葡萄枝干病害(Grape Trunk Diseases,GTDs)是一类危害葡萄枝干的真菌性病害的统称,由多种病原真菌引起,发生复杂,在世界各地葡萄种植区广泛发生,目前包括葡萄衰枯病(ESCA complex disease)、葡萄顶枯病(Eutypa dieback)、葡萄溃疡病(Botryosphaeria dieback)、葡萄黑根病(Black foot disease)和葡萄蔓枯病(Diaporthe dieback)5 种。该类病害的主要症状为枝干变色和坏死,有时在叶片上也会表现不同的症状,严重时表现为整株枝条枯死,甚至整树枯死,每年由于枝干病害导致更换死树的损失高达15亿美元[1]。我国葡萄枝干病害发生以葡萄溃疡病和蔓枯病为主,各地区发生较为普遍,个别年份发生较重,每年仅葡萄溃疡病就造成葡萄减产3%~8%[2]。因此,葡萄枝干病害备受葡萄园区工作者和研究者的关注,很多国家开展了葡萄枝干病害的发生发展和防治措施方面的研究。
1 葡萄枝干病害的发生和危害
葡萄衰枯病是国际上最早被报道的葡萄枝干病害,也是葡萄枝干病害正式被关注的起始[3],随后其他葡萄枝干病害也被相继报道[4-6]。目前已有36 个国家报道了葡萄枝干病害的发生,其中8 个国家均报道了5 种葡萄枝干病害,包括:法国、西班牙、美国、澳大利亚、南非、意大利、捷克和中国,17个国家报道了2~4种葡萄枝干病害(表1)。我国在1991年对葡萄枝干病害中的葡萄蔓枯病进行了报道,近年来,又先后报道了葡萄溃疡病[29]、葡萄衰枯病[2,30]、葡萄黑根病[2]和葡萄顶枯病[31]等。葡萄枝干病害病原菌种类繁多,其症状难以识别,可能存在已有相关病害的发生,但没有完全被科学的研究和报道的情况,导致枝干病害的发生危害可能被低估。
表1 已报导葡萄枝干病害的主要类型及报道病害的国家
Table 1 Countries where the main grape trunk diseases have been reported
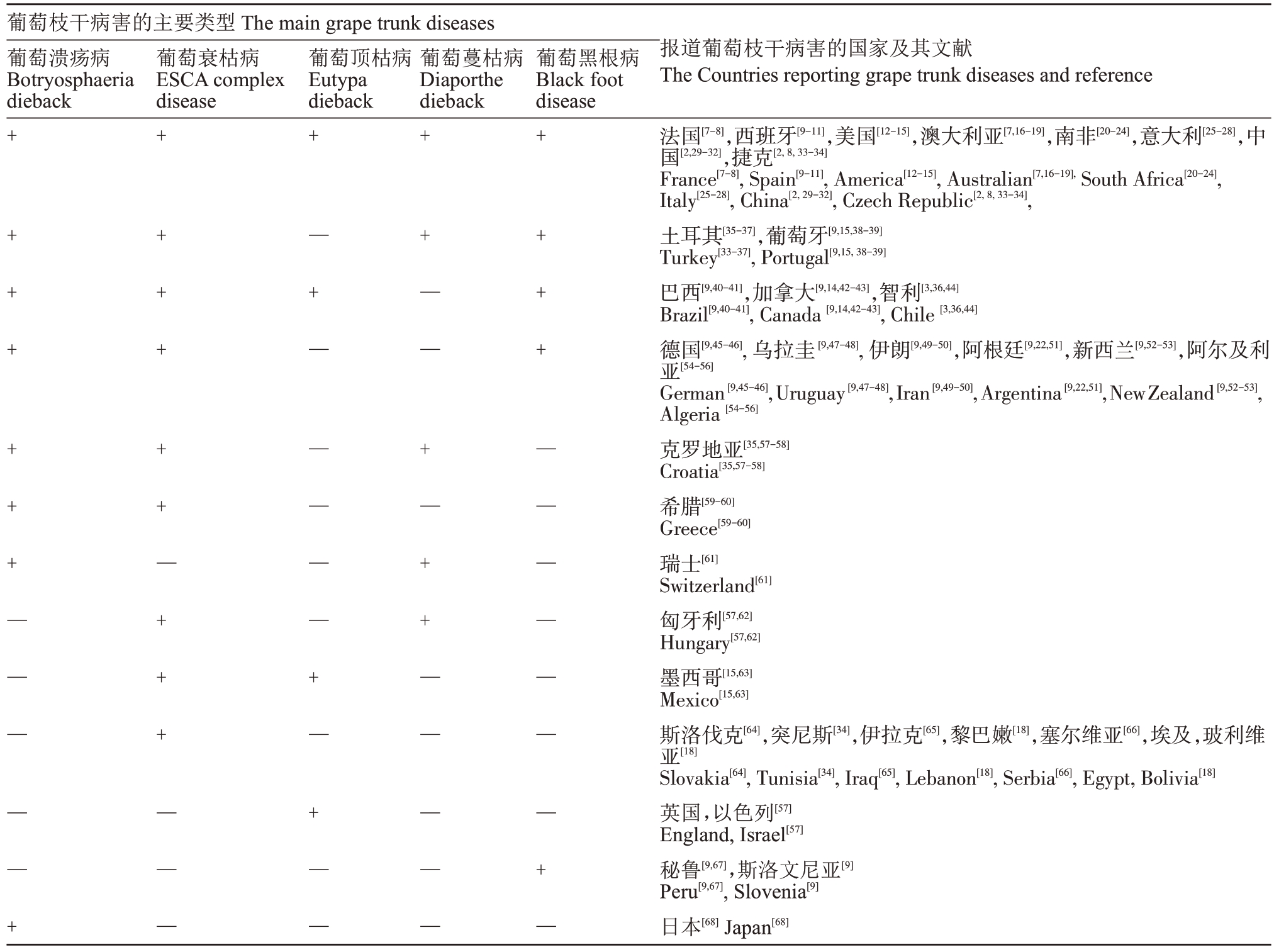
注:+表示该病害在同一行所列出的国家内均有报道,—表示该病害在同一行所列出的国家内均未报道。
Note:+means the disease has been reported in the countries listed in the same line,—means the disease has not been reported in the countries listed in the same line.
葡萄枝干病害的主要类型The main grape trunk diseases葡萄溃疡病Botryosphaeria dieback葡萄衰枯病ESCA complex disease葡萄顶枯病Eutypa dieback葡萄蔓枯病Diaporthe dieback葡萄黑根病Black foot disease报道葡萄枝干病害的国家及其文献The Countries reporting grape trunk diseases and reference法国[7-8],西班牙[9-11],美国[12-15],澳大利亚[7,16-19],南非[20-24],意大利[25-28],中国[2,29-32],捷克[2,8,33-34]France[7-8],Spain[9-11],America[12-15],Australian[7,16-19],South Africa[20-24],Italy[25-28],China[2,29-32],Czech Republic[2,8,33-34],土耳其[35-37],葡萄牙[9,15,38-39]Turkey[33-37],Portugal[9,15,38-39]巴西[9,40-41],加拿大[9,14,42-43],智利[3,36,44]Brazil[9,40-41],Canada[9,14,42-43],Chile[3,36,44]德国[9,45-46],乌拉圭[9,47-48],伊朗[9,49-50],阿根廷[9,22,51],新西兰[9,52-53],阿尔及利亚[54-56]Slovakia[64],Tunisia[34],Iraq[65],Lebanon[18],Serbia[66],Egypt,Bolivia[18]英国,以色列[57]England,Israel[57]秘鲁[9,67],斯洛文尼亚[9]Peru[9,67],Slovenia[9]日本[68]Japan[68]+++++++——+++++++—+++——+—+——+—+——++——+—++——++++——+—German[9,45-46],Uruguay[9,47-48],Iran[9,49-50],Argentina[9,22,51],NewZealand[9,52-53],Algeria[54-56]克罗地亚[35,57-58]Croatia[35,57-58]希腊[59-60]Greece[59-60]瑞士[61]Switzerland[61]匈牙利[57,62]Hungary[57,62]墨西哥[15,63]Mexico[15,63]斯洛伐克[64],突尼斯[34],伊拉克[65],黎巴嫩[18],塞尔维亚[66],埃及,玻利维亚[18]
随着葡萄枝干病害的发生危害日趋严重,较多国家针对该类病害开展了相关研究。欧洲地区[69]研究结果表明,葡萄枝干病害在法国的平均发病率达13%,西班牙为10.5%,意大利是19%,土耳其是2.61%,德国是19%,葡萄牙是19%,匈牙利是11%,一些老的葡萄园区发病率达到60%~80%,例如托斯卡纳、阿普利亚和西西里[70-71]。在2014 年,由7 个成员国(西班牙,德国,意大利,法国,葡萄牙,克罗地亚和匈牙利)合作的‘WINEWORK’项目报告中指出,3种主要的葡萄枝干病害(葡萄衰枯病、葡萄顶枯病和葡萄溃疡病)在7 个成员国的发病率达到15%~20%。此外,Guerin-Dubrana 等在2015—2016 年间调查了欧洲19 个国家和3 个非欧洲国家(阿尔及利亚,黎巴嫩和以色列)主要的葡萄枝干病害,调查范围包括105 个葡萄园区,共4 亿公顷面积[69];调查结果表明,葡萄衰枯病是最频繁发生且发病率显著增加的葡萄枝干病害(38.1%),黑根病和蔓枯病的发病率较轻(低于5%);被调查的15个葡萄品种中,赤霞珠的发病严重度最高,其次为梅鹿辄和霞多丽,黑比诺的发病严重度最低。在美洲,调查发现葡萄枝干病害的症状在树龄8~10 a 时才会出现,占园区的20%,产量损失较小,但在树龄10~15年间,75%的植株均有了葡萄枝干病害的典型症状,如:顶梢枯死,1年生枝条发育不良,叶片显症等。在澳大利亚和新西兰,20 世纪90 年代开始重视葡萄枝干病害的研究,目前上述5种葡萄枝干病害均有报道,其中葡萄顶枯病和溃疡病是主要的葡萄枝干病害,已成为影响葡萄产业的主要病害之一,仅次于白粉病和霜霉病[72]。此外,2018 年对葡萄嫁接绿枝条进行葡萄座腔菌科真菌(Botryosphaeriaceae species)的检测表明,23%的苗木为阳性[73]。在南非,对30个葡萄园区内葡萄枝干病害的发生情况调查显示,葡萄衰枯病发病率为24.5%,葡萄溃疡病发病率为16%[74]。
西亚地区的伊拉克、以色列、伊朗等国,以及东亚地区的日本和中国等国较早开展了葡萄枝干病害的主要病原菌的研究。伊朗主要报道了葡萄溃疡病、葡萄衰枯病和葡萄黑根病病原菌的分子特征和表型特征[9,35]。例如,克尔曼沙希德·巴霍纳尔大学植物保护学院团队鉴定了Phaeomoniella chlamydospore 和Phaeoacremonium spp. 是伊朗葡萄枝干病害的主要致病菌。伊拉克和以色列对葡萄枝干病害也主要集中于相关病原菌的研究,病原菌多为葡萄座腔菌(Botryosphaeria parva)和间座壳属(Diaporthe spp.)。日本主要对葡萄溃疡病的病原菌进行了鉴定,主要致病菌为Botryosphaeria dothidea[68]。我国对葡萄顶枯病、葡萄溃疡病、葡萄蔓枯病和葡萄衰枯病的病原菌及其分布进行了研究。例如,2007年西北农林科技大学的团队从陕西杨凌葡萄园的葡萄病枝干中分离并鉴定出病原菌为葡萄弯孢聚壳(Eutypella vitis),是陕西杨凌地区葡萄顶枯病的主要病原菌[75],但没有完成柯赫氏法则验证。2010年,北京市农林科学院植物保护环境保护研究所团队和中国农科院植保所团队合作首次报道了葡萄溃疡病在我国的发生情况[76];随后在2013 年,北京市农林科院植物保护环境保护研究所团队详细报道了葡萄溃疡病的主要致病菌在我国18 个省的分布情况[7],并揭示了其中4 个优势种群(Botryosphaeria dothidea,Diplodia seriata,Lasiodiplodia theobromae 和Neofusicoccum parvum)存在地域性分布差异的可能性。2019年,北京市农林科学院植物保护环境保护研究所团队还报道了蔓枯病病原菌在我国的遗传多样性[34],2020 年首次报道了国内葡萄衰枯病的病原菌Phaeoacremonium minimum[8],2021年首次报道了国内葡萄黑根病的病原菌[2]。越来越多的国家对葡萄枝干病害展开了更频繁和深入的研究和报道,说明该类病害已成为葡萄园区中影响葡萄经济效益的重要问题。
2 葡萄枝干病害的典型症状和相关病原菌
葡萄枝干病害的发生与树龄关系较大[70]。8 年以上树龄的葡萄树,主要发生有葡萄衰枯病、葡萄顶枯病和葡萄溃疡病3种葡萄枝干病害[71];8年以下树龄的葡萄树,主要有葡萄黑根病和葡萄蔓枯病[77]。葡萄枝干病害主要危害枝干,也可危害叶片和果实,主要是由枝干内部先发生病变,导致维管束内部逐渐产生扇形或梭形或不规则形的坏死斑,又或者根茎木坏死,有时叶片产生萎蔫、褪绿和边缘坏死等症状,或果实皱缩和掉粒。不同枝干病害的典型症状见图1及病原菌种类见表2。

图1 葡萄枝干病害的症状
Fig.1 Symptoms of grape trunk diseases
表2 葡萄枝干病害中5 种主要病害的典型症状和主要病原菌
Table 2 Typical symptoms of five main grape trunk diseases and their related pathogens
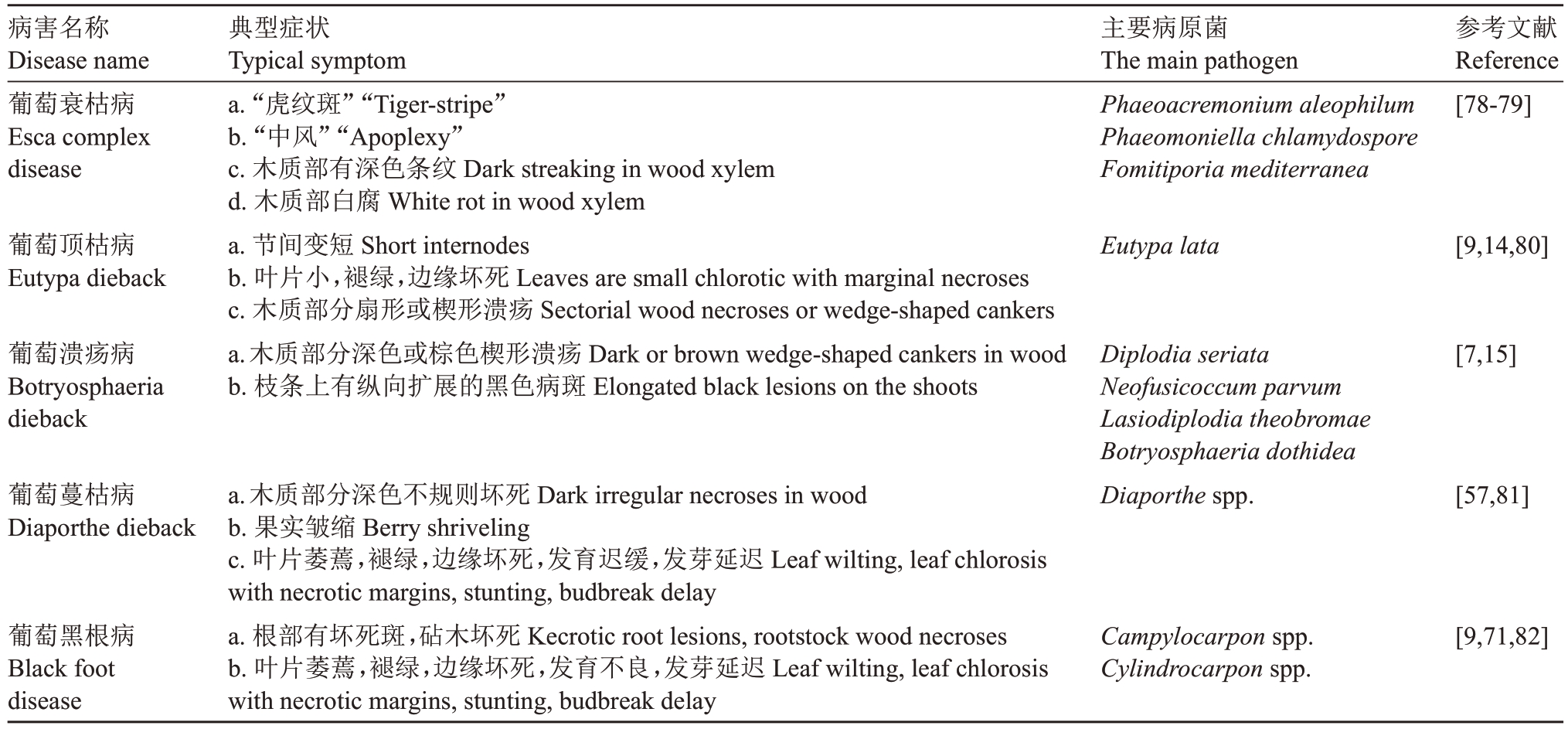
病害名称Disease name葡萄衰枯病Esca complex disease主要病原菌The main pathogen Phaeoacremonium aleophilum Phaeomoniella chlamydospore Fomitiporia mediterranea参考文献Reference[78-79]葡萄顶枯病Eutypa dieback Eutypa lata [9,14,80]葡萄溃疡病Botryosphaeria dieback典型症状Typical symptom a.“虎纹斑”“Tiger-stripe”b.“中风”“Apoplexy”c.木质部有深色条纹Dark streaking in wood xylem d.木质部白腐White rot in wood xylem a.节间变短Short internodes b.叶片小,褪绿,边缘坏死Leaves are small chlorotic with marginal necroses c.木质部分扇形或楔形溃疡Sectorial wood necroses or wedge-shaped cankers a.木质部分深色或棕色楔形溃疡Dark or brown wedge-shaped cankers in wood b.枝条上有纵向扩展的黑色病斑Elongated black lesions on the shoots[7,15]葡萄蔓枯病Diaporthe dieback Diplodia seriata Neofusicoccum parvum Lasiodiplodia theobromae Botryosphaeria dothidea Diaporthe spp.[57,81]葡萄黑根病Black foot disease a.木质部分深色不规则坏死Dark irregular necroses in wood b.果实皱缩Berry shriveling c.叶片萎蔫,褪绿,边缘坏死,发育迟缓,发芽延迟Leaf wilting,leaf chlorosis with necrotic margins,stunting,budbreak delay a.根部有坏死斑,砧木坏死Kecrotic root lesions,rootstock wood necroses b.叶片萎蔫,褪绿,边缘坏死,发育不良,发芽延迟Leaf wilting,leaf chlorosis with necrotic margins,stunting,budbreak delay Campylocarpon spp.Cylindrocarpon spp.[9,71,82]
葡萄衰枯病的典型症状是有枯叶和“中风”现象(整株葡萄几天之内突然萎蔫或死亡,Eskalen and Gubler,2001);叶片上有“虎纹斑”也是葡萄衰枯病的典型症状之一(图1-A)。它主要病原菌是Phaeoacremonium aleophilum、Phaeomoniella chlamydospore和Fomitiporia mediterranea[79]。这些病原菌单独或者复合侵染会导致木质部局部腐烂,逐渐坏死[78]。葡萄顶枯病的典型症状是枝条节间变短,叶片叶绿素缺乏和边缘坏死(图1-C 和F),坏死的叶片组织有时还会进一步影响葡萄果穗大小[80];多在观察到这些症状后的3~5 a 后可能引起植株死亡[9]。该病害的主要病原菌是子囊菌Eutypa lata[14]。葡萄溃疡病的典型症状是枝干横截面具有楔形的病斑,枝条溃疡,不能萌芽,植株枯萎,以及果实腐烂等(图1-D)。目前已报道了45种病原菌可导致该病害,主要包括Diplodia seriata、Neofusicoccum parvum 和Botryosphaeria dothidea[2,15]。葡萄蔓枯病导致枝干内部产生溃疡和维管束变色,与葡萄溃疡病和葡萄顶枯病的症状相似。该病害的典型症状是发芽延迟(图1-E),产生叶斑,与一些毒性较低的种有关,也可能导致溃疡,枝干内部的症状是黑色不规则的坏死斑(图1-G),主要发生在春季和冬季。接近成熟时期,果轴易坏死和变色,果实皱缩。主要病原菌是Diaporthe eres 和D. ampelina[57]。葡萄黑根病与葡萄蔓枯病的叶片症状相似,典型症状包括叶片萎蔫,叶缘坏死,发育迟缓,发芽延迟,生长和活力下降,根部为褐色至黑色坏死(图1-H和I)。引起黑根病的病原菌主要是Campylocarpon fasciculare、Campylocarpon pseudofasciculare、Cylindrocarpon destructans、Cylindrocarpon macrodidymum等[9]。
3 葡萄枝干病害的发生流行规律
3.1 葡萄衰枯病(Esca complex disease)
葡萄衰枯病的病原菌通常在结果母枝的修剪伤口处,副梢和深层裂缝内的枝干表面上被检测到。深层裂缝中的潮湿环境,有利于它们产生分生孢子,然后通过空气传播。P. chlamydospore 的分生孢子释放发生在冬季,P. chlamydospore 和P. aleophilum的分生孢子释放发生在葡萄的发芽期,并且释放高峰期与降雨量密切相关[84]。这些病原菌通过修剪伤口侵入葡萄组织,但还不清楚其侵入条件。研究者认为病原菌侵入寄主时存在一定的顺序;P. chlamydospore 和P. aleophilum 被认为是“先锋菌”,P.chlamydospore 产生毒素(萘酮类、酚类、多糖),P.aleophilum 降解木质部组织[85],F.mediterranea 的侵入则造成枝干腐烂[86]。对病原菌的生物学特性研究(表3)表明,P. aleophilum 的菌丝生长温度是5~40 ℃,适温为25~30 ℃;P.chlamydospore 的菌丝生长适温为20~25 ℃,高于35 ℃生长受抑制;F.mediterranea 的菌丝生长适温为30 ℃,低于15 ℃菌丝生长受抑制。这些病原菌在侵入和定殖初期不会在枝干外部表现明显的症状,但枝干的纵剖面上逐渐形成黑色的条纹,单个或聚集成黑褐色的束状,有时从嫁接处开始,常向上、向下延伸(图1-B)。该病害也危害叶片,初期叶片上形成坏死斑点,严重时叶片坏死斑连接形成坏死条纹与绿色的叶脉间隔,俗称“虎纹斑”。叶片“虎纹斑”症状多出现在凉爽多雨的夏季[71,95],因此,环境因子可能是诱发这些症状的关键因素,但该症状的形成机制目前还不十分清楚[96-101]。目前存在2种解释,一种是病原菌在枝干内部产生的毒素运输至叶片所致;另一种是病原菌对木质部造成的损伤导致维管束堵塞和液压功能障碍,无法运输足够的水分和营养元素所致[102]。
表3 葡萄枝干病害主要病原菌菌丝生长所需的三基点温度
Table 3 Cardinal temperature for mycelium growth of main pathogens of grape trunk diseases
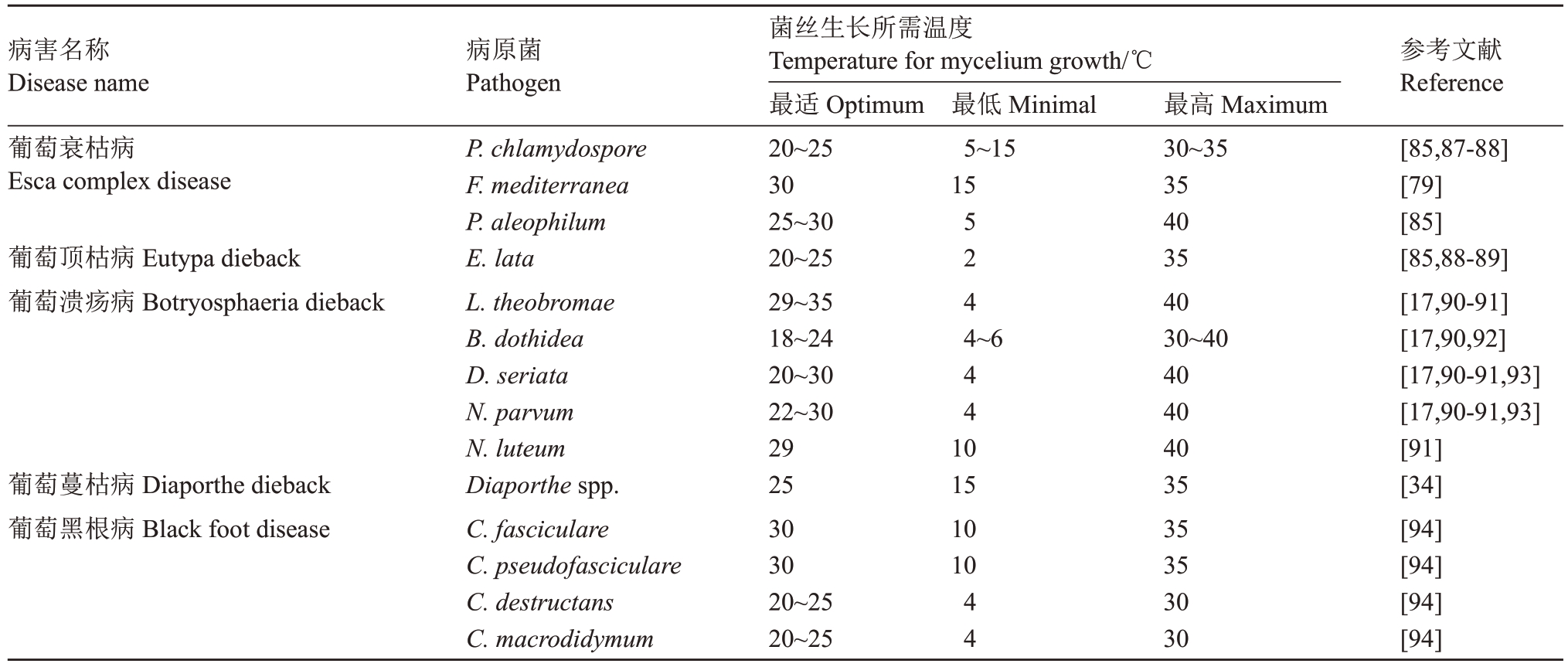
病害名称Disease name病原菌Pathogen参考文献Reference葡萄衰枯病Esca complex disease最低Minimal 5~15 15葡萄顶枯病Eutypa dieback葡萄溃疡病Botryosphaeria dieback 5 2 4 4~6 4 4 1 0葡萄蔓枯病Diaporthe dieback葡萄黑根病Black foot disease P.chlamydospore F.mediterranea P.aleophilum E.lata L.theobromae B.dothidea D.seriata N.parvum N.luteum Diaporthe spp.C.fasciculare C.pseudofasciculare C.destructans C.macrodidymum菌丝生长所需温度Temperature for mycelium growth/℃最适Optimum 20~25 30 25~30 20~25 29~35 18~24 20~30 22~30 29 25 30 30 20~25 20~25 15 10 10 4 4最高Maximum 30~35 35 40 35 40 30~40 40 40 40 35 35 35 30 30[85,87-88][79][85][85,88-89][17,90-91][17,90,92][17,90-91,93][17,90-91,93][91][34][94][94][94][94]
3.2 葡萄顶枯病(Eutypa dieback)
葡萄顶枯病的病原菌(Eutypa lata)主要以菌丝体越冬。在年降雨量达到350 mm或有顶部喷灌设施的葡萄园易于病害发生;子囊孢子的释放需要在降雨量超过2 mm,连续降雨3 h,且温度高于0 ℃的条件下完成;而降雨停止24 h 后,子囊孢子停止传播[103-105];子囊孢子的释放高峰一般在深秋或春季降雨后[104]。其主要病原菌E.lata 通过剪枝切口侵入,逐步扩展至维管束形成层,在树体内的潜伏期可长达3~8 年[105]。E. lata 菌丝在2~35 ℃范围内均可生长,最适温度为20~25 ℃。经过12 d左右,产生新一代可传播的子囊孢子,以每年2~5 cm的平均速度在维管束中向上或向下传导,但不会侵染根部。该病原菌通过降解木质部中贮藏的淀粉、半纤维素和葡聚糖对树体产生危害[106]。几年后枝干有枯死现象,严重时内部木质部细胞全部死亡。这些枯死的枝干作为二次感染源存在于园区中,增加顶枯病的发病率。根据Sosnowski 等[107]建立的降雨量大于4 mm事件或每日最高温度与发病率增长的线性方程(R2= 0.914 和0.724)可知,顶枯病的发病率与温度和降雨呈正相关。
3.3 葡萄溃疡病(Botryosphaeria dieback)
葡萄溃疡病的病原菌在修剪掉的枝干中越冬。翌年,病原菌在降雨或树冠层灌溉时散播,通常在12 月至翌年2 月份[108],在美国加利福尼亚主要是冬季降雨后[109],而在法国是春季到秋季[110],南非[111]和智利[112]是在晚秋至早春。空气中病原菌子囊孢子在全年都能监测到,但子囊孢子量具有显著性差异。Neofusicoccum spp. 子囊孢子量在夏季和冬季最高,3—4月份最低。Diplodia spp.子囊孢子量在1月份和夏季最高,4—5 月份也较高,11 月份最低。子囊孢子散播的最佳温度是8.4~19.9 ℃,最佳相对湿度是77%~94%[113],降雨量达到0.2 mm 以上[112]。子囊孢子在降雨条件下,可随风传播到10 m远的距离,侵染雌性圆锥花序、雄性花朵和幼芽,也通过1年生枝条侵入植株体内。Amponsahnt 等[114]从葡萄树不同部位均分离到了属于葡萄座腔菌的6种病原菌,其中主干部有42%,枝干有17%,1 年生枝条有19%,剪碎的枝干组织有8%,芽有8%,叶片中有3%,萎蔫的花有3%。病原菌在植株内的广泛分布,说明了病原菌从多个部位侵入的可能性。此外,不同病原菌菌丝的最适生长温度存在一定差异(表3),例如:D. seriata,N. luteum 和N. parvum 菌丝最适生长温度相似,在20~30 ℃之间,B.dothideaju菌丝适宜生长温度为10~24 ℃,而L.theobromae 菌丝的适宜生长温度为29~35 ℃;低于4°C和高于40 ℃均会抑制这些病原菌的生长。当病原菌侵入后,7个月左右可在侵入点向下5~10 mm处观察到病变,且枝干横截面出现楔形坏死斑[114]。
3.4 葡萄蔓枯病(Diaporthe dieback)
葡萄蔓枯病的病原菌在枝干内部产生溃疡和维管束褪色症状,与葡萄溃疡病和葡萄顶枯病的症状相似,其形态,eEF1-a、TUB、CAL 和ITS 序列特征[115],以及致病性[57]已有报道,但病害发展的相关信息鲜有报道。已知Phomopsis sp.分生孢子在降雨时散播,通常在11月至翌年2月份较多[116];其菌丝的生长最适温度为25 ℃,低于15 ℃和高于35 ℃均会抑制其生长。
3.5 葡萄黑根病(Black foot disease)
葡萄黑根病的病原菌通过产生黏性的子囊孢子存活于土壤的自由水中,当与根系接触后通过分解皮层细胞来抑制根系吸收水分和养分后向地上部的运输,从而逐渐造成地上部发育不良[117]。其主要致病菌Cylindrocarpon destructans 和C. macrodidymum 菌丝的最适生长温度为20~25 ℃,最低温度和最高温度分别为4 ℃和30 ℃[94];Campylocarpon fasciculare 和C. pseudofasciculare 菌丝则适宜较高温度(30 ℃),但低于10 ℃和高于35 ℃会抑制其生长。有研究者认为该病害的寄主可能是砧木,因为被感染部位通常位于枝干埋土部位的2~12 cm[118]。但在葡萄牙的砧木苗圃中检测黑根病病原菌时,只有0.17%的发病率[119]。在砧木种植时造成的根系伤口以及嫁接苗的接口处暴露被土壤中存在的病原菌感染可能是该病害发生的原因。Aroca和Raposo[120]调查发现15%的嫁接苗中都存在葡萄黑根病的病原菌(Cylindrocarpon spp.)也证实了这一观点。
4 葡萄枝干病害的防控策略
目前,国内外缺乏葡萄枝干病害的高效防控技术,纠其主要原因是尚未明确病原菌的侵入条件及其致病机制。为解决这些问题,研究者需要克服葡萄枝干病害病原菌多样性和葡萄种植区域广泛性的问题。国外对枝干病害重视程度高,投入了大量精力从事相关研究工作,目前国外在农业技术服务团队和科学研究团队之间建立了合作,通过对不同的葡萄枝干病害防治方法进行收集、验证和整合,提出了一些较好的综合防治技术体系,这些办法与我国“预防为主,综合防治”的植保方针相契合,为我国葡萄枝干病害防治工作的开展提供了一定借鉴;在以综合防控技术体系为主开展葡萄枝干病害防治的同时,还应在预防性技术的研究与推广方面有所加强,需要开展大量的探索性和验证性工作来解决我国对该类病害高效防治技术缺乏的问题。
4.1 预防性策略
4.1.1 提前或推迟剪枝时间 葡萄剪枝被认为是为枝干病害病原菌的侵入创造了条件,并且刚经过剪枝的树体比已经修剪一段时间的树体对病原菌更为敏感。通过提前或推迟剪枝时间这一方法避开田间孢子浓度高峰期,从而达到降低病原菌侵染的目的。例如,澳大利亚的种植者将葡萄枝干的修剪工作从历年的12月份推迟至翌年2月份或者更晚来避开高风险的感染时期[121]。二次剪枝也是一种可行的应对措施,通过剪掉可能被感染的枝干部分来达到预防病原菌侵染的目的。它是在休眠期提前进行一次机械剪枝后,在休眠期后再进行一次人工剪枝。研究者在霞多丽和梅鹿辄品种上试用了该方法,结果表明二次剪枝比单次提前剪枝的方法效果较好,葡萄枝干内维管束变色部位平均少0.27~4.67 cm[121]。
此外,剪枝方式也被认为是影响枝干病害偶发性的因素之一。Pascal-Lecomte等[122]在法国连续2 a统计了25 个葡萄园区中葡萄衰枯病的发生情况。研究发现:1)剪枝时如果保留较长的枝条感染率低于保留较短枝条的植株;2)机械剪枝更容易发生枝干病害。而Christian Kraus 等[123]在德国西部的莱茵兰地区连续4 a 观察了葡萄叶片“虎纹斑”症状的发生情况,发现剪枝方式对该病害的发生率没有明显的影响,并跟踪了2015—2018年的2种剪枝方式[短截-即1 年生枝条剪去一段、留下一段的修剪方式;梳剪-即把整个枝蔓(包括一年生和多年生枝蔓)从基部剪除的方式],它们的发病率均在1.8%~6.9%之间;同时指出降雨、葡萄品种和树龄对葡萄枝干病害的发病率有明显影响[123]。
4.1.2 伤口处喷施保护剂 人工或机械喷施保护剂也被认为是一种有效的预防枝干病害发生的方法[124]。在加拿大开展的枝干病害治理建议调查结果表明,除上述调整剪枝时间和方式的方法外,甲基硫菌灵(甲基托布津)Thiophanate-methyl(Topsin M WSB; United Phosphorus, Inc., King of Prussia,PA)被认为是最有效的一种杀菌剂,通过人工或机械喷施在被剪枝的部位而避免因风和雨水引起的孢子飞溅而引起的感染。该杀菌剂可作用于葡萄座腔菌属(Botryosphaeria)引起的顶梢枯死和Eutypa lata引起的顶梢枯死[77]。Brown 等[125]测试了不同保护剂和治疗剂组合对葡萄顶枯病的防治效果,包括氟吡喃+戊康唑、吡唑醚菌酯+啶酰菌胺、甲基硫菌酸+支氯丁尼、解淀粉芽胞杆菌。效果最好的是吡唑醚菌酯+啶酰菌胺,效果较差的是解淀粉芽孢杆菌。
杀菌剂的使用时间也会影响杀菌剂效果[126]。研究者在智利的2个园区中开展了杀菌剂有效性的试验,对赤霞珠的1 年生枝条接种病原菌(D.seriata,Inocutis sp.和P. chlamydospora)前后24 h 施用4种杀菌剂,测量了被感染枝干维管束变色的长度,将其作为杀菌剂施用效果的评价指标。研究结果表明,苯菌灵(BenmoyI)和甲基硫菌灵(Thiophanatemethyl)对2 种病原菌的侵入有抑制作用,并且侵染前24 h施用的效果更佳[127]。
生物制剂也可以抑制病原菌的侵入。例如,深绿木霉Trichoderma atroviride(strain I-1237)用于抑制葡萄溃疡病相关病原菌的侵入[128-130];盖姆斯木霉Trichoderma gamsii(Remedier®)和棘孢木霉Trichoderma asperellum[131]以及寡雄腐霉Pythium oligandrum[132]用于治疗葡萄衰枯病是有效的;噬根球囊Rhizophagus irregularis与黑根病的致病菌有一定拮抗作用[133];砖红镰刀菌Fusarium lateritium 和哈茨木霉Trichoderma harzianum 结合可抑制于葡萄顶枯病的主要病原菌E. lata[134]生长。María del Pilar Martín 等[135]还研究了2 个菌株(Trichoderma atroviride SC1 和T.atroviride I-1237)对P.chlamydospora 和D.seriata 的抑菌效果,结果表明,T.atroviride I-1237 防治效果较好于T.atroviride SC1,但与杀菌剂吡唑醚酯+啶酰菌胺组合相比,防治效果均不明显。
4.1.3 热水处理苗木 热水处理苗木也是一种常用的减少树体内病原菌的方法。准确把握水的温度和处理时间是成功灭菌的关键。通常热水的温度需要达到50 ℃,处理时长不小于30 min[136]。不同的葡萄品种对热水的耐受性是有差异的,通过处理树体剪枝来测试其耐受性可以避免因处理不当而损失苗木。例如,45~47 ℃可以使P.chlamydospora 失活,对其他有耐高温的葡萄衰枯病致病菌需要达到51~53 ℃才有效[137]。50 ℃热水处理30 min可以有效地减少78%的P. chlamydospore[138]。消除葡萄衰枯病和葡萄黑根病的相关病原菌则需要把温度控制在53 ℃下30 min,或者50 ℃下45 min[12,139] ;葡萄溃疡病的相关病原菌的消除条件为51~53 ℃,30 min[140]。
4.2 治疗性策略
4.2.1 “外科手术”治疗 当枝干病害发生后,种植者通过剪掉被感染的枝条,使健康的副梢生长代替原来被感染枝条的方法来治理[18,141];当葡萄主干内部出现枝干病害的症状时,通过刮除主干中被感染的部分,并选择一个侧枝让其生长为主干。为了有效地清除被感染的部分,对于葡萄溃疡病和葡萄顶枯病的感染,建议分别切除与感染部位连接的健康部位的10~20 cm[142]。当被感染部位接近嫁接点时,通常会将整株葡萄树移除,补种新的葡萄树。葡萄衰枯病也可通过该方法来治疗,根据植株受感染的程度来决定是否需要移除整个植株。通常砧木受到病原菌感染的概率较小,且小于接穗的部分[143]。所以培养新的主干是一种有效且经济的补救方式。一些被腐生菌侵入的枝干部位,被叫做“curetage”(法语,用刮匙清除脓肿的操作),通过锯子去除腐烂的木质部组织,保留尚未降解的部分,也是枝干病害补救治疗的方法之一。
4.2.2 注射药剂治疗 利用药剂治疗枝干病害的方式有多种,一种是直接向枝干内注射过氧化氢(Hydrogen peroxide),其目的是通过维管束内流动将其带到被病原菌感染的部位来抑制其生长,从而达到治疗的目的[13]。这项技术已在法国和意大利开展,通过钻孔向受感染的枝干部位注射3~4 mL 的过氧化氢来治疗葡萄衰枯病[144]。而利用刮除腐烂部位和枝干内注射药物结合的方式可以更有效地防止枝干病害的复发[145]。
另一种治疗方式是利用接种了深绿木霉内生菌株(endophytic strains of Trichoderma atroviride)的木钉插入枝干感染部位来治疗枝干病害,该技术是一家新西兰的技术公司发明的[146]。木霉菌可产生多种对植物病原真菌、细菌及昆虫具有拮抗作用的生物活性物质,并能提高农作物的抗逆性[147]。因此该技术也被用于尝试治疗枝干病害,使用方法是在春季发现被感染的枝干后,在其主干地上部和枝蔓上插入3 颗接种深绿木霉菌的木制钉子,但研究者认为这种方法在治疗葡萄衰枯病时并没有起到明显的治疗效果[146]。
4.3 防治策略的比较
上述防治技术在一定程度上对葡萄枝干病害起到了一定控制作用,但不同的防治措施在使用过程中存在一些利弊。例如提前或者推迟剪枝时间的方法与二次剪枝相比,虽然花费相对较少,但由于葡萄品种和地理环境条件差异较大,难以给出准确的的剪枝时间。同时也有研究报道指出与枝干病害相关的病原菌(例如P. chlamydospora,是葡萄衰枯病病害的主要病原菌)一直存在园区中[148]。所以,针对这类终年存在于园区的病原菌,调整剪枝时间可能无法有效降低其对枝干伤口的侵染危害。二次剪枝的方法通过剪掉可能被侵染的部位来减少枝干病害的发生,但被重新剪枝的部分仍会暴露在空气中,存在再次被病原菌侵染的风险。
相比调整剪枝时间,在伤口处喷施保护剂可在一定时间内避免病原菌的侵入。当田间枝干病害发病率在20%~30%,该方法可以达到一定效果。但是,也有一些专家[149]指出杀菌剂的施用对裸露枝干部位的保护是暂时的,在4~26 周的时间内,被剪切的伤口会一直处于较敏感的时期。伤口对葡萄座腔菌属(Botryosphaeria)真菌的敏感期为3~16周[103,150]。同时,该方法需要花费123.5~135.85 美元·hm-2。尽管需要支付较高的费用,但Baumgartner 等[121]表示该方法是预防枝干病害方法中最有效果的,并建议在11~15 年树龄的葡萄园区中用此方法。种植者需要根据自己的田间枝干病害的发生情况,酌情选择适宜的预防方法。但如何采用更加高效的防控药剂,甚至在剂型的创新上还需更多的研究。在种植或补种苗木时,对苗木采用热水处理也是可选方法。
此外,枝干病害对树体的生长发育造成影响时,可在感染处进行刮除治疗来降低病害对树体的进一步危害。虽然这一方法在对种植者调查中被提及或推广,但是很难在短时间内验证其有效性,需要多年连续的观察植株是否会再次发病来验证。相比刮除治疗的方法,向枝干中注射过氧化氢或者插入木霉菌木钉的治疗方法可以降低病原菌量和提高植株抗性,但因品种差异和病原菌的多样性,治疗效果也需要特定地区一段时间的检验。
5 展 望
葡萄枝干病害在全世界的普遍发生且造成严重的经济损失成为研究者关注的热点和难点。目前,全世界报道了5种葡萄枝干病害,包括葡萄衰枯病、葡萄顶枯病、葡萄溃疡病、葡萄黑根病和葡萄蔓枯病。该类病害在欧洲地区发病较重,亚洲地区包括中国发病较轻,平均发病率在15%左右。尽管笔者已经明确了5种葡萄枝干病害的主要致病菌及其形态和流行规律,但其导致枝干、叶片和根部症状的形成机制仍不清楚。主要开展了生物和非生物因素对症状表达影响的研究,包括:1)干旱胁迫、水分胁迫、冻害、移栽对根系的损害以及生长的非最佳环境条件;2)生物胁迫的证明来自植物上寄生的线虫影响一系列病理系统的疾病发展和症状表达。但非生物和生物胁迫因素对该类病害发展的影响在很大程度上仍然是未知的,对病原菌数量和症状表达影响的研究也只是在最近才开始,这些都还需要进一步的深入研究。
随着科学技术的进步,田间孢子监测,分子鉴定技术以及X 光扫描仪器、基因组学技术等可协助实现葡萄枝干病害的症状形成机制的深入研究。可以从以下几个方面考虑:
1)田间孢子监测,分子鉴定技术以及X 光扫描仪器可以辅助定量田间孢子量和判断病原菌是否成功的侵入;2)真菌致病性和毒性的细胞和遗传学知识可以在很大程度上推动葡萄枝干病害的研究深入,明确病原菌在枝干病害发生发展过程中的作用,明确其致病机制,为控制该类病害流行提供不同的视角和策略;3)基因组学技术因其在病原菌致病机制和优异种质资源挖掘方面被广泛研究,该技术也可应用于病原菌重要致病因子解析或筛选对葡萄枝干病害具有抗性的遗传位点或材料研究上,通过改良树体自身抗性来抑制枝干病害的发展。
在葡萄枝干病害的防控技术上,笔者总结了目前已实施的一些措施,包括提前或推迟剪枝时间、热水处理苗木、在伤口处喷洒保护剂/杀菌剂避免病原菌与伤口的直接接触。使用的保护剂/杀菌剂不少还处在试验阶段,还没有针对枝干病害的公认高效药剂。目前多以三唑类杀菌剂(Triazole Fungicides)、甲氧基丙烯酸酯类杀菌剂(Strobilurins)和苯并咪唑类杀菌剂(Benzimidazole fungicides)进行防治。但葡萄枝干病害的发病周期长,且伤口可能一直处于对病原菌敏感的状态,被喷施的保护剂/杀菌剂起到的作用也是暂时的,还需要有更高效的防控措施用于控制枝干病害的发展。近几年,很多研究者以生防真菌木霉属的菌株(Trichoderma spp.)开展了对葡萄枝干病害防治的研究,利用微生物种间或种内的抗生和竞争诱导植物产生抗病性。
笔者认为可以推动免疫诱抗控害技术在葡萄枝干病害上的应用,通过研究寄主作物对病原物的免疫识别机制,找到可以诱导或激活葡萄自身产生葡萄枝干病害病原菌的抗性物质,为该类病害提供绿色高效药剂将成为可能。
[1]HOFSTETTER V,BUYCK B,CROLL D,VIRET O,COULOUX A,GINDRO K. What if esca disease of grapevine were not a fungal disease?[J].Fungal Diversity,2012,54(1):51-67.
[2]叶清桐,李亚萌,周悦妍,李兴红,张玮,孙倩,韩昌坪,燕继晔.国内外葡萄枝干病害的发生危害与病原菌种类[J].果树学报,2021,38(2):278-292.YE Qingtong,LI Yameng,ZHOU Yueyan,LI Xinghong,ZHANG Wei,SUN Qian,HAN Changping,YAN Jiye. Occurrence of grapevine trunk diseases caused by fungal pathogens in the domestic and overseas[J]. Journal of Fruit Science,2021,38(2):278-292.
[3]SURICO G.Towards a redefinition of the diseases within the esca complex of grapevine[J]. Phytopathologia Mediterranea,2009,48(1):5-10.
[4]LEAVITT G M,MUNNECKE D E. The occurrence,distribution,and control of Botryodiplodia theobromae on grapes (Vitis vinifera)in California[J].Phytopathology,1987,77(2):1690.
[5]LEHOCZKY J. Black dead arm disease of grapevine caused by Botryosphaeria stevensii infection[J]. Acta Phytopathologica Acadmiae Scientiarum Hungaricae,1974,9(3/4):319-327.
[6]YAN J X,XIE Y,YAO S W,WANG Z Y,LI X H.Characterization of Botryosphaeria dothidea,the causal agent of grapevine canker in China[J]. Australasian Plant Pathology,2012,41(4):351-357.
[7]HALLEEN F,FOURIE P H,CROUS P W.Control of black foot disease in grapevine nurseries[J].Plant Pathology,2007,56(4):637-645.
[8]LARIGNON P,FULCHIE R,CERE L,DUBOS B. Observations on black dead arm in French vineyards[J]. Phytopathology Mediterranean,2001,40(3):336-342.
[9]AGUSTÍ-BRISACH C,ARMENGOL J. Black-foot disease of grapevine:An update on taxonomy,epidemiology and management strategies[J]. Phytopathologia Mediterranea,2013,52(2):245-261.
[10]FISCHER M,SCHNEIDER P,KARAUS C,MOLNAR M,DUBOIS C,D’AGUIAR D,HAAG N. Grapevine trunk disease in German viticulture:occurrence of lesser known fungi and first report of Phaeoacremonium viticola and P. fraxinopennsylvanicum[J]. Vitis Journal of Grapevine Research,2016,55(4):145-156.
[11]MARTIN M T,MARTIN L,DE-FRANCISCO M T,COBOS R.First report of Lasiodiplodia theobromae and Cryptovalsa ampelina associated with grapevine decline from Castillay Leon,Spain[J].Plant Disease,2009,93(5):545.
[12]LAWRENCE D P,TRAVADON R,BAUMGARTNER K. Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in northern California[J]. Mycologia,2015,107(5):926-940.
[13]RENAUD T,DANIEL P L,SUZANNE R L,WALTER D G,WAYNE F W,PHILIPPE E R. Cadophora species associated with wood-decay of grapevine in north America[J].Fungal Biology,2015,119(1):53-66.
[14]ROLSHAUSEN P E,BAUMGARTNER K,TRAVADON R,FUJIYOSHI P,POUZOULET J,WILCOX W F. Identification of Eutypa spp. causing Eutypa dieback of grapevine in eastern North America[J].Plant Disease,2014,98(4):483-491.
[15]ÚRBEZ-TORRES J R.The status of Botryosphaeriaceae species infecting grapevines[J]. Phytopathologia Mediterranea,2011,50(S):5-45.
[16]PASCOE I,COTTRAL E.Developments in grapevine trunk diseases research in Australia[J]. Phytopathologia Mediterranea,2000,39(1):68-75.
[17]PITT W M,TROUILLAS F P,GUBLER W D,SAVOCCHIA S,SOSNOWSKI M R. Pathogenicity of Diatrypaceous fungi on grapevines in Australia[J].Plant Disease,2013,97(6):749-756.
[18]SAVOCCHIA S,GREEA L A,STEL C C. First report of Phomopsis viticola causing bunch rot of grapes in Australia[J].Plant Pathology,2007,56(4):725.
[19]VAN NIEKERK J,CROUS P W,GRONENEWALD J Z,FOURIZ P H,HALLEEN F. DNA phylogeny,morphology and pathogenicity of Botryosphaeria species on grapevines[J].Mycologia,2004,96(4):781-798.
[20]CLOETE M,FISCHER M,MOSTERT L,HALLEEN F.A novel Fomitiporia species associated with esca on grapevine in South Africa[J].Mycological Progress,2014,13(2):303-311.
[21]CROUS P W,PHILLIPS A J L,BAXTER A P. Phytopathogenic fungi from South Africa[D]. Stellenbos:University of Stellenbosch Printers/Department of Plant Pathology Press,2000.
[22]DHANUSHKA U,LISA A C,AMY Y R,EKACHAI C,KEVIN D H.Insights into the genus Diaporthe:Phylogenetic species delimitation in the D. eres species complex[J]. Fungal Diversity,2014,67(1):203-229.
[23]MOYO P,MOSTERT L,SPIES C F,DAMM U,HALLEEN F.Diversity of Diatrypaceae species associated with dieback of grapevines in South Africa,with the description of Eutypa cre-mea sp.nov[J].Plant Disease,2018,102(1):220-230.
[24]SURICO G,MUGNAI L,MARCHI G. Older and more recent observations on Esca:A critical overview[J]. Phytopathologia Mediterranea,2006,45(4):68-86.
[25]CARLUCCI A,CIBELLT F,LOPAS F,RAIMONDO M L.Characterization of Botryosphaeriaceae species as causal agents of trunk disease on grapevines[J]. Plant Disease,2015,99(12):1678-1688.
[26]CARLUCCI A,LOPS F,MOSTERT L,HALLEEN F,RAIMONDO M L. Occurrence fungi causing black foot on young grapevines and nursery rootstock plants in Italy[J]. Phytopathologia Mediterranea,2017,56(1):10-39.
[27]CROUS P W,GAMS W,WINGFIELD M J,WYK P S V. Phaeoacremonium gen.nov.associated with wilt and decline diseases of woody hosts and human infections[J].Mycologia,1996,88(5):786-796.
[28]LARDNER R,STUMMER B E,SOSNOWSKI M R,SCOTT E S.Molecular identification and detection of Eutypa lata in grapevine[J].Mycological Research,2005,109(7):799-808.
[29]YAN J Y,XIE Y,ZHANG W,WANG Y,LIU J K,HYDE K D,SEEM R C,ZHANG G Z,WANG Z Y,YAO S W,BAI X J,DISSANAYAKE A J,PENG Y L,LI X H. Species of Botryosphaeriaceae involved in grapevine dieback in China[J]. Fungal Diversity,2013,61:221-236.
[30]YE Q T,MANAWASINGHE I,ZHANG W,MUGNAL L,HYDE K,LI X H,YAN J Y. First report of Phaeoacremonium minimum associated with grapevine trunk diseases in China[J].Plant Disease,2020,104(4):1259.
[31]李华.葡萄顶枯病(Eutypa lata)及其合理防治[J].中外葡萄与葡萄酒,2003(4):41-42.LI HUA. Eutypa dieback of grape and its reasonable control[J].Sino-Overseas Grapevine and Wine,2003(4):41-42.
[32]MANAWASINGHE I S,DISSANAYAKE A J,LI X H,LIU M,WANASINGHE D N,XU J P,ZHAO W S,ZHANG W,ZHOU Y Y,HYDE K D,BROOKS S,YAN J Y.High genetic diversity and species complexity of diaporthe associated with grapevine dieback in China[J].Frontiers in Microbiology,2019,10:1936.
[33]MIROSLAV B,JOSEP A,VĚRA H,JAKUB P,FRANCESCO C,ELIŠKA P,MIROSLAV V A E. Incidence of symptoms and fungal pathogens associated with grapevine trunk diseases in Czech vineyards:First example from a north-eastern European grape-growing region[J]. Phytopathologia Mediterranea,2018,57(3):449-458.
[34]PECENKA J,EICHMEIER,A P. First report of dactylonectria torresensis causing black- foot disease on grapevines in the Czech Republic[J].Plant Disease,2018,102(10):2038-2039.
[35]ALANIZ S,AGUSTÍBRISACH C,GRAMAJE D,AGUILAR M I,PÉREZSIERRA A A J. First report of Campylocarpon fasciculare causing black foot disease of grapevine in Turkey[J].Plant Disease,2014,98(9):1277.
[36]DISSANAYAKE A J,PHILLIPS A J L,LI X H.Botryosphaeriaceae:Current status of genera and species[J]. Mycosphere,2016,7(7):1001-1073.
[37]ESSAKHI S,MUGNAI L,CROUS P W,GROENEWALD J Z,SURICO G.Molecular and phenotypic characterization of novel Phaeoacremonium species isolated from esca diseased grapevines[J].Persoonia,2008,21(1):119-134.
[38]MOSTERT L,GROENEWALD J Z,SUMMERBELL R C,GAMS W,CROUS P W. Taxonomy and pathology of Togninia(Diaporthales) and its Phaeoacremonium anamorphs[J]. Studies in Mycology,2006,54(54):1-113.
[39]PHILLIPS A J L. The relationship between Diaporthe perjuncta and Phomopsis viticola on grapevines[J]. Mycologia,1999,91(6):1001-1007.
[40]KAMILA C C,MARCOS P S C,MARIA A G,SALES R. Fungal trunk pathogens associated with table grape decline in Northeastern Brazil[J]. Phytopathologia Mediterranea,2013,52(2):380-387.
[41]MENDES M A S,DA SILVA V L,DIANESE J C,FERREIRA M,SANTOS D,CUNHA-NETO E,URBEN A F,CASTRO J.Fungos em plants no brasil[M].Brasilia:Embrapa-SPI/Embrapa-Cenargen,1998.
[42]FERENTINOS K P,KATSOULAS N,TZOUNIS A,BARTZANAS T,KITTAS C. Wireless sensor networks for greenhouse climate and plant condition assessment[J].Biosystems Engineering,2017,153:70-81.
[43]ÚRBEZ-TORRES J R,HAAG P,O’GORMAN D T.Grapevine trunk diseases in British Columbia:incidence and characterization of the fungal pathogens associated with esca and petri diseases of grapevine[J].Plant Disease,2014,98(4):469-482.
[44]DIAZ G A,PREHN D,LATORRWE B A. First report of Cryptovalsa ampelina and Eutypella leprosa associated with grapevine trunk diseases in Chile[J].Plant Disease,2011,95(4):490.
[45]FISCHER J,COMPANT S,PIERRON R J G,GORFER M,JACQUES A,BERGER E,THINES H. Differing alterations of two Esca associated fungi,Phaeoacremonium aleophilum and Phaeomoniella chlamydospora on transcriptomic level,to cocultured Vitis vinifera L. calli[J]. PLoS One,2016,11(9):e0163344.
[46]VAN NIEKERK J,FOURIE P H,HALLEEN F,CROUS P W.Botryosphaeria spp. as grapevine trunk disease pathogens[J].Phytopathologia Mediterranea,2006,45(4):43-54.
[47]ABREO E,LUPO S,BETTUCCI L. Fungal community of grapevine trunk diseases:A continuum of symptoms[J]. Sydowia,2012,64(1):1-12.
[48]ABREO E,MARTINEZ S,BETTUCCI L,LUPO S. Characterization of Botryosphaeriaceae species associated with grapevines in Uruguay[J].Australasian Plant Pathology,2013,42(3):241-249.
[49]CLOETE M,FISCHER M,MOSTERT L,HALLEEM F.Hymenochaetales associated with esca- related wood rots on grapevine with a special emphasis on the status of esca in South African vineyards[J]. Phytopathologia Mediterranea,2015,54(2):299-312.
[50]MOHAMMADI H,BANIHASHEMI Z,GRAMAJE D,ARMENGOL J. Fungal pathogens associated with grapevine trunk diseases in Iran[J].Journal of Agricultural Science and Technology,2013,15(1):137-150.
[51]RAJCHENBERG M,ROBLEDO G. Pathogenic polypores in Argentina[J].Forest Pathology,2013,43(3):171-184.
[52]BASKARATHEVAN J,JASPERS M V,JONES E E,RIDGWAY H J. Distribution of botryosphaeria species causing grapevine dieback and decline in New Zealand vineyards[J]. New Zealand Plant Protection,2008,61:392.
[53]GRAHAM A B,JOHNSTN P R,WEIR B S.Three new Phaeoacremonium species on grapevines in New Zealand[J]. Australasian Plant Pathology,2009,38(5):505-513.
[54]AIGOUN-MOUHOUS W,ELENA G,CABRAL A,LEÓN M,SABAOU N,ARMENGOL J.Characterization and pathogenicity of cylindrocarpon-like asexual morphs associated with black foot disease in Algerian grapevine nurseries,with the description of Pleiocarpon algeriense sp. nov.[J]. European Journal of Plant Pathology,2019,154(4):887-901.
[55]AMMAD F,BENCHABANE M,TOUMI M,BELKACEM N,GUESMI A,AMEUR C.Occurrence of Botryosphaeriaceae species associated with grapevine dieback in Algeria[J]. Turkish Journal of Agriculture&Forestry,2014,38(6):865-876.
[56]BERRAF-TEBBAL A,BOUZNAD Z,SANTOS J M,COELHO M A,PHILLIPS A J L. Phaeoacremonium species associated with Eutypa dieback and esca of grapevines in Algeria[J].Phytopathologia Mediterranea,2011,50(4):86-97.
[57]GUARNACCIA V,GROENEWALD J Z,WOODHALL J,ARMENGOL J,CINELLI T,EICHMEIER A. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe[J].Persoonia,2018,40(1):135-153.
[58]KALITERNA J,MILICEVIC T,BENCIC D,URALIJA B. First report of Neofusicoccum parvum associated with grapevine trunk diseases in Croatia[J].Plant Disease,2013,97(12):1656.
[59]PANTIDOU M E.Fungus-host index for Greece[M].Greece:Kiphissia,Athens,Benaki Phytopathological Institute,1973.
[60]ZERVAKIS G,DIMOU D,BALIS C.A check-list of the Greek macrofungi including hosts and biogeographic distribution:I.Basidiomycotina[J].Mycotaxon,1998,66:273-336.
[61]CASIERI L,HOFSTETTER V,VIRET O,GINDRO K. Fungal communities living in the wood of different cultivars of young Vitis vinifera plants[J]. Phytopathologia Mediterranea,2009,48(1):73-83.
[62]HORVATH A,SCHWEIGHARDT L. The causes of vine stock decline and experiences of grapevine rejuvenation in Neszmely[J].Novenyvedelem,1991,27(2):83-87.
[63]PAOLINELLI-ALFONSO M,SERRANO-GOMEZ C,HERNANDEZ-MARTINEZ R. Occurrence of Eutypella microtheca in grapevine cankers in Mexico[J]. Phytopathologia Mediterranea,2015,54(1):86-93.
[64]KAKALIKOVA L,JANKURA E,ROBAROVA A. Phaeomoniella chlamydospora:Causal agent of vine decline(Vitis vinifera)in the vineyards of Slovakia[J]. Plant Pathology,2006,55(6):815.
[65]HALEEM R A,ABDULLAH S K,JUBRAEL J M S.Identification and pathogenicity of Botryosphaeria parva associated with grapevine decline in Kurdistan region- Iraq[J].Acta Agrobotanica,2012,65(1):71-78.
[66]ZIVKOVIC S,VASIC T,ANDELKOVIC S,JEVREMOVIC D,TRKULJA V. Identification and characterization of Eutypa lata on grapevine in Serbia[J].Plant Disease,2012,96(6):913.
[67]ROMERO-RIVAS L C,ÁLVAREZ L A,GRAMAJE D,ARMENGOL J.First report of Phaeoacremonium parasiticum causing petri disease of grapevine in Peru[J].Plant Disease,2009,93(2):200.
[68]KOBAYASHI T. Index of fungi inhabiting woody plants in Japan[M]. Tokyo, Japan:Zenkoku-Noson-Kyoiku Kyokai Publishing,2007.
[69]GUERIN-DUBRANA L,FONTAINE F,MUGNAI L. Grapevine trunk disease in European and Mediterranean vineyards:Occurrence,distribution and associated disease-affecting cultural factors[J]Phytopathology,2019,58(1):49-71.
[70]CLAVERIE M,NOTARO M,FONTAINE F. Current knowledge on grapevine trunk diseases with complex etiology:a systemic approach[J]. Phytopathologia Mediterranea,2020,59(1):29-53.
[71]MUGNAI L,GRANITI A,SURICO G. Esca (Black Measles)and brown wood-streaking:Two old and elusive diseases of grapevines[J].Plant Disease,1999,83(5):404-418.
[72]SCHOLEFIELD P,MORISON J.Assessment of economic cost of endemic pests&diseases on the Australian[R].Final report to the grape and wine research and development corporation,GWRDC project,2010.
[73]BILLONES- BAAIJENS R,RIDGWAY H J,JONES E E,CRUICKSHANK R H J.Prevalence and distribution of Botryosphaeriaceae species in New Zealand grapevine nurseries[J]. European Journal of Plant Pathology,2013,135(1):175-185.
[74]VAN NIEKERK J M,BESTER W,HALLEEN F,CROUS P W,FOURIE P H. The distribution and symptomatology of grapevine trunk disease pathogens are influenced by climate[J].Phytopathologia Mediterranea,2011,50(S):98-111.
[75]李华,李茹一,王华.酿酒葡萄新病害:葡萄顶枯病[J].酿酒科技,2007(5):48-50.LI Hua,LI Ruyi,WANG Hua. New disease for wine:making grape-eutypa dieback[J].Liquor-Making Science&Technology,2007(5):48-50.
[76]LI X H,YAN J Y,KONG F F,QIAO G,ZHANG Z W,WANG Z Y. Botryosphaeria dothidea causing canker of grapevine newly reported in China[J].Plant Pathology,2010,59(6):1170.
[77]ROLSHAUSEN P E,ÚRBEZ- TORRES J R,ROONEY-LATHAM S,ESKALEN A,SMITH R J,GUBLER W D.Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases[J].American Journal of Enology and Viticulture,2010,61(1):113-119.
[78]ANDOLFI A,MUGNAI L,LUQUE J,SURICO G,CIMMINO A,EVIDENTE A. Phytotoxins produced by fungi associated with grapevine trunk diseases[J]. Toxins(Basel),2011,3(12):1569-1605.
[79]FISCHER M.A new wood-decaying basidiomycete species associated with esca of grapevine[J]. Fomitiporia Mediterranea,2002,1(3):314-324.
[80]CARTER M V.The status of Eutypa lata as a pathogen[M].Wallingford:Commonwealth Agricultural Bureau International,1991.
[81]VAN NIEKERK J M,GROENEWALD J Z,FARR D F,FOURIE P H,HALLEER F,CROUS P W.Reassessment of Phomopsis species on grapevines[J].Australasian Plant Pathology,2005,34(1):27-39.
[82]BLEACH C M. Management of Cylindrocarpon black foot disease in New Zealand nurseries and vineyards[D].Christchurch:Lincoln University,2012.
[83]YE Q,ZHANG W,JIA J,LI X,ZHOU Y,HAN C,WU X,YAN J Y. Fungal pathogens associated with black foot of grapevine in China[J]. Phytopathologia Mediterranea,2021,60(2):303-319.
[84]ESKALEN A,GUBLER W D.Association of spores of Phaeomoniella chlamydospora,Phaeoacremonium inflatipes,and Pm.aleophilum with grapevine cordons in California[J].Phytopathologia Mediterranea,2001,40(3):429-431.
[85]VALTAUD C,LARIGNON P,ROBLIN G,FLEURAT-LESSARD P. Developmental and ultrastructural features of Phaeomoniella chlamydospora and Phaeoacremonium aleophilum in relation to xylem degradation in esca disease of the grapevine[J].Journal of Plant Pathology,2009,91(1):37-51.
[86]LARIGNON P,DUBOS B. Fungi associated with esca disease in grapevine[J]. European Journal of Plant Pathology,1997,103(2):147-157.
[87]PEDRO W C,WALTER G. Phaeomoniella chlamydospora gen.et comb. nov.,a causal organism of Petri grapevine decline and esca[J].Phytopathologia Mediterranea,2000,39:112-118.
[88]WHITING E C,KHAN A,GUBLER W D. Effect of temperature and water potential on survival and mycelial growth of Phaeomoniella chlamydospora and Phaeoacremonium spp.[J]. Plant Disease,2001,85(2):195-201.
[89]BÉNIGNE ERNEST A,STÉPHANE O,GABRIEL R. Influence of temperature and nutritional requirements for mycelial growth of Eutypa lata,a vineyard pathogenic fungus[J]. Comptes Rendus-Biologies,2005,328(3):263-270.
[90]QIU Y,STEEL C C,ASH G J,SAVOCCHIA S. Effects of temperature and water stress on the virulence of Botryosphaeriaceae spp. causing dieback of grapevines and their predicted distribution using CLIMEX in Australia[J].Acta Horticulturae,2016,1115:171-181.
[91]ÚRBEZ- TORRES J R,LEAVITT G M,VOEGEL T M,GUBLER W D. Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California[J]. Plant Disease,2006,90(12):1490-1503.
[92]COPES W E,HENDRIX J F F.Effect of temperature on sporulation of Botryosphaeria dothidea,B. obtusa,and B. rhodina[J].Plant Disease,2004,88(3):292-296.
[93]BELLÉE A,COMONT G,NIVAULT A. Life traits of four Botryosphaeriaceae species and molecular responses of diferent grapevine cultivars or hybrids[J]. Plant Pathology,2017,66(5):763-776.
[94]HALLEEN F,FOURIE P H,CROUS P W. A review of black foot disease of grapevine[J]. Phytopathologia Mediterranea,2006,45:54-67.
[95]BRACCINI P,CALZARANO F,DI MARCO S. Relation of esca foliar symptoms to rainfall and rainfall-related parameters[J].Phytopathologia Mediterranea,2005,44:107.
[96]DEL FRARI G,GOBBI A,AGGERBECK M R,OLIVEIRA H,HANSEN L H,FERREIRA R B. Characterization of the wood mycobiome of Vitis vinifera in a vineyard affected by esca. Spatial distribution of fungal communities and their putative relation with leaf symptoms[J].Frontiers in Plant Science,2019,10:910.
[97]JUNGES A H,ALMANÇA M A K,FAJARDO T V M,DUCATI J R. Leaf hyperspectral reflectance as a potential tool to detect diseases associated with vineyard decline[J]. Tropical Plant Pathology,2020,45(5):522-533.
[98]MARTOS S,ANDOLFI A,LUQUE J,MUGNAI L,SURICO G,EVIDENTE A. Production of phytotoxic metabolites by five species of Botryosphaeriaceae causing decline on grapevines,with special interest in the species Neofusicoccum luteum and N.parvum[J]. European Journal of Plant Pathology,2008,121(4):451-461.
[99]NERVA L,ZANZOTTO A,GARDIMAN M,GAIOTTI F,CHITARRA W.Soil microbiome analysis in an ESCA diseased vineyard[J].Soil Biology and Biochemistry,2019,135:60-70.
[100]OUADI L,BRUEZ E,BASTIEN S,VALLANCE J,LECOMTE P,DOMEC J C,REY P. Ecophysiological impacts of Esca,a devastating grapevine trunk disease,on Vitis vinifera L.[J].PLoS One,2019,14(9):1-20.
[101]SPARAPANO L,BRUNO G,GRANITI A. Effects on plants of metabolites produced in culture by Phaeoacremonium chlamydosporum,P.aleophilum and Fomitiporia punctata[J].Phytopathologia Mediterranea,2000,39:169-177.
[102]GÓMEZ P,BÁIDEZ A G,ORTUÑO A,DEL RÍO J A. Grapevine xylem response to fungi involved in trunk diseases[J].Annals of Applied Biology,2016,169(1):116-124.
[103]CARTER M V. Eutypa dieback[M]//Compendium of Grape Diseases,Minnesota:APS Press,1988.
[104]MOLLER W J,CARTER M V.Production and dispersal of asco-spores in Eutypa armeniacae[J]. Australian Journal of Biological Sciences 1965,18(1):67-80.
[105]PEARSON R C. Discharge of ascospores of Eutypa armeniacae in New York[J].Plant Disease,1980,64(2):171-174.
[106]RUDELLE J,OCTAVE S,KAID-HARCHE M,ROBLIN G,FLEURAT-LESSARD P. Structural modifications induced by Eutypa lata in the xylem of trunk and canes of Vitis vinifera[J].Functional Plant Biology,2005,32(6):537-547.
[107]SOSNOWSKI M R,LOSCHIAVO A P,WICKS T J,SCOTT E S.Evaluating treatments and spray application for the protection of grapevine pruning wounds from infection by Eutypa lata[J].Plant Disease,2013,97(12):1599-1604.
[108]AMPONSAH N T,JONES E E,RIDGWAY H J,JASPERS M V. Rainwater dispersal of Botryosphaeria conidia from infected grapevines[J].New Zealand Plant Protection,2009,62:228-233.
[109]ÚRBEZ-TORRES J R,BATTANY M,BETTIGA L J,GISPERT C M G,RONCORONI J,SMITH R J,VERDEGAAL P.Botryosphaeriaceae species spore-trapping studies in California vineyards[J].Plant Disease,2010,94(6):717-724.
[110]KUNTZMANN P,VILLAUME S,BERTSCH C. Conidia dispersal of Diplodia species in a French vineyard[J].Phytopathologia Mediterranea,2009,48(1):150-154.
[111]VAN NIEKERK J M,CALITZ F J,HALLEEN F,FOURIE P H.Temporal spore dispersal patterns of grapevine trunk pathogens in South Africa[J]. European Journal of Plant Pathology,2010,127(3):375-390.
[112]VALENCIA D,TORRES C,CAMPS R,LÓPEZ E,CELISDIEZ J,BEOSAIN X.Dissemination of Botryosphaeriaceae conidia in vineyards in the semiarid Mediterranean climate of the Valparaíso region of Chile[J]. Phytopathologia Mediterranea,2015,54:394-402.
[113]SHAFI A,RIDGWAY H J,JASPERS M V,JONES E E.Conidial production of Botryosphaeriaceae species from grapevine shoot lesions in Marlborough vineyards[J]. New Zealand Plant Protection,2017,70:295-300.
[114]AMPONSAH N T,JONES E E,RIDGWAY H J,JASPERS M V. Identification,potential inoculum sources and pathogenicity of Botryosphaeriaceous species associated with grapevine dieback disease in New Zealand[J]. European Journal of Plant Pathology,2011,131(3):467-482.
[115]DISSANAYAKE A J,LIU M,ZHANG W,CHEN Z,UDAYANGA D,CHUKEATIROTE E,LI X H,YAN J Y,HYDE K D.Morphological and molecular characterisation of Diaporthe species associated with grapevine trunk disease in China[J]. Fungal Biology,2015,119(5):283-294.
[116]ESKALEN A,FABER B,BIANCHI M. Spore trapping and pathogenicity of fungi in the Botryosphaeriaceae and DiaporthaceaeassociatedwithavocadobranchcankerinCalifornia[J].Plant Disease,2013,97(3):329-332.
[117]PETIT E,BARRIAULT E,BAUMGARTNER K,WILCOX W F,ROLSHAUSEN P E. Cylindrocarpon species associated with black-foot of grapevine in northeastern United States and southeastern Canada[J].American Journal of Enology and Viticulture,2011,62(2):177-183.
[118]SWEETINGHAM M. Studies on the nature and patho-genicity of soilborne Cylindrocarpon spp.[D]. Hobart:University of Tasmania,1983.
[119]CARDOSO M,DINIZ I,CABRAL A,REGO C,OLIVEIRA H.Unveiling inoculum sources of black foot pathogens in a commercial grapevine nursery[J]. Phytopathologia Mediterranea,2013,52(2):298-312.
[120]AROCA A,RAPOSO R. Occurrence of fungi associated with Petri disease in bench-grafted vines[C]//Setllenbosch,South Africa:4th International Workshop on Grapevine Trunk Diseases,2005.
[121]WEBER E,TROUILLAS F,GUBLER W. Double pruning of grapevines:A cultural practice to reduce infections by Eutypa lata[J].American Journal of Enology and Viticulture,2007,58(1):61-66.
[122]PASCAL L, BARKA D, CARBONNEAU A, PATRICE R,CHRISTEL C. Esca of grapevine and training practices in France:Results of a 10-year survey[J].Phytopathologia Mediterranea,2015,57(3):472-487.
[123]KRAUS C,VOEGELE R T,FISCHER M.The Esca complex in German vineyards:Does the training system influence occurrence of GLSD symptoms? [J]. European Journal of Plant Pathology,2019,155(1):265-279.
[124]HILLIS V,LUBELL M,KAPLAN J,DOLL D,BAUMGARTNER K. The role of pest control advisers in preventative management of grapevine trunk diseases[J]. Phytopathology,2016,106(4):339-347.
[125]BROWN A A,TRAVADON R,LAWRENCE D P,TORRES G,ZHUANG G,BAUMGARTNER K. Pruning-wound protectants for trunk- disease management in California table grapes[J].Crop Protection,2021,141:105490.
[126]KAPLAN J,TRAVADON R,COOPER M,HILLIS V,LUBELL M,BAUMGARTNER K K.Identifying economic hurdles to early adoption of preventative practices:The case of trunk diseases in California Winegrape vineyards[J]. Wine Economic Policy,2016,5(2):127-141.
[127]DÍAZ G A,LATORRE B A. Efficacy of paste and liquid fungicide formulations to protect pruning wounds against pathogens associated with grapevine trunk diseases in Chile[J]. Crop Protection,2013,46:106-112.
[128]MOUNIER E,BOULISSET F,CORTES F,CADIOU M,ESQUIVE W P. Esquive® WP limits development of grapevine trunk diseases and safeguards the production potential of vineyards[M]// Biocontrol of Major Grapevine Diseases:Leading Research,CABI,Wallingford:CABI,2016:160-170.
[129]PAJOT E,MOUNIER E,BLAL B,CORTES F,COUTANT A.Selection steps of the Trichoderma atroviride strain i-1237,and some practical results of the development of the fungi within the cankers diseases of grapevine and soil borne diseases of vegetables[C]// BARKER E A,CLEMENT C. IOBC,Reims,2012:24-27.
[130]REIS P,MAGNIN-ROBERT M,NASCIMENTO T,SPAGNOLO A,MANSOUR E,ABOU-CRISTINA F,CLÉMENT C,REGO C,FONTAINE F. Reproducing Botryosphaeria dieback foliar symptoms in a simple model system[J]. Plant Disease,2016,100(6):1071-1079.
[131]BIGOT G,FRECCERO A,MONTERMINI A,BORTOLOTTI P,NANNINI R.Impiego di Trichoderma contro il mal dell’esca della vite[J].L’Informatore Agrario,2015,6:62-66.
[132]YACOUB A,GERBORE J,MAGNIN N,CHAMBON P,DUFOUR M C,CORIO-COSTET M F,GUYONEAUD R,REY P.Ability of Pythium oligandrum strains to protect Vitis vinifera L.,by inducing plant resistance against Phaeomoniella chlamydospora,a pathogen involved in Esca,a grapevine trunk disease[J].Biological Control,2016,92:7-16.
[133]PETIT E,GUBLER W D. Influence of Glomus intraradices on black foot disease caused by Cylindrocarpon macrodidymum on Vitis rupestris under controlled conditions[J]. Plant Disease,2006,90(12):1481-1484.
[134]JOHN S,WICKS T J,HUNT J S,LORIMER M F,OAKEY H,SCOTT E S. Protection of grapevine pruning wounds from infection by Eutypa lata using trichoderma harzianum and fusarium lateritium[J].Plant Pathology,2005,34(3):569-575.
[135]MARTÍNEZ- DIZ M DEL P,DÍAZ- LOSADA E,DÍAZFERNÁNDEZ Á,BOUZAS-CID Y,GRAMAJE D. Protection of grapevine pruning wounds against Phaeomoniella chlamydospora and Diplodia seriata by commercial biological and chemical methods[J].Crop Protection,2020,143:105465.
[136]WAITE H,GRAMAJE D,WHITELAW-WECKERT M,TORLEY P,HARDIE W J. Soaking grapevine cuttings in water:A potential source of cross contamination by micro-organisms[J].Phytopathologia Mediterranea,2013,52(2):359-368.
[137]BERTSCH C,RAMÍREZ-SUERO M,MAGNIN-ROBERT M,LARIGNON P,CHONG J,ABOU-MANSOUR E,SPAGNOLO A,CLÉMENT C,FONTAINE F. Grapevine trunk diseases:complex and still poorly understood[J]. Plant Pathology,2013,62(2):243-265.
[138]LARIGNON P,FONTAINE F,FARINE S,CLEMENT C,BERTSCH C.Esca et black dead arm:Deux acteurs majeurs des maladies du bois chez la vigne[J]. Comptes Rendus Biologies,2009,332(9):765-783.
[139]GRAMAJE D,GARCIA-JIMENEZ J,ARMENGOL J. Field evaluation of grapevine rootstocks inoculated with fungi associated with Petri disease and Esca[J].American Journal of Enology and Viticulture,2010,61(4):512-520.
[140]ELENA G,DI BELLA V,ARMENGOL J,LUQUE J. Viability of Brotyosphaeriacea species pathogenic to grapevine after hot water treatment[J]. Phytopathologia Mediterranea,2015,54(2):325-334.
[141]CREASER M L,WICKS T J.Short-term effects of remedial surgery to restore productivity to Eutypa lata infected vines[J].Phytopathologia Mediterranea,2004,43(1):105-107.
[142]SOSNOWSKI M R,LARDNER R,WICKS T J,SCOTT E S.The influence of grapevine cultivar and isolate of Eutypa lata on wood and foliar symptoms[J]. Plant Disease,2007,91(8):924-931.
[143]HAUSTEIN M,BECKER A,KORTEKAMP A.Einfluss der Rebengattung auf die intraorganismische Ausbreitung stammassoziierter GTD-Pathogene und Bedeutung für die Langlebigkeit[J].Julius-Kühn-Archiv,2016,454:124-125.
[144]CALZARANO F,DI MARCO S,CESARI A. Benefit of fungicide treatment after trunk renewal of vines with different types of esca necrosis[J]. Phytopathologia Mediterranea,2004,43(1):116-124.
[145]GUILLAUME D,PASCAL L. Evaluation of a trunk injection technique to control grapevine wood diseases[J]. Phytopathology Meditate,2007,46(1):50-57.
[146]MONDELLO V,LARIGNON P,ARMENGOL J,KORTEKAMP A,VACZY K,PREZMAN F,SERRANO E,REGO C,MUGNAI L,FONTAINE f.Management of grapevine trunk diseases:Knowledge transfer,current strategies and innovative strategies adopted in Europe[J]. Phytopathologia Mediterranea,2018,57(3):369-383.
[147]HARMAN G E. Overview of mechanisms and uses of Trichoderma spp.[J].Phytopathology,2006,96(2):190-194.
[148]MOLNAR M,FISCHER R T,VOEGELE M. Grapevine trunk diseases in German viticulture IV.spreading of spores of Phaeomoniella chlamydospora in Esca- affected vineyards[J]. Vitis Geilweilerhof,2020,59(2):63-69.
[149]LARIGNON P,DUBOS B. Preliminary studies on the biology of Phaeoacremonium[J]. Phytopatologia Mediterranea,2000,39(1):184-189.
[150]SERRA S,MANNONI M A,LIGIOS V.Studies on the susceptibility of pruning wounds to infection by fungi involved in grapevine wood diseases in Italy[J]. Phytopatologia Mediterranea,2008,47(3):234-246.