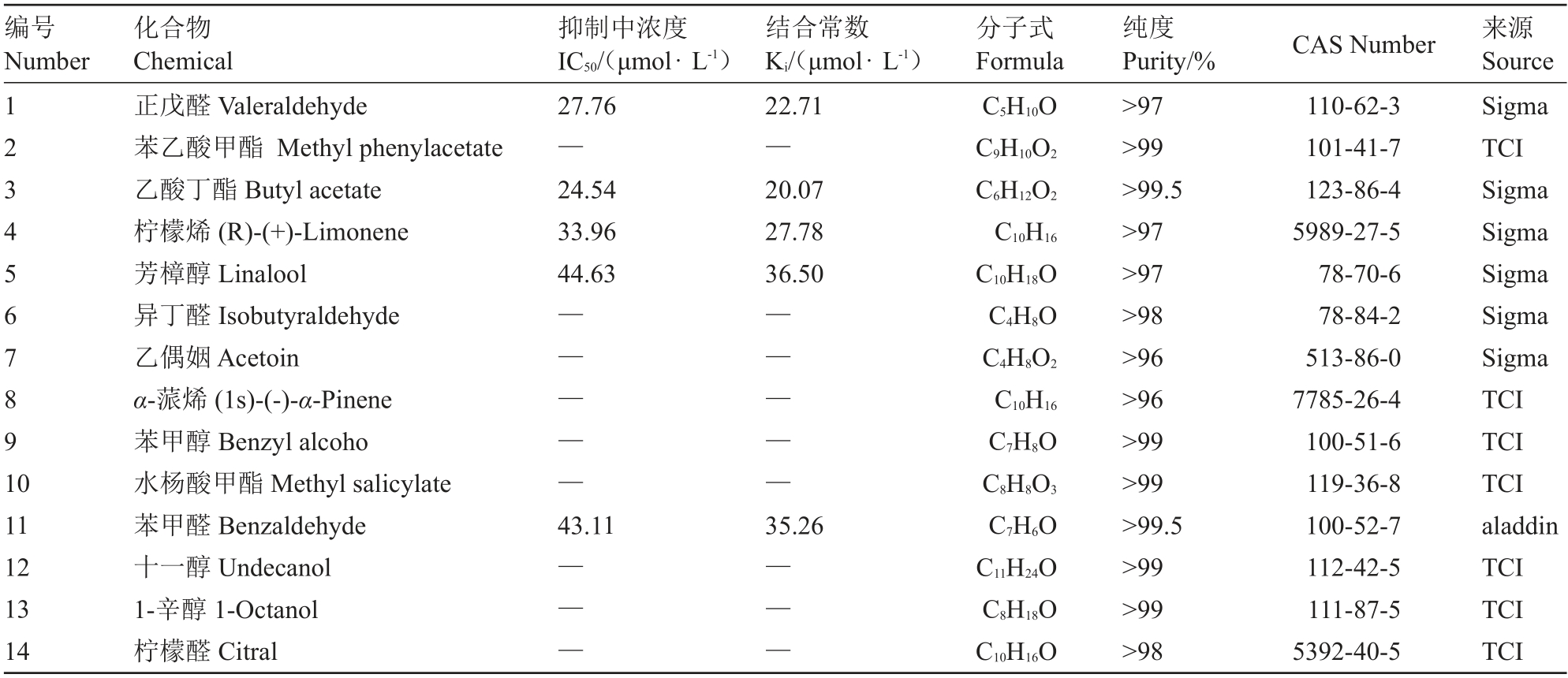
注:—.不能计算出IC50 和Ki。
Note:—.The IC50 and Ki values are unable to be calculated.
在比色皿中加入终浓度为2 μmol · L-1的BminCSP3 蛋白溶液,然后依次加入1-NPN 工作液至终浓度,分别为2、4、6、8、10、12、14、16、18、20、22 μmol·L-1,使荧光值达到饱和,3次重复测定,记录数据。
通过竞争结合实验测定各个气味标样和BminCSP3的结合能力,先加入BminCSP3和1-NPN终浓度均为2 μmol·L-1的混合物,依次加入不同气味标样工作液至终浓度,分别为2、4、6、8、12、16、20、28、36、44、52 μmol·L-1,同时记录最大荧光强度值,每次加入气味标样后反应2 min,记录荧光强度值的变化。每个处理进行3次重复。
假设蛋白活性为100%,在饱和情况下蛋白和配基结合比例为1∶1,根据公式Ki=[IC50]/(1+[1-NPN]/K1-NPN)计算解离常数,IC50为荧光强度值降至初始值1/2 时加入的配基浓度,[1-NPN]为IC50状态下游离的1-NPN的浓度,K1-NPN为BminCSP3/1-NPN的解离常数。解离常数越小,表明气味化合物和蛋白的结合能力越强。一般认为Ki大于20 μmol·L-1则结合能力弱,Ki小于10 μmol·L-1则结合能力强。
2 结果与分析
2.1 柑橘大实蝇BminCSP3基因克隆和序列分析
柑橘大实蝇BminCSP3 基因开放阅读框全长为474 bp,编码157 个氨基酸,编码的BminCSP3 等电点为5.41,不稳定指数为39.51,亲水性总平均值(GRAVY)为-0.397,表明其是亲水性蛋白,带负电荷的残基(Asp+Glu)总数为21个,带正电荷的残基(Arg+Lys)总数为18 个。经预测可知,存在由22 个氨基酸组成的信号肽,成熟肽由135个氨基酸组成,含有4个半胱氨酸残基,无跨膜结构域(图1,图2)。
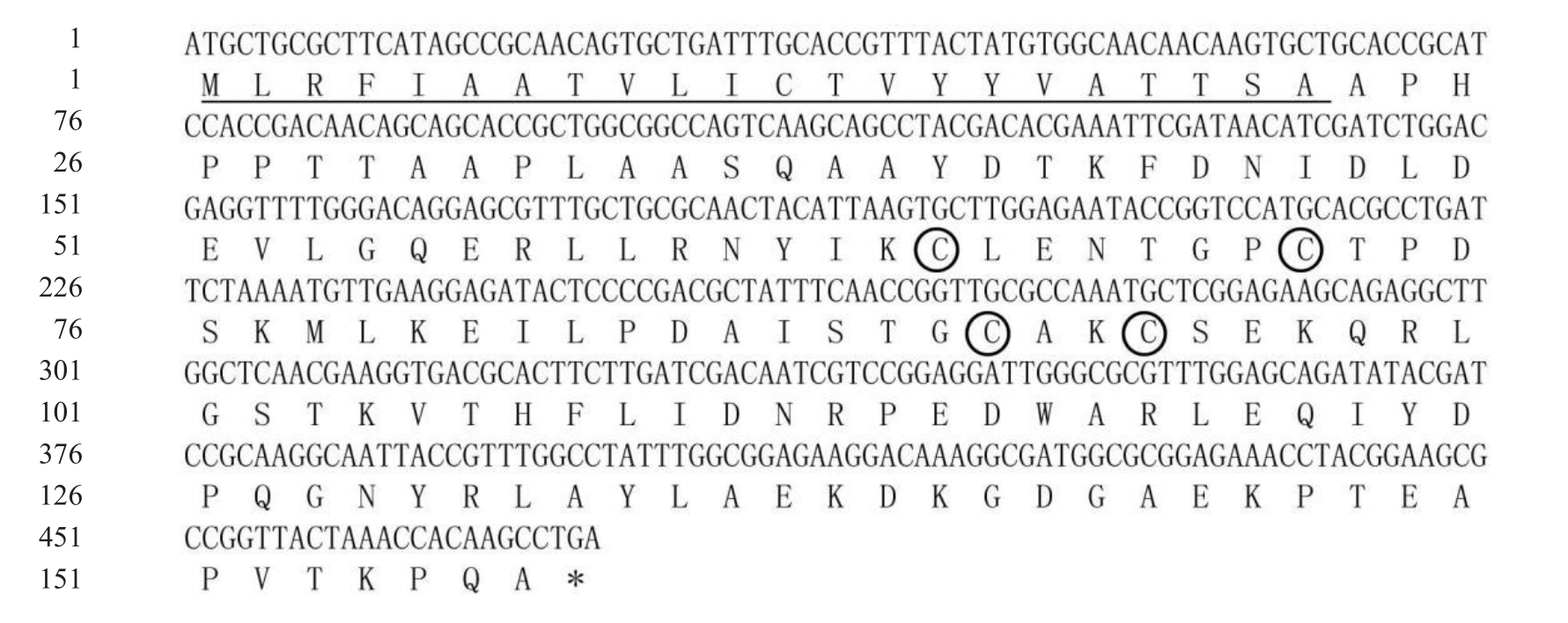
图1 柑橘大实蝇BminCSP3的核苷酸序列及推导的氨基酸序列
Fig.1 Nucleotide acid sequence and deduced amino acid sequence of BminCSP3 in Bactrocera minax
终止密码子用星号显示;下划线标出信号肽序列;保守的半胱氨酸残基用圆圈标注。
The stop codon is showed with an asterisk,the signal peptide is underlined,conserved cysteine residues are marked in circles.
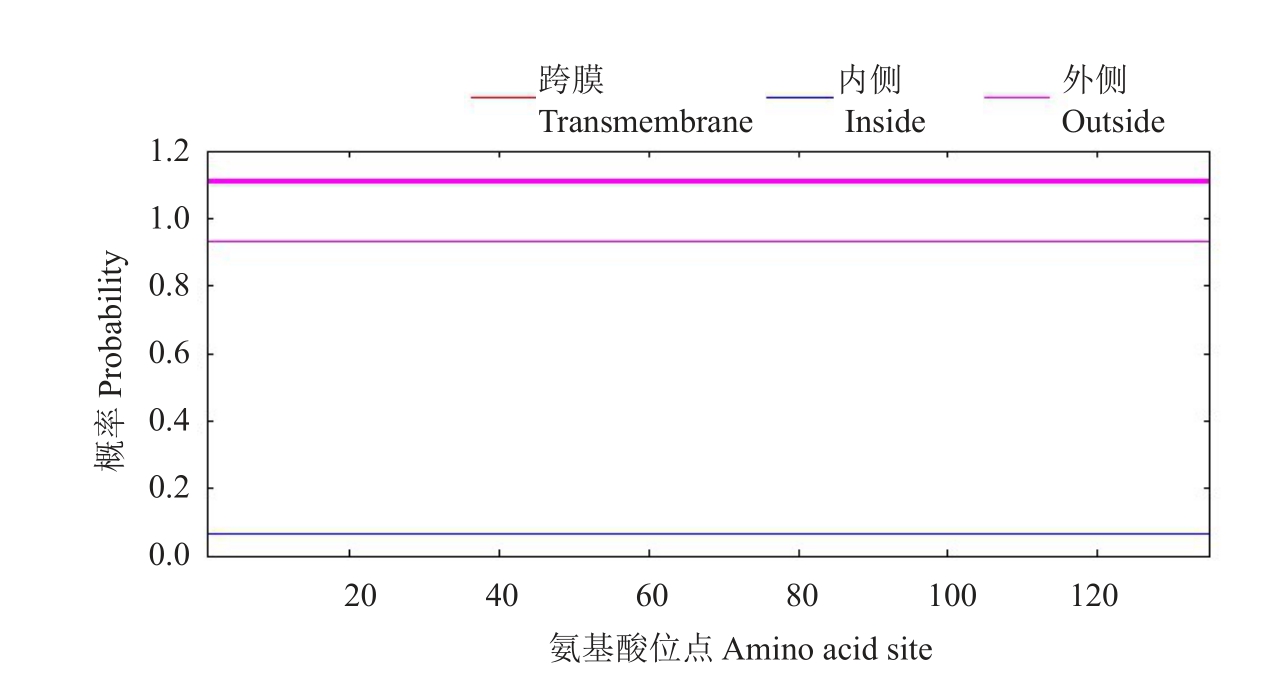
图2 柑橘大实蝇BminCSP3的跨膜结构域
Fig.2 The transmembrane structure of BminCSP3 in Bactrocera minax
2.2 柑橘大实蝇BminCSP3的表达与纯化
对含有表达载体BminCSP3/pET-32a的表达菌株BL21(DE3)通过终浓度为0.5 mmol·L-1的IPTG诱导,结果显示,含有His-Tag的目的蛋白大量存在上清液中(图3,泳道5),而经IPTG诱导的含有空载体pET-32a的BL21(DE3)的菌株在相应位置没有产生条带(图3,泳道2);为了得到纯度较高的BminCSP3,上清液中的蛋白经镍柱纯化后得到较纯的目的蛋白(图3,泳道8);为了避免His-tag对后续荧光竞争试验的影响,纯化后的蛋白经重组肠激酶酶切后,获得不含His-Tag的目的蛋白,条带单一,蛋白分子质量16~17 ku(理论分子质量15.82 ku),从SDSPAGE 结果可以看出蛋白纯度较高,可用于荧光竞争结合实验(图3,泳道7)。
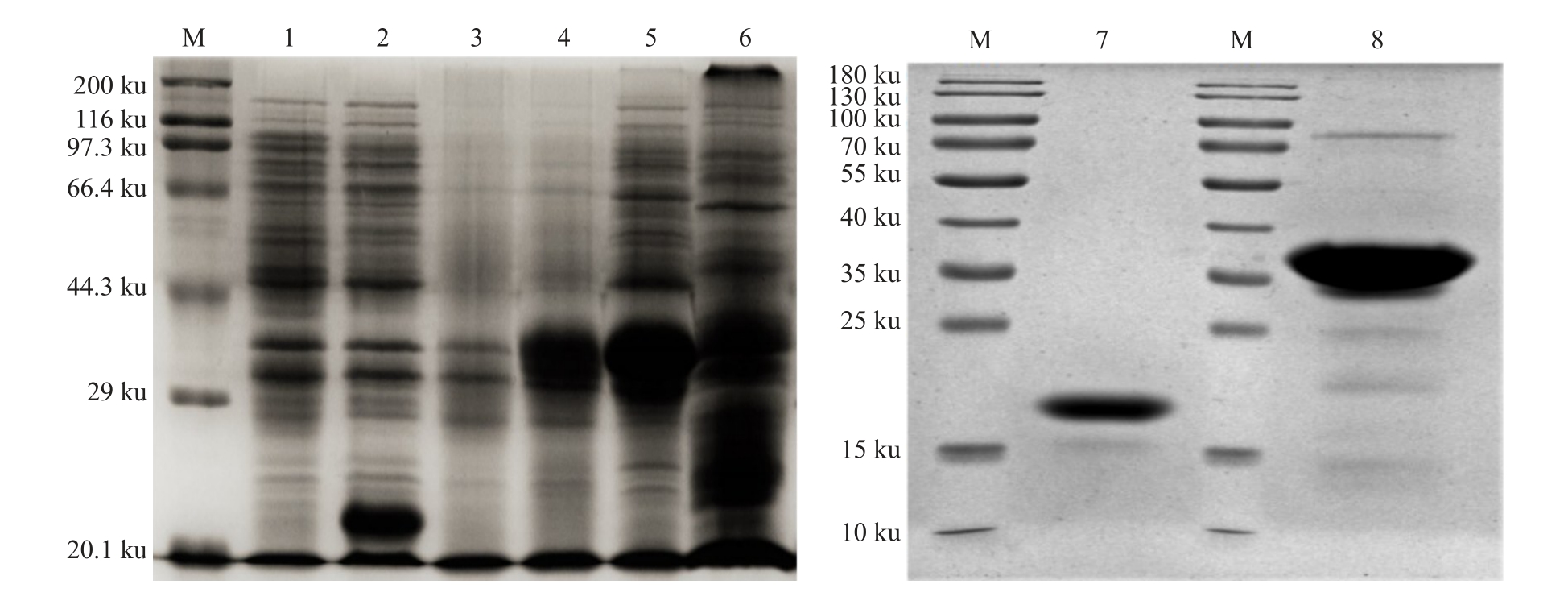
图3 柑橘大实蝇BminCSP3 表达和纯化产物的SDS-PAGE 分析
Fig.3 SDS-PAGE analysis of expressed and purified of BminCSP3 in Bactrocera minax
M.蛋白分子质量标签;1.诱导前的pET-32a;2.诱导后的pET-32a;3.诱导前的BminCSP3/pET-32a;4.诱导后的BminCSP3/pET-32a;5.上清液蛋白;6.包涵体;7.切除His-Tag的目的蛋白;8.含有His-Tag的目的蛋白。
M.Protein molecular weight marker;1.Non-induced pET-32a;2.Induced pET-32a;3.Non-induced BminCSP3/pET-32a;4.Induced BminCSP3/pET-32a;5.Supernatant protein;6.Inclusion body;7.Remove the target protein of His-Tag;8.Target protein containing His-Tag.
2.3 柑橘大实蝇BminCSP3的荧光竞争结合实验
柑橘大实蝇BminCSP3和荧光探针1-NPN的结合特性图由GraphPad Prism 8软件绘制(图4),并计算得出其解离常数为4.49 μmol·L-1。BminCSP3 与14种化合物的荧光竞争试验结果表明,其与不同气味化合物的结合能力均偏弱(表2,图5)。其中,BminCSP3 与正戊醛、乙酸丁酯和柠檬烯的结合能力相对较强,其Ki 值分别为22.71、20.07 和27.78 μmol·L-1。
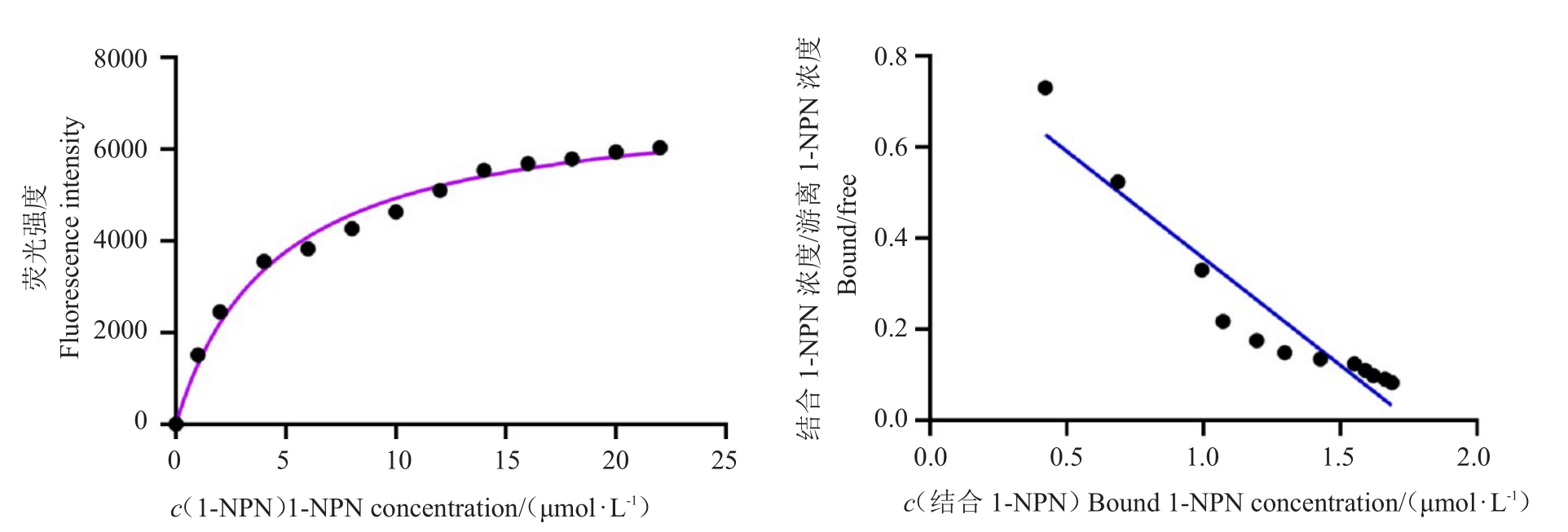
图4 荧光配基1-NPN 与BminCSP3的结合特性
Fig.4 Binding of fluorescent ligand 1-NPN with BminCSP3
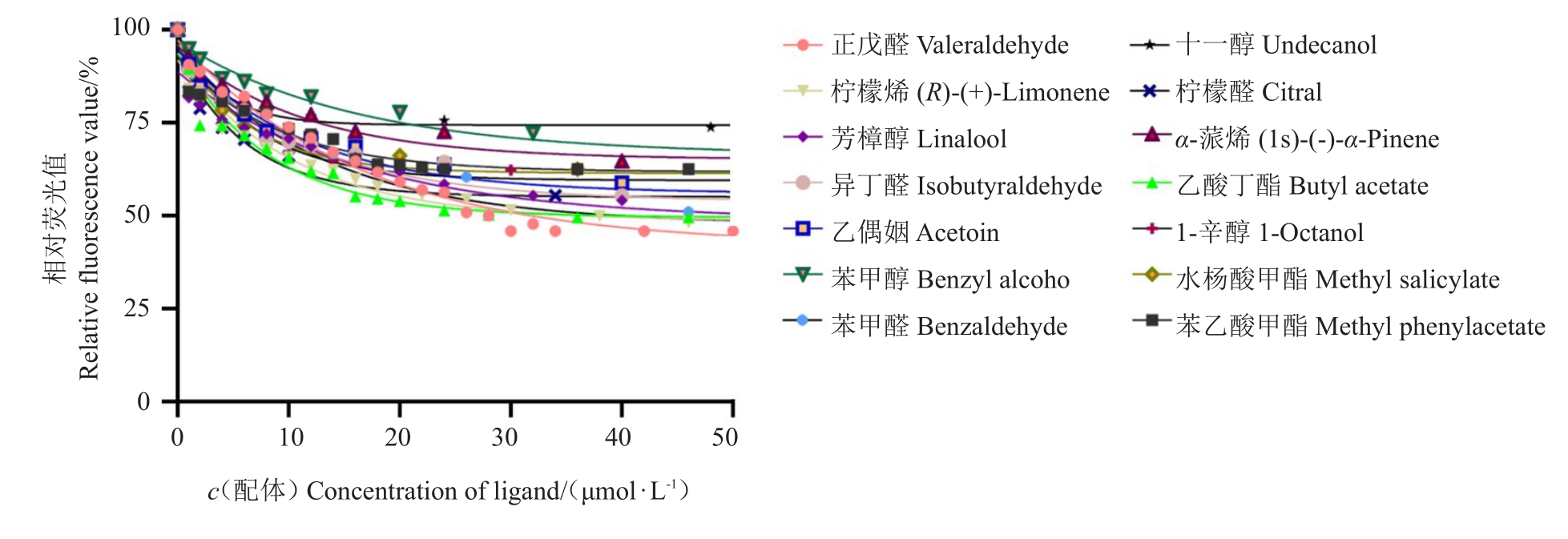
图5 BminCSP3 与配基的竞争性结合
Fig.5 Competitive binding of BminCSP3 and ligands
3 讨论
笔者在本试验中通过基因克隆获得了1个柑橘大实蝇化学感受蛋白基因BminCSP3,其编码的氨基酸序列具有的4 个保守半胱氨酸残基是昆虫CSPs蛋白的典型特征,排列方式为C1-X6-C2-X18-C3-X2-C4,与双翅目、鞘翅目和鳞翅目等昆虫的CSPs蛋白相似[32],这说明化学感受蛋白在昆虫种间和种内具有保守性。BminCSP3 序列分析结果显示,前端编码长为22个氨基酸的信号肽,不存在跨膜结构域,是典型的分泌蛋白;等电点为5.41,是亲水性蛋白,推测该蛋白具有结合并运送外界气味分子穿过淋巴液的功能。
CSPs在昆虫多个组织中均有较高的表达,其功能具有多样性[14-22]。在昆虫触角中高表达的CSPs可能参与了昆虫的嗅觉识别过程,触角为昆虫的主要嗅觉器官,分布着大量的嗅觉感受器,在识别外界信息过程中起着关键作用[33]。
为了验证BminCSP3的功能,笔者通过荧光竞争结合实验测试了BminCSP3与供试的14种气味配体的亲和力。荧光竞争结合实验具有操作简便、价格低廉、安全性高和实验结果稳定的特点,是目前用来测定昆虫OBPs 和CSPs 配体结合特性的典型方法[34],该技术已应用于绿盲蝽(Apolygus lucorum)[35]、中华蜜蜂(Apis cerana cerana)[36]和光肩星天牛(Anoplophora glabripennis)[37]等多种昆虫的OBPs和CSPs 配体结合特性分析。通过对BminCSP3 进行体外原核表达和纯化,获取了可用于进行荧光竞争结合实验的目的蛋白。荧光竞争结合实验结果表明,BminCSP3 与供试14 种气味配体结合能力均偏弱,其Ki值均大于20 μmol·L-1,一般认为Ki值大于20 μmol·L-1,说明蛋白与配体分子间的亲和力比较弱。由此可知,本实验并没有1 个气味配体能与BminCSP3有效结合。这个结果的原因可能有两个方面:一是虽然BminCSP3在触角中高表达,但是该蛋白可能并不参与柑橘大实蝇的嗅觉识别过程,而是具有嗅觉以外的其他生理功能,因为CSPs与OBPs 不同,CSPs的生理功能是非常多样的;二是BminCSP3 可能确实具有结合气味配体的作用,而本次试验未能选中BminCSP3的最适气味配体。
4 结论
根据本研究结果可得,BminCSP3 虽在柑橘大实蝇雌雄成虫触角中高表达,但和已知的可引起柑橘大实蝇成虫行为反应的气味配体无较强的结合活性,由此推测其并不参与柑橘大实蝇成虫的嗅觉识别过程。
[1] 汪兴鉴,罗禄怡.橘大实蝇的研究进展[J].昆虫知识,1995,32(5):310-315.WANG Xingjian,LUO Luyi.Research progress in the chinese citrus fruitfly[J].Entomological Knowlegde,1995,32(5):310-315.
[2] 陈世骧,谢蕴贞.关于橘大实蝇的学名及其种征[J].昆虫学报,1955,5(1):123-126.CHEN Shixiang,XIE Yunzhen.Taxonomic notes on the chinese citrus fly Tetradacus citri[J].Acta Entomologica Sinica,1955,5(1):123-126.
[3] DORJI C,CLARKE A R,DREW R A I,FLETCHER B S,LODAY P,MAHAT K,RAGHU S,ROMIG M C.Seasonal phenology of Bactrocera minax (Diptera:Tephritidae) in western Bhutan[J].Bulletin of Entomological Research,2006,96(5):531-538.
[4] DREW R A I,DORJI C,ROMIG M C,LODAY P.Attractiveness of various combinations of colors and shapes to females and males of Bactrocera minax (Diptera:Tephritidae) in a commercial mandarin grove in bhutan[J].Journal of Economic Entomology,2006,99(5):1651-1656.
[5] 王小蕾,张润杰.橘大实蝇生物学、生态学及其防治研究概述[J].环境昆虫学报,2009,31(1):73-79.WANG Xiaolei,ZHANG Runjie.Review on biology,ecology and control of Bactrocera (tetradacus) minax Enderlein[J].Journal of Environmental Entomology,2009,31(1):73-79.
[6] PILPEL Y,LANCET D.Olfaction:Good reception in fruitfly antennae[J].Nature,1999,398(6725):285-287.
[7] PELOSI P,IOVINELLA I,FELICIOLI A,DANI F R.Soluble proteins of chemical communication:an overview across arthropods[J].Frontiers in Physiology,2014,5:320.
[8] 张坤朋.黏虫嗅觉相关基因的表达模式及MsepOR13 功能研究[D].杨凌:西北农林科技大学,2019.ZHANG Kunpeng.Expression pattern of olfactory relative genes and functional analysis of MsepOR13 in Mythimna separata(Walker)[D].Yangling:Northwest A&F University,2019.
[9] LEAL W S.Odorant Reception in Insects:Roles of receptors,binding proteins,and degrading enzymes[J].Annual Review of Entomology,2013,58:373-391.
[10] PELOSI P,CALVELLO M,BAN L.Diversity of odorant-binding proteins and chemosensory proteins in insects[J].Chemical Senses,2005,30(S1):i291-i292.
[11] ANGELI S,CERON F,SCALONI A,MONTI M,MONTEFORTI G,MINNOCCI A,PETACCHI R,PELOSI P.Purification,structural characterization,cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria[J].European Journal of Biochemistry,1999,262(3):745-754.
[12] DYANOV H M,DZITOEVA S G.Method for attachment of microscopic preparations on glass for in situ hybridization,PRINS and in situ PCR studies[J].BioTechniques,1995,18(5):822-826.
[13] SINGH S,TYAGI C,RATHER I A,SABIR J S M,HASSAN M I,SINGH A,SINGH I K.Molecular modeling of chemosensory protein 3 from Spodoptera litura and its binding property with plant defensive metabolites[J].International Journal of Molecular Sciences,2020,21(11):4073.
[14] WARIS M I,YOUNAS A,AMEEN A,RASOOL F,WANG M Q.Expression profiles and biochemical analysis of chemosensory protein 3 from Nilaparvata lugens (Hemiptera:Delphacidae)[J].Journal of Chemical Ecology,2020,46(4):363-377.
[15] ZHOU X H,BAN L P,LOVINELLA I,ZHAO L J,GAO Q,FELICIOLI A,SAGONA S,PIERACCINI G,PELOSI P,ZHANG L,DANI F R.Diversity,abundance,and sex-specific expression of chemosensory proteins in the reproductive organs of the locust Locusta migratoria manilensis[J].Biological Chemistry,2012,394(1):43-54.
[16] MALESZKA J,FORET S,SAINT R,MALESZKA R.RNAi-induced phenotypes suggest a novel role for a chemosensory protein CSP5 in the development of embryonic integument in the honeybee (Apis mellifera)[J].Development Genes and Evolution,2007,217(3):189-196.
[17] NOMURA A,KAWASAKI K,KUBO T,NATORI S.Purification and localization of p10,a novel protein that increases in nymphal regenerating legs of Periplaneta americana (American cockroach)[J].The International Journal of Developmental Biology,1992,36(3):391-398.
[18] KITABAYASHI A N,ARAI T,KUBO T,NATORI S.Molecular cloning of cDNA for p10,a novel protein that increases in the regenerating legs of Periplaneta americana (American cockroach)[J].Insect Biochemistry and Molecular Biology,1998,28(10):785-790.
[19] CHENG D F,LU Y Y,ZENG L,LIANG G G,HE X F.Si-CSP9 regulates the integument and moulting process of larvae in the red imported fire ant,Solenopsis invicta[J].Scientific Reports,2015,5(1):16340-16347.
[20] XUAN N,GUO X,XIE H Y,LOU Q N,LU X B,LIU G X,PICIMBON J F.Increased expression of CSP and CYP genes in adult silkworm females exposed to avermectins[J].Insect Science,2015,22(2):203-219.
[21] BAUTISTA M A,BHANDARY B,WIJERATNE A J,MICHEL A P,HOY C W,MITTAPALLI O.Evidence for trade-offs in detoxification and chemosensation gene signatures in Plutella xylostella[J].Pest Management Science,2015,71(3):423-432.
[22] GUO W,WANG X H,MA Z Y,XUE L,HAN J Y,YU D,KANG L.CSP and takeout genes modulate the switch between attraction and repulsion during behavioral phase change in the Migratory locust[J].PLoS Genetics,2011,7(2):e1001291.
[23] LIU Y L,GUO H,HUANG L Q,PELOSI P,WANG C Z.Unique function of a chemosensory protein in the proboscis of two Helicoverpa species[J].The Journal of experimental biology,2014,217(10):1821-1826.
[24] WARIS M I,YOUNAS A,ADEEL M M,DUAN S G,QUERSHI S R,KALEEM U R M,WANG M Q.The role of chemosensory protein 10 in the detection of behaviorally active compounds in brown planthopper,Nilaparvata lugens[J].Insect Science,2020,27(3):531-544.
[25] ZHANG Y N,YE Z F,YANG K,DONG S L.Antenna-predominant and male-biased CSP19 of Sesamia inferens is able to bind the female sex pheromones and host plant volatiles[J].Gene,2014,536(2):279-286.
[26] GU S H,WANG S Y,ZHANG X Y,JI P,LIU J T,WANG G R,WU K M,GUO Y Y,ZHOU J J,ZHANG Y J.Functional characterizations of chemosensory proteins of the alfalfa plant bug Adelphocoris lineolatus indicate their involvement in host recognition[J].PLoS ONE,2012,7(8):e42871.
[27] MCKENNA M P,HEKMAT-SCAFE D S,GAINES P,CARLSON J R.Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system[J].The Journal of Biological Chemistry,1994,269(23):16340-16347.
[28] 李慧,顾天滋,陈昌宇,黄开茹,田朔,赵旭东,郝德君.仁扇舟蛾化学感受蛋白cDNA的克隆、序列分析及时空表达[J].林业科学,2018,54(4):67-75.LI Hui,GU Tianzi,CHEN Changyu,HUANG Kairu,TIAN Shuo,ZHAO Xudong,HAO Dejun.cDNA cloning,sequence analysis and expression profile of a chemosensory protein from the Clostera restitura (Lepidoptera:Notodontidae)[J].Scientia Silvae Sinicae,2018,54(4):67-75.
[29] WANG Z X,QI Z H,CHEN J,WANG F L,GUI L Y,ZHANG G H.Molecular characterization of chemosensory protein genes in Bactrocera minax (Diptera:Tephritidae)[J].EntomologicalResearch,2021,51(7):349-356.
[30] 杜田华,华登科,何章章,王福莲,桂连友.柑橘大实蝇成虫对板栗挥发物的行为反应[J].环境昆虫学报,2018,40(2):474-484.DU Tianhua,HUA Dengke,HE Zhangzhang,WANG Fulian,GUI Lianyou.Behavioral responses of adults Bactrocera minax(Diptera:Tephritidae) to volatile organic components from Castanea (Fagales:Fagaceae)[J].Journal of Environmental Entomology,2018,40(2):474-484.
[31] LIU L,ZHOU Q.Olfactory response of female Bactrocera minax to chemical components of the preference host citrus volatile oils[J].Journal of Asia-Pacific Entomology,2016,19(3):637-642.
[32] XU Y L,HE P,ZHANG L,FANG S Q,DONG S L,ZHANG Y J,LI F.Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects[J].BMC Genomics,2009,10:632.
[33] 刘伟,王桂荣.昆虫嗅觉中枢系统对外周信号的整合编码研究进展[J].昆虫学报,2020,63(12):1536-1545.LIU Wei,WANG Guirong.Research progress of integrated coding of peripheral olfactory signals in the central nervous system of insects[J].Acta Entomologica Sinica,2020,63(12):1536-1545.
[34] 彭竹,黄丽,赵慧婷.荧光竞争结合技术在昆虫嗅觉相关蛋白结合能力检测上的应用[J].激光生物学报,2020,29(2):106-112.PENG Zhu,HUANG Li,ZAHO Huiting.Application of fluorescence competitive binding technology in detection of the binding ability of insects olfactory related proteins[J].Acta Laser Biology Sinica,2020,29(2):106-112.
[35] 滑金锋,张帅,崔金杰,王道杰,王春义,雒珺瑜,吕丽敏.绿盲蝽化学感受蛋白基因AlucCSP1的克隆、表达谱分析及结合特征[J].中国农业科学,2012,45(20):4187-4196.HUA Jinfeng,ZHANG Shuai,CUI Jinjie,WANG Daojie,WANG Chunyi,LUO Junyu,LÜ Limin.Cloning,expression analysis and binding characteristics of chemosensory protein gene AlucCSP1 in Apolygus lucorum (Meyer-Dür)[J].Scientia Agricultura Sinica,2012,45(20):4187-4196.
[36] 李红亮,张林雅,倪翠侠,商晗武.中华蜜蜂化学感受蛋白AcerCSP3的配基结合功能分析[J].昆虫学报,2011,54(3):259-264.LI Hongliang.ZHANG Linya,NI Cuixia,SHANG Hanwu.Molecular binding characterization with chemical ligands of a chemosensory protein AcerCSP3 in the Chinese honeybee,Apis cerana cerana Fabricius (Hymenoptera:Apidae)[J].Acta Entomologica Sinica,2011,54(3):259-264.
[37] 李广伟,陈秀琳,尚天翠.光肩星天牛气味结合蛋白AglaOBP12的基因克隆、表达及配体结合特征[J].昆虫学报,2017,60(10):1141-1154.LI Guangwei,CHEN Xiulin,SHANG Tiancui.cDNAcloning,expression and ligand binding properties of the odorant binding protein AglaOBP12 in the Asian longhorned beetle,Anoplophora glabripennis (Coleoptera:Cerambycidae)[J].Acta Entomologica Sinica,2017,60(10):1141-1154.