柑橘轮斑病(Citrus target spot)是由Pseudofabraea citricarpa引起的一种柑橘真菌性病害[1-2],2004年在陕西城固首次报道[3],2018 年冬季于重庆万州突然暴发,造成当地尤力克柠檬树大量感病,呈现由北向南加速传播的趋势[4]。2021年已有湖北宜昌和湖南吉首等地的发病报道[5],与笔者此前预测的该病在国内的适生区范围一致[6]。近年来柑橘轮斑病在我国发生愈加频繁,一旦发生造成枯枝落叶、大量落果甚至绝产,威胁着柑橘产量和柑橘品质。目前主要通过化学药剂和一些农事操作防治柑橘轮斑病菌[4,7],这往往伴随着经济、环境、安全方面的压力以及效果不显著等问题。相反,抗病品种的合理利用则是一种高效且环保的绿色防控手段。
目前关于柑橘轮斑病的研究仍处于初级阶段,主要集中于病原鉴定方法、致病因子鉴定及病害适生区预测等方面。柑橘轮斑病原菌特异SCAR标记引物的开发也为该病害的早期鉴定提供了快速、简便的途径[8]。此外,已有研究采用整合转录组学和分泌蛋白组学结合的方法发现植物细胞壁降解酶、植物-病原互作蛋白以及萜类生物合成途径在Ps.citricarpa 致病性中发挥关键作用[1]。最新研究表明,柑橘轮斑病在我国处于高度风险等级,对长江上中游及鄂西-湘西两大柑橘产区潜在威胁较大[6]。但是,柑橘轮斑病的抗性鉴定并没有一套完善可行的标准化体系,这也导致柑橘轮斑病抗性种质资源筛选及抗病品种选育进程缓慢。
为此,笔者以病斑直径平均值为抗感性评价标准[9],同时以发病率为致病力评价标准[10],根据病斑直径和发病率鉴定不同柑橘品种抗病性,构建抗性评价体系;选择在当地发病严重的尤力克柠檬叶片作为离体接种的材料,参考林月莉等[11]的离体叶片接种法并加以改良,作为抗性鉴定接种方法。建立柑橘轮斑病菌的抗性鉴定体系,进而采用构建好的抗性鉴定体系,研究抗性鉴定的适宜接种部位、接种温度。在最适宜的温度和接种部位下,将病原菌接种到离体叶片,评价病原菌致病力,研究不同柑橘品种的抗病性,筛选出抗耐病品种。
1 材料和方法
1.1 材料
供试菌株:Pc-WZBY1 分离自重庆市万州区白羊镇发病柠檬果园,经过形态学和PCR鉴定为柑橘轮斑病(Ps.citricarpa),在20 ℃于马铃薯葡萄糖琼脂(PDA)培养基避光培养,直至菌丝体覆盖约四分之三的平板,4 ℃保存、备用。
供试柑橘种质27 份来自于重庆市三峡农业科学院柑橘种质资源圃,详见表1。
表1 试验所用柑橘品种
Table 1 Citrus cultivars used in this study

编号Code 1 2 3 4 5 6 7 8 9 1 0 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27品种Cultivar沙田柚Shatianyou北京柠檬Meyer lemon卡拉卡拉Cara cara Navel Orange渝北梨橙Yubei pear orange中柑1号Zhonggan No.1塔罗科血橙Citrus sinensis‘Tarocco’98-1长叶橙98-1 long leaf orange龙都早香柚Longduzaoxiangyou丽朵血橙Liduo Blood Orange沃柑Orah强德勒红心柚Chandler Pummelo 94-1长叶橙94-1 long leaf orange伦晚Lane late Navel Orange葡萄柚Citrus paradisi奉晚Fengwan Navel Orange津香橙Jinxiang orange爱媛30号Aiyuan No.30口之津Koizumi金秋沙糖橘Jinqiushatangju琯溪蜜柚Guanximiyou尤力克Eureka lemon W-默科特W.Murcott Afourer南香Nankou金钱橘Clementines温州蜜柑Satsuma mandarin福本Fukumoto Navel Orange 091无核沃柑Orah 091
1.2 接种方法
选取大小、长势基本一致的健康,成熟的尤力克柠檬叶片,用75%(φ)乙醇表面消毒晾干后放入铺有灭菌纱布的无菌托盘,加蒸馏水浸湿纱布。把直径5 mm 的菌饼接种至托盘内的叶片上,菌丝面紧贴叶片。每叶片放置2 个菌饼,覆上保鲜膜确保湿润度,每隔24~48 h 补充无菌水1 次。接种21 d 后,每隔2 d 测量1次病斑直径并记录数据,直至病斑不继续扩展。
1.3 柑橘轮斑菌离体接种条件探究
1.3.1 创伤对接种效果的影响 采用1.2 节接种方法对离体尤力克柠檬叶片接种,采用无伤接种和针刺接种2 种处理,每个处理10 枚叶片。根据前期开展的预试验以及朱丽[7]针对柑橘轮斑病菌开展的前期研究,选择将接种后的叶片分别置于5 ℃、10 ℃、15 ℃、20 ℃恒温培养箱内黑暗保湿培养。以无菌PDA块接种叶片作为对照,3次重复。
1.3.2 接种温度及部位对接种效果影响 采用1.2节接种方法对离体尤力克柠檬叶片接种,分别对叶片正面、背面接种两种处理,每个处理10 枚叶片。将接种后的叶片分别置于5 ℃、10 ℃、15 ℃、20 ℃恒温培养箱内黑暗保湿培养。以无菌PDA 块接种叶片为对照,3次重复。
1.4 不同柑橘品种抗性鉴定
依据1.3部分确定的最适接种条件,采用离体接种方法对27个柑橘品种叶片进行抗性鉴定,每个处理10 枚叶片,3 次重复。接种21 d 后测量不同品种叶片的病斑直径。
1.5 数据分析
试验数据采用SPSS统计分析,处理间的差异性分析采用多重比较应用中的最小显著差法(LSD)[8]。
2 结果与分析
2.1 创伤对接种效果的影响
在5 ℃、10 ℃、15 ℃、20 ℃温度下分别对离体叶片正面、背面无伤接种无菌PDA 菌丝块,叶片均不发病,而针刺有伤条件下接种病菌时均发病。叶片接种病菌22 d 后,刺伤部位症状表现明显(图1-A)。各温度下发病情况不同,病斑均随接种时间延长表现不同程度扩展,且接种病菌28 d后,发病情况保持稳定,病斑大小几乎不再扩展,此时各接种条件下病斑差异表现显著,接种病菌28 d 是整个观测期内抗性鉴定的最佳时间,也是品种抗性鉴定时观测的最佳时间(图1-B)。除15 ℃外,其他温度下,叶片背面针刺病斑长度大于叶片正面针刺病斑长度。5 ℃叶片病斑呈黑色,边缘油渍状,随着接种时间延长病斑颜色变淡,刺伤部位产生白色条状菌丝。10 ℃叶片病斑呈褐色,具晕圈,随着接种时间延长,病斑老化且中央产生黑色凸起。15 ℃叶片病斑呈红褐色,随后逐渐扩大呈深褐色,刺伤部位产生白色条状菌丝。20 ℃叶片病斑呈深褐色,一些病斑表面覆盖白色菌丝,随着病斑老化其表面产生黑色凸起(图1-A)。

图1 离体柠檬叶片接种病菌后病斑扩展情况
Fig.1 The comparison of lesion expansion on detached leaf inoculated with Pseudofabraea citricarpa
2.2 不同温度及接种部位对接种效果的影响
采用十字交叉法测量病斑直径,在以尤力克柠檬为材料的抗性鉴定试验中,叶片接种病菌28 d 调查发现,在10 ℃、20 ℃下,叶片背面针刺接种产生的病斑直径大于叶片正面且表现显著性差异,而5 ℃、15 ℃下叶片正面、背面病斑大小有差异但并未达到显著性水平。10 ℃下叶片背面接种形成的病斑直径最大,且与其他各温度下叶片正面、背面接种形成的病斑直径存在显著性差异(p <0.05)。因此,10 ℃下叶片背面接种是室内抗性鉴定的最适接种条件(图2)。
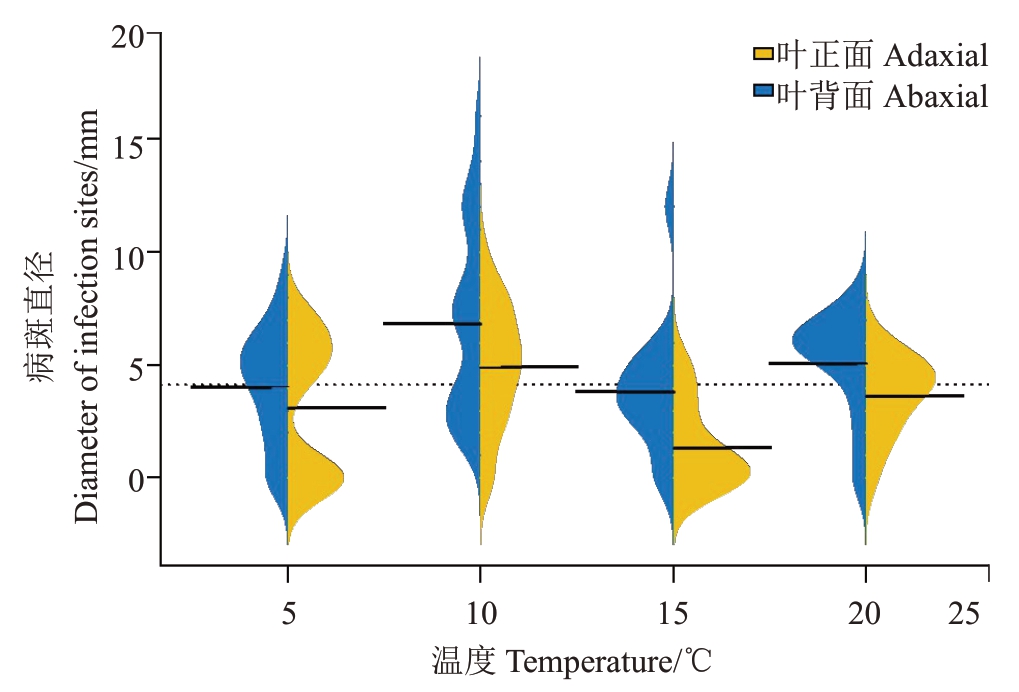
图2 不同温度下接种离体叶片正、背面平均病斑直径
Fig.2 Average lesion diameter on the detached leaves inoculated with Pseudofabraea citricarpa at different temperatures
2.3 不同柑橘品种对柑橘轮斑病的抗性差异
从图3 可知,10 ℃条件下对不同供试柑橘品种的离体叶片背面进行伤口接种后产生的病斑直径和发病率存在不同程度的差异(图3),部分柑橘品种甚至与其他品种的病斑直径达到显著性差异水平(p <0.05)。其中,沙田柚抗性最强,表现为完全免疫。尤力克柠檬平均病斑直径为5.9 mm,部分叶片病斑直径高达12 mm,而091 无核沃柑的平均病斑直径最大,可达13 mm,部分叶片病斑直径可达14 mm,抗性最弱。杂柑类种质中091 无核沃柑抗性最弱,中柑1号抗性最强;脐橙类种质中福本抗性较弱,卡拉卡拉、渝北梨橙、塔罗科血橙抗性相当且不易感病;相比柑类、脐橙类,柚类病斑直径较小,发病不明显,发病率低,抗病力强;除沙田柚表现免疫不发病外,只有北京柠檬的发病率相对较低(20%),其他柑橘品种发病率均在30%以上(图4)。

图3 部分柑橘品种叶片接种后的症状
Fig.3 Symptoms of different citrus varieties after inoculation
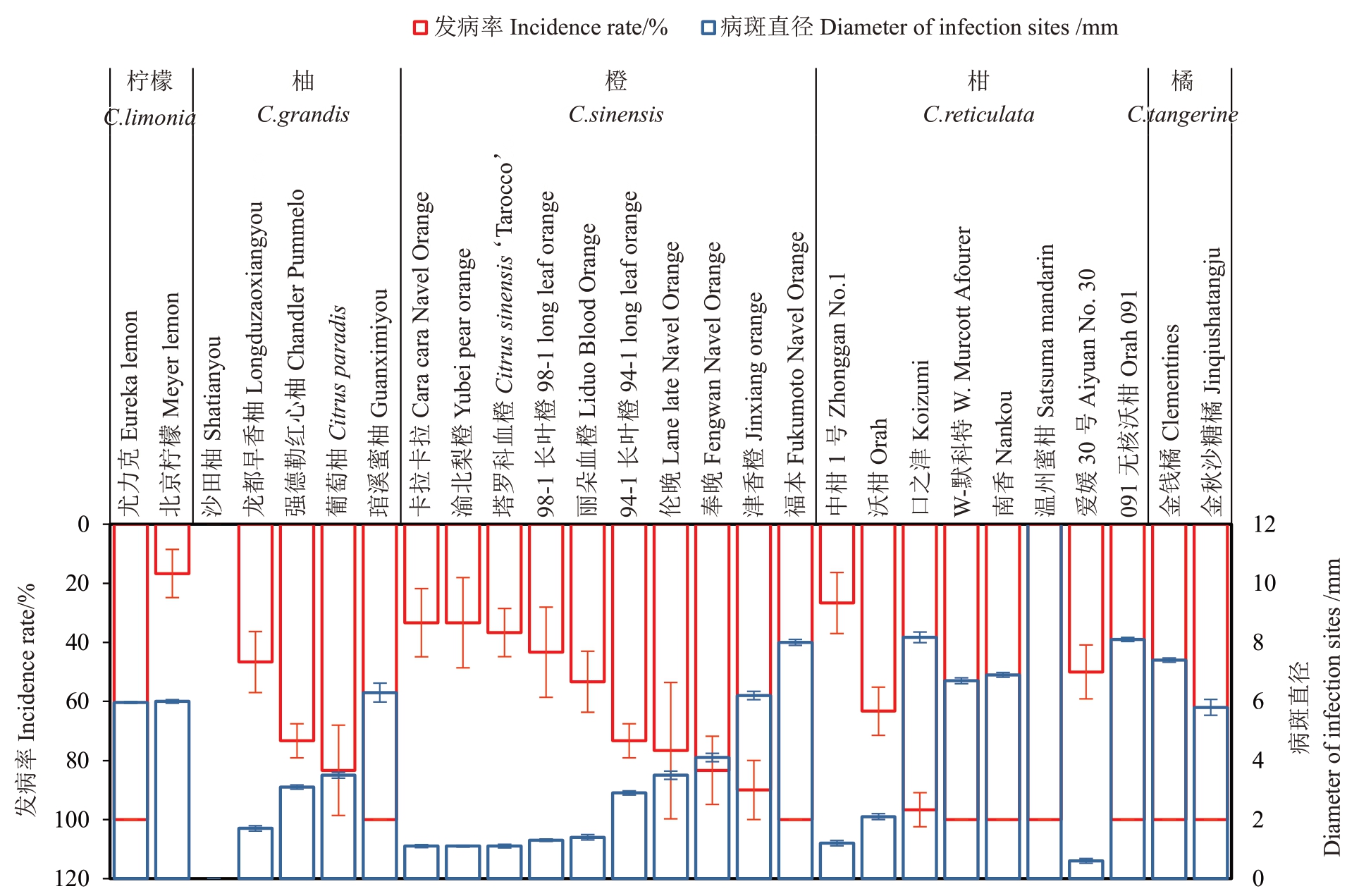
图4 不同柑橘品种接种后发病情况
Fig.4 The disease incidence of different citrus varieties after inoculation
3 讨 论
柑橘轮斑病发病周期较长,病情一旦加重,杀菌剂便难以有效控制,这给防治上造成很大困难。抗性品种筛选是解决防治难题的有效方法,但目前柑橘轮斑病的抗性材料鉴定及药剂筛选方面还没有一套完整的评价体系。剪根浸根法[12]、带菌牙签活体茎秆穿刺接种法[13]、离体叶片喷雾注射法[14]、活体叶片法[15]是目前已有的一些植物病害抗性鉴定接种方法,笔者在本研究中采取离体叶片针刺接种法,该法操作性强,不伤害植株,单株可重复,数据收集简便。除此之外,相较于病情指数法[16]和系统聚类分析法[17-18],根据病斑直径和发病率进行抗性评价,排除了不同严重度分级下病情指数及致病率的差异造成的试验偏差。笔者建立的抗性鉴定体系不但为高效筛选耐病抗病品种提供了技术方案,还可用于药剂筛选,也为其他柑橘病害的抗性鉴定提供了一种思路。由于病原菌存在潜在的致病性分化和变异,因此培育出永远具有抗病性的品种是不可能。笔者通过室内接种试验,选择不同菌株开展寄主-病原的互作研究[19-20],明确柑橘轮斑病菌与柑橘品种间的互作关系,在生产中合理种植柑橘品种,对病害的综合防治具有重要意义。对于研究鉴定的柑橘品种,可采用抗谱分析的方法[21],获得抗柑橘轮斑病基因,从而利用抗性基因培育更加高抗的新品种。另外,本研究中建立的室内接种试验,还可用于病原菌侵染后孢子萌发情况、不同抗性品种的一些生理生化指标、细胞结构等的分析,以研究柑橘轮斑病菌的致病机制。
长江上中游柑橘优势区及鄂西-湘西两大柑橘产区是柑橘轮斑病发生的中高适生区,为减少经济损失,应尽量种植适宜生长且抗性较强的柑橘品种。抗性与感病的界限是模糊的,是相比较而言的,长江上中游柑橘带作为甜橙的最适宜生长区,晚熟品种较早、中熟品种更具生长优势,需要减少福本等易感病脐橙类的种植面积,尽量栽培不易感病且发病率低的脐橙类、柠檬类、柚类,如卡拉卡拉、沙田柚、北京柠檬;鄂西-湘西柑橘带适宜宽皮柑橘的生长,主要种植温州蜜柑、橙类和一些柚类,适当减少易感病的柑类如091 无核沃柑、南香及部分宽皮柑橘如金钱橘的种植面积,同时发挥其自然优势种植抗性较强的柚类如沙田柚、龙都早香柚。
植物为抵御病原菌侵染会建立一系列复杂的抗病机制,植株的形态结构特征如叶型、气孔结构等,都与植物抗病机制有关[22-24],抗性基因是产生抗性的根本原因。根据抗病基因具有保守结构域这一特点,对保守结构域进行序列扩增是鉴定和发掘抗性基因的常用方法[25],这一方法已应用于辣椒[26]、大豆[27]、小麦[28]等作物。研究柑橘种质间抗性差异、柑橘抗病机制甚至柑橘-柑橘轮斑病菌互作的关键是研究柑橘抗性基因,黄代青等[29]在柚的cDNA 中找到10个抗病基因同源序列;谌谋华[30]在柑橘抗病材料中找到25个抗病基因同源序列;研究不同柑橘品种抗性基因差异是了解柑橘抗轮斑病作用机制的基础,这需要今后进行更深入的研究分析。此外,研究还存在一定的局限性,离体叶片可能无法充分反映整个植物在叶龄等方面的变异性,而且一些整株植物的寄主防御反应可能在离体叶片中减弱。因此,需要今后结合田间试验或采取其他抗性鉴定方法加以验证。
笔者采用离体叶片接种法,证明10 ℃条件下叶片背面接种是柑橘轮斑病室内抗性鉴定的最适接种条件,且叶片接种病菌后28 d 是最佳的鉴定时间。利用对27 个柑橘品种抗性鉴定筛选出的抗病耐病材质,如沙田柚、北京柠檬、龙都早香柚等,同时结合柑橘轮斑病适生区,在高中风险区合理地种植和布局抗病品种,对实现农民增收,解决三农问题,迈向乡村振兴具有重大意义。
抗性鉴定方法的建立及主栽品种抗性评价不仅有利于抗轮斑病柑橘品种的鉴定和合理布局,还可用于防治柑橘轮斑病的药剂筛选、病原菌致病机制的研究,以及柑橘与轮斑菌的互作分析。
[1]YANG Y H,FANG A F,YU Y,BI C W,ZHOU C Y. Integrated transcriptomic and secretomic approaches reveal critical pathogenicity factors in Pseudofabraea citricarpa inciting citrus target spot[J].Microbial Biotechnology,2019,12(6):1260-1273.
[2]CHEN C,VERKLEY G J M,SUN G Y,GROENEWALD J Z,CROUS P W. Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera[J]. Fungal Biology,2016,120(11):1291-1322.
[3]ZHU L,WANG X H,HUANG F,ZHANG J Z,LI H Y. A destructive new disease of citrus in China caused by Cryptosporiopsis citricarpa sp.nov[J].Plant Disease,2012,96(6):804-812.
[4]占爽,吴望,胡军华,吴玉珠,乔兴华,陈力,程兰,周彦.重庆万州疑似柑桔轮斑病的病原鉴定及防治药剂筛选[J].中国南方果树,2021,50(1):1-7.ZHAN Shuang,WU Wang,HU Junhua,WU Yuzhu,QIAO Xinghua,CHEN Li,CHENG Lan,ZHOU Yan. Pathogen identification and screening of control agent of suspected citrus target spot in Wanzhou Chongqing[J].South China Fruits,2021,50(1):1-7.
[5]XIAO X E,ZENG Y T,WANG W,CHENG L,QIAO X H,HOU X,LI H Y. First report and new hosts of Pseudofabraea citricarpa causing citrus target spot in China[J]. Plant Health Progress,2021,22(1):26-30.
[6]徐永红,陈力,唐松,丁德宽,杨宇衡.柑橘轮斑病的适生区预测及风险分析[J].中国农业科学,2020,53(21):4430-4439.XU Yonghong,CHEN Li,TANG Song,DING Dekuan,YANG Yuheng. Prediction of suitable area and risk analysis for citrus target spot[J]. Scientia Agricultura Sinica,2020,53(21):4430-4439.
[7]朱丽. 五种柑橘真菌性病害病原鉴定[D]. 杭州:浙江大学,2012.ZHU Li. Identification of five pathogens causing Citrus disease in China[D].Hangzhou:Zhejiang University,2012.
[8]YANG Y H,HU J H,CHEN F J,DING D K,ZHOU C Y.Development of a SCAR marker-based diagnostic method for the detection of the citrus target spot pathogen Pseudofabraea citricarpa[J].BioMed Research International,2018,2018:7128903.
[9]陈志谊,刘永峰,刘凤权,罗楚平,聂亚峰.江苏省水稻品种细菌性条斑病抗性评价与病原菌致病力分化[J].植物保护学报,2009,36(4):315-318.CHEN Zhiyi,LIU Yongfeng,LIU Fengquan,LUO Chuping,NIE Yafeng.Resistant evaluation of rice bacterial leaf streak and virulence differentiation of Xanthomonas oryzae pv.oryzicola in Jiangsu[J].Acta Phytophylacica Sinica,2009,36(4):315-318.
[10]张长伟,丁国祥,倪先林,刘天朋,陈国民,赵甘霖.酿酒高粱品种、组合及亲本的丝黑穗病抗性鉴定[J]. 植物保护学报,2013,40(3):219-224.ZHANG Changwei,DING Guoxiang,NI Xianlin,LIU Tianpeng,CHEN Guomin,ZHAO Ganlin. Resistance identification of the liquor-feedstock sorghum varieties,hybrids and parents to sorghum head smut[J].Acta Phytophylacica Sinica,2013,40(3):219-224.
[11]林月莉,黄丽丽,索朗拉姆,高小宁,陈银潮,康振生.苹果轮纹病室内快速评价体系的建立[J]. 植物保护学报,2011,38(1):37-41.LIN Yueli,HUANG Lili,Suolang Lamu,GAO Xiaoning,CHEN Yinchao,KANG Zhensheng. A rapid laboratory evaluation system for apple ring rot[J]. Acta Phytophylacica Sinica,2011,38(1):37-41.
[12]朱琳,孙素丽,孙菲菲,段灿星,朱振东.绿豆尖镰孢枯萎病抗性鉴定方法[J].植物遗传资源学报,2017,18(4):696-703.ZHU Lin,SUN Suli,SUN Feifei,DUAN Canxing,ZHU Zhendong.The method for evaluation of mung bean resistance to Fusarium wilt[J].Journal of Plant Genetic Resources,2017,18(4):696-703.
[13]聂峰杰,左叶信,黄丽丽,秦虎强,高小宁,韩青梅.陕西省核盘菌不同分离株对油菜的致病性[J].植物保护学报,2010,37(6):499-504.NIE Fengjie,ZUO Yexin,HUANG Lili,QIN Huqiang,GAO Xiaoning,HAN Qingmei.Pathogenicity of Sclerotinia sclerotiorum isolated from rapeseed in Shaanxi province[J].Acta Phytophylacica Sinica,2010,37(6):499-504.
[14]陈莹,柳李旺,谢学文,孟霖,刘博,王晓武,李宝聚,武剑.白菜黑斑病抗性鉴定及QTL 定位[J]. 中国农业科学,2013,46(24):5173-5179.CHEN Ying,LIU Liwang,XIE Xuewen,MENG Lin,LIU Bo,WANG Xiaowu,LI Baoju,WU Jian. Evaluation and QTL mapping for resistance to black spot (Alternaria brassicicola) in Brassica rapa[J]. Scientia Agricultura Sinica,2013,46(24):5173-5179.
[15]高崇,吴国贺,李佰霖,安承荣,高歌农,卢宝慧,高玉亮,田慧敏,郑成叡,崔昌范.延边烟区烟草菌核病病原菌鉴定及抗性种质筛选[J].中国烟草科学,2018,39(2):69-75.GAO Chong,WU Guohe,LI Bailin,AN Chengrong,GAO Genong,LU Baohui,GAO Yuliang,TIAN Huimin,ZHENG Chengrui,CUI Changfan. Identification of tobacco Sclerotinia rot in Yanbian and screening for resistant germplasms[J]. Chinese Tobacco Science,2018,39(2):69-75.
[16]苗红梅,常淑娴,张海洋,黄进勇,段迎辉,曲文文.芝麻营养生长期枯萎病抗性鉴定技术研究[J]. 植物遗传资源学报,2020,21(2):330-337.MIAO Hongmei,CHANG Shuxian,ZHANG Haiyang,HUANG Jinyong,DUANYinghui,QU Wenwen.An evaluation technique of sesame resistance to Fusarium wilt disease at vegetative stage[J].Journal of Plant Genetic Resources,2020,21(2):330-337.
[17]陈红梅,李金花,柴兆祥,郭成,王蒂.35 个马铃薯品种对镰刀菌干腐病优势病原的抗病性评价[J].植物保护学报,2012,39(4):308-314.CHEN Hongmei,LI Jinhua,CHAI Zhaoxiang,GUO Cheng,WANG Di. Resistance evaluation of 35 potato varieties to the dominant pathogens of potato Fusarium dry rot[J]. Acta Phytophylacica Sinica,2012,39(4):308-314.
[18]杨璐嘉,初炳瑶,邓杰,何少清,张怡,马占鸿.宁夏葡萄霜霉病菌致病型鉴定及葡萄品种抗性评价[J]. 植物保护学报,2020,47(6):1321-1332.YANG Lujia,CHU Bingyao,DENG Jie,HE Shaoqing,ZHANG Yi,MA Zhanhong. Pathotype identification of downy mildew pathogen Plasmopara viticola and evaluation of variety resistance in Ningxia[J]. Journal of Plant Protection,2020,47(6):1321-1332.
[19]赵惠,刘崇怀,姜建福,樊秀彩,张颖,房玉林.46 份野生葡萄株系对霜霉病的抗性鉴定及其相关基因的表达[J].植物保护学报,2018,45(6):1242-1250.ZHAO Hui,LIU Chonghuai,JIANG Jianfu,FAN Xiucai,ZHANG Ying,FANG Yulin. Resistance identification and expression of related genes of downy mildew in 46 wild grape strains[J].Journal of Plant Protection,2018,45(6):1242-1250.
[20]刘潮,王慧,刘林,杨静,李成云.水稻防御反应相关基因对稻瘟病菌的响应[J].中国生物防治学报,2017,33(4):504-511.LIU Chao,WANG Hui,LIU Lin,YANG Jing,LI Chengyun.Response of rice defense-related genes to blast fungus[J]. Chinese Journal of Biological Control,2017,33(4):504-511.
[21]蔺瑞明,邱焯,管秀娜,曹丽华,包秀兰,徐世昌.中国小麦条锈病菌鉴别寄主中4 抗病基因遗传组成分析[J].植物保护学报,2007,34(6):573-579.LIN Ruiming,QIU Zhuo,GUAN Xiuna,CAO Lihua,BAO Xiulan,XU Shichang. Genetic analysis on the composition of resistance gene(s)in Chinese differential host Zhong 4 of yellow rust fungi[J].Acta Phytophylacica Sinica,2007,34(6):573-579.
[22]EBRAHIM S,USHA K,SINGH B. Plant architectural traits and their role in defense mechanism against malformation in mango(Mangifera indica L.)[J]. Scientia Horticulturae,2012,139:25-31.
[23]CHATTOPADHYAY S,ALI K A,DOSS S G,DAS N K,AGGARWAL R K,BANDOPADHYAY T K,SARKAR A,BAJPAI A K. Association of leaf micro-morphological characters with powdery mildew resistance in field- grown mulberry (Morus spp.)germplasm[J].AoB Plants,2011,1:plr002.
[24]KULKARNI M,DESHPANDE U. In vitro screening of tomato genotypes for drought resistance using polyethylene glycol[J].African Journal of Biotechnology,2007,6(6):691-696.
[25]FENILLET C,SCHACHERMAYR G,KELLER B. Molecular cloning of a new receptor-like kinase gene encoded at the Lr10 disease resistance locus of wheat[J].The Plant Journal,1997,11(1):45-52.
[26]张丽英,陈儒钢,张俊红,欧阳波,肖景华,李汉霞,叶志彪.辣椒抗病基因同源序列的克隆与分析[J].中国农业科学,2008,41(1):169-175.ZHANG Liying,CHEN Rugang,ZHANG Junhong,OUYANG Bo,XIAO Jinghua,LI Hanxia,YE Zhibiao. Cloning and analysis of resistance gene analogs from pepper (Capsicum annuum L.)[J].Scientia Agricultura Sinica,2008,41(1):169-175.
[27]GARZON L N,OLIVEROS O A,ROSEN B,LIGARRETO G A,COOK D R,BLAIR M W. Isolation and characterization of nucleotide-binding site resistance gene homologues in common bean (Phaseolus vulgaris)[J]. Phytopathology,2013,103(2):156-168.
[28]习玲,王昱琦,杨修,朱微,陈国跃,王益,覃鹏,周永红,康厚扬.243 份云南普通小麦地方品种抗条锈病鉴定及分子标记检测[J].中国农业科学,2021,54(4):684-695.XI Ling,WANG Yuqi,YANG Xiu,ZHU Wei,CHEN Guoyue,WANG Yi,QIN Peng,ZHOU Yonghong,KANG Houyang.Evaluation of resistance to stripe rust and molecular detection of resistance gene(s)in 243 common wheat landraces from the Yunnan Province[J]. Scientia Agricultura Sinica,2021,54(4):684-695.
[29]黄代青,王平,吕柳新.柚cDNA 中NBS-LRR 类R 基因同源序列的分离[J].中国农业科学,2004,37(10):1580-1584.HUANG Daiqing,WANG Ping,LÜ Liuxin. Isolation of NBSLRR class resistance gene analogs from stigma cDNA of pomelo (Citrus grandis cv. Guanxi)[J]. Scientia Agricultura Sinica,2004,37(10):1580-1584.
[30]谌谋华.利用同源序列法克隆柑橘抗病基因类似物及其初步分析[D].武汉:华中农业大学,2003.ZHAN Mouhua.Cloning of resistance gene analog(RGA)of citrus by using homology-based technique and the preliminary analysis[D].Wuhan:Huazhong Agricultural University,2003.