我国属于大陆性季风气侯,冬季寒冷干燥,夏季炎热多雨,因此冬季冻害和生长季节病害是我国葡萄种植面临的两大难题。种间杂交种香百川由于其较好的抗寒和抗病能力在生产上得到了一定规模的栽培,是生产生态葡萄酒的供选品种。但该品种对臭氧敏感,在64.2 μg·m-3臭氧处理下香百川葡萄有18%的叶片受损,而耐臭氧品种威代尔受损叶片低于6%[1]。臭氧胁迫通过抑制葡萄叶片光系统Ⅱ(PSⅡ)的功能而影响光合作用,随着臭氧处理浓度的升高,赤霞珠葡萄叶片单位面积有活性反应中心数目(RC/CSm)以及捕获的激子将电子传递到QA以后其他电子受体的概率(φEo)均显著降低[2]。前人研究发现,光照度和O3浓度之间存在相互作用[3],强光会加剧O3对植物叶片PSⅡ的伤害,随着光强的增加伤害程度逐渐升高。同时有研究表明,遮阴能缓解臭氧胁迫对葡萄叶片的光抑制程度,提高叶片抗氧化能力[4]。由此提示在生产中可以通过栽培措施降低光照度来缓解臭氧伤害。
叶幕是指多年生果树树冠内集中分布并形成一定形状和体积的叶群体[5]。不同的叶幕类型会影响叶际果际的微域气候(包括光照、温度、湿度等),进而对葡萄的生长发育产生重要影响。葡萄生产中常见的叶幕类型有水平叶幕型、直立叶幕型、V形叶幕型、高宽垂叶幕型等。叶幕结构通过调整光照度和时间对植物光合作用产生影响,设施条件下弗雷和夏黑2种葡萄U形叶幕叶片的净光合速率日变化均高于V形叶幕[6]。棚架叶幕比篱架叶幕受光照更均匀,光合产物合成多[7],喜乐和巨玫瑰棚架水平叶幕中部节位的最大光合速率均高于V形叶幕[8]。厂字形整形方式增加了叶幕透光率和果际光合有效辐射,提高了叶片光合速率,并且加强了利用弱光以及抵御强光的能力[9]。因此,可以通过改变葡萄的叶幕结构影响光照来缓解臭氧伤害。在本试验中,笔者研究了棚架下垂叶幕与水平叶幕对香百川葡萄叶片臭氧伤害情况、光合速率及叶绿素荧光参数的影响,以期为筛选有效减缓香百川葡萄臭氧胁迫的叶幕提供一定的理论依据。
1 材料和方法
1.1 试验材料
本试验于2019 年在泰安市岱岳区祝阳镇中青葡萄酒庄基地(北纬36.17°,东经117.21°)进行,该地位于泰山山脉南麓东段,地形为丘陵山地,属暖温带半湿润性季风气候。以5年生的酿酒葡萄品种香百川(Chambourcin:Seyve Villard 12-417×Seibel 7053)为试材,南北行向种植,株行距1.0 m×4.5 m。
1.2 试验方法
试验设置小棚架龙干形水平叶幕和小棚架龙干形下垂叶幕2 种处理。水平叶幕的新梢水平绑缚;下垂叶幕的新梢在7~10枚叶片时向下引缚,使新梢与地面呈垂直状态。试验按照随机区组设计,每个处理3 个重复,每个重复50 株树。2 种处理除整形方式不同外,生长期内修剪及水肥管理技术均一致,周年无大规模病虫害发生。于6月21日对2种叶幕主梢进行保留20枚叶片摘心,于7月3号对2种叶幕副梢保留3 枚叶片摘心,于7 月20 日雨季来临前去除2种叶幕果穗节位以下所有副梢。
1.3 测定指标及方法
1.3.1 臭氧浓度的监测及叶片臭氧伤害程度分级使用深圳沃赛特公司生产的臭氧监测仪(DR70C-臭氧型)对泰安中青葡萄酒庄基地内大气臭氧浓度进行实时监测。监测仪通过RS485将臭氧浓度数据传输到电脑,每隔5 min保存1组。
如图1所示,根据香百川叶片表观伤害(棕褐色斑点面积)不同将叶片臭氧伤害程度分为0级(无表观伤害)、1 级(表观伤害面积在30%以下)、2 级(表观伤害面积为30%~60%)、3 级(表观伤害面积在60%以上)。

图1 臭氧伤害程度分级
Fig.1 Classification of ozone damage
1.3.2 叶片叶面积及叶绿素总含量的测定 分别采集2 种叶幕成熟期的主副梢叶片,将被测叶片平铺在坐标纸上,拍照并用Digimizer 软件测定叶面积。用乙醇-丙酮浸提比色法测定叶绿素含量[10]。
1.3.3 叶片净光合速率的测定 分别于2 种叶幕的转色期和成熟期选择晴天的9:00—10:00,采用CIRAS-3便携式光合作用测定仪(PPSystems,英国)测定新梢偶数节位叶片净光合速率(Pn)。每个叶幕随机选取向阳面且长势相同的中庸枝重复测定3次。
1.3.4 快速叶绿素荧光诱导曲线的测定 用连续激发式荧光仪(Handy PEA,Hansatech,英国)测量快速叶绿素荧光诱导曲线(O-J-I-P曲线),从O-J-I-P曲线中可以得到以下参数[11]:Fo:O 点(20~50 μs)最小荧光;Fk:K 点(300 μs)的荧光;Fj:J 点(2 ms)的荧光;Fi:I点(30 ms)的荧光;Fm:P点(0.3~2 s)最大荧光。
通过JIP-text 参考李鹏民等[12]的计算方式获得如下参数:PSⅡ最大光化学效率(Fv/Fm)= φPo=TRo/ABS=[1-(Fo/Fm)];单位面积内有活性的反应中心的数量(RC/CSm)=Fm×φPo×(Vj/Mo);反应中心吸收的光能用于电子传递的量子产额(φEo)=ETo/TRo=(1–Vj);J 点的相对可变荧光(Vj)=(Fj-Fo)/(Fm/Fo);进行(Fm-Fo)标准化后K 点的相对可变荧光(Wk)=(Ft-Fo)/(Fm-Fo);O-J-I-P 荧光诱导曲线的初始斜率(Mo)=4(F300μs-Fo)(Fm-Fo)。
1.4 数据统计及处理方法
使用Microsoft Excel 软件处理数据和制图,采用SPSS 17.0 软件进行方差分析,Duncan’s 方法进行多重比较,显著水平为0.05。
2 结果与分析
2.1 棚架下垂叶幕与水平叶幕对香百川葡萄叶片臭氧伤害的影响
2.1.1 葡萄园环境臭氧浓度监测 试验期间对泰安中青葡萄酒庄基地(北纬36.17°,东经117.21°)微环境臭氧浓度进行实时监控,图2 显示了从2019 年6月至9 月该基地的每日最高臭氧浓度。由图可知,从6月至9月日最高臭氧浓度(φ)的变化范围在14~56 nL·L-1之间,其中最高臭氧浓度出现在7月4日,最低臭氧浓度出现在6月28日;6月至9月的日最高臭氧浓度的平均值为30.6 nL·L-1,7月下旬进入雨季后,臭氧浓度降低。
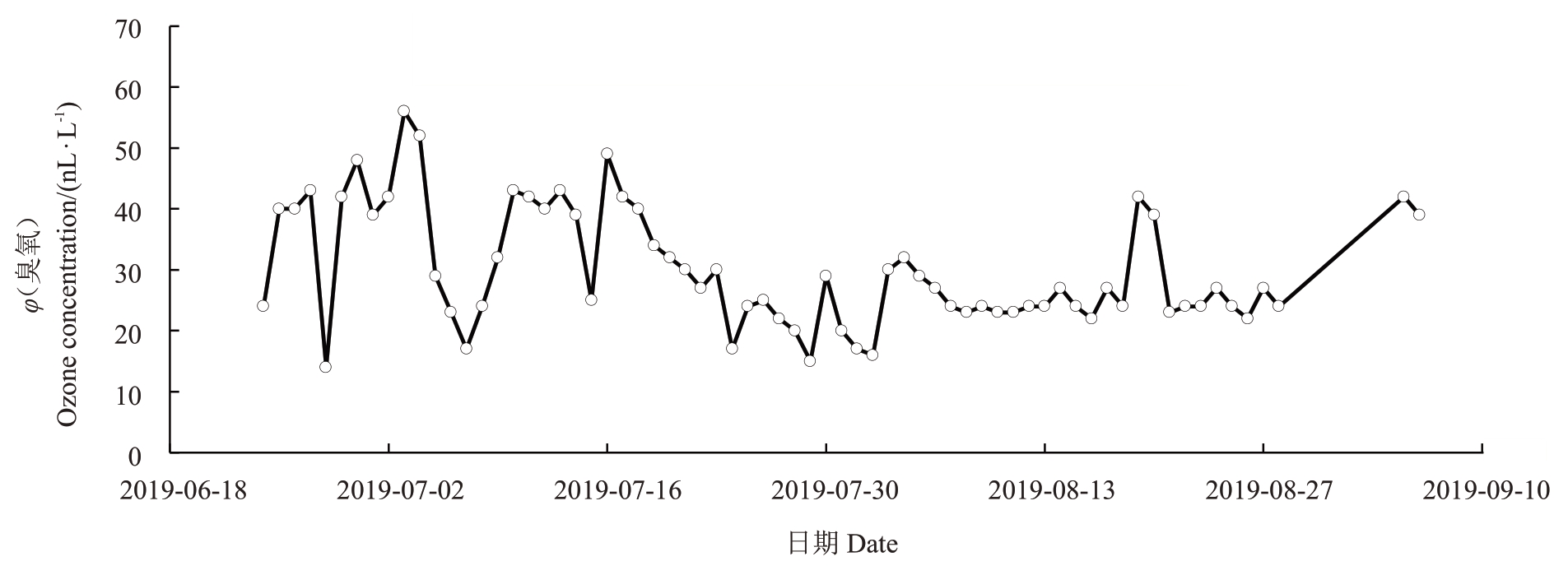
图2 葡萄园微环境臭氧浓度实时监控
Fig.2 Real time monitoring of ozone concentration in vineyard microenvironment
2.1.2 棚架下垂叶幕与水平叶幕香百川葡萄叶片臭氧伤害情况 如图3所示,6月份下垂叶幕的主副梢叶片受臭氧伤害程度显著低于水平叶幕,主梢和副梢受臭氧伤害程度0级叶片比例分别比水平叶幕高169.34%、4.66%;下垂叶幕主梢受臭氧伤害程度1级、2 级、3 级的比例分别比水平叶幕低63.07%、100%、61.12%。7月份,2种叶幕发生臭氧伤害的叶片均有所增加。下垂叶幕发生臭氧伤害的主梢叶片比例由6 月份的33.54% 上升到83.15%,发生臭氧伤害的副梢叶片比例由6 月份的8.72%上升到77.86%。水平叶幕发生臭氧伤害的主梢叶片比例由6月份的75.32%上升到90.83%,发生臭氧伤害的副梢叶片比例由6月份的12.46%上升到64.00%。
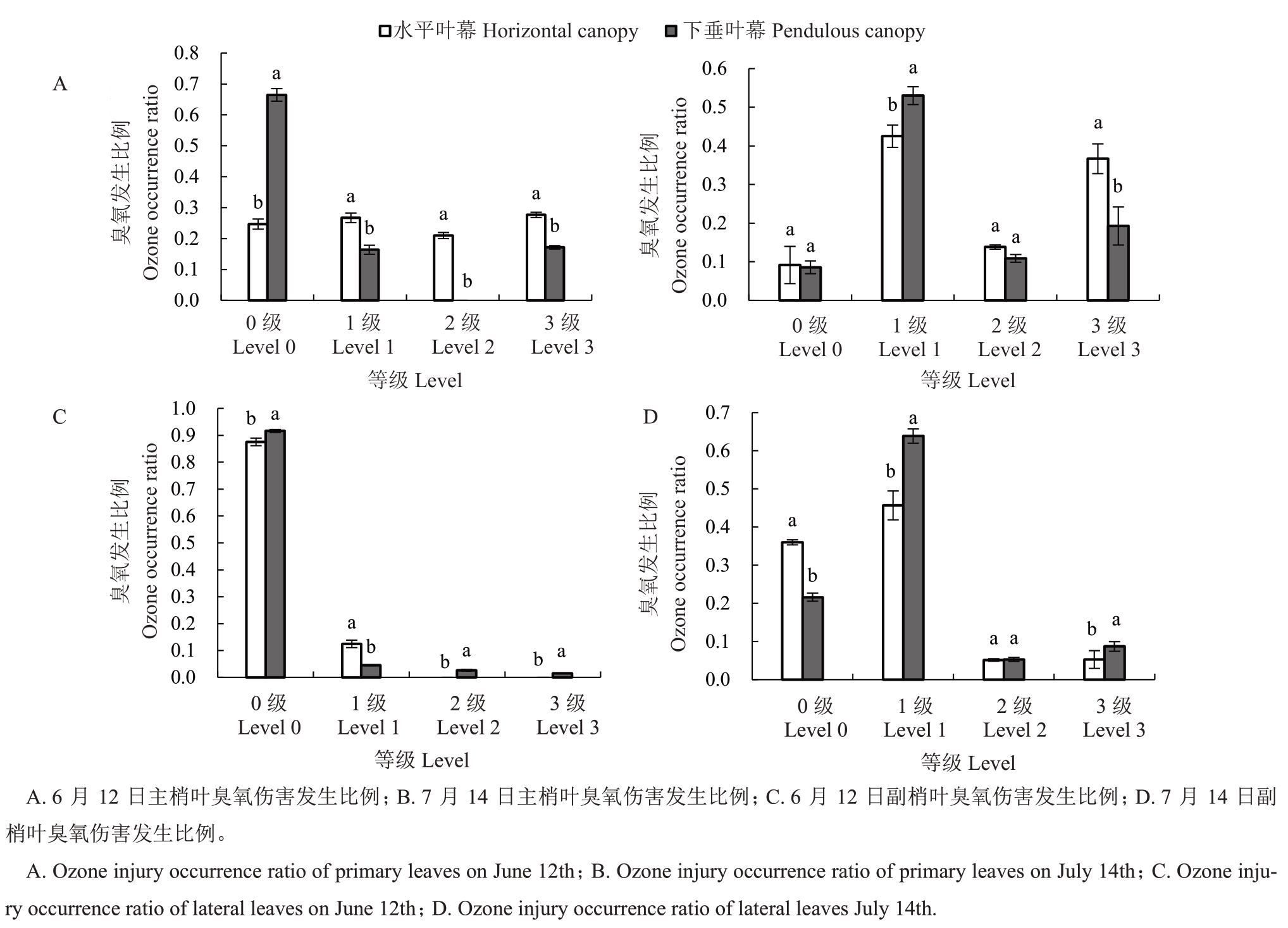
图3 棚架下垂叶幕与水平叶幕香百川叶片臭氧伤害情况
Fig.3 Ozone damage situation in Chambourcin leaves of the pendulous canopy and the horizontal canopy
2.2 棚架下垂叶幕与水平叶幕对香百川葡萄叶片叶面积和叶绿素含量的影响
成熟期时2种叶幕主梢和副梢叶片的叶面积及叶绿素总含量如图4 所示。2 种叶幕的主梢叶面积差异不显著,而下垂叶幕的副梢叶面积显著高于水平叶幕53.25%。2种叶幕的副梢叶片叶绿素含量均高于主梢叶片,下垂叶幕的主副梢叶片叶绿素含量分别比水平叶幕显著高131.18%、59.52%。

图4 棚架下垂叶幕与水平叶幕对香百川叶片叶面积和叶绿素含量的影响
Fig.4 Leaf area and chlorophyll content of Chambourcin leaves in the pendulous canopy and horizontal canopy
2.3 棚架下垂叶幕与水平叶幕对香百川葡萄叶片净光合速率的影响
由图5-A可知,在转色期,下垂叶幕主梢中部功能叶片净光合速率高于水平叶幕13%~56%,新梢叶片基部叶片与末端叶片低于水平叶幕11%~27%。下垂叶幕新梢的第2、12、14节位的叶片净光合速率分别比水平叶幕高出13.44%、28.29%、55.56%;第6、10、18 节位的叶片净光合速率分别比水平叶幕低24.81%、26.19%、11.36%;第4、8、16节位无显著性差异。由图5-B可知,在成熟期,下垂叶幕主梢的中部功能叶片净光合速率比水平叶幕高出28%~99%,新梢基部与末端净光合速率比水平叶幕低15%~60%。下垂叶幕新梢第12、14、16、18、20 节位分别比水平叶幕高28.57%、94.12%、67.55%、98.25%、92.13%;而第8、10、22、24节位新梢叶分别比水平叶幕低51.67%、59.83%、30.46%、15.33%;第2 节位老化,第4、6节位无显著性差异。
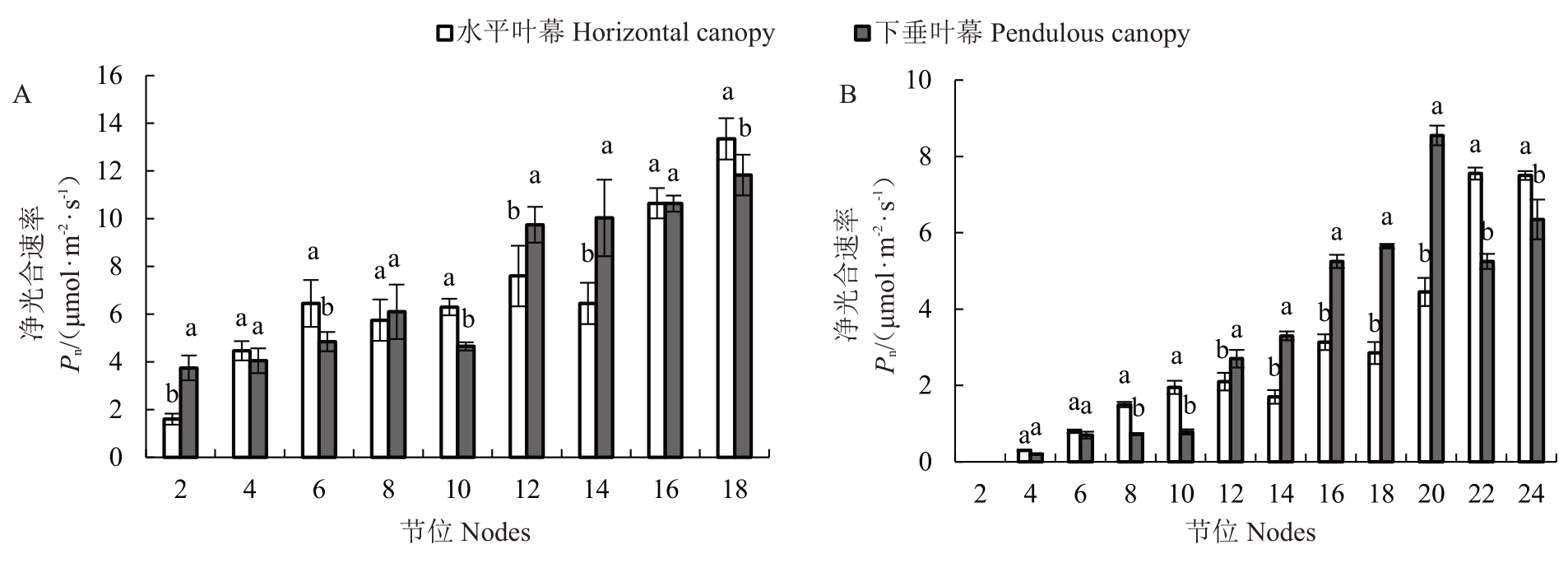
图5 棚架下垂叶幕与水平叶幕对转色期(A)和成熟期(B)香百川叶片净光合速率的影响
Fig.5 Net photosynthetic rate of Chambourcin leaves in the pendulous canopy and the horizontal canopy at veraison and berry maturity stages
2.4 棚架下垂叶幕与水平叶幕对香百川葡萄快速叶绿素荧光诱导曲线及参数的影响
叶绿素荧光蕴含着丰富的与光合原初反应有关的信息,快速叶绿素荧光诱导曲线(O-J-I-P)已经被广泛用于检测PSⅡ活性[12]。下垂叶幕与水平叶幕的香百川葡萄叶片O-J-I-P曲线差异显著,图6-A与图6-B是在转色期(7月31日)与成熟期(9月6日)时香百川葡萄叶片实际荧光曲线的变化,下垂叶幕P点的实际荧光(Fm)在2 个日期均高于水平叶幕,表明下垂叶幕叶片单位面积吸收光能的能力较强。为了更加直观地表现2 种叶幕之间的差异,对原始的O-J-I-P 曲线进行Vt=(Ft-Fo)/(Fm-Fo)标准化后得到相对荧光变化曲线(图6-C~D),从中可以看出2 种叶幕在7 月31 日与9 月6 日时均出现了明显的J 点(2 ms),水平叶幕的J点均高于下垂叶幕,表明水平叶幕叶片PSⅡ受体侧伤害程度显著高于下垂叶幕,电子从Q-A向Q-B 的传递受到限制。
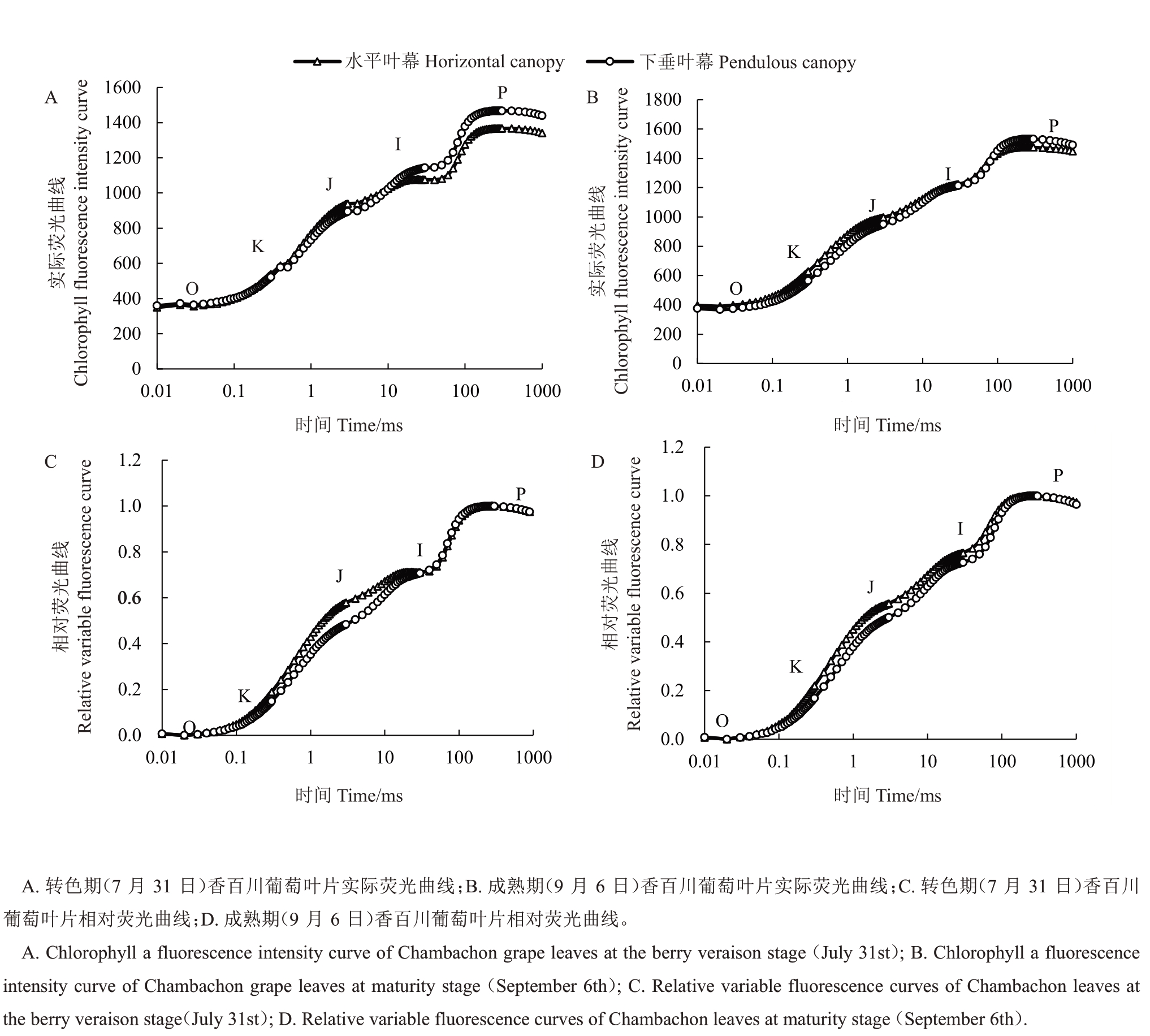
图6 棚架下垂叶幕与水平叶幕对香百川葡萄快速叶绿素荧光诱导曲线的影响
Fig.6 The O-J-I-P curve of Chambourcin leaves in the pendulous canopy and the horizontal canopy
通过JIP-text后得到的荧光动力学曲线参数,能够反映更多关于PSⅡ光化学反应的信息。Fv/Fm 是PSⅡ最大光化学量子产量,可以直观地反映PSⅡ活性,水平叶幕Fv/Fm 在7 月31 日比下垂叶幕低3.08%,而在9月6日无显著性差异。RC/CSm反映了叶片单位面积内有活性反应中心的数量,φEo反映了反应中心吸收的光能用于电子传递的量子产额,水平叶幕在7 月31 日与9 月6 日的RC/CSm与φEo均显著低于下垂叶幕,其RC/CSm在2 个日期分别比下垂叶幕低12.79%、23.58%,φEo 分别比下垂叶幕低19.02%、26.08%。Vj是在J点的相对可变荧光,可用来反映PSⅡ受体侧受到的伤害程度[13],水平叶幕在7 月31 日和9 月6 日Vj分别比下垂叶幕高12.25%、24.98%。Wk是进行Vt=(Ft-Fo)/(Fm-Fo)标准化后K点相对可变荧光的大小,能够反映出PSⅡ供体侧放氧复合体伤害程度[14]。由图7可知,在7月31日和9月6日水平叶幕的Wk均高于下垂叶幕,在7月31日下垂叶幕和水平叶幕Wk呈显著性差异,水平叶幕比下垂叶幕高5.92%,而在9 月6 日时无显著性差异。Mo是相对荧光的初始斜率,可用来表示QA被还原的相对速率[12],2 种叶幕对其影响较小,在7 月31 日和9月6日均无显著差异。
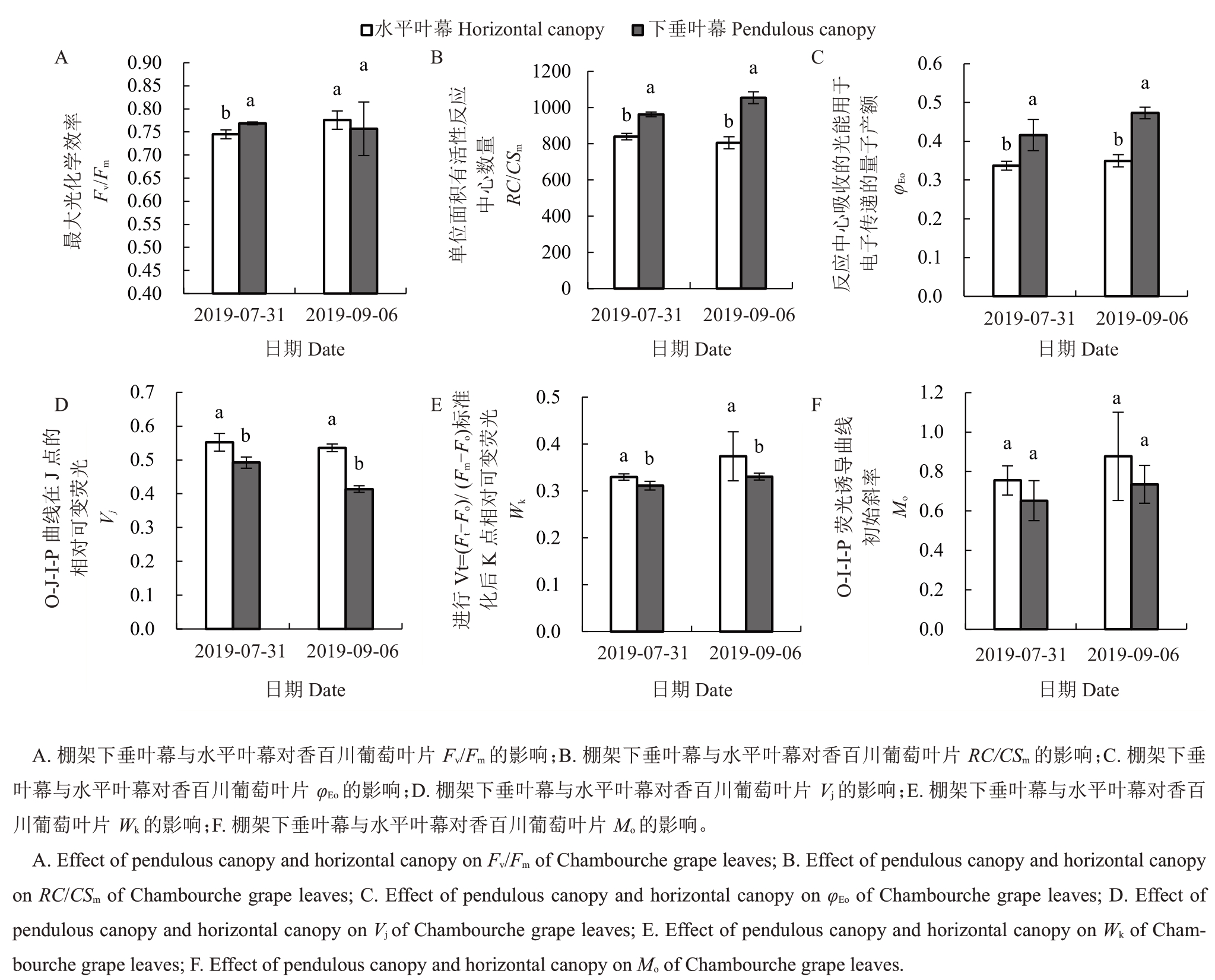
图7 棚架下垂叶幕与水平叶幕对香百川葡萄快速叶绿素荧光诱导曲线参数的影响
Fig.7 The fluorescent parameters of O-J-I-P curve of Chambourcin leaves in the pendulous canopy and the horizontal canopy
水平叶幕9 月6 日RC/CSm 比7 月31 日降低4.01%,但并无显著差异,而下垂叶幕则显著升高9.54%;水平叶幕φEo升高不显著,仅比7 月31 日高3.24%,而下垂叶幕显著升高13.68%;水平叶幕Vj仅降低3.00%,下垂叶幕显著降低15.90%。
3 讨 论
臭氧作为近地面重要的光化学污染物之一,其强氧化性会对植物正常生理活动的进行造成不良的影响[15-16]。叶片是植物与外界进行气体交换的门户,也是最先受到臭氧伤害的场所[17],叶片中叶绿体对臭氧较为敏感,叶绿体数量在臭氧处理后显著减少,并且臭氧胁迫导致的植物蛋白质表达差异也主要存在于叶绿体中[18]。叶绿体蛋白质的下调会限制叶绿素的生物合成,从而破坏光合作用并促进叶片衰老[19]。此外,强光会进一步加剧臭氧胁迫对植物的伤害程度,1000µmol·m-2·s-1光强与臭氧的共同胁迫处理显著大于单一臭氧胁迫处理对菜豆叶片的影响[20]。葡萄树形结构是决定光照的重要因素[21],本试验中香百川葡萄叶片因受到臭氧胁迫呈现棕褐色斑点的伤害症状,水平叶幕主梢叶片在6月和7月受臭氧伤害程度均高于下垂叶幕。同时,水平叶幕主梢叶片叶绿素含量在成熟期时相较于下垂叶幕也明显降低。由此说明下垂叶幕受到的臭氧伤害较轻可能与下垂叶幕接受到强光直射时间短有关,水平叶幕全天受光直射时间显著高于下垂叶幕。
臭氧胁迫会破坏叶片的叶绿素含量,进而降低植株的光合能力[22]。光合作用对臭氧引起的氧化应激十分敏感[23],臭氧诱导植物体产生的活性氧(ROS)是光合作用产生抑制的重要原因[24],同时活性氧的过量积累会影响光合作用受到光抑制时的修复,从而进一步加剧叶片受光抑制程度[25]。也有研究认为,臭氧胁迫对植物光合系统造成的抑制是由于植物气孔导度[26]或Rubisco 酶活性的降低所引起的[27]。Chen等[18]发现,在臭氧胁迫下PSⅠ和PSⅡ结构蛋白PsbR、PsaK、PsaA 表达下调,导致PSⅡ[Y(Ⅱ)]的实际光化学效率和PSI[Y(Ⅰ)]的实际光化学效率显著降低。PSⅡ是臭氧胁迫下电子传递链中最敏感的部分[28]。PSⅡ反应中心D1 蛋白在保护光合机构免受光损伤方面起着重要作用,在臭氧胁迫下PSⅡ反应中心蛋白D1蛋白表达下调,D1蛋白周转也受到抑制,进而加剧了PSⅡ的光损伤程度[18]。研究中可以通过JIP-test 来分析光系统各部分的损伤程度[12]。在7 月31 日葡萄转色期时,水平叶幕Fv/Fm比下垂叶幕低3.08%,表明臭氧胁迫对水平叶幕PSⅡ造成更严重的光损伤。邢浩[17]发现臭氧胁迫主要对赤霞珠叶片PSⅡ的受体侧造成伤害,在本试验中水平叶幕在J 点相对可变荧光(Vj)显著高于下垂叶幕,其PSⅡ受体侧受到的伤害程度更高。Wk的大小受到PSⅡ供体侧和受体侧伤害程度的共同影响,可用来表示PSⅡ供体侧相较于受体侧的受伤害程度[29]。研究发现水平叶幕Wk显著高于下垂叶幕,所以臭氧胁迫除对受体侧造成伤害外,还抑制了供体侧放氧复合体的活性,并且水平叶幕受到抑制的程度更高。同时,水平叶幕RC/CSm与φEo也均显著低于下垂叶幕,表明臭氧胁迫对水平叶幕PSⅡ反应中心造成了更严重的伤害并抑制了PSⅡ电子传递[30-31]。由此可见,水平叶幕光系统受伤害程度高于下垂叶幕。
晴朗的高光强天气时臭氧浓度通常较高,试验期间对臭氧浓度进行监测发现,6、7月份光照强、臭氧浓度高,强光协同臭氧对葡萄叶片的光系统造成了严重的损伤,显著影响葡萄叶片的光合能力[32]。但在光抑制恢复过程中D1 蛋白可以被修复,PSⅡ活性也得到恢复[33]。因此,8 月份进入雨季,环境臭氧浓度有所下降。9 月6 日成熟期水平叶幕RC/CSm、φEo、Vj与7月31日相比无显著升高或降低,而下垂叶幕RC/CSm与φEo显著升高,Vj显著降低,下垂叶幕PSⅡ反应中心、供受体侧伤害程度均小于水平叶幕,表明下垂叶幕在经历低臭氧浓度后光系统恢复能力较强。此现象可能是由于下垂叶幕每天受直射光时间较短,降低了部分葡萄叶表面的光照度进而降低了臭氧伤害程度,使得葡萄叶片PSⅡ修复速率较其破坏速率得到一定的提升,因此葡萄叶片光合能力得到较好恢复。
4 结 论
棚架下垂叶幕减少了强光直射时间,从而缓解了臭氧对PSⅡ的伤害,增加了叶片的叶绿素含量和叶片净光合速率。
[1]BOOKER F,MUNTIFERING R,MCGRATH M,BURKEY K,DECOTEAU D,FISCUS E,MANNING W,KRUPA S,CHAPPELKA A,GRANTZ D. The ozone component of global change:Potential effects on agricultural and horticultural plant yield,product quality and interactions with invasive species[J].Journal of Integrative Plant Biology,2009,51(4):337-351.
[2]孙永江,王金欢,耿庆伟,邢浩,翟衡,杜远鹏.不同浓度臭氧处理对赤霞珠葡萄叶片光系统Ⅱ功能的影响[J].植物生理学报,2015,51(11):1947-1954.SUN Yongjiang,WANG Jinhuan,GENG Qingwei,XING Hao,ZHAI Heng,DU Yuanpeng. Effects of different concentrations of ozone stress on photosynthetic system II in Vitis vinifera cv.Cabernet Sauvignon[J].Plant Physiology Journal,2015,51(11):1947-1954.
[3]GENG Q W,XING H,SUN Y J,HAO G M,ZHAI H,DU Y P.Analysis of the interaction effects of light and O3 on fluorescence properties of Cabernet Sauvignon grapes based on response surface methodology[J]. Scientia Horticulturae,2017,225:599-606.
[4]王金欢,耿庆伟,邢浩,孙永江,王杨,翟衡,杜远鹏.遮阴对臭氧胁迫下赤霞珠葡萄叶片光合功能及活性氧代谢的影响[J].果树学报,2016,33(7):823-831.WANG Jinhuan,GENG Qingwei,XING Hao,SUN Yongjiang,WANG Yang,ZHAI Heng,DU Yuanpeng.Effects of shading on photosynthesis and reactive oxygen metabolism in Vitis vinifera‘Cabernet Sauvignon’leaves under ozone stress[J]. Journal of Fruit Science,2016,33(7):823-831.
[5]XIE T T,SU P X,AN L Z,SHAN L S,ZHOU Z J,CHAI Z P.Physiological characteristics of high yield under cluster planting photosynthesis and canopy microclimate of cotton[J]. Plant Production Science,2016,19(1):165-172.
[6]王青风,郁松林,杨双双,赵宝龙,樊新民,于坤,许雯博.设施葡萄不同叶幕类型对果实发育及品质的影响[J].新疆农垦科技,2013,36(6):14-17.WANG Qingfeng,YU Songlin,YANG Shuangshuang,ZHAO Baolong,FAN Xinmin,YU Kun,XU Wenbo. Effect of different canopy types on fruit development and quality in grapevine facilities[J]. Xinjiang Farm Research of Science and Technology,2013,36(6):14-17.
[7]段长青,刘崇怀,刘凤之,王忠跃,刘延琳,徐丽明.新中国果树科学研究70 年:葡萄[J]. 果树学报,2019,36(10):1292-1301.DUAN Changqing,LIU Chonghuai,LIU Fengzhi,WANG Zhongyue,LIU Yanlin,XU Liming. Fruit scientific research in New China in the past 70 years:Grape[J]. Journal of Fruit Science,2019,36(10):1292-1301.
[8]张昱,李秀杰,韩真,李晨,李勃.不同形状叶幕形对葡萄生长的影响[J].落叶果树,2015,47(5):22-23.ZHANG Yu,LI Xiujie,HAN Zhen,LI Chen,LI Bo. Effect of different shapes of leaf curtain shape on grape growth[J].Deciduous Fruits,2015,47(5):22-23.
[9]成果,陈立业,王军,陈武,张振文.2 种整形方式对赤霞珠葡萄光合特性及果实品质的影响[J]. 果树学报,2015,32(2):215-224.CHENG Guo,CHEN Liye,WANG Jun,CHEN Wu,ZHANG Zhenwen. Effect of training system on photosynthesis and fruit characteristics of Cabernet Sauvignon[J]. Journal of Fruit Science,2015,32(2):215-224.
[10]赵世杰,苍晶.植物生理学实验指导[M].北京:中国农业出版社,2016.ZHAO Shijie,CANG Jing. Experimental guide to plant physiology[M].Beijing:China Agriculture Press,2016.
[11]STRASSER B J. Donor side capacity of photosystem Ⅱprobed by chlorophyll a fluorescence transients[J]. Photosynthesis Research,1997,52(2):147-155.
[12]李鹏民,高辉远,STRASSER R J.快速叶绿素荧光诱导动力学分析在光合作用研究中的应用[J].植物生理与分子生物学学报,2005,31(6):559-566.LI Pengmin,GAO Huiyuan,STRASSER R J. Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study[J].Journal of Plant Physiology and Molecular Biology,2005,31(6):559-566.
[13]MEHTA P,ALLAKHVERDIEV S I,JAJOO A. Characterization of photosystem II heterogeneity in response to high salt stress in wheat leaves(Triticum aestivum)[J].Photosynthesis Research,2010,105(3):249-255.
[14]罗海波,马苓,段伟,李绍华,王利军.高温胁迫对赤霞珠葡萄光合作用的影响[J].中国农业科学,2010,43(13):2744-2750.LUO Haibo,MA Ling,DUAN Wei,LI Shaohua,WANG Lijun.Influence of heat stress on photosynthesis in Vitis vinifera L. cv.Cabernet Sauvignon[J]. Scientia Agricultura Sinica,2010,43(13):2744-2750.
[15]FENG Z Z,HU E Z,WANG X K,JIANG L J,LIU X J.Groundlevel O3 pollution and its impacts on food crops in China:A review[J].Environmental Pollution,2015,199:42-48.
[16]LEISNER C P,AINSWORTH E A. Quantifying the effects of ozone on plant reproductive growth and development[J]. Global Change Biology,2012,18(2):606-616.
[17]邢浩.臭氧与光照胁迫对葡萄叶片生理功能的影响[D].泰安:山东农业大学,2018.XING Hao.The Influence of ozone and light stress on physiological function of grape leaf[D]. Tai’an:Shandong Agricultural University,2018.
[18]CHEN Z W,GAO Z,SUN Y J,WANG Y F,YAO Y X,ZHAI H,DU Y P.Analyzing the grape leaf proteome and photosynthetic process provides insights into the injury mechanisms of ozone stress[J].Plant Growth Regulation,2020,91(1):143-155.
[19]XU X B,GUO K,LIANG W W,CHEN Q F,SHI J,SHEN B.Quantitative proteomics analysis of proteins involved in leaf senescence of rice (Oryza sativa L.)[J]. Plant Growth Regulation,2018,84(2):341-349.
[20]GUIDI L,TONINI M,SOLDATINI G F. Effects of high light and ozone fumigation on photosynthesis in Phaseolus vulgaris[J].Plant Physiology and Biochemistry,2000,38(9):717-725.
[21]张抗萍,李荣飞,常耀栋,梁国鲁,陆智明,易佑文,胡涛,鲁振华,郭启高.果树树形的形成机制与调控技术研究进展[J].果树学报,2017,34(4):495-506.CHEN Kangping,LI Rongfei,CHANG Yaodong,LIANG Guolu,LU Zhiming,YI Youwen,HU Tao,LU Zhenhua,GUO Qigao. A review of the canopy architecture formation mechanism and regulation technology in fruit trees[J]. Journal of Fruit Science,2017,34(4):495-506.
[22]LÖW M,HERBINGER K,NUNN A J,HABERLE K H,LEUCHNER M,HEERDT C,WERNER H,WIPFLER P,PRETZSCH H,TAUSZ M,MATYSSEK R. Extraordinary drought of 2003 overrules ozone impact on adult beech trees(Fagus sylvatica)[J].Trees,2006,20(5):539-48.
[23]CALATAYUD A,RAMIREZ J W,IGLESIAS D J,BARRENO E. Effects of ozone on photosynthetic CO2 exchange,chlorophyll a fluorescence and antioxidant systems in lettuce leaves[J].Physiologia Plantarum,2002,116(3):308-316.
[24]DEGL'INNOCENTI E,GUIDI L,SOLDATINI G F. Characterisation of the photosynthetic response of tobacco leaves to ozone:CO2 assimilation and chlorophyll fluorescence[J]. Journal of Plant Physiology,2002,159(8):845-853.
[25]MOHANTY P,ALLAKHVERDIEV S I,MURATA N.Application of low temperatures during photoinhibition allows characterization of individual steps in photodamage and the repair of photosystem Ⅱ[J].Photosynthesis Research,2007,94(2/3):217-224.
[26]MAGGIO A,DE PASCALE S,FAGNANO M,BARBIERI G.Can salt stress-induced physiological responses protect tomato crops fro mozone damages in Mediterranean environments?[J].European Journal of Agronomy,2007,26(4):454-461.
[27]PELL E J,ECKARDT N,ENYEDI A J. Timing of ozone stress and resulting status of ribulose bisphosphate carboxylase/oxygenase and associated net photosynthesis[J]. New Phytologist,1992,120(3):397-405.
[28]TRAN T A,VASSILEVA V,PETROV P,POPOVA L P. Cadmium-induced structural disturbances in Pisum sativum leaves are alleviated by nitric oxide[J]. Doga Turkish Journal of Botany,2013,37(4):698-707.
[29]金立桥,车兴凯,张子山,高辉远.高温、强光下黄瓜叶片PSII供体侧和受体侧的伤害程度与快速荧光参数Wk 变化的关系[J].植物生理学报,2015,51(6):969-976.JIN Liqiao,CHE Xingkai,ZHANG Zishan,GAO Huiyuan. The relationship between the changes in Wk and different damage degree of PSⅡdonor side and acceptor side under high temperature with high light in Cucumber[J]. Plant Physiology Journal,2015,51(6):969-976.
[30]YAMASAKI T,YAMAKAWA T,YAMANE Y,KOIKE H,SATOH K,KATOH S. Temperature acclimation of photosynthesis and related changes in photosystem Ⅱelectron transport in winter wheat[J].Plant Physiology,2002,128(3):1087-1097.
[31]耿庆伟,邢浩,翟衡,蒋恩顺,杜远鹏.臭氧胁迫下不同光强与温度处理对赤霞珠葡萄叶片PSⅡ光化学活性的影响[J].中国农业科学,2019,52(7):1183-1191.GENG Qingwei,XING Hao,ZHAI Heng,JIANG Enshun,DU Yuanpeng.Effects of different light intensity and temperature on PS Ⅱ photochemical activity in Cabernet Sauvignon grape leaves under ozone stress[J]. Scientia Agricultura Sinica,2019,52(7):1183-1191.
[32]邢浩,郝桂梅,卞凤娥,陈钲文,王辉,翟衡,孙永江,杜远鹏.臭氧胁迫及恢复过程中葡萄叶片光系统活性的变化[J].园艺学报,2018,45(12):2321-2330.XING Hao,HAO Guimei,BIAN Feng’e,CHEN Zhengwen,WANG Hui,ZHAI Heng,SUN Yongjiang,DU Yuanpeng. The response of light system activity in grapevine leaves during ozone stress and recovery periods[J].Acta Horticulturae Sinica,2018,45(12):2321-2330.
[33]JIANG Y P,HUANG L F,CHENG F,ZHOU Y H,XIA X J ,MAO W H,SHI K,YU J Q. Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress by balancing the electron partitioning,carboxylation and redox homeostasis in cucumber[J].Physiologia Plantarum,2013,148(1):133-145.