甜度作为水果风味的重要组成部分,在果实品质形成中扮演重要角色,也是影响水果经济效益和市场竞争力的关键。甜度主要由糖含量、糖酸比和糖的类型决定[1]。其中,可溶性糖含量和比例是果实品质的重要组成部分,主要受碳水化合物转运和代谢的高度调控。
在大部分植物中,光合同化物是以蔗糖为主要碳源形式进行运输的,而在蔷薇科果树中,山梨醇则是主要的运输形式[2]。在苹果、梨等果树中,山梨醇甚至占运输总碳流的60%~80%,它们分别被蔗糖转运蛋白(sucrose transporter,SUT)和山梨醇转运蛋白(sorbitol transporter,SOT)直接转运进入胞质中进行不同的代谢活动;或者蔗糖也可以被细胞壁转化酶(cell wall invertase,CWINV)分解为己糖,然后通过己糖转运蛋白(hexose transporter, HT)转运进入胞质中[3]。胞质中的可溶性糖一部分用于代谢,一部分通过液泡糖转运蛋白转运至液泡中储存起来,如ERDL6[4]等。研究表明,糖转运蛋白与果实中糖积累密切相关。Zhu等[4]的研究发现,过表达葡萄糖外排蛋白基因MdERDL6 可引起液泡中葡萄糖外排,促进葡萄糖和果糖在液泡中的积累。然而,转运蛋白介导的糖在液泡中的积累与细胞质中糖的可利用性调控有关,而胞质中糖的可利用性及其种类受糖代谢的高度调控。
糖代谢是植物整个生物代谢的中心,可以为生命活动提供能量和碳源。包括蔗糖代谢、山梨醇代谢和己糖代谢[5]。因此,了解果实糖代谢,对科学调控果实品质至关重要。笔者分别从糖的组成、糖代谢与糖含量的关系以及环境因素对糖积累的影响三部分进行综述,重点介绍了糖代谢酶和相关基因在果实糖含量调控中的关键作用以及糖信号响应不同环境变化的生理和生化过程,旨在为改良果实风味品质和提高果实经济效益提供理论参考。
1 糖的组成
果实中可溶性糖主要包括蔗糖、果糖和葡萄糖。三种糖的组成与比例是决定果实甜度的重要因素[6]。其中,果糖的甜度是蔗糖的1.73倍,而葡萄糖仅是蔗糖的0.74 倍。因此,水果变甜的原因可能与蔗糖、果糖和葡萄糖在液泡中的积累有关。
不同类型的园艺作物果实内占优势的糖组分不同,比如柑橘[7]主要的糖组分是蔗糖,而葡萄[8]的糖组分主要是葡萄糖。在苹果果实发育早期,碳水化合物主要以淀粉的形式存在;进入成熟后期,果糖含量约占可溶性糖的60%[9]。说明果糖在液泡中积累是苹果甜度形成的关键。
2 糖代谢与含量的关系
源叶中合成的光合产物是果实糖物质积累的重要来源,主要以山梨醇和蔗糖的形式通过韧皮部长距离运输,最后卸载进入果实内进行代谢(包括山梨醇代谢、蔗糖代谢和己糖代谢),最终以蔗糖、果糖和葡萄糖等形式分散在果实的不同部位,使得果实具有独特的风味品质。
2.1 山梨醇代谢
山梨醇是蔷薇科植物中较为特殊的碳水化合物,约占光合同化物的80%[10]。山梨醇在细胞质中合成,通过光合作用生成的磷酸丙糖(TP)穿过叶绿体膜进入到胞质中,经一系列的反应合成果糖-6-磷酸(Fru-6-phosphate, F6P),生成的F6P 在醛糖-6-磷酸还原酶(aldose-6-phosphate reductase,A6PR; EC 1.1.1.200)作用下最终催化生成山梨醇[11](图1)。

图1 源叶中蔗糖和山梨醇合成和韧皮部装载
Fig.1 Sucrose and sorbitol synthesis and phloem loading in source leaves
合成的山梨醇经长距离运输,通过胞间连丝以共质体方式卸载进入库细胞中进行代谢;或者通过山梨醇转运蛋白(sorbitol transporter,SOT)介导从筛管/伴胞复合体(SE-CC, sieve element/companion cell)中直接吸收进入库细胞中[2]。研究表明,苹果成熟果实中山梨醇含量仅占可溶性糖的3%~8%[12]。推测山梨醇在库细胞中可能被山梨醇脱氢酶(sorbitol dehydrogenase,SDH;EC1.1.1.14)催化生成果糖而进入碳代谢或在液泡中贮存。SDH 是植物山梨醇降解的主要酶,可以将山梨醇氧化生成甜度较高的果糖,在维持果实库强和糖分转化等过程中起关键作用[13]。目前,已从多种植物中分离、克隆并鉴定出了SDH基因[14-16]。Yamada等[17]首先从苹果果实中克隆了SDH 基因全长,其mRNA序列长度为1433 bp,编码379 个氨基酸。Li 等[14]进一步对苹果中山梨醇脱氢酶基因MdSDHs 进行mRNA 和蛋白水平研究,发现SDH 活性与蛋白含量变化一致,均在未成熟果实中高表达;同时,MdSDH1基因在花后112 d 出现峰值,暗示着SDH 活性可能在转录水平受到严格调控,并且这种调控作用在桃果实成熟阶段同样存在。最新研究表明,在苹果中降低山梨醇合成关键酶MdA6PR 的表达后,转基因苹果叶片中的山梨醇含量降低了近80%,蔗糖含量升高。在整个果实发育期,山梨醇输入的降低和蔗糖输入的增加显著提高了转基因苹果果实中葡萄糖含量[18]。相应的转基因苹果中山梨醇代谢下调(尤其SDH 和FRK),而蔗糖的分解能力上调,以补偿源自山梨醇的果糖减少量。在转基因苹果中,这种糖代谢和运输系统(TST表达上调)的变化导致了果糖和蔗糖含量的稳态,并且引起了葡萄糖积累。说明在含有山梨醇合成的蔷薇科植物果实中糖代谢具有明显的可选择性和灵活性,且蔗糖循环和糖转运在果实含量调控中起重要作用。
2.2 蔗糖代谢
蔗糖是高等植物光合作用的主要终产物,在植物生长发育、品质改良及逆境防御等过程中起重要作用。蔗糖及其代谢产物不仅为植物生长提供能量基础,而且具有信号分子功能,是细胞代谢的主要调控因子[19]。
叶片叶绿体固定CO2后,形成的磷酸丙糖(TP)穿过叶绿体膜进入细胞质,经过一系列的反应合成果糖-6-磷酸(Fru-6-phosphate,F6P)和UDP-葡萄糖(UDP-glucose, UDPG),最终在蔗糖磷酸合酶(sucphosphate synthase,SPS;EC 2.4.1.14)作用下合成蔗糖[5]。源叶中合成的蔗糖被转运至库器官中储存起来或者在细胞质中被分解用于能量代谢、淀粉合成及重新合成蔗糖。SPS 在蔗糖代谢中起重要作用,其活性与植物中淀粉和蔗糖积累的平衡有关[20]。研究表明,许多果实成熟过程中蔗糖积累与SPS 活性呈正相关。成熟杧果中,SPS 活性在蔗糖快速积累阶段增加了10 倍[21]。此外,编码SPS 基因的过量表达也会增加果实中碳水化合物的积累。利用反义转基因方法抑制甜瓜蔗糖合成关键酶CMSPS1基因的表达,成熟果实中SPS活性和蔗糖浓度显著降低[22]。
库器官中的蔗糖可以在蔗糖合成酶(sucrose synthase, SUSY; EC 2.4.1.13)催化作用下生成果糖和UDP-葡萄糖,或被胞质中性转化酶催化生成果糖和葡萄糖。液泡中的蔗糖可以在液泡酸性转化酶(vAINV)催化作用下生成果糖和葡萄糖[5](图2)。SUSY 可逆地催化蔗糖分解与合成,是蔗糖进入各种代谢途径所必需的关键酶之一。通常认为SUSY主要起分解蔗糖的作用[23]。目前,SUSY基因已在苹果[14]、桃[24]等果树中克隆得到,并且直接影响蔗糖的生理功能及代谢。沉默草莓中蔗糖合成酶Fasusy1的表达导致果实中SUSY 活性和蔗糖含量降低,推测fasusy1 下调表达导致韧皮部蔗糖转运能力受到抑制[25]。同时,SUSY 也可以作为衡量库强的一种“指示器”[26]。与其他蔗糖分解酶相比,SUSY参与调节胞质内外蔗糖浓度梯度,从而控制输入和储存在果实中可溶性糖含量。在番茄中降低蔗糖合成酶SUSY 的表达后,转基因番茄果实中蔗糖积累不受影响,猜测SUSY 可能在调节番茄果实库强中起重要作用[27]。最新研究表明,过表达MdFRK2 能够改变苹果中碳水化合物代谢,增加SUSY 的活性和蔗糖的裂解,促进碳水化合物有效地转化为生物质参与次生韧皮部的形成[28]。
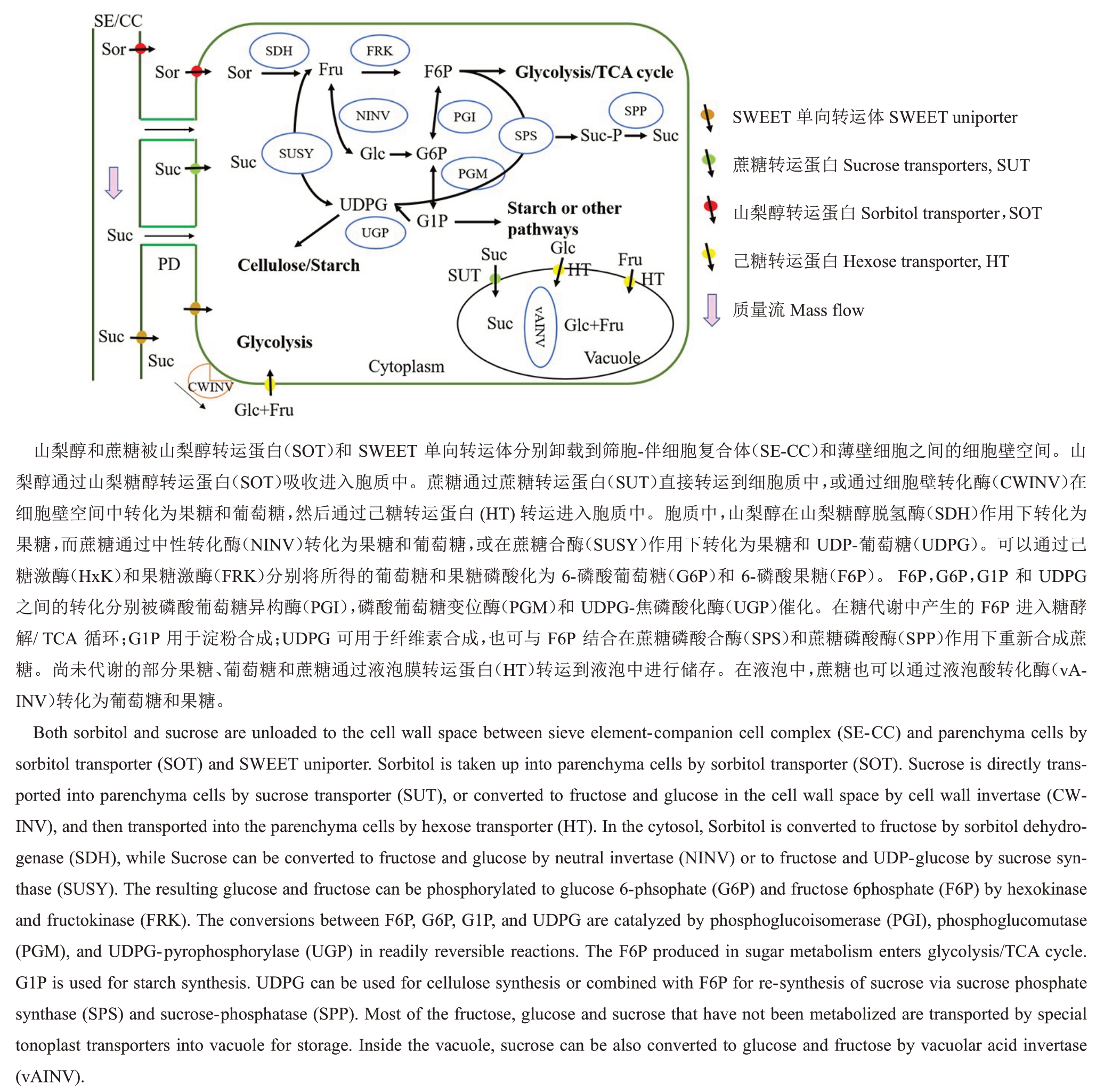
图2 韧皮部卸载和果实中糖代谢
Fig.2 Phloem unloading and sugar metabolism in fruits
转化酶不可逆地催化蔗糖水解产生果糖和葡萄糖,进而作为重要能源物质和信号分子在植物生长发育、胁迫响应、碳水化合物分配及源/库调节等过程中发挥重要作用[5]。转化酶根据其作用位置分为酸性转化酶(acid invertase,AINV)和胞质中性转化酶(neutral invertase, NINV; EC 3.2.1.26)两类。其中,酸性转化酶按其功能分为细胞壁转化酶(cell wall invertase, CWINV)和液泡酸性转化酶(vacuolar acid invertase,vAINV)两种。Wan 等[29]通过进化分析发现,两种酶由同一祖先进化而来,且CWINs的进化主要发生在维管植物的韧皮部中。CWINV通过保持源/库之间蔗糖浓度梯度在植物韧皮部卸载、碳分配和库发育等方面发挥重要功能[19]。研究表明,CWINV 主要在韧皮部中特异表达,被认为是质外体卸载的关键酶。在番茄幼果中,提高CWINV活性可以降低质外体空间的蔗糖浓度,促进蔗糖的质外体卸载[30]。最新研究表明,Wang等[31]在番茄中异源表达苹果己糖转运蛋白基因MdHT2.2,发现成熟果实中CWINV活性与其编码基因SlLIN5表达量显著增加可能与蔗糖含量的降低相关。说明己糖转运蛋白可通过调控质外体己糖信号来调控CWINV活性及转录水平,进而影响蔗糖运输分配和果实糖含量。vAINV 虽然与CWINV 由同一祖先进化而来,但前者在高等植物进化过程中被诸多因素限制,这种进化差异表明vAINVs主要在液泡中将蔗糖水解成葡萄糖和果糖,使蔗糖的渗透效应加倍,从而维持细胞内水和离子平衡[29]。另外,vAINV 也参与液泡中己糖积累与细胞膨大,为果实发育提供营养[32]。因此,果实中己糖的积累常伴随着高活性的vAINV。反义抑制vAINV活性,使得转基因番茄中蔗糖含量提高了5 倍,改变了果实中可溶性糖的组成[33],说明vAINV 活性可能受到了翻译转化酶基因表达的抑制,使得番茄果实中蔗糖大量积累。NINV可将细胞质中蔗糖分解产生果糖和葡萄糖[5]。并且,NINV在分解蔗糖过程中,使筛管-伴胞中的蔗糖浓度稳定地形成一个梯度,方便蔗糖卸载。因此,NINV 在库器官中表现出较高活性,参与库细胞中糖分组成、渗透调节和细胞增大。上述结果解析了蔗糖代谢关键酶及相关基因在不同果实中糖含量的差异调控,为利用分子手段调节果实中糖积累提供了重要的思路。
2.3 己糖代谢
多数植物中,光合产物主要以蔗糖形式在源-库之间转运,进入果实后仅有一半的蔗糖转化为果糖。而在苹果等蔷薇科果树中,叶片光合长距离运输的产物包括山梨醇和蔗糖,其中山梨醇占到近80%。经过长距离运输,山梨醇在苹果库细胞中经山梨醇脱氢酶(SDH)催化下生成果糖而进入碳代谢或在液泡中贮存,使得胞质中至少90%的总碳流以果糖形式进入代谢中[34]。
生成的果糖和葡萄糖在进入糖酵解途径之前会被磷酸化。其中,己糖激酶(hexokinase, HxK; EC 2.7.1.1)和果糖激酶(fructokinase, FRK; EC 2.7.1.4)分别是催化己糖磷酸化的两种关键酶[35]。图2 所示,果糖和葡萄糖分别在FRK和HxK作用下生成果糖-6-磷酸(F6P)和葡萄糖-6-磷酸(glucose 6-phsophate, G6P)。F6P、G6P、G1P 和UDPG 之间可以在酶的催化下发生可逆的相互转化。F6P进入糖酵解和TCA循环;G1P 被转运进入质体中用于淀粉合成;UDPG 可作为纤维素合成前体;F6P和UDPG在SPS 和SPP 作用下再合成蔗糖,储存在液泡中。研究表明,FRK 和HxK 两种酶都可以磷酸化果糖,但FRK 对果糖的亲和力远高于HxK,所以通常认为FRK是催化果糖磷酸化的关键酶,在果糖分解代谢中起关键作用[36]。
FRK是果糖代谢开始的“起点”,已从苹果[14]、柑橘[37]等多种物种中鉴定出具有不同动力学特性的FRK 基因。植物FRK 属于pfkB 碳水化合物激酶家族(phosphofructokinase B type family of carbohydratekinase),其氨基酸序列高度保守,含有FRK 家族特有的ATP 结合位点和底物识别结合域[38]。通常,FRK2同源基因对果糖的亲和力远高于其他FRK基因,在控制果糖利用和调节碳分配方面扮演了重要角色。通过RNAi干扰杨树中FRK2基因的表达,使得叶片中果糖、葡萄糖和蔗糖浓度增加,影响了碳水化合物转化为生物质参与次生木质部形成[39]。在苹果等蔷薇科果树中,由于山梨醇的存在,使得经过果糖代谢的碳流高于85%,远高于其他植物的50%,这就需要苹果库器官中拥有较强的FRK 活性以利用果糖。Yang 等[34]的研究发现,编码苹果中的果糖激酶(FRK)的MdFRK2基因在苹果库器官中高度表达,与苹果库器官中果糖的高效利用有关(苹果库器官中经过果糖的碳流占总碳流的80%以上,而大多数植物为50%)。与其他植物FRK2 相比,MdFRK2蛋白不仅对果糖亲和力高,而且过表达MdFRK2 能够改变苹果中碳水化合物代谢,增加SUSY 的活性和蔗糖的裂解,促进碳水化合物有效地转化为生物质参与次生韧皮部的形成[28]。苹果果糖激酶2(Md-FRK2)氨基酸序列具有pfkB 碳水化合物激酶家族的显著特征,包含高度保守的ATP 和糖结合域[34]。说明MdFRK2是苹果中果糖激酶蛋白的关键编码基因之一。研究发现,MdFRK2 和CuFRK2 分别在苹果[14]和柑橘[40]果实发育早期高表达,在成熟后期转录水平下降,与成熟期果实中果糖积累正好相反。说明果糖在果实中的积累和代谢受果糖激酶(FRK)活性及其转录水平的高度调控。Dai 等[41]反义表达番茄中SlFRK2 基因,发现果实中果糖含量显著提高。同时,番茄幼果中的果糖激酶活性与淀粉积累具有较强的相关性,推测FRK可能在淀粉积累过程中起重要作用。然而,番茄中反义表达LeFRK2 基因,幼果中淀粉合成并没有受到影响,可能与蔗糖含量增加有关[42]。上述结果表明了FRK具有调节果糖信号和果实中淀粉合成的能力,为改善果实甜度和提高果实品质等方面提供了理论依据。最新研究表明,坐果过程部分依赖于果糖激酶的活性,果糖激酶的诱导可以从液泡中释放果糖,从而控制胞质中的果糖浓度及比例,并为下游的生物合成途径提供营养[43]。
植物中HxK多数以二聚体形式存在,首次在拟南芥中证明了HxK是一个双功能酶,不仅可以催化己糖,而且可作为葡萄糖传感器感知和传递葡萄糖[44]。果实中,HxK 可以将葡萄糖转变为6-磷酸葡萄糖(G6P),调控贮藏糖和游离糖的利用效率,同时也影响糖酵解和氧化戊糖磷酸等途径的代谢速率[45]。苹果[46]、桃[47]等多种蔷薇科果树中的HxKs 基因已得到克隆。研究表明,苹果己糖激酶1(Md-HxK1)基因与已知的葡萄糖感受器AtHxK1 基因高度同源,具有高度保守的氨基酸序列,包含hexokinase_1 和hexokinase_2 两个保守结构域,推测是酶进行催化反应的区域[48]。Zhao等[49]在番茄中异源表达梨的己糖激酶基因PbHxK1,发现显著增加了HxK 活性并且降低了葡萄糖含量。然而,赵锦等[50]的研究发现苹果MdHxK1基因的表达水平与其果实不同发育时期的HxK 活性和葡萄糖含量表现并不一致,推测存在其他己糖激酶基因参与了葡萄糖磷酸化过程。
综上,果实糖代谢是一个较为复杂的生理过程,有必要从分子生物学角度研究基因对果实糖积累和代谢的调控作用,考虑采用多组学联用或开发新的表型分析模型等方式分析代谢关键酶基因在果实糖分积累和代谢中的作用方式,为有效改善果实品质、提高果品商业价值提供更多的可能性。
3 环境因素与糖积累的关系
糖是果树生长发育的重要物质,其不仅作为能源物质参与果树的生长发育过程,而且可以作为响应外界环境的一种信号物质参与成花与果实成熟控制基因的表达调节[51]。以下笔者将从温度、光照、水分、土壤以及病虫害等五个方面进一步解析不同环境条件下果实中糖含量变化的生理和分子机制,从而为改善果实品质提供理论依据。
3.1 温度
适宜的环境温度是植物正常生长的基础,也是植物自然地理分布的主要限制因素[52]。许多植物不能在北方或高寒地区生存,就是由于低温的限制。可溶性糖不仅作为渗透调节物质平衡细胞质内外,而且能够提供植物耐冷性所需要的能量和次级代谢产物的前体[53]。通常情况下,可溶性糖积累可以提高植物抗寒性。
研究表明,苹果[54]和猕猴桃[55]在低温环境下果实中可以积累较多的可溶性糖。Maruyama 等[56]的研究发现,某些响应耐低温的基因是通过影响糖类物质积累而起作用的。过表达脱水应答基因DREB1A 可以增加植物对低温的耐受性,DREB1A可引起下游不同糖代谢基因的表达,结果使植物积累较多的可溶性糖,这可能是植物抗性增加的原因。所以,可溶性糖作为渗透调节物质,在植物发生冷害和冻害时对细胞具有一定的保护作用。然而,不同植物品种在低温条件下积累的可溶性糖种类是不一样的,如云莓遭受低温后会积累蔗糖,通过脂质磷酸化直接保护细胞膜、降低膜的渗透性[57]。另外,可溶性糖除了在渗透调节中起作用外,它还可以保护蛋白质活性,为植物安全越冬和春季返青提供代谢所需能量。可以说,可溶性糖积累是植物安全越冬的必要保障。
植物在低温下可溶性糖发生变化需要相关的糖代谢酶的参与。一些糖代谢关键酶基因的表达又会受低温的调控。Wang等[58]的研究发现,桃果实中蔗糖含量在0 ℃低温下显著积累,而葡萄糖和果糖水平维持在较低水平,猜测这与蔗糖水解相关的转化酶NINV 和vAINV 活性较低有关。有趣的是,桃果实中与蔗糖合成关键基因PpeSPS1在0 ℃低温下显著上调表达,说明桃遭受低温胁迫后通过提高SPS活性促进果实中蔗糖的合成,提高果实对低温的耐受性。因此,SPS 及其产物蔗糖可能是植物适应冷环境的一种机制。这一结果与Agopian 等[59]在香蕉中的低温研究结果相似,低温胁迫下蔗糖水平提高与淀粉水解有关,淀粉水解的产物在果实中通过SPS 作用进一步转化生成蔗糖。说明除蔗糖外,淀粉也是香蕉果实冷适应机制中的关键部分。
适宜的昼夜温差也会影响碳水化合物的分配、转化和代谢途径,进一步影响果实品质和产量[60]。Jing 等[61]的研究发现,适当增大昼夜温差可增加柑橘果实中可溶性糖含量[61]。Yuan[62]的研究也证实,10 ℃昼夜温差下有利于番茄果实可溶性糖的积累和产量的提高。说明昼夜温差越大,日间温度越高,植株光合强度增加;夜间温度越低,植株呼吸强度降低,促进碳水化合物积累。然而,当温差过大则造成不良影响,虽然白天光合作用旺盛,夜间呼吸作用同样加强,消耗过多的有机物,造成果实的同化物供应不足,产量下降。另外,昼夜温差大,夜间温度低可促进糖分向花青苷转化,有利于果实着色[63]。
3.2 光照
光照度、光周期和光质均能影响同化物合成、转运和代谢[64]。笔者主要探讨光照度和光质在糖含量调控中的应用,为合理密植,改善光照条件,从而提高果实品质奠定理论基础。
光照度会影响植物光合作用,与光合速率密切相关[65]。光合作用的增强有利于光合产物在源/库器官之间的转运与分配。可溶性糖是植物的主要光合产物,也是碳水化合物代谢和储存的主要形式。果实中的可溶性糖主要以果糖、葡萄糖和蔗糖为主,这些糖在不同库中的分配、合成及代谢决定了果实品质和风味形成。研究表明,适度遮光处理可以提高叶片光合能力,减轻中午光抑制现象,从而改善果实品质,增加产量[66]。如套袋可增加枇杷果实中蔗糖和葡萄糖含量[67]。但是,也有相反的报道。在瑞光油桃上进行套袋试验发现果实中可溶性固形物含量下降了0.5%~1.5%[68]。因此,在果实发育期,通过修剪、摘叶等方式改善植株光照条件,可有效提高果实中可溶性糖含量。果实作为一个强大的代谢库,最终进入果实中的同化产物是由细胞代谢决定。果实中可溶性糖含量一方面与叶片中合成的碳水化合物运输有关,另一方面受相关代谢酶活性调控,包括蔗糖磷酸合成酶(SPS),蔗糖合成酶(SUSY),中性转化酶(NINV)等。遮光处理降低了桃果实整个发育过程中SUSY、SPS 和NINV 活性,同时减少了果实中果糖和蔗糖含量[69]。Goldschmid等[70]的研究发现白天光照时,SPS酶因脱磷酸化使得酶活性提高,光合产物主要用于蔗糖合成;晚上黑暗时,蔗糖合成因SPS 磷酸化而减弱,此时维持代谢的产物主要是淀粉。说明SPS可响应光/暗信号,通过磷酸化修饰作用进行酶活性的调节。
不同光质触发不同光受体,从而影响植物光合特性、果实品质等[71]。红光处理能提高番茄的糖、酸含量[72]。3.0 kJ·m-2 UV-C(短波紫外线)处理采后草莓,可抑制草莓果实的呼吸速率,维持了整个贮藏期间较高的FRK 和HXK 活性,促进后期果实中果糖和葡萄糖含量的下降[73]。研究表明,通过光质调节果实糖代谢的作用机制有可能是通过影响碳水化合物吸收来影响糖含量,也可能通过对代谢酶活性的调节来调控糖含量[74]。Interdonato等[75]用UV-B照射柠檬后,发现可溶性糖含量的提高与蔗糖代谢酶活性的变化相关,推测光质可能直接调控代谢酶活性和关键酶基因来影响果实中糖含量。
3.3 水分
水分是细胞中信号传递和物质运输的基本介质,参与细胞分裂和糖分转化等生理过程[76]。实际生产中,在需水非关键时期让植株维持适度的水分亏缺,能够促进可溶性糖积累[77]。如苹果在果实膨大前期承受一定程度的水分胁迫,可溶性固形物含量得到提高,表明果实承受水分胁迫期间可溶性糖积累[78]。另外,发现旱后复水的桃果实快速生长[79],推测也与光合同化物优先转运到果实有关。
研究表明,一定时期,一定程度的水分亏缺会使果实中积累较多的可溶性糖,分别在苹果、桃中已得到证实[80-81]。最新研究表明,苹果蔗糖转运蛋白Md-SUT2.2在ABA诱导的可溶性糖积累中具有重要作用[82]。发现干旱诱导的蛋白激酶MdCIPK22与Md-SUT2.2 相互作用并使其磷酸化,提高了MdSUT2.2蛋白稳定性,促进可溶性糖在液泡中的积累,提高抗逆性和果实品质。糖含量变化过程复杂,其中一些重要代谢酶在植物应对水分亏缺反应中发挥着重要调节作用。Wang 等[80]发现苹果果实中山梨醇氧化酶(SOX)和酸性转化酶(AI)活性受水分胁迫诱导,使得山梨醇和蔗糖代谢增强,导致果糖和葡萄糖在果实中积累,促进苹果抗旱性的提高。说明山梨醇和蔗糖代谢在响应干旱胁迫中起重要作用。Wei等[83]发现干旱引起四倍体枸橘中液泡转化酶(VINV)活性及mRNA 水平均上调表达,加速了INV 介导蔗糖的分配和利用,而蔗糖水解产生的己糖可以作为渗透调节剂起作用并且减轻缺水对植物造成的影响。果糖激酶(FRK)是植物果糖代谢的“开关”,在调节碳分配和果糖利用方面具有重要作用[34]。编码苹果果糖激酶(FRK)的MdFRK2基因受干旱胁迫诱导,引起果糖代谢加快,山梨醇(SDH上调表达)和蔗糖代谢(SUSY 上调表达)分解能力提高,以补偿源自山梨醇的果糖减少量[84]。这种糖代谢和运输系统(HT 表达上调)的变化导致了果糖稳态,并且引起果糖和葡萄糖的积累,促进了果实抗旱性的提高。
3.4 土壤
植物生长发育所需的水分和营养物质主要通过根系从土壤中吸收,是仅次于气候对果实品质起重要作用的生态因素[85]。土壤酸碱度是土壤重要的理化性质之一,其pH过低或过高都会使得同化产物吸收受阻,影响果实品质和产量[86]。
研究表明,H+-焦磷酸酶(H+-pyrophosphatase,VHP)能够调节液泡渗透势,有利于细胞吸收水分,并伴随矿质元素和碳水化合物积累[87]。Amemiya等[88]和Yao等[89]分别在番茄和苹果中发现VHP在可溶性糖积累中起关键作用,并且Yao 等[89]的研究结果表明,苹果中过表达MdVHP1 基因不仅促进果实中苹果酸的积累,而且通过调节转运蛋白NHX1 和SUT1来增加可溶性糖的积累。最新研究表明,蔗糖转运蛋白MdSUT2.2 在盐胁迫中起到正调控作用。发现盐胁迫诱导的蛋白激酶MdCIPK13 与Md-SUT2.2相互作用并发生磷酸化,促进可溶性糖在液泡中的积累,提高抗逆性和果实品质[90]。所以,可溶性糖含量增加对碳储存、渗透保护和清除活性氧(ROS)等起着至关重要的作用。进一步研究发现,糖含量变化与糖代谢过程中关键酶活性改变有关,并且这些糖代谢关键酶基因的表达又会受土壤盐碱度的影响。最新研究表明,番茄中异源表达苹果己糖转运蛋白基因MdHT2.2,使得与蔗糖合成相关基因SlSPS显著上调表达,促进了番茄中蔗糖、果糖和葡萄糖积累,通过平衡细胞质与细胞间的Na+/K+离子浓度,清除活性氧(ROS)来响应番茄耐盐机制[91]。
可溶性糖含量不仅与遗传特性相关,也受施肥管理的影响。氮素(N)作为植物生长发育所必须的元素,与果实糖含量密切相关[92]。丁宁等[93]的研究发现,晚秋叶面追氮提高了矮化苹果的可溶性糖含量。但是,氮水平过高会导致蜜柑汁囊中蔗糖积累减少,推测可溶性含氮化合物增多可能限制了葡萄糖向蔗糖转化[94]。可溶性糖含量依赖于糖代谢和转运相关基因的调控。糖主要在液泡中积累,液泡膜糖转运蛋白负责糖在液泡和胞质中的转运。李凡等[95]的研究发现,成熟期施氮处理后,富士苹果液泡膜糖转运蛋白基因MdTMT1,MdTST2 表达下调,果糖和葡萄糖在液泡中积累受到抑制。同时,施氮后果实中果糖激酶基因(MdFRK2)和己糖激酶(Md-HXK1)上调表达,促进了果糖和葡萄糖转化,蔗糖随之增加(MdSPS1,MdSPS6 表达上调),说明成熟期施氮后果实糖代谢和转运相关基因表达发生变化,己糖含量降低,蔗糖含量增加,改变了果实中可溶性糖的组成,导致果实甜度和品质降低。因此,果实采收前不宜施氮。
3.5 病虫害
植物在生长发育过程中经常受到如真菌、细菌等病原物的侵害。当病原菌侵染寄主后,必须从寄主细胞内获取营养物质供其生长所需[96]。糖类物质是病原菌从宿主细胞吸收的主要能源物质。
糖作为营养物质不仅在植物防御反应中提供能量,而且与免疫信号物质相互作用参与植物免疫。真菌攻击植物后,可溶性碳水化合物组成和比例通过植物调节机制发生改变[97]。因此,在植物-病原菌相互作用中,可观察到感染组织中可溶性糖的增加或减少。例如,柑橘黄龙病菌侵染的甜橙果实中,蔗糖和果糖含量均表现为增多[98],推测蔗糖和果糖含量升高可能是受褐斑病病原菌诱导产生的。说明糖含量在植物抗病防御机制中发挥重要作用。
研究表明,植物受病原菌侵染后,宿主体内糖代谢发生显著变化,其中与蔗糖代谢相关的转化酶活性增强[99]。细胞壁转化酶(CWINV)能够水解质外体途径中的蔗糖其活性增强可促进从韧皮部到库器官己糖的供应,该过程对于调节糖分配并提供病原体发育所必需的糖源是必不可少的[100]。同时,增强的转化酶活性也可改变胞质外己糖/蔗糖的比例,并引起己糖介导的防御反应[101]。Lecompte 等[102]研究发现,番茄茎对坏死性真菌灰葡萄孢的敏感性变化与果糖含量的特定调节有关,并且提出栽培上可通过改变果糖含量来实现抗病的思路,为深入研究病害控制方面提供了新的途径。近年来,测序结果分析发现,与糖代谢相关基因的表达上调或下调在植物与病原菌互作过程中扮演了重要角色。山梨醇脱氢酶(SDH)基因在苹果上接种褐斑病后,mRNA 水平先升高后降低,推测褐斑病病原菌入侵后引起苹果叶片光合作用下降[103]。另外,糖转运蛋白在植物中参与韧皮部卸载、生长发育及宿主-病原菌的互作反应等多个生理过程[96]。糖转运蛋白SWEETs的表达受真菌病原体诱导,表明SWEET 转运蛋白对糖的转运功能可能与病原体中对可溶性糖的需求有关。Chong等[104]在葡萄上鉴定了参与寄主植物糖分输出并影响菌根共生的SWEET 蛋白VvSWEET4,VvSWEET4 蛋白基因的转录本受到坏死性病原体的活性氧诱导,将葡萄糖单向转运,促进了病原体从细胞中获取糖分。这些结果进一步加深了对SWEET 转运蛋白在抗病过程中糖分的积累与分配的相关认识。
4 总结与展望
果实中几乎所有的山梨醇和50%的蔗糖水解产生果糖进入代谢流,可以确定的是果糖与果实变甜密切相关。此外,糖在韧皮部装载和卸载机制可以为食物、纤维和生物燃料实际生产提供可靠的理论指导,对于解决人类目前所面临的粮食短缺、能源不可再生等问题至关重要。
糖是果实风味等品质形成的关键因素,其在果实中代谢和积累容易受到环境的影响,如水分、光照和温度等。它们影响果实中糖含量变化主要与关键代谢酶和基因有关,如SPS、SUSY 和FRK 等、一些转化酶和转运蛋白等。因此,需要进行更多的研究和技术来了解如何控制糖代谢酶,以在适当的时间、空间和数量上为植物生长发育提供充足的营养物质,如韧皮部转运的动态放射性示踪成像技术,动态解读糖在不同环境条件下的转运、代谢和积累[105]。植物还面临真菌病害带来的挑战,如白粉病、褐斑病等,尤其在果树上越发严重。病原菌侵染植物后,糖含量变化主要与一些转化酶和转运蛋白相关,如CWINV、SWEET。增加转化酶活性能改变胞质外己糖/蔗糖的比例,引起己糖介导的防御反应。通过基因组工程方法,改变转录调控或蛋白质修饰影响病原体靶向的转化酶活性或糖转运蛋白[106],在不影响果实品质的情况下对病原体产生抗性,从而为植物防御病害提供重要的策略。
总之,综合研究环境因子与果实糖含量的关系,可以为果实糖代谢的生态调控提供理论基础,达到通过有效的生态调控措施提高果实品质的目的;进一步探讨糖代谢相关酶和关键基因表达对果实糖含量的调控机制,为改善果实甜度、风味及着色等方面提供了理论依据。
[1]LI M J,LI P M,MA F W,DANDEKAR A M,CHENG L L.Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis[J]. Horticulture Research,2018,5(1):60.
[2]LI J,WU L M,FOSTER R,RUAN Y L.Molecular regulation of sucrose catabolism and sugar transport for development,defence and phloem function[J].Journal of Integrative Plant Biology,2017,59(5):322-335.
[3]ZHANG L Y,PENG Y B,PELLESCHI-TRAVIER S,FAN Y,LU Y F,LU Y M,GAO X P,SHEN Y Y,DELROT S,ZHANG D P. Evidence for apoplasmic phloem unloading in developing apple fruit[J].Plant Physiology,2004,135(1):574-586.
[4]ZHU L C,LI B Y,WU L M,LI H X,WANG Z Y,WEI X Y,MA B Q,ZHANG Y F,MA F W,RUAN Y L,LI M J.MdERDL6-mediated glucose efflux to the cytosol promotes sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato[J]. Proceedings of the National Academy of Sciences,2021,118(1):e2022788118.
[5]RUAN Y L. Sucrose metabolism:gateway to diverse carbon use and sugar signaling[J].Annual Review of Plant Biology,2014,65(1):33-67.
[6]COLARIC M,VEBERIC R,STAMPAR F,HUDINA M.Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes[J]. Journal of the Science of Food and Agriculture,2005,85(15):2611-2616.
[7]CARVALHO D U D,CRUZ M A D,COLOMBO R C,WATANABE L S,TAZIMA Z H,NEVES C S V J. Determination of organic acids and carbohydrates in‘Salustiana’orange fruit from different rootstocks[J].Brazilian Journal of Food Technology,2020,23(1).DOI:10.1590/1981-6723.32918
[8]GROSS K C,SAMS C E. Changes in cell wall neutral sugar composition during fruit ripening:A species survey[J]. Phytochemistry,1984,23(11):2457-2461.
[9]TAO H X,SUN H Q,WANG Y F,SONG X N,GUO Y P. New insights on‘Gala’apple fruit development:Sugar and acid accumulation:a transcriptomic approach[J]. Journal of Plant Growth Regulation,2020,39(2):680-702.
[10]LOESCHER W H,MARLOW G C,KENNEDY R A. Sorbitol metabolism and sink-source interconversions in developing apple leaves[J].Plant Physiology,1982,70(2):335-339.
[11]ZHOU R,CHENG L L,WAYNE R. Purification and characterization of sorbitol-6-phosphate phosphatase from apple leaves[J].Plant Science,2003,165(1):227-232.
[12]BERUTER J. Sugar accumulation and changes in the activities of related enzymes during development of the apple fruit [J]Journal of Plant Physiology,1985,121(4):331-341.
[13]YANG J J,ZHANG J,LI C,ZHANG Z,MA F W,LI M J. Response of sugar metabolism in apple leaves subjected to shortterm drought stress[J].Plant Physiology and Biochemistry,2019,141:164-171.
[14]LI M J,FENG F J,CHENG L L.Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development[J].PloS One,2012,7(3):e33055.
[15]KIM H Y,AHN J C,CHOI J H,HWANG B,CHOI D W. Expression and cloning of the full-length cDNA for sorbitol-6-phosphate dehydrogenase and NAD-dependent sorbitol dehydrogenase from pear(Pyrus pyrifolia N.)[J].Scientia Horticulturae,2007,112(4):406-412.
[16]DUANGSRISAI B S,YAMADA K,BANTOG N A,SHIRATAKE K,KANAYAMA Y,YAMAKI S. Presence and expression of NAD+-dependent sorbitol dehydrogenase and sorbitol-6-phosphate dehydrogenase genes in strawberry[J].The Journal of Horticultural Science and Biotechnology,2007,82(2):191-198.
[17]YAMADA K,OURA Y,MORI H,YAMAKI S. Cloning of NAD-dependent sorbitol dehydrogenase from apple fruit and gene expression[J]. Plant and Cell Physiology,1998,39(12):1375-1379.
[18]CHENG L,ZHOU R,REIDEL E J,SHARKEY T D,DANDEKAR A M.Antisense inhibition of sorbitol synthesis leads to upregulation of starch synthesis without altering CO2 assimilation in apple leaves[J].Planta,2005,220(5):767-776.
[19]RUAN Y L. Signaling role of sucrose metabolism in development[J].Molecular Plant,2012,5(4):763-765.
[20]HUANG D L,QIN C X,GUI Y Y,ZHAO L H,CHEN Z L,WANG M,SUN Y,LIAO Q,LI Y R,LAKSHMANAN P. Role of the SPS gene families in the regulation of sucrose accumulation in sugarcane[J].Sugar Tech,2017,19(2):117-124.
[21]LI L,WU H X,MA X W,XU W T,LIANG Q Z,ZHAN R L,WANG S B.Transcriptional mechanism of differential sugar accumulation in pulp of two contrasting mango (Mangifera indica L.)cultivars[J].Genomics,2020,112(6):4505-4515.
[22]TIAN H M,MA L Y,ZHAO C,HAO H,GONG B,YU X Y,WANG X F.Antisense repression of sucrose phosphate synthase in transgenic muskmelon alters plant growth and fruit development[J]. Biochemical and Biophysical Research Communications,2010,393(3):365-370.
[23]TONG X L,WANG Z Y,MA B Q,ZHANG C X,ZHU L C,MA F W,LI M J. Structure and expression analysis of the sucrose synthase gene family in apple[J].Journal of Integrative Agriculture,2018,17(4):847-856.
[24]ASLAM M M,DENG L,WANG X B,WANG Y,PAN L,LIU H,NIU L,LU Z H,CUI G C,ZENG W F,WANG Z Q.Expression patterns of genes involved in sugar metabolism and accumulation during peach fruit development and ripening[J]. Scientia Horticulturae,2019,257:108633.
[25]HUA L N,ZANG M,WANG S F,LI Y Z,SHEN Y Y,GUO J X. Cloning,silencing,and prokaryotic expression of strawberry sucrose synthase gene FaSus1[J]. The Journal of Horticultural Science and Biotechnology,2017,92(1):107-112.
[26]ZHAO H Y,SUN S M,ZHANG L H,YANG J J,WANG Z Y,MA F W,LI M J.Carbohydrate metabolism and transport in apple roots under nitrogen deficiency[J].Plant Physiology and Bio-Chemistry,2020,155:455-463.
[27]D’AOUST M A,YELLE S,QUOC N B.Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit[J].The Plant Cell,1999,11(12):2407-2418.
[28]SU J,ZHANG C X,ZHU L C,YANG N,YANG J J,MA B Q,MA F W,LI M J.MdFRK2-mediated sugar metabolism accelerates cellulose accumulation in apple and poplar[J]. Biotechnology for Biofuels,2021,14(1):1-13.
[29]WAN H J,WU L M,YANG Y J,ZHOU G Z,RUAN Y L. Evolution of sucrose metabolism:the dichotomy of invertases and beyond[J].Trends in Plant Science,2018,23(2):163-177.
[30]JIN Y,NI D A,RUAN Y L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level[J].The Plant Cell,2009,21(7):2072-2089.
[31]WANG Z Y,WEI X Y,YANG J J,LI H X,MA B Q,ZHANG K K,ZHANG Y F,CHENG L L,MA F W,LI M J. Heterologous expression of the apple hexose transporter MdHT2.2 altered sugar concentration with increasing cell wall invertase activity in tomato fruit[J]. Plant Biotechnology Journal,2020,18(2):540-552.
[32]RUAN Y L,JIN Y,YANG Y J,LI G J,BOYER J S. Sugar input,metabolism,and signaling mediated by invertase:Roles in development,yield potential,and response to drought and heat[J].Molecular Plant,2010,3(6):942-955.
[33]QIN G Z,ZHU Z,WANG W H,CAI J H,CHEN Y,LI L,TIAN S P.A tomato vacuolar invertase inhibitor mediates sucrose metabolism and influences fruit ripening[J].Plant Physiology,2016,172(3):1596-1611.
[34]YANG J J,ZHU L C,CUI W F,ZHANG C,LI D X,MA B Q,CHENG L L,RYAN Y L,MA F W,LI M J. Increased activity of MdFRK2,a high-affinity fructokinase,leads to upregulation of sorbitol metabolism and downregulation of sucrose metabolism in apple leaves[J].Horticulture Research,2018,5(1):1-12.
[35]GRANOT D,SCHWARTZ D R,KELLY G.Hexose kinases and their role in sugar-sensing and plant development[J]. Frontiers in Plant Science,2013,4:44.
[36]GRANOT D,KELLY G,STEIN O,SCHWARTZ D R. Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development[J]. Journal of Experimental Botany,2014,65(3):809-819.
[37]QIN Q P,CUI Y Y,ZHANG L L,LIN F F,LAI Q X. Isolation and induced expression of a fructokinase gene from loquat[J].Russian Journal of Plant Physiology,2014,61(3):289-297.
[38]STEIN O,GRANOT D.Plant fructokinases:evolutionary,developmental,and metabolic aspects in sink tissues[J]. Frontiers in Plant Science,2018,9:339.
[39]ROACH M,GERBER L,SANDQUIST D,GORZSAS A,HEDENSTROM M,KUMAR M,STEINHAUSER M C,FEIL R,DANIEL G,STITT M,SUNDBERG B,NIITTYLA T.Fructokinase is required for carbon partitioning to cellulose in aspen wood[J].The Plant Journal,2012,70(6):967-977.
[40]QIN Q P,ZHANG S L,CHEN J W,XIE M,JIN Y F,CHENG K S,ASGHAR S. Isolation and expression analysis of fructokinase genes from citrus[J]. Journal of Integrative Plant Biology,2004,46(12):1408.
[41]DAI N,GERMAN M A,MATSEVITZ T,HANAEL R,SWARTZBERG D,YESELSON Y,PETREIKOV M,SCHAFFER A A,GRANOT D. LeFRK2,the gene encoding the major fructokinase in tomato fruits,is not required for starch biosynthesis in developing fruits[J].Plant Science,2002,162(3):423-430.
[42]SCHAFFER A A,PETREIKOV M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation[J].Plant Physiology,1997,113(3):739-746.
[43]SHINOZAKI Y,BEAUVOIT B P,TAKAHARA M,HAO S,EZURA K,ANDRIEU M H,NISHIDA K,MORI K,SUZUKI Y,KUHARA S,ENOMOTO H,KUSANO M,FUKUSHIMA A,MORI T,KOJIMA M,KOBAYASHI M,SAKAKIBARA H,SAITO K,OHTANI Y,BENARD C,PRODHOMME D,GIBON Y,EZURA H,ARIIZUMI T. Fruit setting rewires central metabolism via gibberellin cascades[J]. Proceedings of the National Academy of Sciences,2020,117(38):23970-23981.
[44]XIAO W Y,SHEEN J,JANG J C. The role of hexokinase in plant sugar signal transduction and growth and development[J].Plant Molecular Biology,2000,44(4):451-461.
[45]JANG J C,LEON P,ZHOU L,SHEEN J. Hexokinase as a sugar sensor in higher plants[J].The Plant Cell,1997,9(1):5-19.
[46]ZHU L C,JING S,JIN Y R,ZHAO H Y,TIAN X C,ZHANG C,MA F W,LI M J,MA B Q.Genome-wide identification,molecular evolution,and expression divergence of the hexokinase gene family in apple[J].Journal of Integrative Agriculture,2021,20(8):2112-2125.
[47]XU W J,WEI Y Y,WANG X X,HAN P P,CHEN Y,XU F,SHAO X F. Molecular cloning and expression analysis of hexokinase genes in peach fruit under postharvest disease stress[J].Postharvest Biology and Technology,2021,172:111377.
[48]ZHAO J,SUN M H,HU D G,HAO Y J.Molecular cloning and expression analysis of a hexokinase gene,MdHXK1 in apple[J].Horticultural Plant Journal,2016,2(2):67-74.
[49]ZHAO B Y,QI K J,YI X R,CHEN G D,XING L,QI X X,ZHANG S L. Identification of hexokinase family members in pear (Pyrus × bretschneideri) and functional exploration of Pb-HXK1 in modulating sugar content and plant growth[J]. Gene,2019,711:143932.
[50]赵锦,孙美红,胡大刚,郝玉金.苹果己糖激酶基因MdHXK1的克隆与表达分析[J].园艺学报,2015,42(8):1437-1447.ZHAO Jin,SUN Meihong,HU Dagang,HAO Yujin. Molecular cloning and expression analysis of a hexokinase gene MdHXK1 in apple[J].Acta Horticulturae Sinica,2015,42(8):1437-1447.
[51]LASTDRAGER J,HANSON J,SMEEKENS S. Sugar signals and the control of plant growth and development[J]. Journal of Experimental Botany,2014,65(3):799-807.
[52]HATFIELD J L,PRUEGER J H. Temperature extremes:Effect on plant growth and development[J]. Weather and Climate Extremes,2015,10:4-10.
[53]MA Y Y,ZHANG Y L,LU J,SHAO H B.Roles of plant soluble sugars and their responses to plant cold stress[J]. African Journal of Biotechnology,2009,8(10).DOI:10.1186/1471-2164-10-234
[54]YANG G X,XU H F,ZOU Q,ZHANG J,JIANG S H,FANG H C,WANG Y C,SU M Y,WANG N,CHEN X S. The vacuolar membrane sucrose transporter MdSWEET16 plays essential roles in the cold tolerance of apple[J].Plant Cell,Tissue and Organ Culture,2020,140(1):129-142.
[55]MITALO O W,TOKIWA S,KONDO Y,OTSUKI T,GALIS I,SUEZAWA K,KATAOKA I,DOAN A T,NAKANO R,USHIJIMA K,KUBO Y. Low temperature storage stimulates fruit softening and sugar accumulation without ethylene and aroma volatile production in kiwifruit[J]. Frontiers in Plant Science,2019,10:888.
[56]MARUYAMA K,TAKEDA M,KIDOKORO S,YAMADA K,SAKUMA Y,URANO K,FUJITA M,YOSHIWARA K,MATSUKURA S,MORISHITA Y,SASAKI R,SUZUKI H,SAITO K,SHIBATA D,SHINOZAKI K,SHINOZAKI K Y. Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcripts regulated by DREB1A and DREB2[J].Plant Physiology,2009,150(4):1972-1980.
[57]KAURIN A,JUNTTILA O,HANSON J. Seasonal changes in frost hardiness in cloudberry (Rubus chamaemorus) in relation to carbohydrate content with special reference to sucrose[J].Physiologia Plantarum,1981,52(2):310-314.
[58]WANG T,WRIGHT D,XU H,YANG Y,ZHENG R,SHI J,CHEN R,WANG L. Expression patterns,activities and sugar metabolism regulation of sucrose phosphate synthase,sucrose synthase,neutral invertase and soluble acid invertase in different Goji cultivars during fruit development[J]. Russian Journal of Plant Physiology,2019,66(1):29-40.
[59]AGOPIAN R G D,PERONI-OKITA F H G,SOARES C A,MAINARDI J A,NASCIMENTOJ R O D,CORDENUNSI B R,LAJOLO F M,PURGATTO E. Low temperature induced changes in activity and protein levels of the enzymes associated to conversion of starch to sucrose in banana fruit[J]. Postharvest Biology and Technology,2011,62(2):133-140.
[60]WAGENMAKERS P S,CALLESEN O.Light distribution in apple orchard systems in relation to production and fruit quality[J].Journal of Horticultural Science,1995,70(6):935-948.
[61]JING P P,WANG D,ZHU C W,CHEN J Q. Plant physiological,morphological and yield-related responses to night temperature changes across different species and plant functional types[J].Frontiers in Plant Science,2016,7:1774.
[62]YUAN X K. Effect of day/night temperature difference on chlorophyll content,photosynthesis and fluorescence parameters of tomato at fruit stage[J].Photosynthetica,2016,54(3):475-477.
[63]YAN Y F,SONG C Z,FALGINELLA L G,CASTELLARIN S D.Day Temperature has a stronger effect than night temperature on anthocyanin and flavonol accumulation in‘Merlot’(Vitis vinifera L.) grapes during ripening[J]. Frontiers in Plant Science,2020,11:1095.
[64]NTAGKAS N,WOLTERING E,BOURAS S,VOS D R C H,DIELEMAN J A,NICOLE C C S,LABRIE C,MARCELIS L F M. Light-induced vitamin C accumulation in tomato fruits is independent of carbohydrate availability[J].Plants,2019,8(4):86.
[65]CARDONA T,SHAO S X,NIXON P J.Enhancing photosynthesis in plants:The light reactions[J]. Essays in Biochemistry,2018,62(1):85-94.
[66]KANSKI L,KAHLE H,NAUMANN M,HAGENGUTH J,ULBRICH A,PAWELZIK E. Cultivation systems,light intensity,and their influence on yield and fruit quality parameters of tomatoes[J].Agronomy,2021,11(6):1203.
[67]NI Z J,ZHANG Z,GAO Z H,GU L P,HUANG L F.Effects of bagging on sugar metabolism and the activity of sugar metabolism related enzymes during fruit development of Qingzhong loquat[J]. African Journal of Biotechnology,2011,10(20):4212-4216.
[68]叶正文,李雄伟,马亚萍,刘盼,苏明申,周京一,杜纪红.桃果实糖代谢研究进展[J].上海农业学报,2019,35(4):144-150.YE Zhengwen,LI Xiongwei,MA Yaping,LIU Pan,SU Mingshen,ZHOU Jingyi,DU Jihong.Research progress of sugar metabolism in peach[J]. Acta Agriculturae Shanghai,2019,35(4):144-150.
[69]WANG X Q,HUANG W D,ZHAN J C. Effects of low light on phloem ultrastructure and subcellular localization of sucrose synthase in Prunus persica L. var. nectarina Ait. fruit[J]. Russian Journal of Plant Physiology,2009,56(4):462-469.
[70]GOLDSCHMID E E,HUBER S C. Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch,sucrose,and hexose sugars[J].Plant Physiology,1992,99(4):1443-1448.
[71]THWE A A,KASEMSAP P,VERCAMBER G,GAY F,PHATTARALERPHONG J,GAUTIER H.Impact of red and blue nets on physiological and morphological traits,fruit yield and quality of tomato (Solanum lycopersicum Mill.)[J]. Scientia Horticulturae,2020,264:109185.
[72]LIU H,FU Y,HU D W,YU J,LIU H. Effect of green,yellow and purple radiation on biomass,photosynthesis,morphology and soluble sugar content of leafy lettuce via spectral wavebands‘knock out’[J].Scientia Horticulturae,2018,236:10-17.
[73]蔡艳,施丽愉,陈伟,苏新国,杨震峰.UV-C 处理对采后草莓果实品质和活性氧代谢的影响[J]. 中国食品学报,2015,15(3):128-136.CAI Yan,SHI Liyu,CHEN Wei,SU Xinguo,YANG Zhenfeng.Effect of UV-C treatment on fruit quality and active oxygen metabolism of postharvest strawberry fruit[J]. Journal of Chinese Institute of Food Science and Technology,2015,15(3):128-136.
[74]ZHANG B B,XU J L,ZHOU M,YAN D H,MA R J. Effect of light quality on leaf photosynthetic characteristics and fruit quality of peach (Prunus persica L. Batch)[J]. Photosynthetica,2018,56(4):1113-1122.
[75]INTERDONATO R,ROSA M,NIEVA C B,GONZALEZ J A,HILAL M,PRADO F E.Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons[J].Environmental and Experimental Botany,2011,70(2/3):204-211.
[76]LUFU R,AMBAW A,OPARA U L.Water loss of fresh fruit:Influencing pre-harvest,harvest and postharvest factors[J]. Scientia Horticulturae,2020,272:109519.
[77]ZHONG Y,FEI L J,LI Y B,ZENG J,DAI Z G. Response of fruit yield,fruit quality,and water use efficiency to water deficits for apple trees under surge-root irrigation in the Loess Plateau of China[J]. Agricultural Water Management,2019,222:221-230.
[78]LOTTER J D V,BEUKES D J,WEBER H W.Growth and quality of apples as affected by different irrigation treatments[J].Journal of Horticultural Science,1985,60(2):181-192.
[79]LI S H,HUGUET J G,SCHOCH P G,ORLANDO P. Response of peach tree growth and cropping to soil water deficit at various phenological stages of fruit development[J].Journal of Horticultural Science,1989,64(5):541-552.
[80]WANG Y J,LIU L,WANG Y,TAO H X,FAN J L,ZHAO Z Y,GUO Y P. Effects of soil water stress on fruit yield,quality and their relationship with sugar metabolism in‘Gala’apple[J].Scientia Horticulturae,2019,258:108753.
[81]KOBASHI K,GEMMA H,IWAHORI S. Abscisic acid content and sugar metabolism of peaches grown under water stress[J].Journal of the American Society for Horticultural Science,2000,125(4):425-428.
[82]MA Q J,SUN M H,LU J,KANG H,YOU C X,HAO Y J.An apple sucrose transporter MdSUT2.2 is a phosphorylation target for protein kinase MdCIPK22 in response to drought[J]. Plant Biotechnology Journal,2019,17(3):625-637.
[83]WEI T L,WANG Y,XIE Z Z,GUO D Y,CHEN C W,FAN Q J,DENG X D,LIU J H. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata[J]. Plant Biotechnology Journal,2019,17(7):1394-1407.
[84]詹瑞玲.MdFRK2 过量表达对苹果抗旱性的影响[D].杨凌:西北农林科技大学,2020.ZHAN Ruiling. Effects of over-expression MdFRK2 on drought resistance of apple[D]. Yangling:Northwest A&F University,2020.
[85]COWAN I R. Transport of water in the soil-plant-atmosphere system[J].Journal of Applied Ecology,1965,2(1):221-239.
[86]ACOSTAMOTOS J R,ORTUNO M F,BERNALVICENTE A,DIAZVIANCOS P,SANCHEZBLANCO M J,HERNANDEZ J A. Plant responses to salt stress:Adaptive mechanisms[J].Agronomy,2017,7(1):18.
[87]SILVA P,FACANHA A R,TAVARES R M,GEROS H. Role of tonoplast proton pumps and Na+/H+ antiport system in salt tolerance of Populus euphratica Oliv[J]. Journal of Plant Growth Regulation,2010,29(1):23-34.
[88]AMEMIYA T,KANAYAMA Y,YAMAKI S,YAMADA K,SHIRATAKE K. Fruit-specific V-ATPase suppression in antisense-transgenictomatoreducesfruitgrowthandseedformation[J].Planta,2006,223(6):1272-1280.
[89]YAO Y X,DONG Q L,YOU C X,ZHAI H,HAO Y J. Expression analysis and functional characterization of apple MdVHP1 gene reveals its involvement in Na+,malate and soluble sugar accumulation[J]. Plant Physiology and Biochemistry,2011,49(10):1201-1208.
[90]MA Q J,SUN M H,KANG H,LU J,YOU C X,HAO Y J. A CIPK protein kinase targets sucrose transporter MdSUT2.2 at Ser254 for phosphorylation to enhance salt tolerance[J]. Plant,Cell&Environment,2019,42(3):918-930.
[91]WANG Z Y,LIANG Y H,JIN Y R,TONG X L,WEI X Y,MA F W,MA B Q,LI M J.Ectopic expression of apple hexose transporter MdHT2.2 reduced the salt tolerance of tomato seedlings with decreased ROS-scavenging ability[J].Plant Physiology and Biochemistry,2020,156:504-513.
[92]CARRANCA C,BRUNETTO G,TAGLIAVINI M.Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns[J].Plants,2018,7(1):4.
[93]丁宁,沙建川,丰艳广,陈建明,张民,姜远茂.晚秋叶施尿素提高矮化苹果翌春生长及果实品质的效果[J].植物营养与肥料学报,2016,22(6):1665-1671.DING Ning,SHA Jianchuan,FENG Yanguang,CHEN Jianming,ZHANG Min,JIANG Yuanmao. Foliage application of urea in late autumn will improve growth in the following spring and fruit quality of dwarfed apple [J]. Journal of Plant Nutrition and Fertilizer,2016,22(6):1665-1671.
[94]LIAO L,DONG T T,QIU X,RONG Y,WANG Z H,ZHU J.Nitrogen nutrition is a key modulator of the sugar and organic acid content in citrus fruit[J].PloS One,2019,14(10):e0223356.
[95]李凡,魏桦,戚建国,孙思敏,邹养军,李明军.成熟期施氮对富士苹果糖含量及相关基因表达的影响[J].西北农林科技大学学报(自然科学版),2021,49(8):111-119.LI Fan,WEI Hua,QI Jianguo,SUN Simin,ZOU Yangjun,LI Mingjun. Effects of nitrogen application at mature stage on sugar contents and related gene expression of Fuji apple[J]. Journal of Northwest A&F University (Natural Sciences),2021,49(8):111-191.
[96]HUAI B,YANG Q,QIAN Y R,QIAN W H,KANG Z S,LIU J.ABA-induced sugar transporter TaSTP6 promotes wheat susceptibility to stripe rust[J]. Plant Physiology,2019,181(3):1328-1343.
[97]FORMELA M,SAMARDAKIEWICZ S,MARCZAK Ł,NOWAK W,NAROZNA D,BEDNARSKI W,KASPROWICZ M A,MORKUNAS I.Effects of endogenous signals and Fusarium oxysporum on the mechanism regulating genistein synthesis and accumulation in yellow lupine and their impact on plant cell cytoskeleton[J].Molecules,2014,19(9):13392-13421.
[98]FAN J,CHEN C,BRLANSKY R H,JR F G G,LI Z G.Changes in carbohydrate metabolism in Citrus sinensis infected with‘Candidatus Liberibacter asiaticus’[J]. Plant Pathology,2010,59(6):1037-1043.
[99]VOEGELE R T,WIRSEL S,MOLL U,LECHNER M,MENDGEN K. Cloning and characterization of a novel invertase from the obligate biotroph Uromyces fabae and analysis of expression patterns of host and pathogen invertases in the course of infection[J]. Molecular Plant-Microbe Interactions,2006,19(6):625-634.
[100]ROJAS C M,SENTHIL K M,TZIN V,MYSORE K. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense[J]. Frontiers in Plant Science,2014,5:17.
[101]PROELS R K,HUCKELHOVEN R. Cell-wall invertases,key enzymes in the modulation of plant metabolism during defence responses[J].Molecular Plant Pathology,2014,15(8):858-864.
[102]LECOMPTE F,NICOT P C,RIPOLL J,ABRO M A,RAIMBAULT A K,LOPEZ L F,BERTIN N. Reduced susceptibility of tomato stem to the necrotrophic fungus Botrytis cinerea is associated with a specific adjustment of fructose content in the host sugar pool[J].Annals of Botany,2017,119(5):931-943.
[103]同晓蕾.苹果叶片可溶性碳水化合物与褐斑病抗性的关系研究[D].杨凌:西北农林科技大学,2017.TONG Xiaolei. The study of the relationship between soluble carbohydrate and marssonina coronaria resistance in apple leaves[D].Yangling:Northwest A&F University,2017.
[104]CHONG J,PIRON M C,MEYER S,MERDINOGLU D,BERTSCH C,MESTRE P. The SWEET family of sugar transporters in grapevine:VvSWEET4 is involved in the interaction with Botrytis cinerea[J]. Journal of Experimental Botany,2014,65(22):6589-6601.
[105]MATHAN J,SINGH A,RANJAN A. Sucrose transport and metabolism control carbon partitioning between stem and grain in rice[J]. Journal of Experimental Botany,2021,72(12):4355-4372.
[106]HUAI B,YANG Q,QIAN Y,QIAN W,KANG Z,LIU J.ABA-induced sugar transporter TaSTP6 promotes wheat susceptibility to stripe rust[J].Plant Physiology,2019,181(3):1328-1343.