猕猴桃原产中国,属于猕猴桃科(Actinidiaceae)猕猴桃属(Actinidia)的重要果树[1],因其富含维生素C(Vc,抗坏血酸)被称为水果中的“维C之王”[2]。Vc是不仅是一种重要的商品性状,而且作为一种功能性的代谢物质,在植物的生长发育、光合作用和延缓衰老方面有着十分重要的作用[3]。植物通过合成维生素C直接清除植株体内的活性氧[4],增强植物的抗逆性。已有研究表明,施用外源Vc可以提高小麦[5]、玉米[6]、甜瓜[7]、黄瓜[8-9]、水稻[10]、大白菜[11]、紫花苜蓿[12]等植物的抗盐性及干旱胁迫下玉米[13]的抗氧化酶活性,不同光质处理可显著影响草莓果实维生素C的含量[14-15]。对于高维生素C含量的猕猴桃来说,探究猕猴桃通过调控Vc 合成响应胁迫(如干旱、高盐、光质)具有重要科学意义。
维生素C 生物合成途径包括D-半乳糖醛酸途径[16]、L-半乳糖途径[17]、L-古洛糖途径[18]和肌醇途径[19],其中L-半乳糖途径是植物中Vc合成的主要途径[17]。相关研究发现不同物种控制维生素C的关键基因不同,在番茄中,GMP和GME基因的表达量影响着维生素C的含量[20],且过表达GME基因明显增强非生物胁迫的耐受性[21]。在苹果和番茄叶片中,GPP基因对维生素C积累起主要的调控作用[22]。在猕猴桃中,L-半乳糖途径中的4个基因GME、GMP、GGP和GPP具有较高的转录水平,是猕猴桃维生素C合成的主要限速因子[23]。在高维生素C含量的毛花猕猴桃中,GGP基因的表达量明显高于低维生素C 含量的山梨猕猴桃[24],被认为是猕猴桃维生素C代谢的关键基因。但是,目前胁迫如何影响猕猴桃维生素C 合成的关键基因的表达进而调控Vc 的合成机制仍不清楚。
笔者在本研究中通过设置不同的盐、干旱和光质条件,采用高效液相色谱法测定毛花猕猴叶片维生素C 含量的变化。现有研究表明,在遗传背景相同的猕猴桃后代中,可以用成熟叶片的维生素C 含量预测成熟果实中的维生素C含量[25]。通过研究上述胁迫下猕猴桃叶片的维生素C 含量,同时通过实时荧光定量PCR 方法测定不同处理后维生素C 合成相关基因的表达情况,探究盐和干旱胁迫及光质对猕猴桃维生素C合成的影响。
1 材料和方法
1.1 材料
供试品种为毛花猕猴桃华特(Actinidia eriantha Benth.),由浙江省农科院园艺所选育,试验材料取自中国科学院武汉植物园国家猕猴桃种质资源圃,盐和干旱胁迫试验组取1 年生枝条(粗1~2 cm,长50~100 cm)水培萌发出叶片后进行试验,光质试验以新鲜的毛花猕猴桃叶片为外植体,经过植物组织培养诱导分化形成植株,置于不同光质下,25 ℃环境下进行培养。
1.2 方法
1.2.1 盐胁迫试验 试验于2020—2021 年春夏季在中国科学院武汉植物园进行,从毛花猕猴桃枝条经过水培抽芽发出若干片嫩叶开始,进行不同程度盐胁迫试验,育苗室内盐胁迫组设置清水水培处理(CK)、轻度盐胁迫处理(3 g·L-1 NaCl溶液)、中度盐胁迫处理(6 g·L-1 NaCl 溶液)和重度盐胁迫处理(9 g·L-1 NaCl溶液),每组处理设置5个重复,水培溶液体积均为1 L,水培溶液每2 d 进行1 次更换。盐胁迫组在处理前(0 d)和处理后2、4、6 d 各取1 次样品,每根水培枝条分别取3枚叶片装入茶包,浸入液氮后放入-80 ℃冰箱保存样品,测定和比较不同盐处理下猕猴桃叶片的Vc含量。
1.2.2 干旱胁迫试验 育苗室内干旱胁迫组设置清水水培处理(CK)、轻度干旱胁迫处理(5%PEG6000溶液)、中度干旱胁迫处理(10% PEG6000 溶液)和重度干旱胁迫处理(20%PEG6000 溶液),每组处理设置5 个重复,水培溶液体积均为1 L,水培溶液每2 d 进行1 次更换。从毛花猕猴桃枝条经过水培抽芽发出若干片嫩叶开始,进行不同程度干旱胁迫,盐胁迫处理后0、2、4、6 d,分别收集样品并测定和比较不同程度干旱胁迫处理下猕猴桃叶片的Vc含量,其他样品处理方法及Vc 含量测定方法与盐胁迫组保持一致。
1.2.3 光质试验 组培室内设置白光、红光、蓝光和红蓝光(红光∶蓝光=1∶1)4 种不同光质(图1),白光波长450~465 nm、红光波长650~700 nm、蓝光波长465~480 nm,光照度为(200±5)μmol·m-2·s-1,环境温度为(24±1)℃,每组设置5个重复。为测定不同光质对猕猴桃叶片Vc合成的影响,保持试验材料一致性,首先将通过外植体诱导分化的组培幼苗材料放置在黑暗条件下进行暗培养,暗培养处理48 h 后分别放入白光、红光、蓝光和红蓝混合光4种不同光质条件下进行光照培养,取样时间点是暗培养处理0 h、24 h、48 h和光照培养后1 h、2 h、6 h、12 h,在这7个时间点分别取猕猴桃叶片后测定其Vc含量,比较不同光质条件下猕猴桃叶片的Vc含量的变化,其他样品处理方法及Vc含量测定方法与盐胁迫组保持一致。

图1 不同光质试验
Fig.1 Different light quality test
1.2.4 维生素C含量的测定 依照李国秀等[26]的高效液相色谱法测定猕猴桃中Vc 含量的方法并加以改进,色谱柱:Shim-pack GIST C18 柱(4.6 mm×250 mm,5 μm);流动相:V0.1%偏磷酸溶液∶V 甲醇=98∶2;流速:1.0 mL·min-1;检测波长:243 nm;柱温:40 ℃;进样量:10 μL。
1.2.5 猕猴桃维生素C合成相关基因表达分析 利用实时荧光定量PCR 技术,分析盐胁迫处理后2 d、干旱胁迫处理后2 d和光质试验中光照2 h维生素C合成途径相关基因表达水平。使用植物RNA 提取试剂盒(天根生化科技有限公司)分别提取不同处理叶片的总RNA,反转录成cDNA。以猕猴桃中Actin为内参基因进行qPCR 分析,实时荧光定量PCR 检测系统型号为7500FAS,由Applied Biosystems 公司提供;反应体系中qPCR Mix加5 μL,cDNA加1 μL,正反向引物各加0.2 μL,加无菌水补齐到10 μL;反应步骤为94 ℃预变性30 s,然后进行45 个循环的94 ℃变性5 s、60 ℃退火30 s。每个基因3个技术重复,基因相对表达量的计算公式为:2(-∆∆CT)。所用引物见表1。
表1 实时荧光定量PCR 分析所用引物
Table 1 Primers for real-time PCR analysis
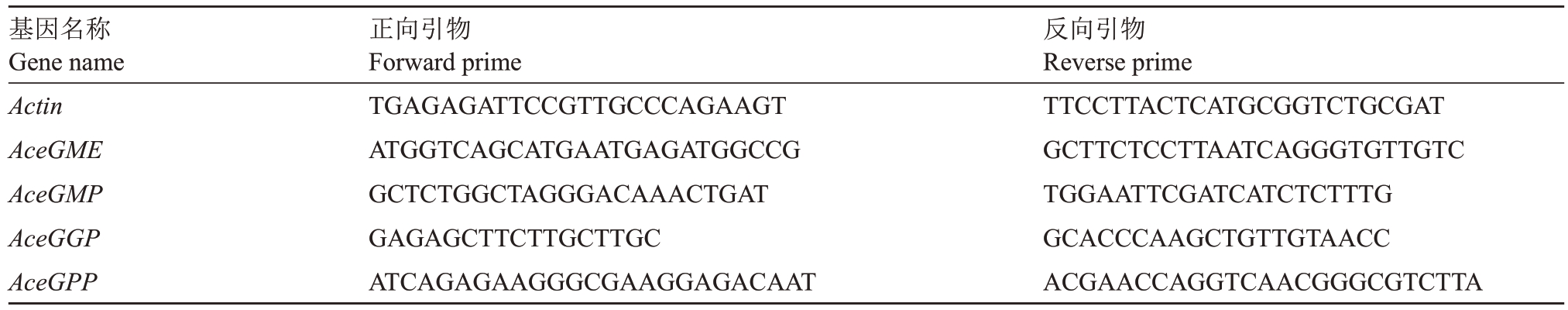
基因名称Gene name Actin AceGME AceGMP AceGGP AceGPP正向引物Forward prime TGAGAGATTCCGTTGCCCAGAAGT ATGGTCAGCATGAATGAGATGGCCG GCTCTGGCTAGGGACAAACTGAT GAGAGCTTCTTGCTTGC ATCAGAGAAGGGCGAAGGAGACAAT反向引物Reverse prime TTCCTTACTCATGCGGTCTGCGAT GCTTCTCCTTAATCAGGGTGTTGTC TGGAATTCGATCATCTCTTTG GCACCCAAGCTGTTGTAACC ACGAACCAGGTCAACGGGCGTCTTA
1.3 数据处理
试验数据采用Excel 2016软件进行常规统计分析,用SPSS 21.0 软件对数据进行单因素方差分析,多重比较采用LSD 法、Tukey 法、Duncan 法,不同小写字母表示差异显著(p<0.05),使用Photoshop软件进行绘图。
2 结果与分析
2.1 不同程度盐胁迫对猕猴桃叶片维生素C 合成的影响
由图2 可知,相对于对照处理的猕猴桃叶片随着盐胁迫处理时间增加,轻度盐胁迫均显著促进猕猴桃叶片的Vc合成(p<0.05),中度盐胁迫对Vc的积累没有明显的作用,而重度胁迫对猕猴桃叶片Vc的积累在胁迫4 d时有所增加,在6 d时下降。上述结果表明,轻度盐胁迫(3 g·L-1 NaCl溶液)显著促进猕猴桃叶片Vc的合成。
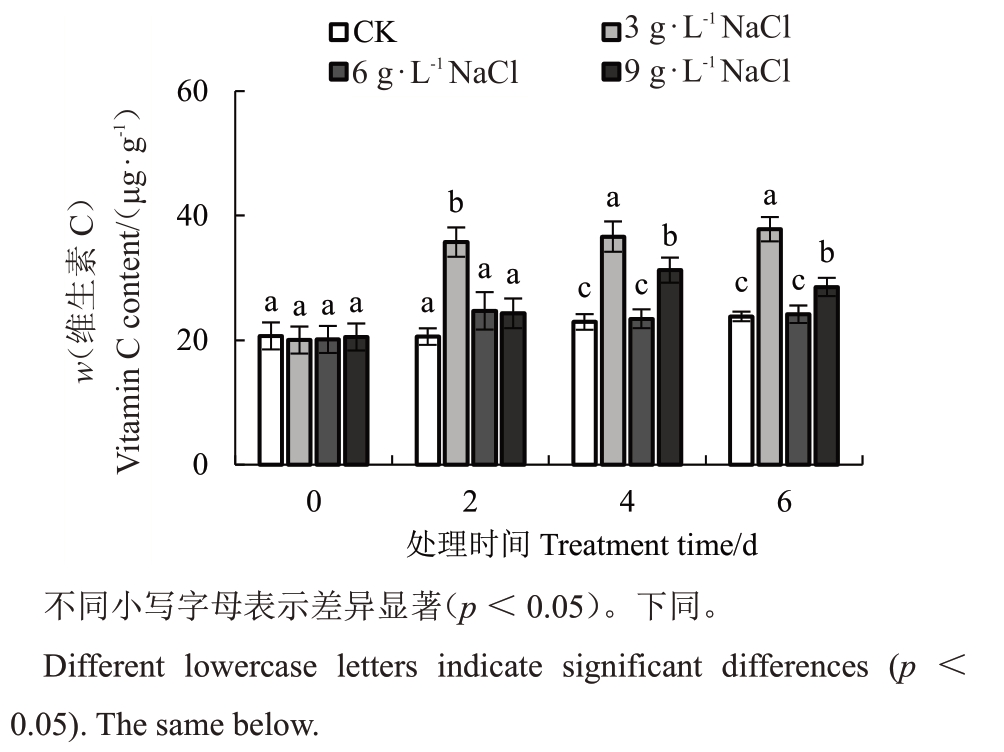
图2 不同程度盐胁迫处理猕猴桃叶片的维生素C 含量
Fig.2 Vitamin C content of kiwifruit leaves treated with different salt concentrations
2.2 不同程度干旱胁迫对猕猴桃叶片维生素C 合成的影响
由图3可知,相对于对照组猕猴桃叶片,不同程度的干旱胁迫均能显著促进叶片Vc 的积累(p<0.05)。随着干旱胁迫时间的增加,轻度干旱胁迫对猕猴桃叶片Vc含量的影响相对稳定,而中度和重度干旱胁迫随着胁迫时间的增加,对Vc的积累有轻微的增加。上述结果表明,干旱环境下会增加猕猴桃叶片Vc的积累。
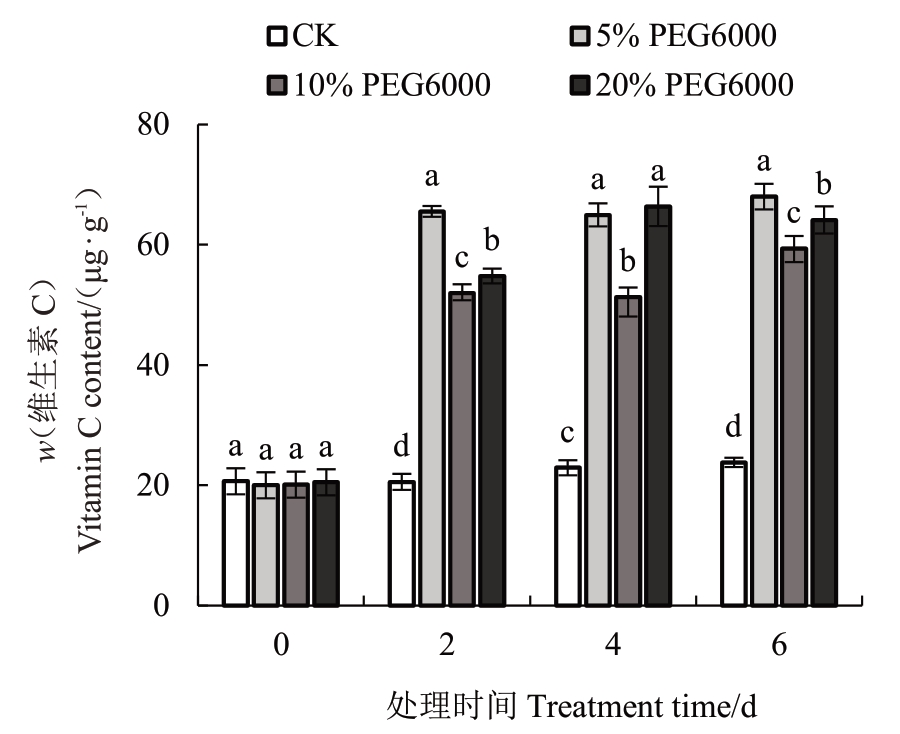
图3 不同程度干旱胁迫猕猴桃叶片的维生素C 含量
Fig.3 Vitamin C content of kiwifruit leaves treated with different drought levels
2.3 不同光质条件下猕猴桃叶片维生素C 含量的变化
如图4 所示,黑暗条件下,猕猴桃叶片Vc 含量显著低于光照条件,并且随着黑暗处理时间的增加,Vc的合成逐渐降低;当猕猴桃从黑暗转移到光照条件后,其Vc含量迅速积累,并且在光照后2 h达到峰值。在4 种不同光照条件中,相对于黑暗条件,4 种不同光质均能促进猕猴桃叶片Vc合成,其中红光的促进作用较弱。相对于白光,红蓝混合光对猕猴桃叶片Vc合成的促进作用最明显,而蓝光和红光2种单色光对Vc 积累的促进作用不如白光。上述结果表明,黑暗条件抑制猕猴桃叶片Vc 的积累,而光照条件促进Vc合成,其中红蓝混合光的促进作用比单色光强。
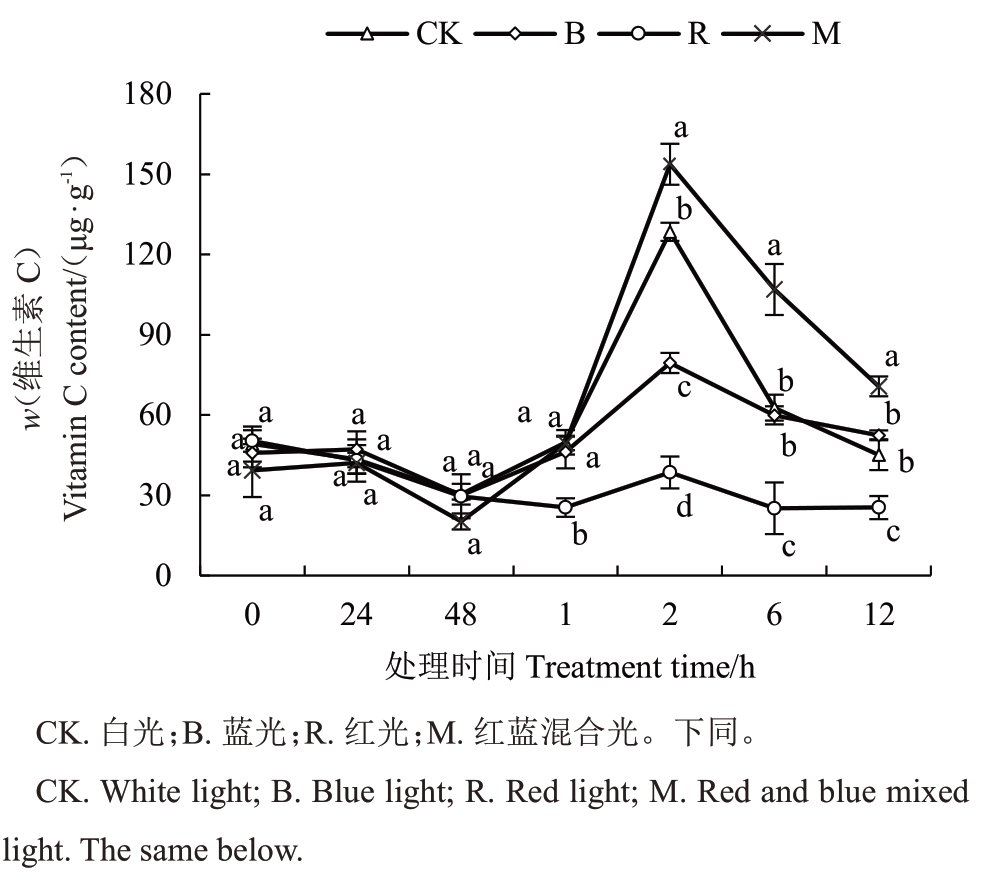
图4 不同光质处理猕猴桃叶片的维生素C 含量变化
Fig.4 Vitamin C variation of kiwifruit leaves under different light conditions
2.4 盐、干旱胁迫和不同光质条件对猕猴桃维生素C合成相关基因表达的影响
如图5 所示,L-半乳糖途径的AceGME、Ace-GMP、AceGGP 和AceGPP 基因在一定程度上呈现出与Vc 相似的表达趋势。在盐胁迫处理中,Ace-GMP 的相对表达量比对照组低,AceGME 和Ace-GPP 基因则无明显差异,而AceGGP 的相对表达量在重度盐胁迫时高于对照组,表明盐胁迫促进猕猴桃叶片Vc 含量的积累可能与AceGGP 基因的上调表达有关。
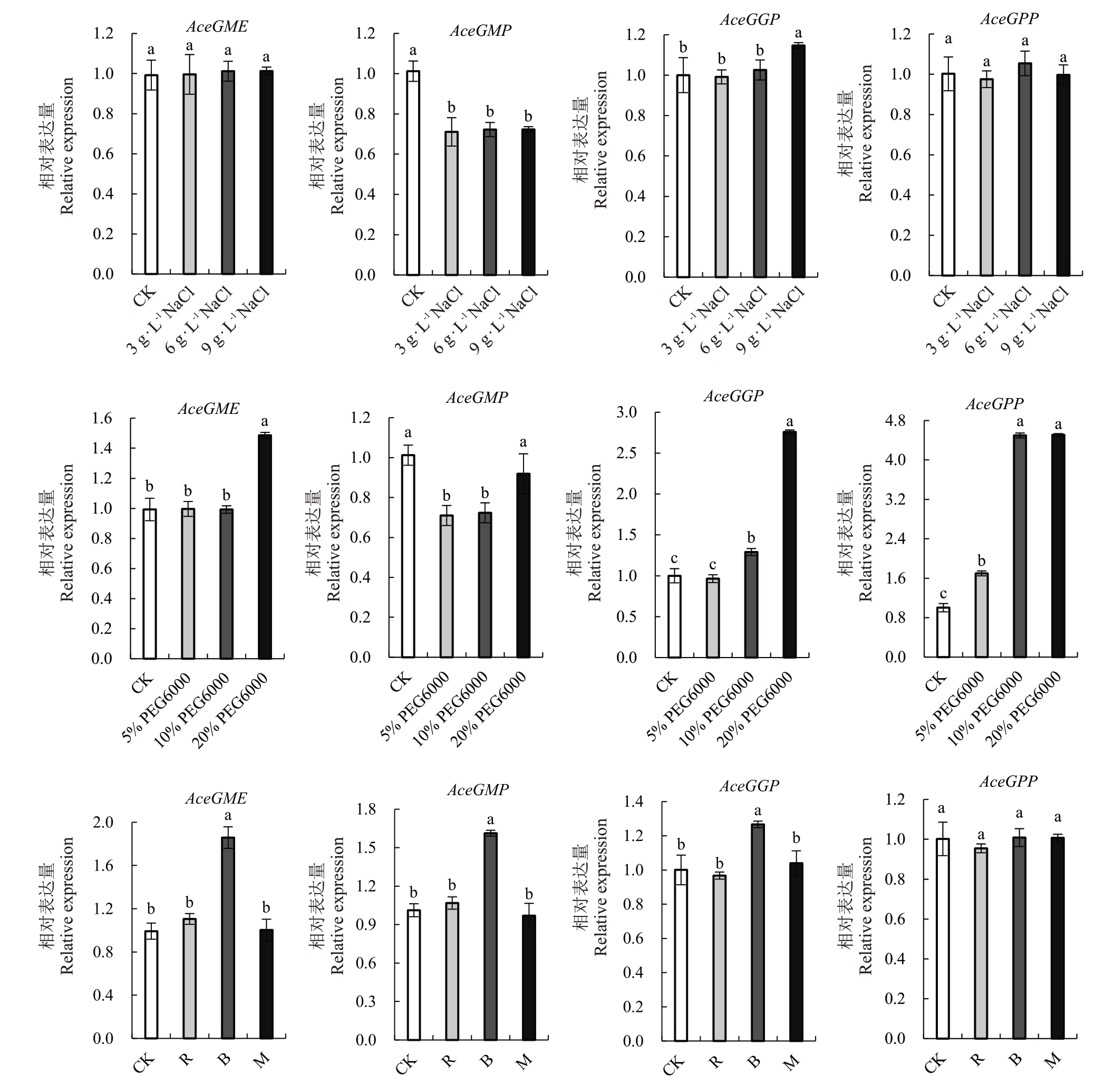
图5 盐、干旱胁迫和不同光照条件下维生素C 合成途径相关基因的表达情况
Fig.5 The expression of Vitamin C synthesis pathway-related genes under abiotic stress and different light conditions
在干旱胁迫处理中,相对于对照组,AceGMP基因随着干旱程度的增加,表达水平先降低后上升,AceGME、AceGGP 和AceGPP 在干旱胁迫后均呈现表达水平上升的趋势。干旱胁迫下AceGME、AceG-GP 和AceGPP 基因表达上调能够较好地解释猕猴桃叶片Vc积累增加的现象。
通过分析不同光照条件下AceGME、AceGMP、AceGGP 和AceGPP 基因的表达水平,相对于白光,发现在蓝光条件下,AceGME、AceGMP 和AceGGP基因的表达水平显著上升(p<0.001),AceGPP基因则无明显差异;红光和红蓝混合光对Vc合成相关基因表达的影响与对照组没有明显的差异,蓝光可能是影响维生素C合成的一个重要因子。
3 讨 论
笔者探究了不同盐浓度、干旱程度以及不同光质条件处理下猕猴桃维生素C 含量的变化,发现在低盐环境下,猕猴桃维生素C含量上升,随着盐浓度的增加,维生素C 含量逐渐降低。推测是因为在盐胁迫环境中,体内活性氧产生速率增加,自由基浓度上升,而Vc 被认为是活性氧的清除剂[27],在低盐环境下,由于活性氧的增加,刺激了Vc的合成,但是随着盐浓度不断上升,膜脂过氧化作用增强,破坏了细胞的结构和功能,导致Vc含量下降[28]。在干旱环境中,猕猴桃叶片Vc 的积累增加,在其他的研究中也发现适度的干旱有利于辣椒果实中Vc 含量的增加[29]。在光照条件下,红蓝混合光下Vc含量积累最高,这与彭鑫[30]的研究一致,不同光质处理可显著影响植株Vc 含量。对遗传背景相同的猕猴桃果实与叶片维生素C 的相关性分析研究中,叶片与果实维生素C 含量的相关系数较高[25],以上胁迫环境可以改变猕猴桃叶片中维生素C 含量,也代表了猕猴桃果实的维生素C含量也可能会受到以上胁迫环境的影响。通过这些结果可以明显发现不同胁迫环境中猕猴桃维生素C 含量的变化,推测维生素C 也可以提高猕猴桃在胁迫环境中的抗性。
通过分析盐和干旱胁迫和不同光质条件猕猴桃维生素C 合成相关基因的表达,发现猕猴桃中的AceGME、AceGMP、AceGGP 和AceGPP 基因表达水平与Vc合成水平相似。相关研究表明,在水淹胁迫下,不结球白菜中的GME基因表达量变化趋势与维生素C 含量变化一致[31]。通过抑制GGP 基因的表达,可以降低番茄抵抗低温胁迫的能力[32]。在本研究中,笔者发现盐胁迫中猕猴桃叶片中Vc含量的增加主要与AceGGP 基因的表达上调有关,干旱胁迫下猕猴桃叶片Vc 积累增加与AceGME、AceGGP 和AceGPP 基因的表达上调有关。这些结果表明,维生素C 含量与Vc 合成基因表达量的相关性会随着胁迫环境的变化而改变,但Vc合成基因受到非胁迫环境影响后的反馈机制还有待进一步研究。
4 结 论
通过设计盐和干旱胁迫及不同光质条件试验,首次发现盐、干旱和光质影响毛花猕猴桃Vc合成并改变其关键基因表达,这说明适合的环境条件能够促进猕猴桃Vc积累,为未来通过设施栽培生产高营养猕猴桃提供了可能。
[1]钟彩虹,黄文俊,李大卫,张琼,李黎.世界猕猴桃产业发展及鲜果贸易动态分析[J].中国果树,2021(7):101-108.ZHONG Caihong,HUANG Wenjun,LI Dawei,ZHANG Qiong,LI Li. Dynamic analysis of global kiwifruit and industry development and fresh fruit trade[J].China Fruits,2021(7):101-108.
[2]陈璐,王小玲,张小丽,高柱.猕猴桃AsA 生物合成途径及调控机制研究进展[J].江西科学,2021,39(2):224-232.CHEN Lu,WANG Xiaoling,ZHANG Xiaoli,GAO Zhu. Advance in biosynthesis pathway and regulation mechanism of AsA in kiwifruit[J].Jiangxi Science,2021,39(2):224-232.
[3]殷学仁.猕猴桃果实后熟进程中乙烯信号转导元件功能及其对非生物胁迫的应答[D].杭州:浙江大学,2010.YIN Xueren. The function of ethylene signal transduction element and its response to abiotic stress during the ripening process of kiwi fruit[D].Hangzhou:Zhejiang University,2010.
[4]刘拥海,俞乐,王若仲,彭新湘,萧浪涛.抗坏血酸对植物生长发育的作用及其缺失突变体的研究进展[J].植物生理学报,2011,47(9):847-854.LIU Yonghai,YU Le,WANG Ruozhong,PENG Xinxiang,XIAO Langtao.Role of ascorbic acid in plant growth and development and its deficient mutants in higher plants[J].Plant Physiology Communications,2011,47(9):847-854.
[5]周春菊,王林权,李生秀,赵伯善.有机酸和维生素对小麦某些生理生化特性的影响[J]. 西北植物学报,1999,19(4):623-628.ZHOU Chunju,WANG Linquan,LI Shengxiu,ZHAO Boshan.Effects of organic acids and vitamins on some physiological and biochemical characteristics of wheat[J]. Acta Botanica Boreali-Occidentalia Sinica,1999,19(4):623-628.
[6]孙西丽,赵方贵,张英昊,侯丽霞,刘洪庆.外源维生素对盐胁迫下玉米种子萌发和某些生理指标的影响[J].青岛农业大学学报(自然科学版),2009,26(4):313-317.SUN Xili,ZHAO Fanggui,ZHANG Yinghao,HOU Lixia,LIU Hongqing.Effects of exogenous vitamins on corn seed germination and some physiological indexes under salt stress[J]. Journal of Qingdao Agricultural University (Natural Science Edition),2009,26(4):313-317.
[7]王薇,王亚君,陈志刚.维生素C 浸种对盐胁迫下甜瓜种子萌发的影响[J].吉林蔬菜,2013(8):33-34.WANG Wei,WANG Yajun,CHEN Zhigang.Effect of vitamin C seed soaking on melon seed germination under salt stress[J]. Jilin Vegetables,2013(8):33-34.
[8]杨萍,罗庆熙,张珂珂,柳翠霞,刘健伟.外源维生素对盐胁迫下黄瓜种子发芽特性的影响[J].西南师范大学学报(自然科学版),2010,35(3):103-105.YANG Ping,LUO Qingxi,ZHANG Keke,LIU Cuixia,LIU Jianwei.Effects of exogenous vitamins on the germination characteristics of cucumber seeds under salt stress[J]. Journal of Southwest China Normal University (Natural Science Edition),2010,35(3):103-105.
[9]张洪涛,安旺盛,曾福礼.维生素C 对黄瓜叶绿体膜脂过氧化的影响[J].西北植物学报,1999,19(2):321-324.ZHANG Hongtao,AN Wangsheng,ZENG Fuli. Effect of vitamin C on peroxidation of chloroplast membrane lipid from cucumber[J].Acta Botanica Boreali-Occidentalia Sinica,1999,19(2):321-324.
[10]谭新中,曹赐生,肖用森.维生素C 对渗透胁迫下杂交稻幼苗膜脂过氧化作用的影响[J].杂交水稻,2003,18(3):59-61.TAN Xinzhong,CAO Cisheng,XIAO Yongsen. Effect of vitamin C on peroxidation of membrane lipid in hybrid rice seedlings under osmotic stress[J].Hybrid Rice,2003,18(3):59-61.
[11]唐祖君,宋明.PEG CaCl2和维生素C 对大白菜种子活力的影响[J].西南农业大学学报,1999,21(5):430-432.TANG Zujun,SONG Ming.Effects of PEG CaCl2 and vitamin C on seed vigor of Chinese cabbage[J].Journal of Southwest Agri-cultural University,1999,21(5):430-432.
[12]申玉华,徐振军,李文辉,包楠.维生素C 浸种对盐胁迫下紫花苜蓿种子发芽特性的影响[J].种子,2009,28(7):42-44.SHEN Yuhua,XU Zhenjun,LI Wenhui,BAO Nan. Effect of vitamin C seed soaking on the germination characteristics of alfalfa seed under salt stress[J].Seeds,2009,28(7):42-44.
[13]张仁和,薛吉全,浦军,赵兵,张兴华,郑友军,卜令铎.干旱胁迫对玉米苗期植株生长和光合特性的影响[J]. 作物学报,2011,37(3):521-528.ZHANG Renhe,XUE Jiquan,PU Jun,ZHAO Bing,ZHANG Xinghua,ZHENG Youjun,BU Lingduo. Effects of drought stress on plant growth and photosynthetic characteristics of maize seedlings[J]. Acta Agronomica Sinica,2011,37(3):521-528.
[14]徐凯,郭延平,张上隆,戴文圣,符庆功.不同光质膜对草莓果实品质的影响[J].园艺学报,2007,34(3):585-590.XU Kai,GUO Yanping,ZHANG Shanglong,DAI Wensheng,FU Qinggong. Effect of light quality on the fruit quality of‘Toyonoka’strawberry (Fragaria ×ananassa Duch.)[J]. Acta Horticulturae Sinica,2007,34(3):585-590.
[15]李岩,王丽伟,文莲莲,魏珉,史庆华,杨凤娟,王秀峰.红蓝光质对转色期间番茄果实主要品质的影响[J].园艺学报,2017,44(12):2372-2382.LI Yan,WANG Liwei,WEN Lianlian,WEI Min,SHI Qinghua,YANG Fengjuan,WANG Xiufeng. Effects of red and blue light qualities on main fruit quality of tomato during color-turning period[J].Acta Horticulturae Sinica,2017,44(12):2372-2382.
[16]DAVEY M W,GILOT C,PERSIAU G,ØSTERGAARD J,HAN Y,BAUW G C,VAN MONTAGU M C.Ascorbate biosynthesis in Arabidopsis cell suspension culture[J]. Plant Physiology,1999,121:535-543.
[17]WHEELER G L,JONES M A,SMIRNOFF N.The biosynthetic pathway of vitamin C in higher plants[J]. Nature,1998,393(6683):365-369.
[18]WOLUCKA B A,MONTAGU M V.GDP-mannose 3’,5’-epimerase forms GDP-L-gulose,a putative intermediate for the de novo biosynthesis of vitamin C in plants[J]. Journal of Biological Chemistry,2003,278(48):47483-47490.
[19]VALPUESTA V,BOTELLA M. Biosynthesis of L-ascorbic acid in plants:new pathways for an old antioxidant[J].Trends in Plant Science,2004,9(12):573-577.
[20]CONKLIN P L,NORRIS S R,WHEELER G L,WILLIAMS E H,SMIRNOFF N,LAST R L. Genetic evidence for the role of GDP-mannose in plant ascorbic acid(vitamin C)biosynthesis[J].Proceedings of the National Academy of Sciences,1999,96(7):4198-4203.
[21]ZHANG C J,LIU J X,ZHANG Y Y,CAI X F,GONG P J,ZHANG J H,WANG T T,LI H X,YE Z B. Overexpression of SlGMEs leads to ascorbate accumulation with en-hanced oxidative stress,cold,and salt tolerance in tomato[J]. Plant Cell Reports,2011,76(3):389-398.
[22]IOANNIDI E,KALAMAKI M S,ENGINEER C,PATERAKI I,ALEXANDROU D, MELLIDOU I,GIOVANNONNI J,KANELLIS A K. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions[J]. Journal of Experimental Botany,2009,60(2):663-678.
[23]LI J,LI M J,LIANG D,MA F W,LEI Y S. Comparison of expression pattern, genomic structure, and promoter analysis of the gene encoding GDP-L-galactose phosphorylase from two Actinidia species[J].Scientia Horticulturae,2014,169(2):206-213.
[24]LI M J,MA F W,LIANG D,LI J,WANG Y L.Ascorbate biosynthesis during early fruit development is the main reason for its accumulation in kiwi[J].PLoS One,2010,5(12):e14281.
[25]张蕾,王彦昌,黄宏文.猕猴桃(Actinidia Lindl.)叶片与果实维生素C 含量的相关性研究[J].武汉植物学研究,2010,28(6):750-755.ZHANG Lei,WANG Yanchang,HUANG Hongwwen.Investigation of the correlations between the leaf and fruit vitamin C content in Actinidia [J]. Journal of Wuhan Botanical Research,2010,28(6):750-755.
[26]李国秀,刘小宁,李劼.高效液相色谱法测定猕猴桃中的VC含量[J].保鲜与加工,2016,16(5):89-93.LI Guoxiu,LIU Xiaoning,LI Jie. Determination of VC in kiwifruit by high performance liquid chromatography[J]. Storage and Process,2016,16(5):89-93.
[27]周小华,谷照虎,徐慧妮,陈丽梅,李昆志.外源抗坏血酸AsA对铝胁迫下水稻光合特性的影响[J].扬州大学学报(农业与生命科学版),2015,36(3):73-78.ZHOU Xiaohua,GU Zhaohu,XU Huini,CHEN Limei,LI Kunzhi. Effects of exogenous ascorbic acid AsA on photosynthetic characteristics of rice under aluminum stress[J].Journal of Yangzhou University (Agriculture and Life Sciences),2015,36(3):73-78.
[28]LU J L,GAO Y J,CHANG Y J. Effects of exogenous Vc on seed germination and seedling physiological characteristics of oil sunflower (Helianthus annuus) under salt stress[J]. Agricultural Science&Technology,2015,16(3):500-503.
[29]李莉,田士林,姜俊.干旱胁迫对辣椒叶片水势及果实Vc 含量的影响[J].河南科技学院学报(自然科学版),2016,44(4):8-11.LI Li,TIAN Shilin,JIANG Jun.Effects of drought stress on leaf water potential and fruit Vc content of pepper[J]. Journal of Henan Institute of Science and Technology (Natural Science),2016,44(4):8-11.
[30]彭鑫.不同光照条件对草莓光合性能与果实品质的影响[D].杭州:浙江农林大学,2019.PENG Xin.Effects of different light conditions on photosynthetic performance and fruit quality of strawberry[D]. Hangzhou:Zhejiang Agriculture and Forestry University,2019.
[31]李妍,王雪花,陈忠文,段伟科,侯喜林,李英.不结球白菜抗坏血酸合成基因BcGME 的同源克隆及胁迫下的表达分析[J].南京农业大学学报,2016,39(2):205-212.LI Yan,WANG Xuehua,CHEN Zhongwen,DUAN Weike,HOU Xilin,LI Ying. Homologous cloning of ascorbic acid synthesis gene BcGME in non-heading Chinese cabbage and its expression analysis under stress[J]. Journal of Nanjing Agricultural University,2016,39(2):205-212.
[32]王丽燕,孟庆伟.抑制GDP-L-半乳糖磷酸酶基因(S/GGP)的表达降低番茄抵抗低温胁迫的能力[C]//山东植物生理学会第七次代表大会暨植物生物学与现代农业研讨会,2012:6-7.WANG Liyan,MENG Qingwei. Inhibiting the expression of GDP-L-galactose phosphatase gene (S/GGP) reduces the ability of tomato to resist low temperature stress[C]//Shandong Plant Physiology Society No. 7 Proceedings of the Second Congress and Symposium on Plant Biology and Modern Agriculture,2012:6-7.