柠檬[Citrus limon (L.) Burm. f.]属芸香科柑橘属常绿果树,在世界柑橘产区中广泛栽培,产量仅次于橙类和宽皮橘,营养价值高,经济效益好。香水柠檬和白花柠檬均是柠檬栽培品种之一,其中香水柠檬1 a 可多次开花结果,花紫红色,果实成熟后香气浓郁,具有无籽、自交不亲和、单性结实率高等众多优良性状;白花柠檬花白色,果实无浓郁香气、有籽自交亲和,单性结实率低[1]。目前,我国柠檬产业存在品种结构单一、种性退化等问题,对柠檬品种进行品质改良及优化、选育高品质柠檬品种极为重要。杂交育种是培育新品种的重要手段,选用无核品种香水柠檬为母本、有核品种白花柠檬为父本构建F1群体,获得152株杂种后代,由本研究组前期研究结果可知,经简单重复序列标记(simple sequence repeats,SSR)分子标记鉴定结果均为真杂种,为柠檬遗传连锁图谱构建及重要性状功能基因挖掘提供研究基础。同时,香水柠檬与白花柠檬杂交后代群体的创制,可以利用母本优良特性及杂种优势,选育优良单株,推动柠檬新品种培育。
遗传多样性分析一般指种内个体之间或一个群体内不同个体的遗传变异总和[2]。目前,遗传多样性的分析主要来自形态学、细胞学、生理生化及分子层次[3]。其中,形态学和分子标记被广泛应用于苹果[4]、柚[5]、金柑[6]、龙眼[7]和杜鹃花[8]等园艺植物的表型变异分析和种质资源遗传多样性分析研究。SSR为共显性标记,具有多态性高、重复性和稳定性好等优点,在果树杂种鉴定[9]、种质资源遗传多样性分析[10-11]及亲缘关系分析[12-14]中广泛应用。此外,在遗传多样性分析中,目标起始密码子多态性(start codon targeted polymorphism,SCoT)分子标记作为目标分子标记,与功能基因相关,能对部分性状相关的DNA序列进行差异扩增,从而为分子标记辅助育种提供新的参考[15-16]。SCoT 标记已广泛应用于扁柑[17]、柑橘[18]、杧果[19]、樱桃[20]等果树的遗传多样性分析中。
目前,关于柑橘遗传多样性的研究,主要集中在不同柑橘种质资源多样性评价、亲缘关系分析等方面,且杂种群体遗传多样性分析多从单一水平开展,而结合表型多样性和分子标记遗传多样性的分析在柠檬中鲜有报道。笔者在本研究中利用叶、花、果等15 个表型性状和SSR、SCoT 这2 种分子标记,对香水柠檬×白花柠檬杂种群体进行遗传多样性分析,了解柠檬主要性状的遗传规律,为后续开展遗传连锁图谱构建、重要性状的数量性状座位(quantitative trait locus,QTL)定位和遗传育种研究提供参考。
1 材料和方法
1.1 供试材料
用于遗传多样性分析的材料母本为无核品种香水柠檬、父本为有核品种白花柠檬及其152 株F1杂种。该群体于2011年人工杂交后播种至育苗小盆,2012 年换40 cm 直径大盆,2014 年定植于广西大学农学院果树科研基地,常规管理。
1.2 表型性状测定
1.2.1 描述型性状测定 在2020 年3—7 月对稳定开花结果的香水柠檬、白花柠檬及152 株柠檬杂种后代单株进行采样观察,嫩叶色、花色性状采用直接观察法评定。
1.2.2 数值型性状测定 对数值型性状采用常规测量法测量并计算。每株树冠外围随机选取春梢成熟完整且无虫害的叶片10枚,进行叶长度、叶宽度、叶形指数的测定;果实性状测定:于7月柠檬果实成熟期,每株树中上部随机选取6个果实,分别测定计算果实纵径、横径、果形指数、单果质量、果皮厚度、可溶性固形物含量、总酸含量、固酸比、维生素C含量、果汁率等性状,测定的标准参考《柑橘种质资源描述规范和数据标准》[21]。
果实纵径、横径、果皮厚度使用数显电子游标卡尺测量;单果质量使用电子天平(0.01 g)进行称量;可溶性固形物和总酸含量使用ATAGO(爱拓)柑橘糖酸度计测定;维生素C 含量采用2,6-二氯酚靛酚滴定法测定;果汁率测定使用手动榨汁机榨取果汁,计算公式:果汁率/%=果汁质量/单果质量×100。每个性状测定均3次重复。
1.3 基因组DNA提取及检测
于2019 年10 月采集群体及亲本新梢顶端幼嫩叶片,编号后放置于装有冰袋的保温箱中保存带回实验室。经蒸馏水洗净擦拭干后立即在液氮中进行研磨,利用改良十六烷基三甲基溴化铵(cetyltrimethylammonium bromide,CTAB)法提取样品DNA[22],采用1.5%(w)琼脂糖凝胶检测DNA 质量,用核酸定量仪检测DNA的纯度和质量浓度,并将所有样品质量浓度统一稀释到50 ng·μL-1,-20 ℃保存备用。
1.4 SSR分子标记
SSR引物来源于笔者课题组前期基于香水柠檬转录组开发的SSR 标记(表1),由擎科生物有限公司合成。利用2个亲本和随机选取的4个杂交后代进行引物筛选,再用筛选出的具有多态性的SSR引物对亲本和152 株杂交后代进行PCR 扩增。SSRPCR体系10 μL:DNA模板(50 mg·L-1)1 μL,上下游引物(10 μmol)各0.5 μL,2×Es Taq Master Mix(Dye)5 μL,最后用ddH2O 补足至10 μL。扩增程序:94 ℃预变性3 min;94 ℃变性30 s,60 ℃退火30 s,72 ℃延伸30 s,30 个循环;最后72 ℃延伸5 min,4 ℃保存。扩增反应结束后,PCR 产物在8%(w,后同)非变性聚丙烯酰胺凝胶电泳中检测,条件为220 V、60 min,扩增产物使用银染显色检测,人工统计电泳条带。

扩Product size/bp度长物产增15813214013215715211213812814613511811115315982101105155116基碱otif (6*11)(6*5))A(3*7)5)C(5*5)7)*6))复AA )5)(6*4 R重T(5*T(5*4)(4*6*7))SS Repeat m GC C(3*GC CT TGTG T(3*5)TA G AGA CTAT AT G(3*(4CA GC AG(2*7(4*5)4)AA TG CG GT TC GT GT G(3*7)T(3*CT AT GT AC TTTC AG C(3*6)G(3*6)AA (2(2*6 CT AG CGA TGCATAAAGT CGT AC TA AAA AT G TGAT AG AC TTTG TC GA CTAAAC G TTCTA ACCC AGTAT AAAT CTAG GA AATT CA AG TGAG CAAC TTGA CC CGGGAA GT CAAAGCGT CTTT CTGAAC 3’)TC CC ATGC CATAATTT CT TC GA AA dy GTGA GT TC GTTC AT GG GATACTAAGG CC AA TC AT AC GTAT TTGCAT 3’)AA AA GGGG ACAC TTTT AA ACCC CTGATC CT AA TG AA AGAC CT CT AACA CCAC AGAC CT(5’–TTAA CGCG CGTG GC AC CGAG CTTAAC TA AG CAAA CG TT CGAAGTCC TGAT AAAA TA AA sed in this stu TACA TACT AC TA TCGA TTTG AGGA物引A AGA TT TGAA TC列TT CT AA TAAA TC CA CG CA GG AAAGCA ACCG CT GT R 引序ers u TG反Reverse primer(5’–向GC GG TT TA AC CT CTGT CAGAGA TTCA GG CG AC AA CA AA GG CT CCAATAAC物AC TT AA CT TC TA AC GC CA CA TC CG TC TG SSprim的SR G中CGACT AAAT uences of the S GT TTTTCGAGGA T究TCT GTG GC TTAG GC ACCT GCATC GTGT G A GG研TC TACT本C TAA CC GA CG TAAA TCTGCG GCTC GCTTTA ATGGAG GGCTGA 3’)AA AT 1 A TCA TA TT ACAA AC GTGT AT CGATTT TAAG表GCTAAG GTAGAT TG AAGGACAA TGAT TGTGAC eq TA CA GC CA 3’)GGCAAAGA AC ATCTAA TT AT ble 1 S TC T GCCTC GTTC TG TACG GCTTCA TAAA GA AA引ard primer(5’–TACG GT GGGA TG CTTT GCGGTT CAGGTT TG GA GGAT CTGA TGGTAG TTCC TAAA GT GA AC TGTG(5’–GGTATT GA TCGT TATA AT TTTCCA AA GT A ATC CGAAAT AT AA AA GT TT TT AATC TG Ta 物CTAGGA TA CT AAGA AT ACCT ATGA CT GA GT GG GA AC GG正Forw向CA CC TT CGGA TG TTGT AT AT GA CT AA TA CA TT TT TT TGGAGT GA GA GCAT ATAG ATCG GATG AGTG TGGG ACGC位e position置始R起SS R homSS 28915759874917283645 181811210585128775858 352315 574771976881191245 3216 tig3 tig 1on onntig4 onntig3tig3 tig 4on onononononononononntig4 tig7 tig2 tig1 tig1 tig3 tig3 tig1 tig3 tig1 tig2 tig2 ene ID onntig4 ontig7 Unig 1273.C CL 141.Co CL 1498.C CL 1941.C CL 216.Co CL 2206.C CL 2213.C CL 2297.C CL 2346.C CL 2449.C CL 2557.C CL 2971.C CL 3039.C CL 3149.C CL 3154.C CL 370.Co CL 4026.C CL 432.Co CL 4333.Con CL 43.C CL序er cod e号物引Prim C3-860-5-3 CL C3-119CL C1-995-29-3 CL C3-116CL C 4-15 9-2CL C 4-13 15-3CL C 7-13 21-3CL C 2-13 51-2CL C 1-13 95-4CL C 3-14 38-3CL C 3-14 86-3CL C 3-16 57-3CL C 1-16 66-3CL C 3-17 06-4CL C 1-17 09-2CL C 1-32 8-4CL C 2-20 10-4CL C 4-37 3-4CL C 3-21 27-3CL C 7-49-1CL
1.5 SCoT分子标记
SCoT引物参考前期研究结果[22],由擎科生物有限公司合成。利用2个亲本和随机选取的6个杂交后代进行引物筛选,再用筛选出的具有多态性的引物对亲本和152 株杂交后代进行PCR 扩增。SCoTPCR 体系20 μL:DNA 模板(50 mg·L-1)1μL,引物(10 μmol)各1 μL,2×Es Taq Master Mix(Dye)10 μL,最后用ddH2O 补足至20 μL。扩增程序:95 ℃预变性3 min;95 ℃变性50 s,55 ℃退火1 min,72 ℃延伸2 min,38 个循环;最后72 ℃延伸8 min,10 ℃保存。扩增反应结束后,PCR产物在2.0%琼脂糖凝胶电泳检测,紫外灯下拍照记录。
1.6 数据统计与分析
利用Excel 2013 对观测的表型数据进行整理,利用SPSS 18.0进行频率描述统计、正态性检验及相关性分析,绘制频率分布直方图。相关计算公式参考闫忠业等[23]方法。
对扩增后的清晰且易于辨认的条带利用Excel 2013进行统计,SSR标记采用A、B、C、D共4个字母对亲本及F1代单株的不同等位基因进行标注,SCoT标记采用0/1系统记录位置。利用POPGENE V.1.32软件计算SSR 标记和SCoT 标记的遗传多样性参数,生成亲本和杂交后代之间的Nei’s(72)遗传距离矩阵。基于遗传距离,利用MEGA 7.0软件,采用非加权组平均法(unweighted pair-group method with arithmetic means,UPGMA)构建聚类图,最后使用iTOL 软件(iTOL: Interactive Tree Of Life (embl.de)在线编辑美化。利用NTSYS PC v.2.1 中Mantel 检验对SSR 和SCoT 分子标记得到的Nei’s 遗传距离矩阵进行相关性分析。
2 结果与分析
2.1 表型性状遗传多样性分析
2.1.1 数值型性状离散特征值分析 香水柠檬×白花柠檬杂种群体F1代的叶、果等13个表型性状的变异幅度较大(图1),变异系数范围为9.76%~45.37%(表2)。其中,单果质量、维生素C含量和叶形指数的变异系数最大,分别为45.37%、43.63%和43.51%;其次为果汁率、果皮厚度和固酸比,变异系数在20.15%~26.73%之间;仅可溶性固形物含量的变异系数小于10%。杂交群体中叶长度、纵径、单果质量和维生素C 含量性状分离范围较广,多数性状存在广泛的遗传变异。
表2 杂种群体表型性状描述统计
Table 2 Descriptive statistics of phenotypic traits of hybrid population
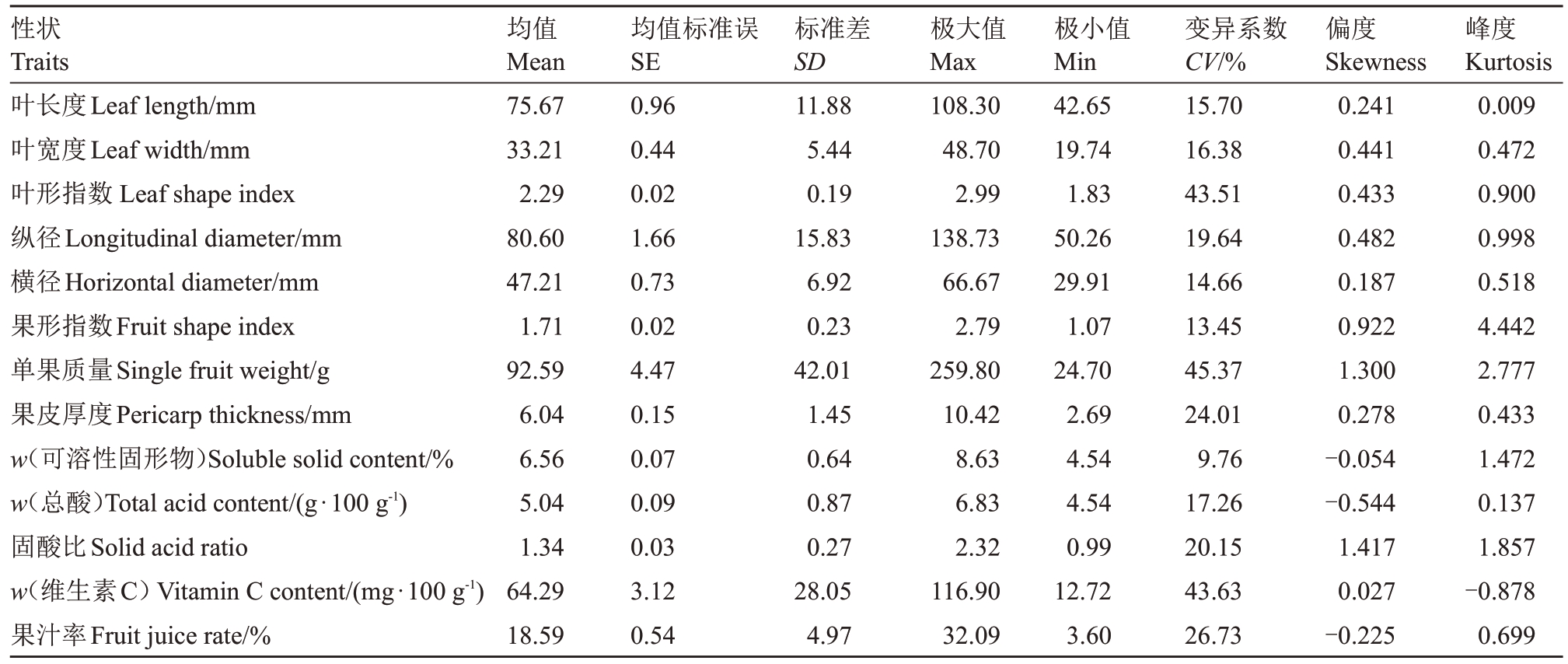
性状Traits叶长度Leaf length/mm叶宽度Leaf width/mm叶形指数Leaf shape index纵径Longitudinal diameter/mm横径Horizontal diameter/mm果形指数Fruit shape index单果质量Single fruit weight/g果皮厚度Pericarp thickness/mm w(可溶性固形物)Soluble solid content/%w(总酸)Total acid content/(g·100 g-1)固酸比Solid acid ratio w(维生素C)Vitamin C content/(mg·100 g-1)果汁率Fruit juice rate/%均值Mean 75.67 33.21 2.29 80.60 47.21 1.71 92.59 6.04 6.56 5.04 1.34 64.29 18.59均值标准误SE 0.96 0.44 0.02 1.66 0.73 0.02 4.47 0.15 0.07 0.09 0.03 3.12 0.54标准差SD 11.88 5.44 0.19 15.83 6.92 0.23 42.01 1.45 0.64 0.87 0.27 28.05 4.97极大值Max 108.30 48.70 2.99 138.73 66.67 2.79 259.80 10.42 8.63 6.83 2.32 116.90 32.09极小值Min 42.65 19.74 1.83 50.26 29.91 1.07 24.70 2.69 4.54 4.54 0.99 12.72 3.60变异系数CV/%15.70 16.38 43.51 19.64 14.66 13.45 45.37 24.01 9.76 17.26 20.15 43.63 26.73偏度Skewness 0.241 0.441 0.433 0.482 0.187 0.922 1.300 0.278-0.054-0.544 1.417 0.027-0.225峰度Kurtosis 0.009 0.472 0.900 0.998 0.518 4.442 2.777 0.433 1.472 0.137 1.857-0.878 0.699
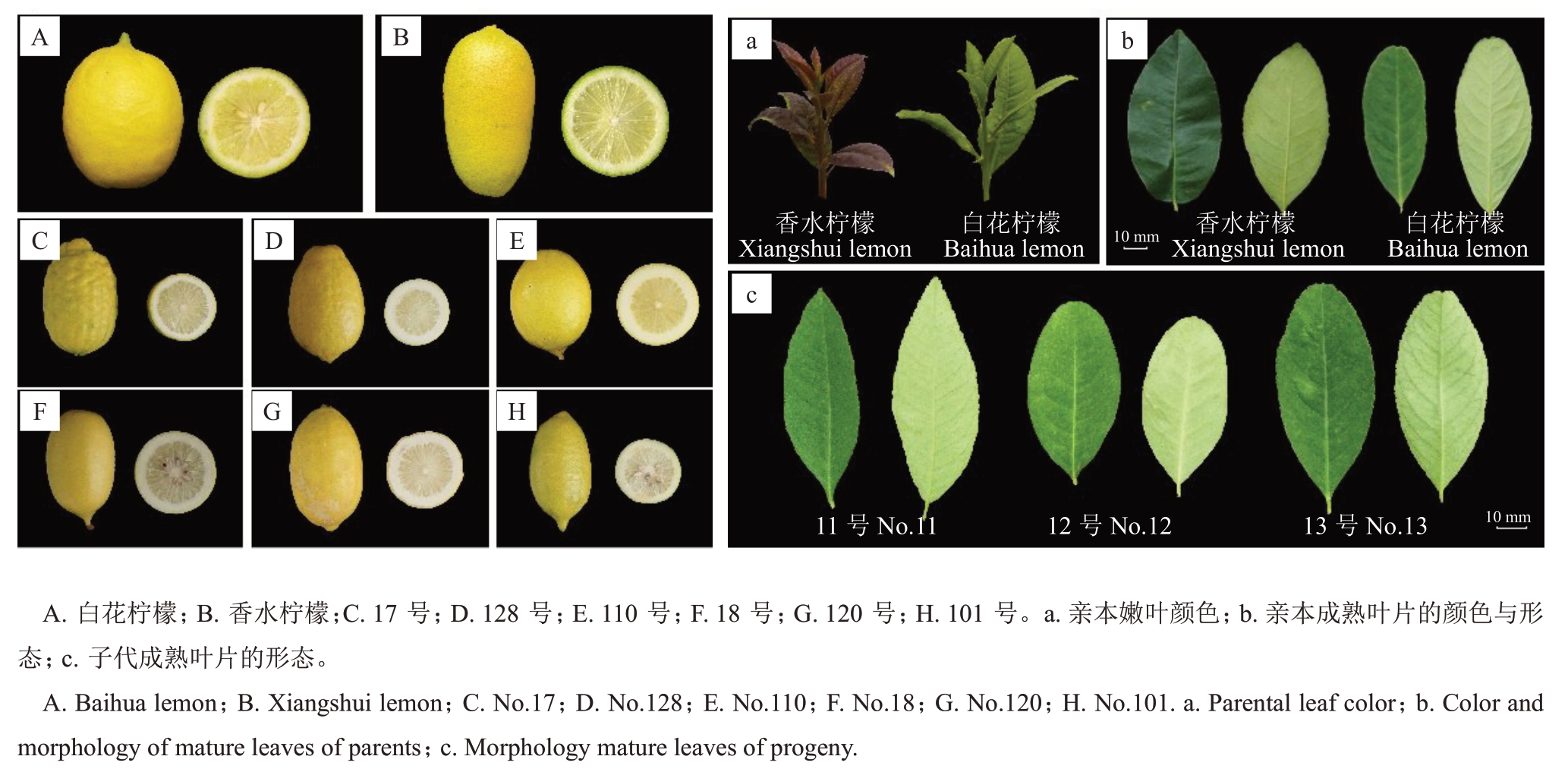
图1 香水柠檬与白花柠檬杂种后代部分叶片和果实形态
Fig.1 Leaf and fruit morphology of Xiangshui lemon,Baihua lemon and their hybrids
对杂种群体的表型性状进行频率分析(图2 和表2),结果表明,叶长度、叶宽度、叶形指数、纵径、横径、果形指数、单果质量、果皮厚度、可溶性固形物含量、总酸含量、固酸比、维生素C 含量和果汁率都呈现单峰分布,接近正态分布,符合数量性分布的特点。其中,果形指数和可溶性固形物含量的峰度大于1,偏度小于1,呈正态分布;单果质量和固酸比的峰度和偏度均大于1,呈现明显的偏正态性分布;叶长度、叶宽度、叶形指数、纵径、横径、果皮厚度、总酸含量、维生素C 含量和果汁率的偏度和峰度均小于1,呈正态分布。
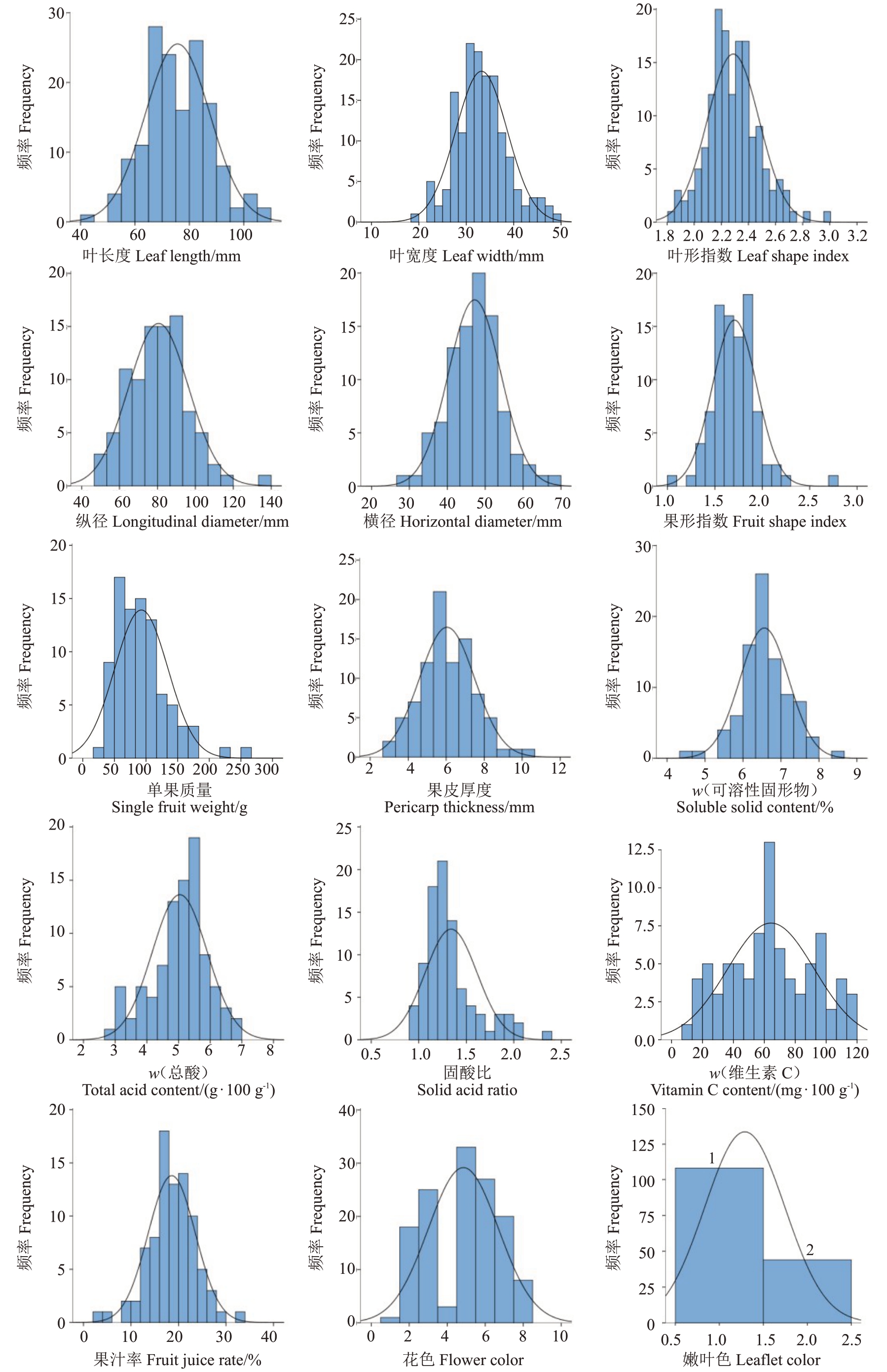
图2 杂种后代中15 个表型性状频率分布直方图
Fig.2 Frequency distribution histogram of 15 phenotypic traits in hybrid population
2.1.2 数量性状遗传分析 对杂种后代叶和果实的数量性状进行遗传分析(表3),结果表明,13个数量性状遗传传递力的范围在34.27%~152.27%之间,最大的是总酸含量;其中果皮厚度、果形指数、叶长度、叶形指数的遗传传递力均在100%以上。性状之间杂种优势率差异较大,在-65.73%~52.27%之间,其中叶长度、叶形指数、果形指数、果皮厚度及总酸含量性状表现为正向优势率,叶宽度、纵径、横径、单果质量、可溶性固形物含量、固酸比、维生素C 含量和果汁率等性状表现为负向优势率。此外,在13个数量性状中均存在低于低亲或高于高亲的杂种单株,果形指数、总酸含量和果皮厚度3 个性状总体表现为较强的偏高亲变异;纵径、横径、单果质量、固酸比和果汁率性状的遗传倾向表现为较强的趋小变异;叶宽度、叶形指数和可溶性固形物含量表现为趋中变异;叶长度和维生素C含量分离广泛。总体来说,叶片和果实的13 个性状是典型的由多个基因共同控制的数量性状,杂种后代群体遗传变异丰富。
表3 杂种群体数量性状遗传分析
Table 3 Genetic analysis of quantitative traits in hybrid populations
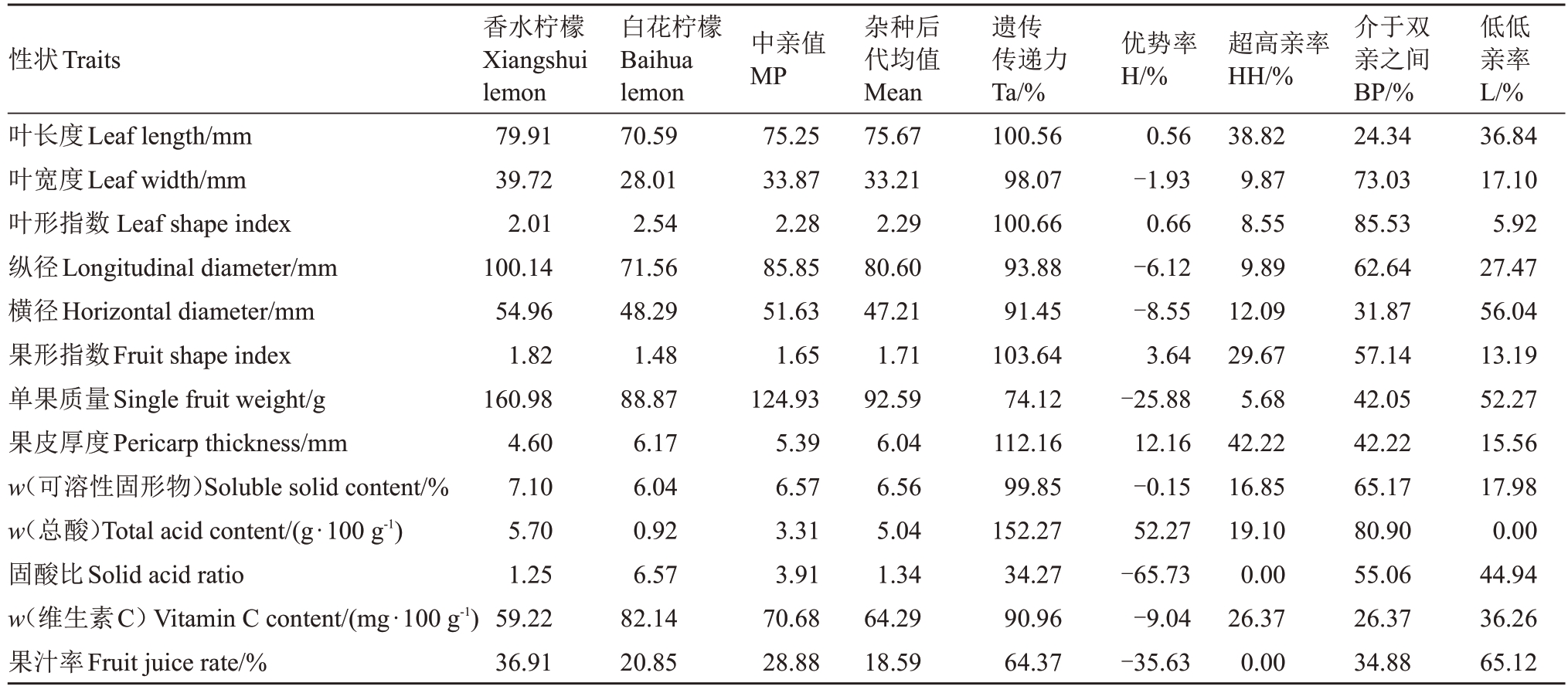
性状Traits叶长度Leaf length/mm叶宽度Leaf width/mm叶形指数Leaf shape index纵径Longitudinal diameter/mm横径Horizontal diameter/mm果形指数Fruit shape index单果质量Single fruit weight/g果皮厚度Pericarp thickness/mm w(可溶性固形物)Soluble solid content/%w(总酸)Total acid content/(g·100 g-1)固酸比Solid acid ratio w(维生素C)Vitamin C content/(mg·100 g-1)果汁率Fruit juice rate/%香水柠檬Xiangshui lemon 79.91 39.72 2.01 100.14 54.96 1.82 160.98 4.60 7.10 5.70 1.25 59.22 36.91白花柠檬Baihua lemon 70.59 28.01 2.54 71.56 48.29 1.48 88.87 6.17 6.04 0.92 6.57 82.14 20.85中亲值MP 75.25 33.87 2.28 85.85 51.63 1.65 124.93 5.39 6.57 3.31 3.91 70.68 28.88杂种后代均值Mean 75.67 33.21 2.29 80.60 47.21 1.71 92.59 6.04 6.56 5.04 1.34 64.29 18.59遗传传递力Ta/%100.56 98.07 100.66 93.88 91.45 103.64 74.12 112.16 99.85 152.27 34.27 90.96 64.37优势率H/%0.56-1.93 0.66-6.12-8.55 3.64-25.88 12.16-0.15 52.27-65.73-9.04-35.63超高亲率HH/%38.82 9.87 8.55 9.89 12.09 29.67 5.68 42.22 16.85 19.10 0.00 26.37 0.00介于双亲之间BP/%24.34 73.03 85.53 62.64 31.87 57.14 42.05 42.22 65.17 80.90 55.06 26.37 34.88低低亲率L/%36.84 17.10 5.92 27.47 56.04 13.19 52.27 15.56 17.98 0.00 44.94 36.26 65.12
2.1.3 质量性状遗传分析 从表4和图2可知,杂交后代群体中嫩叶色变异系数为35.66%,遗传传递力为86%,相较于青绿色,紫红色嫩叶占比较大,后代群体中紫红色与青绿色嫩叶表型分离比趋于3∶1,偏母本香水柠檬遗传较多。花色性状变异丰富(图3),变异系数为38.14%,遗传传递力为80.83%,双亲花色分别为紫红色和白色,杂种后代中花色分离广泛,与母本花色相近的数量最多,划分为9 种花色,其中紫色花所占比例最多,为24.4%,未观察到白色花性状。
表4 杂种群体质量性状描述统计
Table 4 Descriptive statistics on quality traits of hybrid populations

注:嫩叶色-1=紫红色,2=青绿色;花色-1=紫黑色,2=深紫红色,3=紫红色,4=深紫色,5=紫色,6=淡紫色,7=紫白色,8=白紫色,9=白色。
Note:Leaflet color:1=purple red,2=green.Flower color:1=purple black,2=deep purple red,3=purple red,4=deep purple,5=purple,6=pale purple,7=purple white,8=white purple,9=white.
性状Traits嫩叶色Leaflet color花色Flower color香水柠檬Xiangshui lemon白花柠檬Baihua lemon 23456789 13 29中亲值MP 1.5平均值Mean 1.29标准差SD 0.46变异系数CV/%35.66遗传传递力Ta/%86.00杂交群体分布频率Distribution frequency/%1 71.128.9 6.04.851.8538.1480.830.713.318.52.224.42014.85.90.0

图3 香水柠檬与白花柠檬杂种后代部分花形态
Fig.3 Flower morphology of Xiangshui lemon,Baihua lemon and their hybrids
2.1.4 表型性状相关性分析 叶、花和果实之间存在一定的相关性(表5),结果显示,叶长度与叶宽度、花色呈极显著正相关,与叶形指数呈显著性正相关,与嫩叶色和可溶性固形物含量呈显著负相关,且相关性不高;叶宽度与叶形指数呈极显著负相关,与花色呈极显著正相关,与果实纵径呈显著负相关;叶形指数与总酸含量呈显著正相关,与固酸比呈显著负相关;嫩叶色与维生素C 含量和果汁率呈显著负相关,且相关性不高;纵径与横径、果形指数、单果质量、果皮厚度和总酸含量呈极显著正相关,与固酸比呈极显著负相关;横径与果皮厚度、单果质量和总酸含量呈极显著正相关,与固酸比呈极显著负相关;果形指数与单果质量呈显著正相关,与维生素C 含量呈极显著负相关;果皮厚度与单果质量呈极显著正相关;单果质量与总酸含量呈极显著正相关,与固酸比呈极显著负相关;可溶性固形物含量与总酸含量呈、固酸比呈显著正相关,与维生素C含量呈极显著正相关;总酸含量与固酸比、果汁率呈极显著负相关,与维生素C含量呈极显著正相关;固酸比与果汁率呈极显著负相关;维生素C 含量与果汁率相关性不显著。
表5 杂种后代表型性状相关性分析
Table 5 Correlation analysis of phenotypic traits in hybrid population
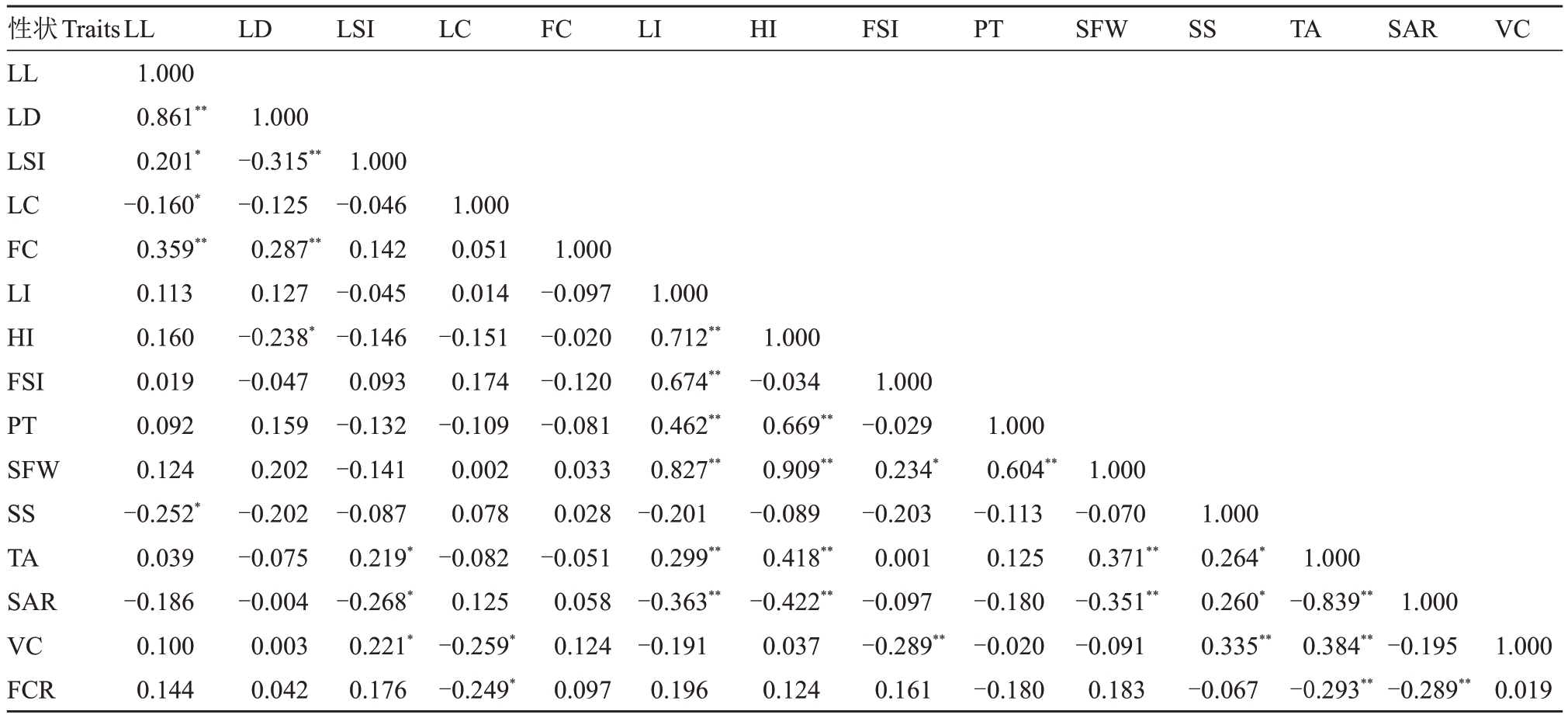
注:**. 在0.01 水平(双侧)上显著相关;*. 在0.05 水平(双侧)上显著相关。LL. 叶长度;LD. 叶宽度;LSI. 叶形指数;LI. 纵径;HI. 横径;FSI. 果形指数;SFW. 单果质量;PT. 果皮厚度;SS. 可溶性固形物含量;TA. 总酸含量;SAR. 固酸比;VC. 维生素C 含量;FCR. 果汁率;LC. 嫩叶色;FC. 花色。
Note: ** present significant correlation at 0.01 level; * present signification correlation at 0.05 level. LL. Leaf length; LD. Leaf width; LSI. Leaf shape index; LI. Longitudinal; HI. Horizontal; FSI. Fruit shape index; SFW. Single fruit weight; PT. Pericarp thickness; SS. Soluble solid;TA.Total acid;SAR.Solid acid ratio;VC.Vitamin C;FCR.Fruit juice ratio;LC.Leaflet color;FC.Flower color.
1.000 0.019性状Traits LL LD LSI LC FC LI HI FSI PT SFW SS TA SAR VC FCR LL 1.000 0.861**0.201*-0.160*0.359**0.113 0.160 0.019 0.092 0.124-0.252*0.039-0.186 0.100 0.144 LD LSI LC FC LI HI FSI PT SFW SS TA SAR VC 1.000-0.315**-0.125 0.287**0.127-0.238*-0.047 0.159 0.202-0.202-0.075-0.004 0.003 0.042 1.000-0.046 0.142-0.045-0.146 0.093-0.132-0.141-0.087 0.219*-0.268*0.221*0.176 1.000 0.051 0.014-0.151 0.174-0.109 0.002 0.078-0.082 0.125-0.259*-0.249*1.000-0.097-0.020-0.120-0.081 0.033 0.028-0.051 0.058 0.124 0.097 1.000 0.712**0.674**0.462**0.827**-0.201 0.299**-0.363**-0.191 0.196 1.000-0.034 0.669**0.909**-0.089 0.418**-0.422**0.037 0.124 1.000-0.029 0.234*-0.203 0.001-0.097-0.289**0.161 1.000 0.604**-0.113 0.125-0.180-0.020-0.180 1.000-0.070 0.371**-0.351**-0.091 0.183 1.000 0.264*0.260*0.335**-0.067 1.000-0.839**0.384**-0.293**1.000-0.195-0.289**
2.2 杂种后代SCoT标记遗传多样性分析
对80 条SCoT 引物进行多态性筛选,最终筛选出11条引物,其在双亲和杂种后代中表现出多态性高、重复性好的特点,得出的部分扩增结果见图4。11条SCoT引物共扩增出49条清晰条带,平均每条引物扩增出4.46个条带,其中共有多态性条带35个,多态性比例为71.43%。

图4 引物SCoT2、SCoT14、SCot23 和SCoT25 对亲本及F1杂种群体的扩增结果
Fig.4 Amplification results of parent and F1 hybrid population by primer SCoT2,SCoT14,SCoT23 and SCoT25
SCoT分子标记为显性标记,采用POPGENE软件对扩增结果条带进行分析。平均等位基因数为1.714 3,平均有效等位基因数为1.270 7;常用Nei’s基因多样性指数和Shannon’s信息指数的大小作为衡量基因多样性的指标,Nei’s基因多样性指数均值为0.158 3,不同位点Shannon’s 信息指数均值为0.272 1;不存在明显的遗传分化系数和基因流。
2.3 杂种后代SSR标记遗传多样性分析
SSR 分子标记为共显性标记,利用筛选出的20对多态性SSR引物对152株杂交子代进行PCR扩增和电泳检测(图5),采用POPGENE软件对数据进行标准化分析(表6)。20对引物共扩增出57个等位基因,每对引物扩增出的等位基因数2~4个不等,多态性位点百分比为100%,平均等位基因数为2.85 个,平均有效等位基因数为1.915 4 个。20 对引物的Shannon’s 信息指数平均值为0.722 8(<1.00),其中CLC1-1709-2最大,为1.098 2,最小的是引物CLC2-1351-2,为0.541 2;观测杂合度范围为0.396 1(CLC2-1351-2)~0.954 5(CLC1-1666-3),平均值为0.638 6,杂合程度中等;期望杂合度大小范围为0.326 3(CLC2-1351-2)~0.658 4(CLC1-1709-2),平均值为0.462 4,且20对引物中均出现观测杂合度大于期望杂合度;Nei’s 基因多样性指数的范围为0.325 3(CLC2-1351-2)~0.656 2(CLC1-1709-2),平均值为0.460 9;遗传分化系数的范围为0.081 3(CLC3-860-5)~0.485 9(CLC3-1706-4),平均值为0.312 7(>0.25),表明杂种群体内有很明显的遗传分化;基因流的变动范围为0.264 5~2.825 9,平均值为0.549 4。
表6 杂交F1代20 对SSR 引物的遗传参数
Table 6 Genetic parameters of 20 pairs of SSR primers in F1 hybrids
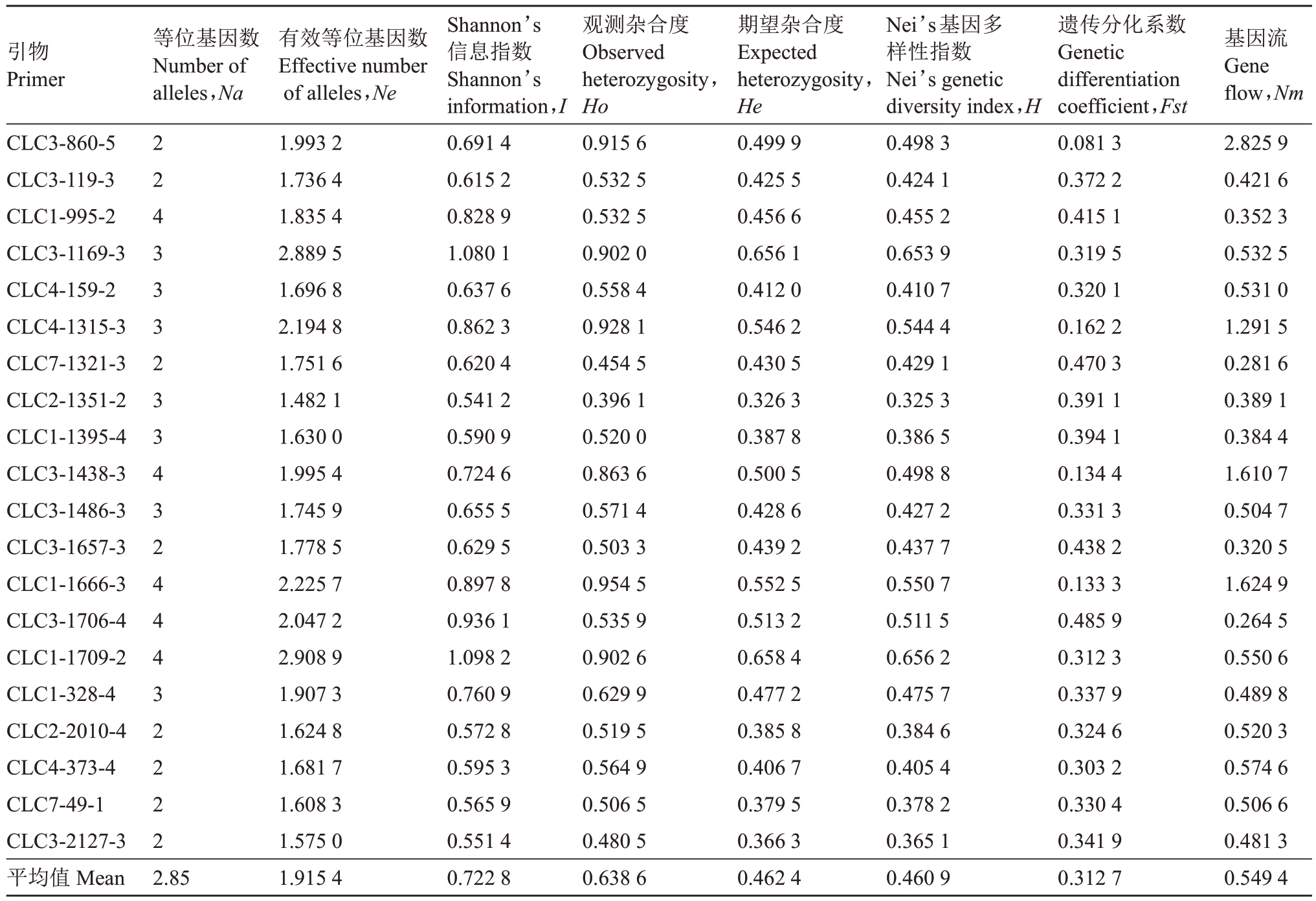
注:基因流数值估算来自遗传分化系数,公式:基因流=0.25(1-遗传分化系数值)/遗传分化系数值。
Note:Nm=Gene flow estimated from Fst=0.25(1-Fst)/Fst.
引物Primer等位基因数Number of alleles,Na CLC3-860-5 CLC3-119-3 CLC1-995-2 CLC3-1169-3 CLC4-159-2 CLC4-1315-3 CLC7-1321-3 CLC2-1351-2 CLC1-1395-4 CLC3-1438-3 CLC3-1486-3 CLC3-1657-3 CLC1-1666-3 CLC3-1706-4 CLC1-1709-2 CLC1-328-4 CLC2-2010-4 CLC4-373-4 CLC7-49-1 CLC3-2127-3平均值Mean基因流Gene flow,Nm 2.825 9 0.421 6 0.352 3 0.532 5 0.531 0 1.291 5 0.281 6 0.389 1 0.384 4 1.610 7 0.504 7 0.320 5 1.624 9 0.264 5 0.550 6 0.489 8 0.520 3 0.574 6 0.506 6 0.481 3 0.549 4 22433323343244432222 2.85有效等位基因数Effective number of alleles,Ne 1.993 2 1.736 4 1.835 4 2.889 5 1.696 8 2.194 8 1.751 6 1.482 1 1.630 0 1.995 4 1.745 9 1.778 5 2.225 7 2.047 2 2.908 9 1.907 3 1.624 8 1.681 7 1.608 3 1.575 0 1.915 4 Shannon’s信息指数Shannon’s information,I 0.691 4 0.615 2 0.828 9 1.080 1 0.637 6 0.862 3 0.620 4 0.541 2 0.590 9 0.724 6 0.655 5 0.629 5 0.897 8 0.936 1 1.098 2 0.760 9 0.572 8 0.595 3 0.565 9 0.551 4 0.722 8观测杂合度Observed heterozygosity,Ho 0.915 6 0.532 5 0.532 5 0.902 0 0.558 4 0.928 1 0.454 5 0.396 1 0.520 0 0.863 6 0.571 4 0.503 3 0.954 5 0.535 9 0.902 6 0.629 9 0.519 5 0.564 9 0.506 5 0.480 5 0.638 6期望杂合度Expected heterozygosity,He 0.499 9 0.425 5 0.456 6 0.656 1 0.412 0 0.546 2 0.430 5 0.326 3 0.387 8 0.500 5 0.428 6 0.439 2 0.552 5 0.513 2 0.658 4 0.477 2 0.385 8 0.406 7 0.379 5 0.366 3 0.462 4 Nei’s基因多样性指数Nei’s genetic diversity index,H 0.498 3 0.424 1 0.455 2 0.653 9 0.410 7 0.544 4 0.429 1 0.325 3 0.386 5 0.498 8 0.427 2 0.437 7 0.550 7 0.511 5 0.656 2 0.475 7 0.384 6 0.405 4 0.378 2 0.365 1 0.460 9遗传分化系数Genetic differentiation coefficient,Fst 0.081 3 0.372 2 0.415 1 0.319 5 0.320 1 0.162 2 0.470 3 0.391 1 0.394 1 0.134 4 0.331 3 0.438 2 0.133 3 0.485 9 0.312 3 0.337 9 0.324 6 0.303 2 0.330 4 0.341 9 0.312 7
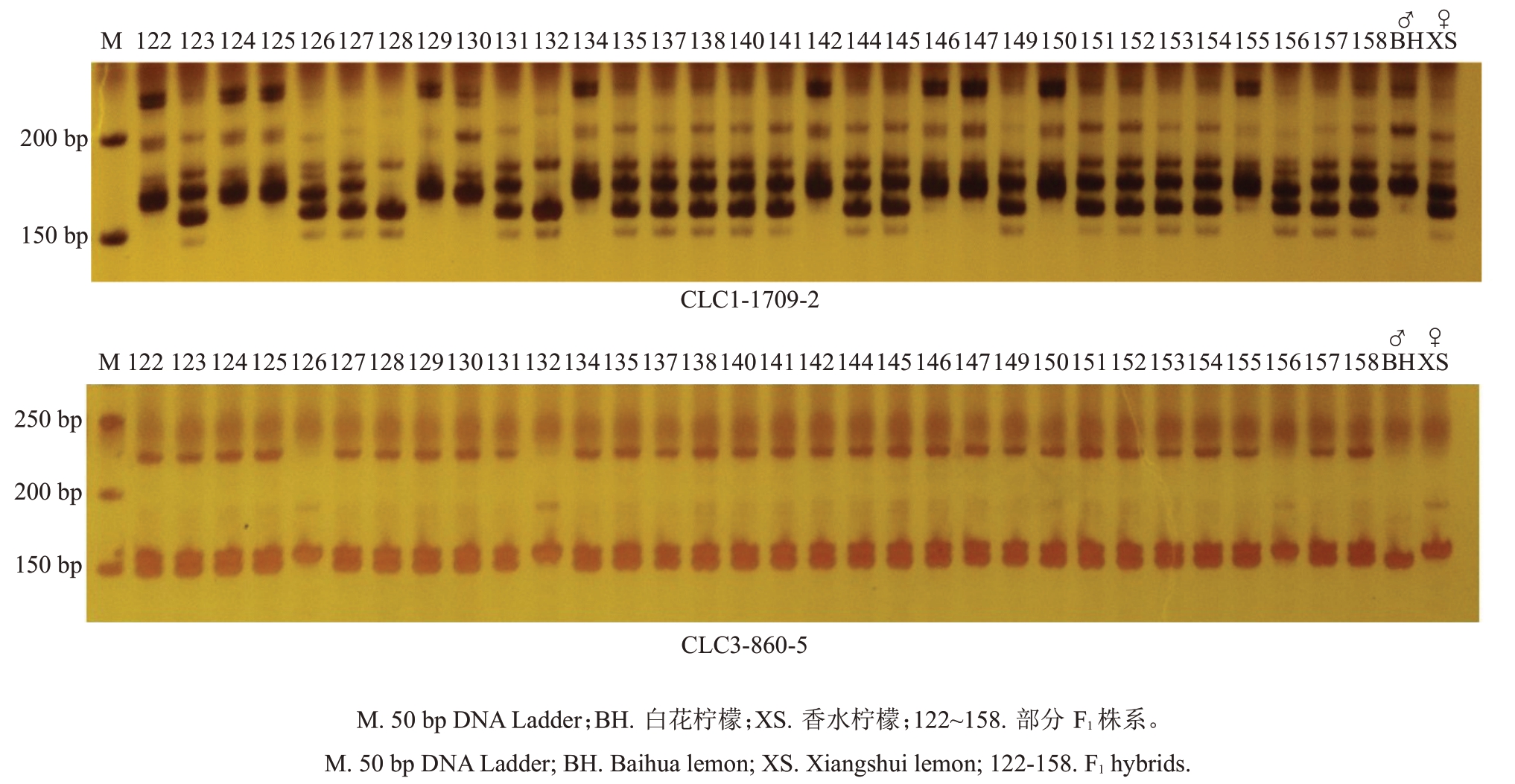
图5 SSR 引物CLC1-1709-2 和CLC3-860-5 对亲本及F1杂种群体的扩增结果
Fig.5 Amplification results of parent and F1 hybrid population by SSR primer CLC1-1709-2 and CLC3-860-5
2.4 亲本与杂种后代群体的聚类分析
利用SSR 标记和SCoT 标记扩增出的条带计算Nei’s(72)遗传距离,根据该遗传距离对亲本及F1群体进行UPGMA聚类分析。结果显示,2个标记计算出的亲本遗传距离存在较大差异,在SCoT 分子标记中,亲本之间的遗传距离为0.202 9,杂种群体平均遗传距离为0.176 2。SCoT 聚类分析图(图6)显示,在遗传距离为0.11 处,可将152 株划分为6 大类。第Ⅰ大类中115号和117号聚类在一起;第Ⅱ和Ⅲ大类64 号和129 号自成一类;第Ⅳ大类有9 株聚成一类,占5.84%,与亲本的遗传距离较大;第Ⅴ大类与母本聚类在一起,共30株,占19.48%;第Ⅵ大类与父本聚类在一起,共111株,占总数的72.08%。在遗传距离为0.07 处可将第Ⅵ大类细分为6 个亚类,杂种群体后代多样性丰富。
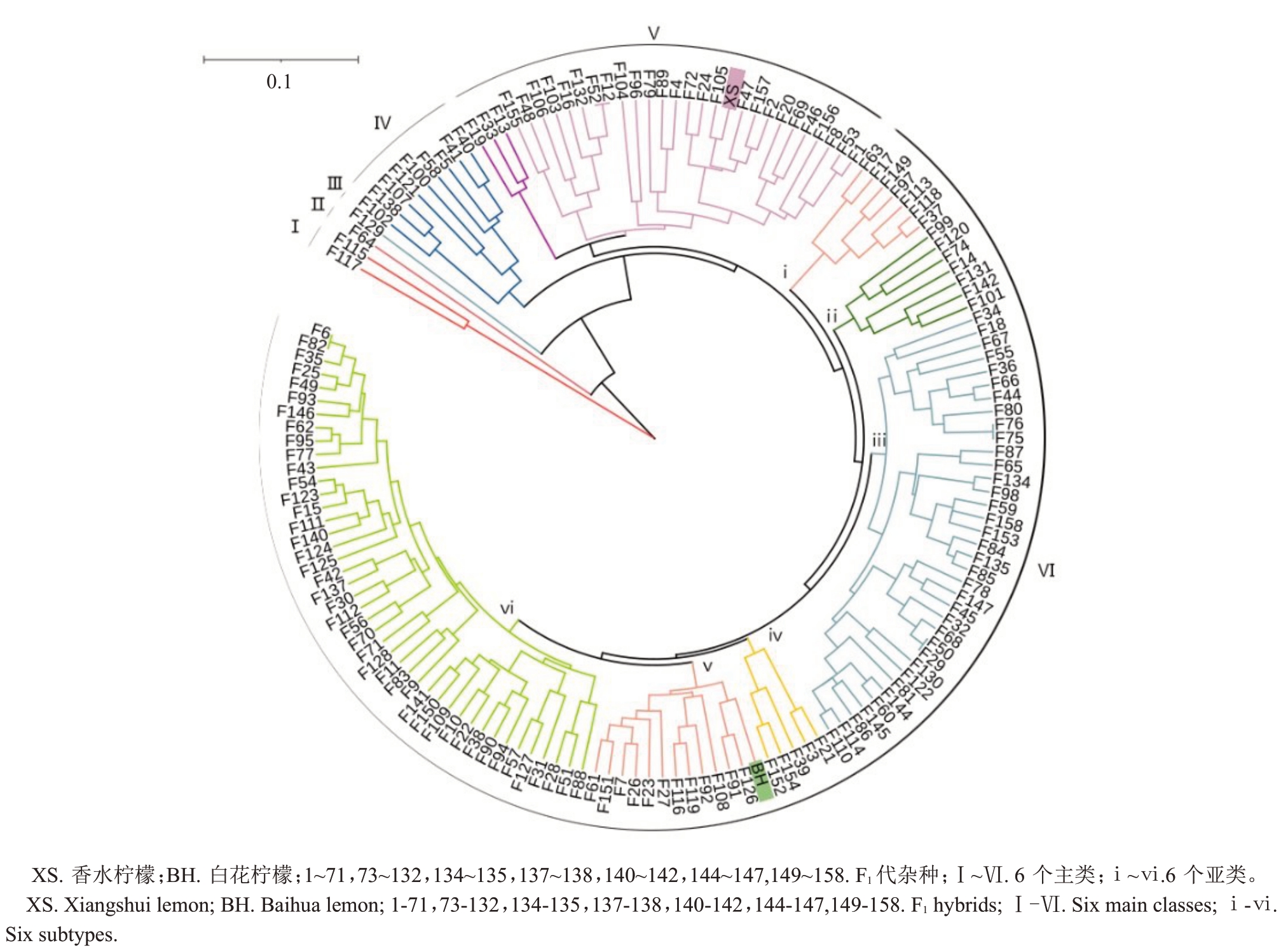
图6 亲本与152 株杂种后代SCoT 分析的遗传距离聚类分析
Fig.6 Clustering analysis of the parents and 152 hybrids based on SCoT markers
在SSR标记分析中,亲本间遗传距离为0.836 2,杂种群体间平均遗传距离为0.247 7。如构建的遗传聚类图(图7)所示,在遗传距离为0.13处,聚类图可分为6 大类。第Ⅰ大类中64 号单独聚类;第Ⅱ大类中115 号和117 号聚类在一起;第Ⅲ大类中72 号单独聚类;第Ⅳ大类有5株杂种单株聚成一类;第Ⅴ大类有17株与母本聚成一类;第Ⅵ大类与父本聚类在一起,共127 株,占总数的82.47%;在遗传距离0.09处,第Ⅵ大类可以分为7个亚类,其中父本白花柠檬、86 号、127 号均单独聚类,与亲本聚类在一起的单株较少。综合2 个标记的聚类图,从整体看杂种F1代中双亲遗传背景较大,杂种群体间平均遗传距离SSR 标记大于SCoT 标记;当遗传距离小于0.1时,与亲本聚在一起的杂种单株较少,多数单株间遗传变异广泛,多样性丰富。

图7 亲本与152 株杂种后代SSR 分析的遗传距离聚类分析
Fig.7 Clustering analysis of the parents and 152 hybrids based on SSR markers
利用Mantel 检验[24]对SSR 和SCoT 这2 种分子标记分析所产生的Nei’s遗传距离矩阵进行相关性分析,结果表明,这2个分子标记得到的遗传距离矩阵之间相关性不显著,相关系数r 值为0.302 1,p 值为1.000(>0.05)。
3 讨 论
3.1 F1群体叶、花遗传变异分析
遗传多样性是物种遗传潜力和适用环境变化能力的重要指标,能从基因水平上反映出群体的变异性,对优良子代初步筛选具有重要的指导意义[25]。柑橘类果树性状遗传较复杂,表型性状一般受到基因和环境因素的共同影响。胡哲等[26]对沙田柚和枸橼C-05杂交后代新叶颜色进行遗传分析,结果表明新叶紫色对绿色为显性,新叶颜色呈现绿色到紫色不同程度的分布,可能涉及到多对基因的互作。本研究中嫩叶色性状在杂种后代分离比约为3∶1,紫红色嫩叶对青绿色为显性,紫红色嫩叶成熟转色至绿色,同样涉及到多基因互作。在花色性状中,杂种后代紫红色花比例较高,表现出明显的偏母性遗传,同时子代花色变异丰富,出现不同深浅的紫色,具备数量性状遗传特征,与杜鹃花研究结果相一致[8]。双亲的花色差距越大,F1代花色分离越广泛,本研究中亲本花色相对性状差异显著,后代花色有超亲现象,这与非洲菊F1代花色分离遗传表现研究结果一致[27]。
3.2 F1群体果实遗传变异分析
果实外观、品质等性状是柑橘类果树重要的经济性状,多表现为复杂的数量遗传。本研究中果实性状遗传分析结果显示,正态性检验中10个果实性状分布均呈正态分布或偏正态分布,是典型多基因控制的数量遗传。果实性状都表现出广泛的性状分离现象,存在一定数量高于高亲和低于低亲的杂种单株,具有选育优良柠檬单株的潜力,这与苹果[28]、梨[29]等研究结果相一致。相关性分析结果显示,叶、花、果性状存在一定的相关性,与柚类研究结果部分一致[30],由于研究群体不同,各性状之间相关性存在差异。在本研究中,叶片大小与花色呈极显著正相关,推测可能与花青素积累相关,嫩叶、成熟叶片及花蕾、花瓣中花青素含量之间的相关性需要进一步研究。
3.3 SSR和SCoT标记遗传多样性分析比较
本研究表明利用SSR 和SCoT 标记对柠檬杂种群体进行遗传多样性分析是可行的。在SCoT标记中,11 条引物共扩增出49 条条带,多态性条带35条,占71.43%;20 对SSR 引物共扩增出57 条条带,多态性为100%,说明杂种群体具有丰富的遗传多样性。衡量群体中等位基因频率是否偏离遗传平衡论比例的指标即为遗传分化系数;而基因流则指具有某一基因频率的群体的一部分移入基因频率与其不同的另一群体,并杂交定居,引起迁入群体的基因频率发生改变。遗传分化系数和基因流的大小反映群体分化程度[25]。本研究中,SCoT标记中F1代不存在明显的基因流和遗传分化系数,符合理论研究结果。而在SSR 标记分析中,F1代遗传分化系数平均值为0.312 7,基因流均值为0.549 4,表明杂交组合间可能出现了遗传分化,且处于高等程度(>0.25)。这一现象的原因可能是杂交亲本组合香水柠檬×白花柠檬均为柠檬类品种,种内杂交存在基因交流现象,这与江锡兵等[25]对栗杂交F1代遗传多样性的研究结果类似。
3.4 SSR和SCoT标记聚类分析比较
在杂种后代与亲本的聚类分析中,SSR和SCoT标记的聚类图中均出现64 号单株独自聚为一类,115号和117号聚为一类,在叶色、叶长度、叶宽度性状方面,64、115和117号均出现明显变异,聚类结果与表型性状相一致。在SCoT聚类结果中与母本香水柠檬遗传距离最近的是46 号,而SSR 标记中105号与母本遗传距离最近,存在明显的差异;Mantel检验显示2 个标记遗传距离矩阵相关性不显著,推测由于2种分子标记在基因组上分布不均,且SCoT和SSR 标记扩增原理不同,扩增片段为基因组中不同区域,因而计算得到不同的遗传距离,由此聚类结果存在不一致,与前人研究结果一致[31-32]。不同分子标记具备不同功能,本研究中综合SCoT 和SSR 分子标记联合分析相互补充,减少单一标记产生的误差,有助于准确了解杂种后代群体遗传多样性。
4 结 论
本研究结果表明,叶、花和果实等15 个性状均存在一定程度的分离,杂种后代变异广泛,各性状中出现一定比例超亲个体;结合SSR 标记和SCoT 标记进行遗传多样性分析发现,F1代存在较多的基因重组类型,遗传多样性丰富,为后续遗传图谱构建、QTL 定位、重要基因挖掘及遗传育种研究提供参考。
[1]张树伟.香水柠檬无籽成因和相关基因的分离与鉴定[D].南宁:广西大学,2014.ZHANG Shuwei. Causes of seedless forming and identification of seedless related genes from‘Xiangshui’lemon [D]. Nanning:Guangxi University,2014.
[2]沈浩,刘登义.遗传多样性概述[J].生物学杂志,2001,18(3):5-7.SHEN Hao,LIU Dengyi.Overview of genetic diversity[J].Journal of Biology,2001,18(3):5-7.
[3]冯夏莲,何承忠,张志毅,安新民,王冬梅.植物遗传多样性研究方法概述[J].西南林学院学报,2006,26(1):69-74.FENG Xialian,HE Chengzhong,ZHANG Zhiyi,AN Xinmin,WANG Dongmei. Overview of plant genetic diversity research methods[J].Journal of Southwest Forestry College,2006,26(1):69-74.
[4]韩婷婷,杨天资,赵培磊,祝军,张玉刚,孙晓红.‘金冠’和‘红勋1 号’杂交后代遗传多样性分析[J].青岛农业大学学报(自然科学版),2021,38(1):1-6.HAN Tingting,YANG Tianzi,ZHAO Peilei,ZHU Jun,ZHANG Yugang,SUN Xiaohong.Analysis of genetic diversity of the hybrid populations of‘Golden Delicious’בHongxun No.1’[J].Journal of Qingdao Agricultural University (Natural Science),2021,38(1):1-6.
[5]刘冬峰,陈巍,林绍生,徐文荣,郭秀珠,黄品湖.基于SSR 标记的浙江地方柚类种质资源遗传关系分析[J]. 果树学报,2017,34(2):166-174.LIU Dongfeng,CHEN Wei,LIN Shaosheng,XU Wenrong,GUO Xiuzhu,HUANG Pinhu.Analysis of genetic relationship of pummelo germplasms by SSR markers in Zhejiang province[J].Journal of Fruit Science,2017,34(2):166-174.
[6]黄桂香,郭丽英,张树伟,何新华,周瑞阳,陈虎,杨春奖.中越金柑种质资源的ISSR 分析[J]. 果树学报,2011,28(4):563-567.HUANG Guixiang,GUO Liying,ZHANG Shuwei,HE Xinhua,ZHOU Ruiyang,CHEN Hu,YANG Chunjiang.Genetic relationship analysis of Fortunella germplasm resources from China and Vietnam by ISSR markers[J]. Journal of Fruit Science,2011,28(4):563-567.
[7]陈虎,何新华,黄桂香,李峰,姜建初,朱建华.不同龙眼资源遗传多样性的SCoT 和ISSR 比较分析[J].广西植物,2012,32(4):536-541.CHEN Hu,HE Xinhua,HUANG Guixiang,LI Feng,JIANG Jianchu,ZHU Jianhua. Comparison and analysis of SCoT and ISSR markers for genetic diversity of longan[J]. Guihaia,2012,32(4):536-541.
[8]苏鸣,夏溪,张春英,奉树成,倪穗.杜鹃花品种间杂交F1代主要形态性状变异[J].东北林业大学学报,2021,49(5):6-11.SU Ming,XIA Xi,ZHANG Chunying,FENG Shucheng,NI Sui.Variation of main morphological characters in F1 generation of Azalea varieties[J]. Journal of Northeast Forestry University,2021,49(5):6-11.
[9]韩国辉,向素琼,汪卫星,魏旭,何波,李晓林,梁国鲁.沙田柚杂交后代群体的SSR 鉴定与遗传多样性分析[J].中国农业科学,2010,43(22):4678-4686.HAN Guohui,XIANG Suqiong,WANG Weixing,WEI Xu,HE Bo,LI Xiaolin,LIANG Guolu. Identification and genetic diversity of hybrid progenies from Shatian pummelo by SSR[J]. Scientia Agricultura Sinica,2010,43(22):4678-4686.
[10]AHMED S,RATTANPAL H S,SINGH G. Diversity,characterization and evaluation in pummelo (Citrus maxima Merr.) cultivars using SSR markers and quality parameters[J]. Indian Journal of Genetics and Plant Breeding,2019,79(3):594-605.
[11]RANA M K,SINGH S. Assessment of genetic diversity and DNA profiling of linseed(Linum usitatissimum subsp.usitatissimum L.)germplasm using SSR markers[J].Journal of Plant Biochemistry and Biotechnology,2017,26(3):293-301.
[12]刘通,邓崇岭,程玉芳,李秋景,陈传武,刘冰浩,伊华林.利用SSR 和SRAP 技术分析广西柑橘种质遗传多样性[J].华中农业大学学报,2016,35(2):23-29.LIU Tong,DENG Chongling,CHENG Yufang,LI Qiujing,CHEN Chuanwu,LIU Binghao,YI Hualin. Analyzing genetic diversity of citrus germplasm in Guangxi province with SSR and SRAP markers[J].Journal of Huazhong Agricultural University,2016,35(2):23-29.
[13]王旭,彭洁,朱延松,杨胜男,张晓楠,余洪,江东,梁大成.基于SSR 分子标记的68 份柚类种质资源亲缘关系分析[J].安徽农业科学,2021,49(4):100-103.WANG Xu,PENG Jie,ZHU Yansong,YANG Shengnan,ZHANG Xiaonan,YU Hong,JIANG Dong,LIANG Dacheng.Analysis of genetic relationship of 68 pummelo germplasm resources based on SSR molecular marker[J]. Journal of Anhui Agricultural Sciences,2021,49(4):100-103.
[14]KONGSRI S,BOOMPRAKOB U. Assessment of genetic relationships among pummelo cultivars [Citrus maxima (Burm.)Merrill]using simple sequence repeat markers[J].Maejo International Journal of Science and Technology,2016,10(2):209-219.
[15]熊发前,蒋菁,钟瑞春,韩柱强,贺梁琼,李忠,庄伟建,唐荣华.目标起始密码子多态性(SCoT)分子标记技术在花生属中的应用[J].作物学报,2010,36(12):2055-2061.XIONG Faqian,JIANG Jing,ZHONG Ruichun,HAN Zhuqiang,HE Liangqiong,LI Zhong,ZHUANG Weijian,TANG Ronghua.Application of SCoT molecular marker in genus Arachis[J].Acta Agronomica Sinica,2010,36(12):2055-2061.
[16]AYMEN M,GHADA B,AMEL O,AMEL S. Start Codon Targeted (SCoT) markers provide new insights into the genetic diversity analysis and characterization of Tunisian Citrus species[J].Biochemical Systematics and Ecology,2015,61:390-398.
[17]郭丽英,郭雁君,吉前华,何新华,黄桂香,陈虎.扁柑种质资源的SCoT 分析[J].西南农业学报,2013,26(2):428-431.GUO Liying,GUO Yanjun,JI Qianhua,HE Xinhua,HUANG Guixing,CHEN Hu. SCoT analysis of Citrus reticulate Blanco cv. Bian Gan germplasm resources[J]. Southwest China Journal of Agricultural Sciences,2013,26(2):428-431.
[18]韩国辉,向素琼,汪卫星,贾志刚,洪棋斌,梁国鲁.柑橘SCoT分子标记技术体系的建立及其在遗传分析中的应用[J].园艺学报,2011,38(7):1243-1250.HAN Guohui,XIANG Suqiong,WANG Weixing,JIA Zhigang,HONG Qibin,LIANG Guolu. Establishment and application of SCoT molecular marker system for citrus[J].Acta Horticulturae Sinica,2011,38(7):1243-1250.
[19]LUO C,HE X,CHEN H,HU Y,OU S.Genetic relationship and diversity of Mangifera indica L.:Revealed through SCoT analysis[J]. Genetic Resources and Crop Evolution,2012,59(7):1505-1515.
[20]彭芳芳,龙治坚,魏召新,李勋兰,罗友进,韩国辉.樱桃种质SCoT 分子标记与叶片表型性状关联分析[J].园艺学报,2021,48(2):325-335.PENG Fangfang,LONG Zhijian,WEI Zhaoxin,LI Xunlan,LUO Youjin,HAN Guohui.Association analysis of sCoT markers and leaf phenotypic traits in cherry germplasm[J].Acta Horticulturae Sinica,2021,48(2):325-335.
[21]江东,龚桂芝.柑橘种质资源描述规范和数据标准[M].北京:中国农业出版社,2006.JIANG Dong, GONG Guizhi. Specification for description and data standards of Citrus germplasm resources[M]. Beijing:China Agriculture Press,2006.
[22]罗聪.芒果SCoT 分子标记与逆境和重要开花时间相关基因研究[D].南宁:广西大学,2012.LUO Cong. Study on SCoT marker and analysis on genes of stress-related and important flowering time in mango[D]. Nanning:Guangxi University,2012.
[23]闫忠业,伊凯,刘志,王冬梅,吕天星,李春敏,陈东玫.‘红玉’ב金冠’苹果杂交后代果实糖酸组分遗传分析[J].果树学报,2017,34(2):129-136.YAN Zhongye,YI Kai,LIU Zhi,WANG Dongmei,LÜ Tianxing,LI Chunmin,CHEN Dongmei.A study of genetic trend of sugar and acid components in the fruits of apple hybrid progeny of‘Jonathan’בGolden Delicious’[J]. Journal of Fruit Science,2017,34(2):129-136.
[24]MANTEL N. The detection of disease clustering and a generalized regression approach[J]. Cancer Research,1967,27(2):209-220.
[25]江锡兵,章平生,徐阳,吴聪连,张东北,龚榜初,吴开云,赖俊声. 栗杂交F1 代SSR 标记遗传多样性分析[J]. 园艺学报,2021,48(5):897-907.JIANG Xibing,ZHANG Pingsheng,XU Yang,WU Conglian,ZHANG Dongbei,GONG Bangchu,WU Kaiyun,LAI Junsheng. Genetic diversity of F1 hybrids of chestnut based on SSR markers[J].Acta Horticulturae Sinica,2021,48(5):897-907.
[26]胡哲,龙桂友,谭李梅,李大志,符红艳,盛玲,马先锋,刘冰浩,邓子牛.‘沙田柚’与枸橼杂交后代叶形态学性状遗传分析[J].果树学报,2018,35(5):531-538.HU Zhe,LONG Guiyou,TAN Limei,LI Dazhi,FU Hongyan,SHENG Ling,MA Xianfeng,LIU Binghao,DENG Ziniu. Leaf morphology inheritance analysis of hybrids between‘Shatian’pumelo and citron[J].Journal of Fruit Science,2018,35(5):531-538.
[27]董雪娜,陈希,蒋甲福,陈俊律,管志勇,史玉娇,房伟民.非洲菊F1代观赏性状的遗传表现[J].南京农业大学学报,2015,38(2):226-232.DONG Xuena,CHEN Xi,JIANG Jiafu,CHEN Junlü,GUAN Zhiyong,SHI Yujiao,FANG Weimin. Heredity of ornamental traits in F1 of Gerbera jamesonii Bolus[J]. Journal of Nanjing Agricultural University,2015,38(2):226-232.
[28]王亚杰,孟蕊,武月妮,杨亚州,赵政阳.秦冠、富士苹果杂交后代果实性状遗传趋势分析[J].西北农业学报,2014,23(4):52-59.WANG Yajie,MENG Rui,WU Yueni,YANG Yazhou,ZHAO Zhengyang. Genetic tendency of fruit characters in apple hybrids of Qinguan and Fuj[J].Acta Agriculturae Boreali-Occidentalis Sinica,2014,23(4):52-59.
[29]白牡丹,郝国伟,张晓伟,杨盛,郭黄萍.‘玉露香梨’与‘黄冠’梨杂交后代果实性状遗传倾向的初步研究[J].中国果树,2017(S1):13-16.BAI Mudan,HAO Guowei,ZHANG Xiaowei,YANG Sheng,GUO Huangping. Primary research on genetic tendency of fruit characters in hybrid progenies between‘Yuluxiangli’and‘Huangguan’pear cultivars[J].China Fruits,2017(S1):13-16.
[30]卢华琼,苏智先.部分柚类品种主要果实性状变异及相关性研究[J].中国农学通报,2006(12):220-222.LU Huaqiong,SU Zhixian.Variation and correlation research of main fruit characters of pumelo cultivars[J]. Chinese Agricultural Science Bulletin,2006(12):220-222.
[31]藕丹,樊军锋,高建社,周永学. SSR 和SCoT 标记在美洲黑杨×青杨派杂种无性系遗传差异性分析上的比较[J].西北农林科技大学学报(自然科学版),2017,45(4):79-85.OU Dan,FAN Junfeng,GAO Jianshe,ZHOU Yongxue.Comparison of genetic diversity of Popolus deltoides ×Section tacamahaca hybrids based on SSR and SCoT marker[J]. Journal of Northwest A&F University(Natural Science Edition),2017,45(4):79-85.
[32]李永祥,李斯深,李立会,杨欣明,李秀全.披碱草属12 个物种遗传多样性的ISSR 和SSR 比较分析[J]. 中国农业科学,2005,38(8):1522-1527.LI Yongxiang,LI Sishen,LI Lihui,YANG Xinming,LI Xiuquan. Comparison of genetic diversity of twelve Elymus species using ISSR and SSR markers[J]. Scientia Agricultura Sinica,2005,38(8):1522-1527.