无子瓯柑(Citrus suavissima‘Wuzi Ougan’)是野生型瓯柑(Citrus suavissima)的无核芽变,具有野生型瓯柑的酸甜适中、汁多微苦、风味独特以及储藏性极佳等优良性状[1]。果实无核一直是柑橘品质改良的重要目标[2]。导致柑橘果实无核的因素包括雄性不育、胚中途败育、胚囊败育以及自交不亲和等,而其中雄性不育影响最大[3]。前期形态学、细胞学和组织化学层面的研究表明,雄性不育是无子瓯柑无核的重要原因[4-6]。笔者课题组前期对无子瓯柑及其野生型小孢子母细胞时期的花药进行多组学测序研究,发现差异表达基因与蛋白显著富集于苯丙素生物合成、黄酮类化合物生物合成和苯丙氨酸代谢通路,而苯丙素生物合成(phenylpropanoid pathway)是富集基因数目最多的生物代谢途径[7-8],该途径的差异代谢物则关联到在无子瓯柑中显著上调表达的RING型E3泛素连接酶基因RNF217。
泛素化(ubiquitination)是真核生物重要的翻译后修饰机制,对细胞周期、转录调控、DNA修复以及信号转导等生物过程具有重要的调控作用[9]。泛素连接酶E3(简称E3)将泛素蛋白连接至目标蛋白的赖氨酸残基,是泛素化的关键步骤[10],在蛋白质降解过程中的作用至关重要[11]。根据E3 的结构域和作用方式,目前植物中已鉴定的E3 主要分为4 种,分别是HECT 型、RING 型、U-box 型和CRLs 型[12]。其中,RING 型E3 由基本结构域RING(really interesting new gene)加上40~60 个氨基酸残基组成,并根据这些残基的差异,主要分为RING-HC(C3HC4)和RING-H2(C3H2C3)2个亚家族[13]。研究表明,RING型E3 在生物胁迫[14]与非生物胁迫[15-17]、调控激素响应[18-19]、抗病防御反应[20-21]和植物花粉发育[22-25]等生理活动中发挥重要作用。其中,在水稻和拟南芥中已分离鉴定的RING 型E3 基因,如PTB1、DAF、APD1-4以及SINAT1基因等在花粉发育方面都表现出重要作用[22-25]。另外,RING 型E3 家族成员中的Cullin(CUL)基因和CCCH型锌指蛋白基因也被证实参与梨和油菜有性生殖过程中的花粉发育过程[26-27]。
笔者在本研究中采用同源克隆法分离无子瓯柑RING 型E3 泛素连接酶基因CsRNF217 全长编码序列(coding sequence,CDS),采用生物信息学方法进行序列分析和系统发育树构建,利用烟草瞬时表达系统分析其亚细胞定位。通过实时荧光定量PCR(real-time quantitative PCR,qRT-PCR)技术分析Cs-RNF217 基因在无子瓯柑和野生型瓯柑中的时空表达特性,并分析CaMV 35S::CsRNF217 过表达的烟草植株表型变化,为进一步揭示CsRNF217 基因在无子瓯柑雄性不育过程中的重要作用奠定基础。
1 材料和方法
1.1 植物材料
试验材料无子瓯柑(N)和野生型瓯柑(Y)植株种植于浙江农林大学校内试验基地。2021 年3—4月,分别采集长势良好的无子瓯柑和野生型瓯柑不同发育时期的花蕾并迅速分离出花药,置于液氮中速冻后保存于-80 ℃。采集盛花期的花瓣、雄蕊、雌蕊和花托立即置于液氮中速冻并于-80 ℃保存。以上速冻的植物材料用于基因克隆与qRT-PCR分析。
利用实验室自有的本氏烟草(Nicotiana benthamiana)实生苗进行CsRNF217基因亚细胞定位分析,并以普通烟草(Nicotiana tabacum)无菌组培苗为材料进行转基因研究。
1.2 植物材料总RNA的抽提及反转录cDNA
采用TaKaRa 公司MiniBEST Plant RNA Extraction Kit试剂盒分别抽提冻存于-80 ℃的植物材料总RNA,经反转录试剂盒(TransGen Biotech,code#AE311-03)反转录成cDNA,以Actin GU911361(citrus sinensis)为内参基因(表1)并检验合格后,于-20 ℃保存备用。
表1 引物信息
Table 1 Specific primers used in this study
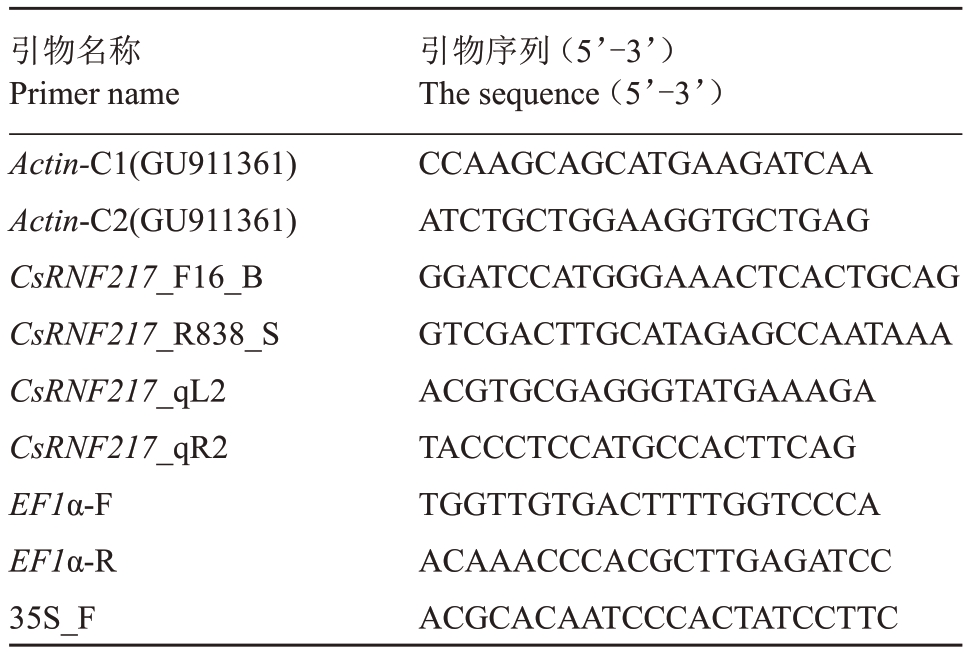
引物名称Primer name Actin-C1(GU911361)Actin-C2(GU911361)CsRNF217_F16_B CsRNF217_R838_S CsRNF217_qL2 CsRNF217_qR2 EF1α-F EF1α-R 35S_F引物序列(5’-3’)The sequence(5’-3’)CCAAGCAGCATGAAGATCAA ATCTGCTGGAAGGTGCTGAG GGATCCATGGGAAACTCACTGCAG GTCGACTTGCATAGAGCCAATAAA ACGTGCGAGGGTATGAAAGA TACCCTCCATGCCACTTCAG TGGTTGTGACTTTTGGTCCCA ACAAACCCACGCTTGAGATCC ACGCACAATCCCACTATCCTTC
1.3 无子瓯柑CsRNF217基因的克隆及序列分析
以克里曼丁橘基因组(https://phytozome.jgi.doe.gov/)数据库为参考,以E3 基因RNF217(XM_024187476.1)为模板设计基因特异引物Cs-RNF217_F16_B/CsRNF217_R838_S进行克隆(表1),引物序列送杭州有康生物科技有限公司合成。PCR扩增产物经凝胶回收,送杭州有康生物科技有限公司进行测序。采用DNAMAN 分析无子瓯柑的Cs-RNF217 基因的全长cDNA,氨基酸数目(aa)和开放阅读框(open reading frame,ORF)数目,利用NCBI Conserved Domains Search 在线软件和IBS 在线软件(http://ibs.biocuckoo.org/)进行蛋白结构域预测和制图。
1.4 无子瓯柑CsRNF217基因的系统发育树构建
利用NCBI 中的Blastp,以无子瓯柑CsRNF217基因的氨基酸序列作为参考,在不同物种中寻找该蛋白的同源基因,通过MEGA X软件进行系统发育树分析,方法采用邻接法(neighbor-joining method),置信度检测参数bootstrap值为1000次。
1.5 无子瓯柑CsRNF217基因的过表达载体构建
以快切酶Q.Cut BamHⅠ和Q.Cut SalⅠ(TaKa-Ra)对测序质粒进行双酶切。将酶切产物割胶回收,连接pCAMBIA 2300s 载体,提取正向阳性克隆抽提质粒,采用冻融法转化农杆菌菌株EHA105。
1.6 无子瓯柑和野生型瓯柑CsRNF217 基因的时空表达特性
使用GenScript 在线软件设计基因特异性定量分析引物CsRNF217_qL2/CsRNF217_qR2(表1),以无子瓯柑和野生型瓯柑的花瓣、雄蕊、雌蕊和花托等花器官的不同部分和小孢子不同发育时期(Ⅰ时期-小孢子母细胞时期、Ⅱ时期-减数分裂时期、Ⅲ时期-四分体时期、Ⅳ时期-花粉粒成熟期)花药的cDNA 为模板,参照NovoStart®SYBR qPCR Super-Mix plus 试剂盒(上海近岸科技有限公司)说明书,以Actin GU911361 为内参基因在qTOWER3G 仪器中进行qRT-PCR扩增,每个反应进行3次重复,采用2-ΔΔCt法分别以Y的花托和Ⅰ时期的花蕾表达水平为标准,计算相对表达量情况,利用SPSS 软件进行显著性分析,并在Excel 和GraphPad Prism 8 中绘制图表。
1.7 无子瓯柑CsRNF217的转基因功能初步验证
采用农杆菌介导的叶盘转化方法[28]侵染普通烟草;对再生植株采用35S_F和基因特异下游引物Cs-RNF217_R838_S(表1)进行PCR 检测筛选阳性植株;采用CsRNF217 基因定量分析引物和烟草内参基因(EF1α)特异引物(表1)进行相对表达量的半定量PCR检测,扩增循环程序为:95 ℃预变性5 min;95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s,28个循环;72 ℃延伸10 min;12 ℃保存。采用亚历山大红染色法[29]对转基因阳性株系(1#~65#)和对照WT分别进行花粉活力检测,每个花朵取10个视野,每个视野至少含50个花粉粒,每棵转基因阳性植株取3朵花进行生物学重复试验。
1.8 无子瓯柑CsRNF217基因的亚细胞定位
将无子瓯柑CsRNF217 基因的ORF 序列,经双酶切连接到含有绿色荧光蛋白(green fluorescent protein,GFP)的双元表达载体pLGFP1301 中构建CaMV35S::CsRNF217::GFP 融合表达载体,并转化农杆菌GV3101(上海唯地生物技术有限公司)。采用农杆菌介导的烟草瞬时转化法[30]注射本氏烟叶片下表皮,以转化农杆菌的空载体作为阴性对照,在激光共聚焦显微镜(OLYMPUS FV3000)20 倍镜头下观察目的基因荧光在烟草表皮细胞中的分布。
2 结果与分析
2.1 无子瓯柑CsRNF217 基因的克隆和编码蛋白序列分析
以无子瓯柑花药cDNA 为模板,经PCR 扩增获得长度为750~1000 bp 的特异条带(图1)。测序结果显示,cDNA 全长为768 bp,编码由255 个氨基酸组成的蛋白(图2-A),其中包含1 个ORF 序列。该ORF序列包含1个RING-HC_RBR结构域(26~79个氨基酸)和1 个环指间结构域IBR(in between ring)结构域(97~157个氨基酸)(图2-B)。由于IBR结构是典型的环指结构域(ring finger domain)的一半,通常存在于2个环指(ring fingers)之间,因此该基因编码的蛋白具备RING-HC E3 亚家族的典型结构特征,遂命名为CsRNF217基因。
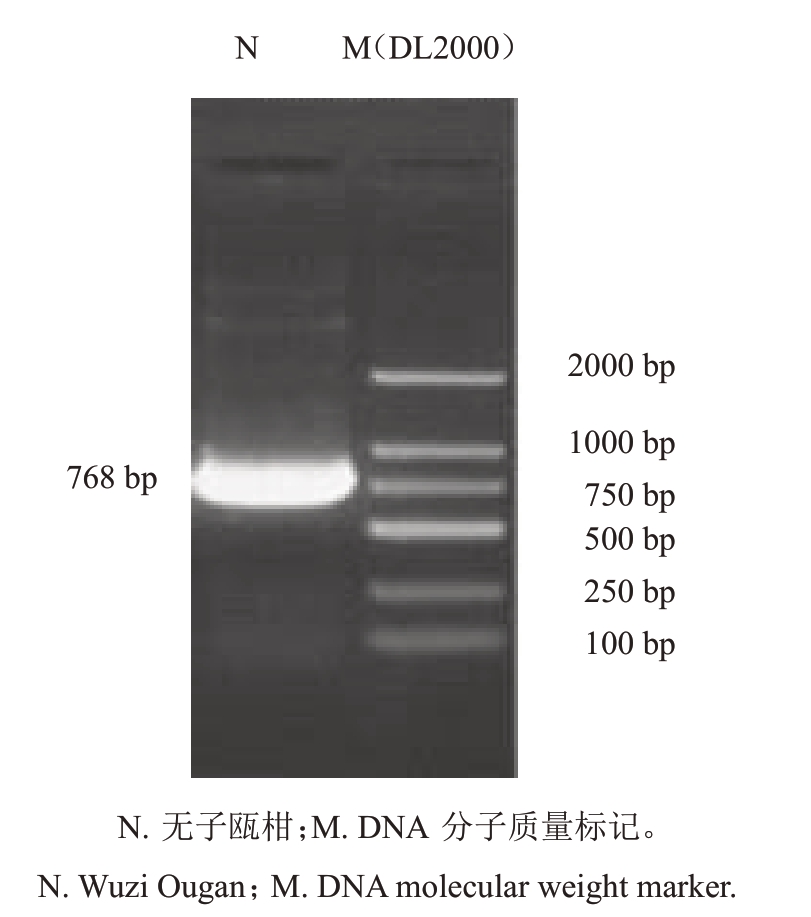
图1 无子瓯柑CsRNF217 基因的PCR 扩增产物电泳检测
Fig.1 Electrophoresis detection PCR product of CsRNF217 in Wuzi Ougan
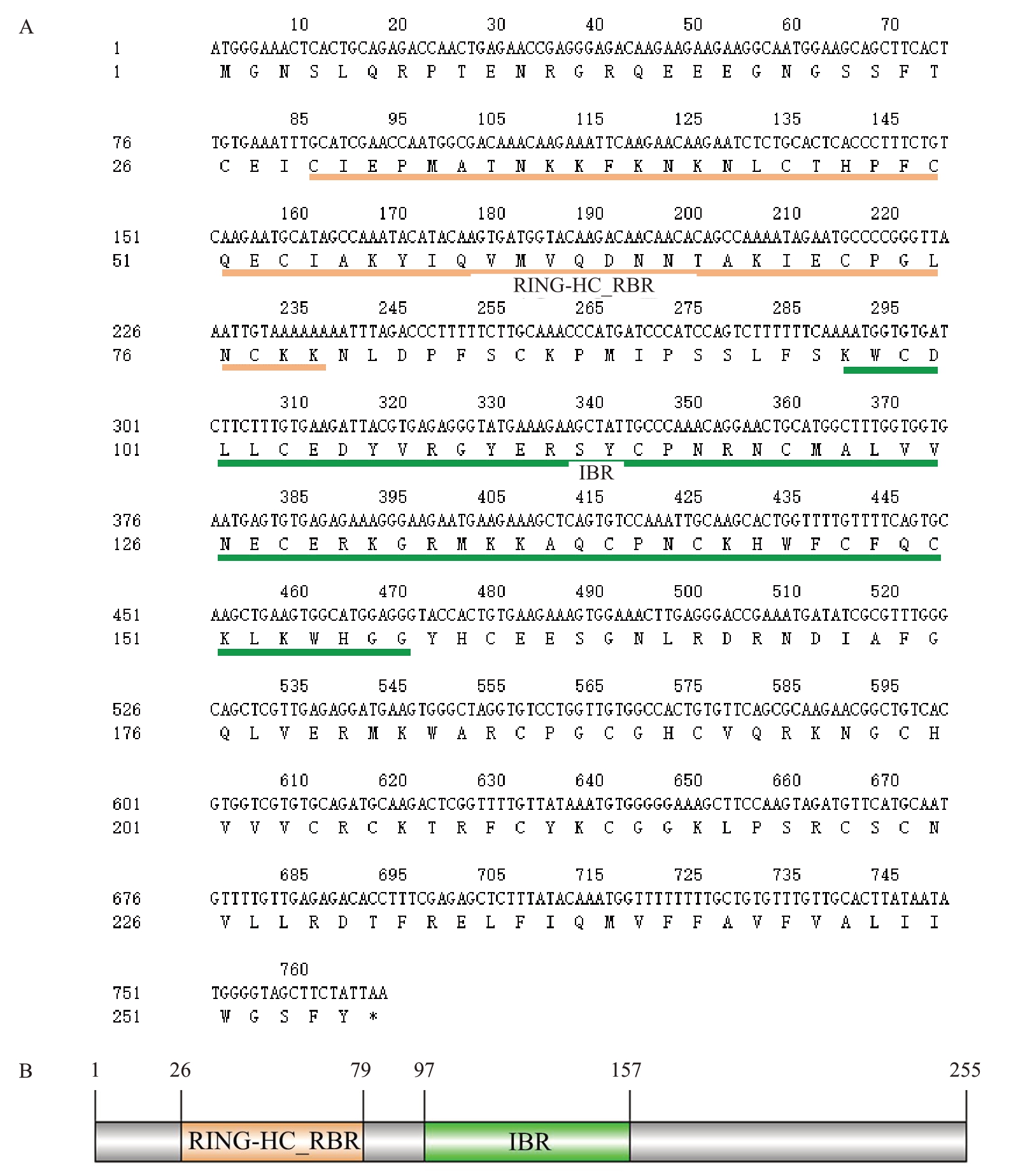
图2 无子瓯柑CsRNF217 基因的核苷酸及其预测的氨基酸序列(A)和结构域(B)
Fig.2 Nucleotide and putavite amino acid sequence(A)and predicted domains(B)of CsRNF217 in Wuzi Ougan
2.2 无子瓯柑CsRNF217基因的系统发育树构建
在NCBI 数据库中对无子瓯柑CsRNF217 基因编码的蛋白进行Blastp分析。结果显示,该蛋白与克里曼丁橘(Citrus clementina)、甜橙(Citrus sinensis)、榴莲(Durio zibethinus)、哥伦比亚锦葵(Herrania umbratica)和可可树(Theobroma cacao)等具有较高的同源率,其相似度分别是97.65%、97.56%、60.66%、59.67%和59.67%。为了分析该蛋白与其他物种的亲缘关系,从NCBI 和甜橙数据库(http://citrus.hzau.edu.cn/orange/)筛选获得拟南芥、玉米、水稻、烟草以及克里曼丁橘等13种植物的24个氨基酸序列,进行多序列对比和系统发育树分析。结果显示,这些氨基酸序列分别属于RING型E3的2个亚家族RINGHC和RING-H2;无子瓯柑CsRNF217基因与来自柑橘属的克里曼丁橘(XP_024043244.1)、甜橙(XP_024953912.1)、木锦科的榴莲(XP_022734107.199)、锦葵科的哥伦比亚锦葵(XP_021291308.1)和梧桐科的可可树(XP_007033813.1)的E3 基因编码的蛋白均被划分在RING-HC亚家族中(图3)。
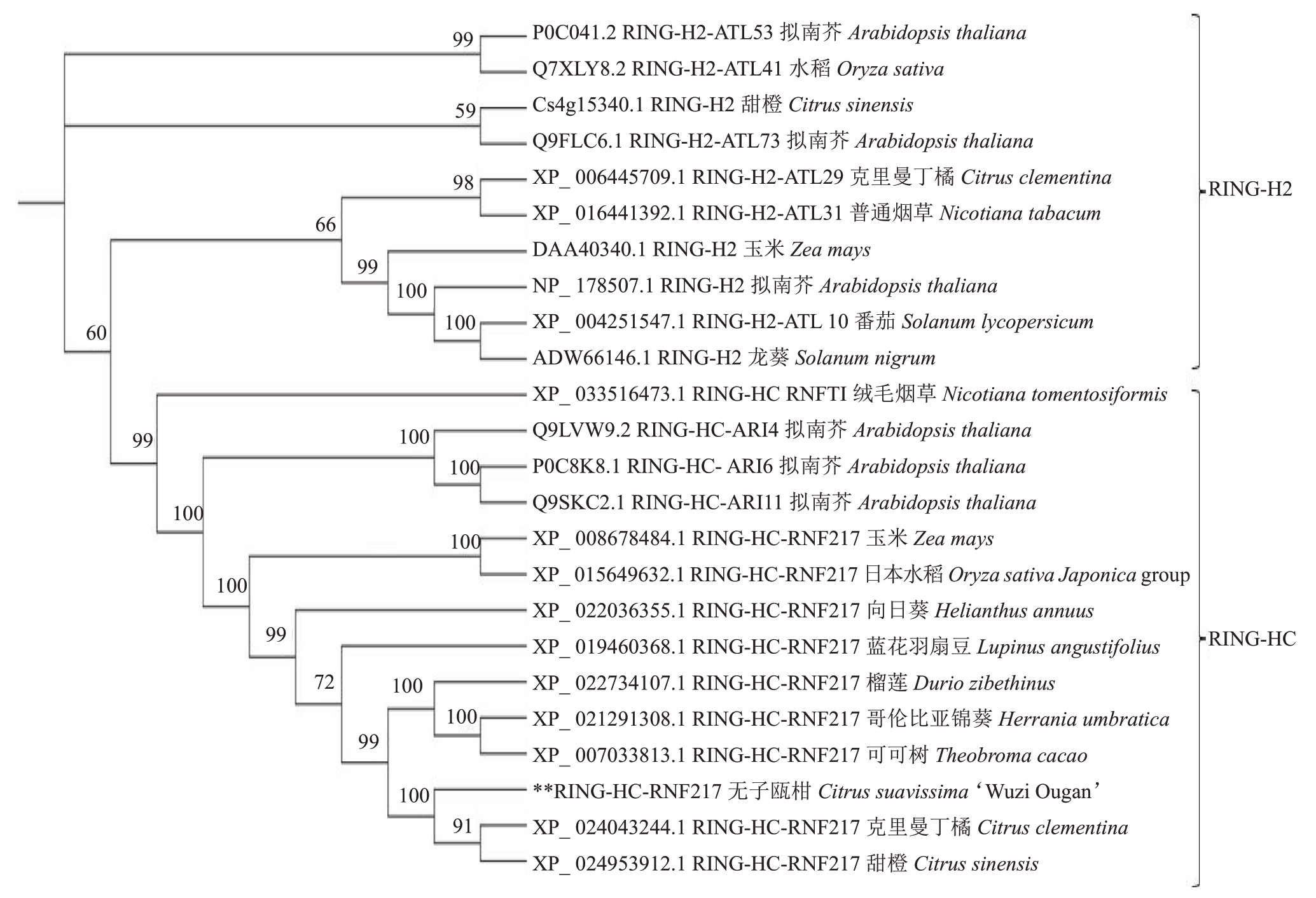
图3 无子瓯柑与其他物种的E3 基因系统发育树分析
Fig.3 Phylogenetic analysis of E3 in Wuzi Ougan and other species
2.3 无子瓯柑和野生型瓯柑CsRNF217 基因的时空表达特性
分别采集无子瓯柑(图4-A)和野生型瓯柑(图4-B)盛花期的花朵并分离出花瓣、雄蕊、雌蕊和花托等花的不同部分(图4-C~D),采用qRT-PCR分析Cs-RNF217 基因在无子瓯柑和野生型瓯柑花器官的不同部分的表达水平。结果表明,CsRNF217 基因的相对表达量在无子瓯柑和野生型瓯柑的雄蕊中均显著高于雌蕊、花瓣和花托,表现为一定的组织特异性。对无子瓯柑和野生型瓯柑小孢子不同发育时期的花药进行CsRNF217 基因表达水平的检测,结果显示,CsRNF217 基因在无子瓯柑和野生型的小孢子不同发育时期均有表达,并在无子瓯柑花粉粒成熟期(Ⅳ)显著上调表达(图4-E)。

图4 CsRNF217 在无子瓯柑和野生型瓯柑花的不同部分和小孢子不同发育时期的表达
Fig.4 Relative expression of CsRNF217 in different parts of flower different stages of microspore development in Wuzi Ougan and Ougan
2.4 无子瓯柑CsRNF217基因的转基因功能研究
将无子瓯柑CsRNF217基因的过表达载体通过农杆菌介导的叶盘法导入普通烟草,经侵染、共培养、筛选培养、壮芽培养和生根培养(图5-A~E)和PCR鉴定(图5-F)获得12株阳性株系。阳性株系与野生型普通烟草的花器官形态无明显差异,但转基因阳性株系成熟花粉的自然散粉量明显减少(图5-G)。利用亚历山大花粉染色液对转基因阳性株系进行花粉活力检测。结果表明,以野生型烟草WT植株为对照,共有7 个转基因阳性株系花粉活力下降。其中,6#、35#、63#株系花粉活力下降显著,分别下降了59.05%、63.75%、61.22%(图5-H~L)。这些株系均可产生种子,种子数量明显减少(图5-M)。半定量RT-PCR 分析显示,CsRNF217 基因在6#、35#、63#转基因株系的花药中可以高效表达(图5-N)。
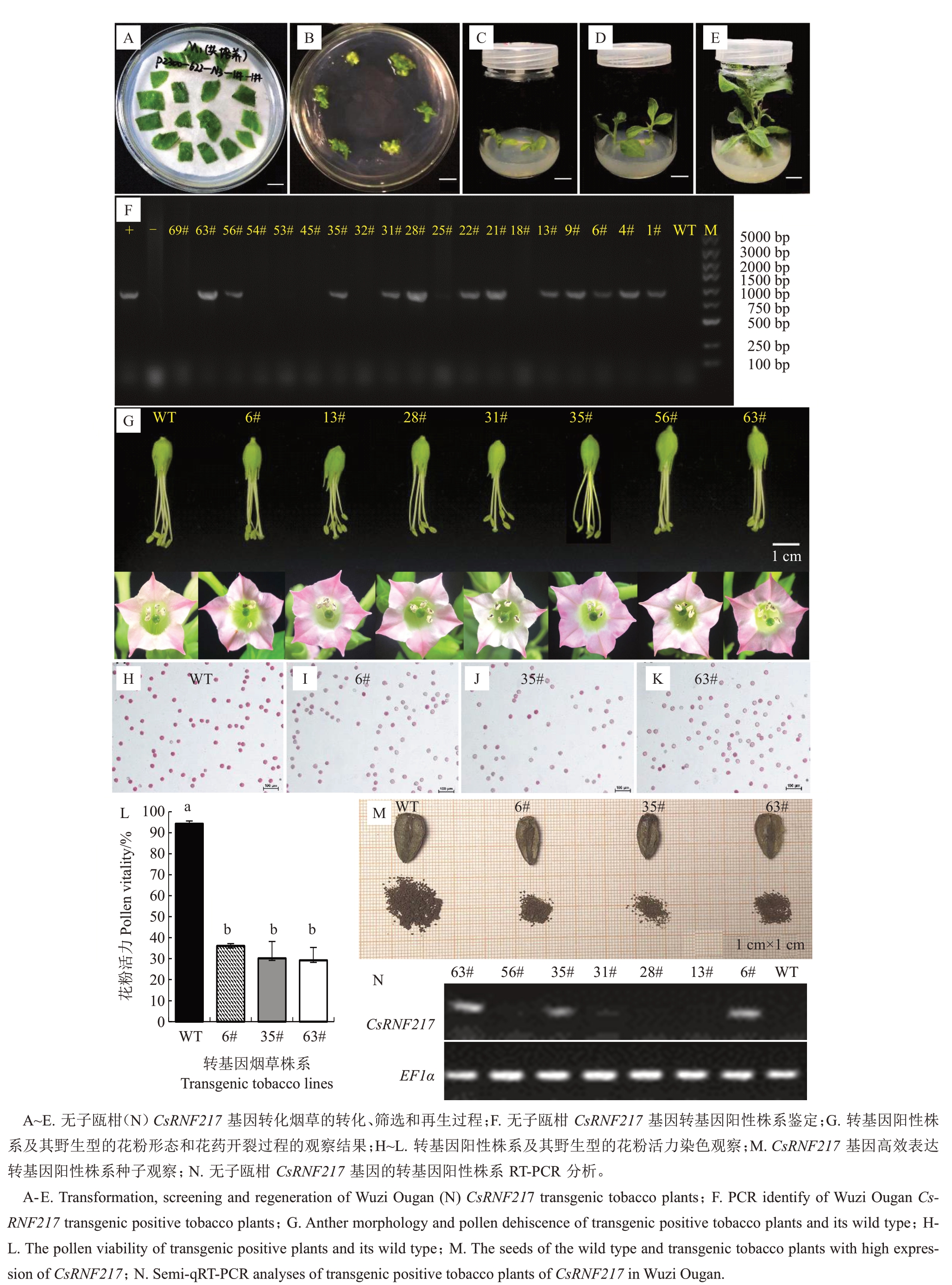
图5 无子瓯柑CsRNF217 基因的转基因功能分析
Fig.5 Functional analysis of transgenic tobacco plants of CsRNF217 in Wuzi Ougan
2.5 无子瓯柑CsRNF217基因亚细胞定位分析
烟草瞬时表达结果显示,对照的GFP 随机分布于烟草表皮细胞的质膜和细胞核中,而CaMV35S::CsRNF217::GFP 融合蛋白荧光特异地分布于烟草表皮细胞的细胞核中(图6)。
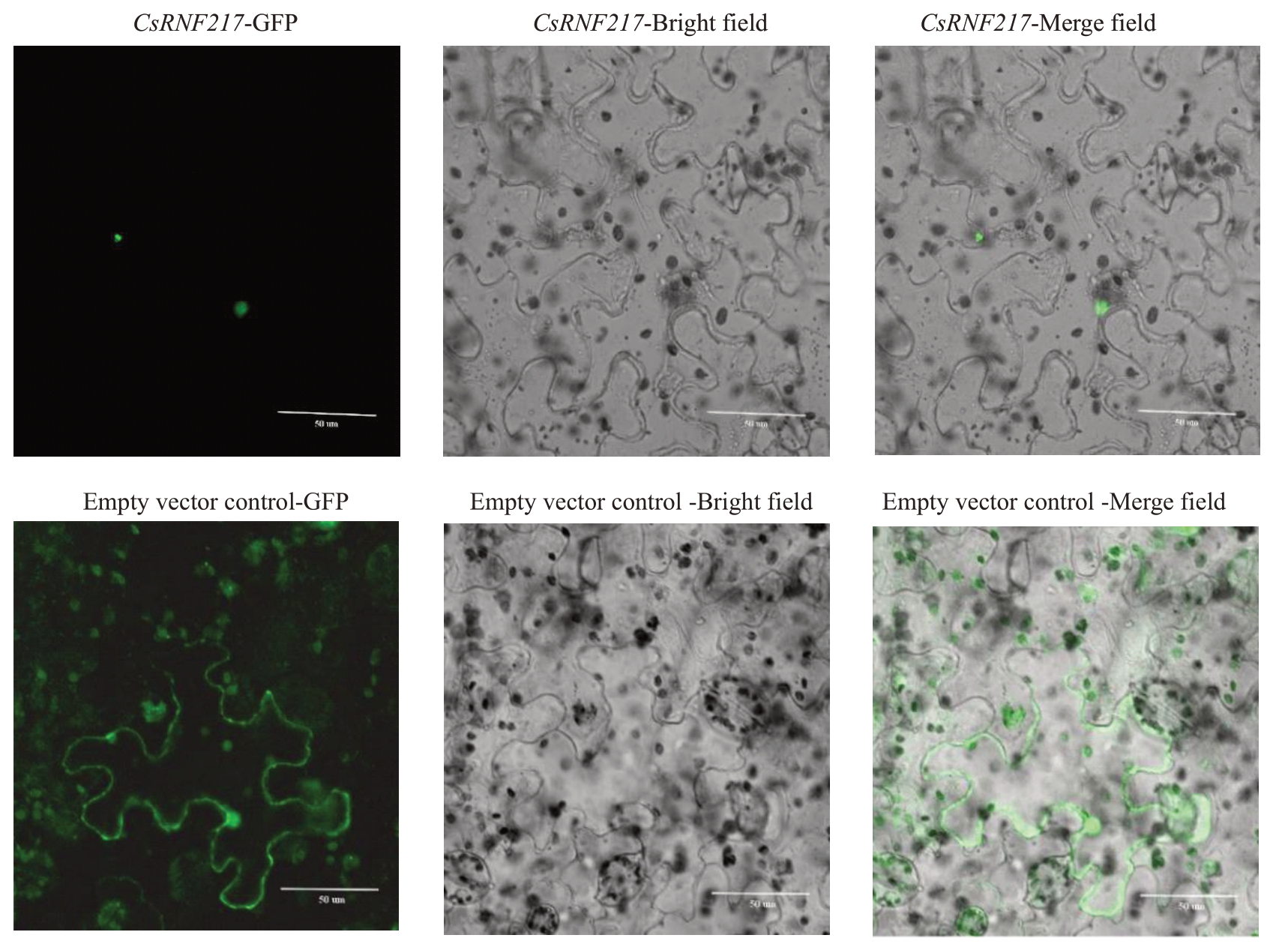
图6 无子瓯柑CsRNF217 基因的亚细胞定位
Fig.6 Subcellular localization of CsRNF217 in Wuzi Ougan
3 讨 论
植物雄性不育指植物在有性繁殖过程中雄蕊发育不正常,不能产生正常生物学功能的花粉,是植物生殖生物学的重要现象,在自然界开花植物中广泛存在,是果实无核的重要原因[31]。雄性不育的原因较多,在柑橘中主要包括减数分裂异常[32]、花药退化[33]或开裂受阻[34]以及花粉部分或完全败育等[35]。花粉发育是复杂的基因网络调控的过程,明确无子瓯柑花粉发育相关基因的功能和调控机制,有利于深入解析无子瓯柑雄性不育分子机制。
E3作为整个泛素化过程的最后一环,是目标蛋白特异性辨识的关键决定步骤。大部分E3 基因都含有1个RING锌指结构域,因此RING型E3数量庞大且功能复杂[36]。经生物信息学分析显示,本研究获得的CsRNF217 基因编码的蛋白具有RING 型E3家族典型结构特征,属于RING-HC亚家族。亚细胞定位分析显示,CaMV35S::CsRNF217::GFP 融合蛋白定位在细胞核,与大多数植物的RING 型锌指蛋白定位的结果一致[37]。
近年来,关于RING 型E3 功能研究表明,其对应的基因参与包括植物花粉发育、花粉管生长等许多细胞生理过程。基因PTB1 是控制水稻结实率的主效基因,Li等[22]发现RING型E3基因PTB1相对表达量水平显著上调可以促进水稻花粉管生长从而正向调节水稻结实率。王剑峤[23]发现拟南芥中RING型E3基因SINAT1的过量表达会诱导花粉管生长去极化,并通过降解花粉管生长相关蛋白RopGAPs来抑制花粉管的生长,从而导致不育。Guo 等[24]通过鉴定发现4 个拟南芥中RING 型E3 基因APD1-4 均与花粉发育异常相关,这些APD功能的缺失抑制了花粉细胞有丝分裂过程,从而导致植株不育。Peng等[25]发现拟南芥DAF基因是RING 型E3基因,该基因可通过正向调节茉莉酸生物合成途径中DAD1基因(DEFECTIVE IN ANTHER DEHISCENCE 1)的表达来控制花药开裂,影响花粉的自然散粉并导致不育。
Cullin 基因家族是RING 型E3 的重要组成部分。Zhou 等[26]从蔷薇科基因组筛选分离获得了57个Cullin 基因,并对这些基因在梨的不同组织(根、茎、叶、果实、花柱和花粉)中的表达水平和花粉管生长过程的表达谱进行分析,发现PbCUL1C1、Pb-CUL3B、PbCUL3C 和PbCUL4 这4 个基因表达模式一致,在梨的花粉和花粉管中的表达显著高于其他基因,且在花粉管生长过程中均有不同程度的表达,说明这些基因参与了梨花粉管的生长过程。拟南芥E3 基因AtMMS21 在拟南芥小孢子转变为双核花粉时期起到重要的调控作用[38],该基因与含有β-葡萄糖苷酸酶基因(β-glucuronidase,GUS)的融合蛋白可在花药等生殖器官中高效表达[39],缺失则会导致突变体减数分裂的染色体行为异常,从而产生异常四分体或二分体并最终造成种子数量显著下降[40]。本研究的表达分析显示,无子瓯柑CsRNF217 基因在雄蕊中高效表达,在花粉发育的成熟花粉粒时期显著上调,这一结果表明CsRNF217 基因在无子瓯柑花粉发育的后期可能存在重要的调控作用。Xu等[27]对油菜中的RING型E3家族中的2个编码蛋白中含有CCCH型的锌指蛋白的基因BcMF30a和Bc-MF30c 进行过表达研究,GUS 染色结果显示这2 个基因的启动子活性在花药中的双核后期达到峰值,且仅在花药和雌蕊中检测到;而通过光电子显微镜等方法观察过表达转基因植株的小孢子发育进程发现,BcMF30a和BcMF30c基因的过表达导致晚期单核小孢子花粉发育异常。张云[41]对13 个番茄E3 基因进行时空表达分析时发现,RING型E3基因SLL8在雄蕊中的相对表达量显著高于其他组织,且在成熟花粉粒期显著高于小孢子发育其他时期,推测SLL8基因参与调控番茄花粉发育过程,其过量表达可影响番茄花粉活力和花粉萌发过程。因此,无子瓯柑小孢子败育可能与其发育晚期CsRNF217基因的上调表达关系密切。
无子瓯柑CsRNF217基因的转基因烟草花药自然散粉量明显下降和花粉活力显著降低的表现,与无子瓯柑的雄性不育表现相似[6];而且CsRNF217基因高效表达的转基因烟草株系的种子数量也明显减少。Xu 等[27]对油菜中过表达BcMF30a 和BcMF30c基因大白菜的转基因植株进行亚历山大花粉活力检测,发现过表达株系花粉活性也显著下降。但是,Liu等[39]则发现AtMMS21基因的缺失突变体的结实率和种子数量均显著下降,雌雄配子体的减数分裂均出现异常,并推测该E3基因在拟南芥减数分裂和雌雄配子体发育的过程中起到重要作用。因此,无子瓯柑的E3 基因CsRNF217 的过量表达对转基因烟草小孢子的育性可能存在负调控作用,并由此推测其在无子瓯柑雄性不育的发生过程中可能存在相似的调控作用。
4 结 论
无子瓯柑CsRNF217 基因编码蛋白为RING 型锌指蛋白,亚细胞定位于细胞核,在无子瓯柑雄蕊中相对表达量最高,并在花粉发育晚期上调表达。异源转基因研究表明,CsRNF217 基因过表达的转基因烟草的花药不能正常散粉,花粉活力和种子数量显著下降,表现出部分雄性不育的特征,为进一步揭示CsRNF217基因在无子瓯柑小孢子发育中的重要作用和雄性不育的机制提供了依据。
[1]徐象华,斯金平,谢建秋,蓝云龙,曾燕如,江伟林,叶文伟.柑橘新品种无子瓯柑的选育[J].果树学报,2006,23(5):781-782.XU Xianghua,SI Jinping,XIE Jianqiu,LAN Yunlong,ZENG yanru,JIANG Weilin,YE Wenwei.Ougan seedless,a new mandarin cultivar[J].Journal of Fruit Science,2006,23(5):781-782.
[2]邓秀新.世界柑橘品种改良的进展[J].园艺学报,2005,32(6):1140-1146.DENG Xiuxin. Advances in worldwide citrus breeding[J]. Acta Horticulturae Sinica,2005,32(6):1140-1146.
[3]胡志勇,伊华林,邓秀新.国庆一号温州蜜柑小孢子发育过程及其分期[J].果树学报,2005,22(5):567-569.HU Zhiyong,YI Hualin,DENG Xiuxin. Microspore development and its stages in Citrus unshiu[J].Journal of Fruit Science,2005,22(5):567-569.
[4]曾燕如,张炼炳,斯金平,徐象华.无籽瓯柑无核原因的研究[J].浙江林学院学报,2005,22(4):359-362.ZENG Yanru,ZHANG Lianbing,SI Jinping,XU Xianghua.Preliminary study on seedlessness of seedless Ougan[J]. Journal of Zhejiang Forestry College,2005,22(4):359-362.
[5]HU Z Y,XU X H,ZHANG M,WEN Q G,WEI J,YI H L,DENG X X.Abnormal microspore development leads to pollen abortion in a seedless mutant of‘Ougan’mandarin (Citrus suavissima Hort.ex Tanaka)[J].Journal of the American Society for Horticultural Science,2007,132(6):777-782.
[6]张迟,张敏,朱铨,刘志辉,颜福花,吴连海,徐象华,周晓音,陈翔.‘瓯柑’及其无子突变体花粉发育的细胞学观察[J].果树学报,2014,31(2):265-269.ZHANG Chi,ZHANG Min,ZHU Quan,LIU Zhihui,YAN Fuhua,WU Lianhai,XU Xianghua,ZHOU Xiaoyin,CHEN Xiang.Cytological observation of pollen development in‘Ougan’(Citrus suavissima Hort. ex Tanaka) and its seedless mutant[J].Journal of Fruit Science,2014,31(2):265-269.
[7]ZHANG C,YU D H,KE F Z,ZHU M M,XU J G,ZHANG M.Seedless mutant‘Wuzi Ougan’(Citrus suavissima Hort. ex Tanaka‘Seedless’) and the wild type were compared by iTRAQ-based quantitative proteomics and integratedly analyzed with transcriptome to improve understanding of male sterility[J].BMC Genetics,2018,19(1):106.
[8]俞狄虎.转录组和蛋白质组关联分析无子瓯柑雄性不育分子机制[D].杭州:浙江农林大学,2018.YU Dihu. An integrative analysis of the transcriptome and proteome of‘Wuzi Ougan’(Citrus suavissima Hort. ex Tanaka‘Seedless’) and the wild type improves our understanding of male sterility[D].Hangzhou:Zhejiang A&F University,2018.
[9]HO S R,LEE Y J,LIN W C.Regulation of RNF144A E3 ubiquitin ligase activity by self-association through its transmembrane domain[J].The Journal of Biological Chemistry,2015,290(38):23026-23038.
[10]DOMINGO J L,FRANCISCO M B,JUAN MANUEL G P,VICTOR A H,PLINIO G. Repertoire of plant RING E3 ubiquitin ligases revisited:New groups counting gene families and single genes[J].PLoS One,2018,13(8):e0203442.
[11]CRAIG A,EWAN R,MESMAR J,GUDIPATI V,SADANADOM A.E3 ubiquitin ligases and plant innate immunity[J].Journal of Experimental Botany,2009,60(4):1123-1132.
[12]IRENE S,CAMPOS L,RIVAS S. Roles of E3 ubiquitin-ligases in nuclear protein homeostasis during plant stress responses[J].Frontiers in Plant Science,2018,9:139.
[13]QANMBER G,YU D Q,LI J,WANG L L,MA S Y,LU L L,YANG Z R,LI F G.Genome-wide identification and expression analysis of Gossypium RING-H2 finger E3 ligase genes revealed their roles in fiber development,and phytohormone and abiotic stress responses[J]. Journal of Cotton Research,2018,1(6):1364-1381.
[14]LUO H L,LALUK K,LAI Z B VERONESE P,SONG F M,MENGISTE T. The Arabidopsis Botrytis Susceptible1 interactor defines a subclass of RING E3 ligases that regulate pathogen and stress responses[J]. Plant Physiology,2010,154(4):1766-1782.
[15]LIM S D,CHO H Y,PARK Y C,HAM D J,LEE J K,JANG C S. The rice RING finger E3 ligase,OsHCI1,drives nuclear export of multiple substrate proteins and its heterogeneous overexpression enhances acquired thermos tolerance[J]. Journal of Experimental Botany,2013,64(10):2899-2914.
[16]CHENG M C,HSIEH E J,CHEN J H,CHEN H Y,LIN T P.Arabidopsis RGLG2,functioning as a RING E3 ligase,interacts with AtERF53 and negatively regulates the plant drought stress response[J].Plant Physiology,2012,158(2):363-375.
[17]LIU Z B,WANG J M,YANG F X,YANG L,YUE Y F,XIANG J B,GAO M,XIONG F J,DONG L,WU X J,LIU N,ZHANG X,LI X F,YANG Y.A novel membrane-bound E3 ubiquitin ligase enhances the thermal resistance in plants[J]. Plant Biotechnology Journal,2014,12(1):93-104.
[18]RAGHAVENDRA A S,GONUGUNTA V K,CHRISTMANN A,GRILL E.ABA perception and signalling[J]. Trends in Plant Science,2010,15(7):395-401.
[19]ZHANG X R,GARRETON V,CHUA N H.The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation[J]. Genes & Development,2005,19(13):1532-1543.
[20]LEE D H,CHOI H W,HWANG B K. The pepper E3 ubiquitin ligase RING1 gene,CaRING1,is required for cell death and the salicylic acid-dependent defense response[J]. Plant Physiology,2011,156(4):2011-2025.
[21]LIU H X,RAVICHANDRAN S,TEC O K,MCVEY S,LILLEY C,TERESINSKI H J,GONZALEZ-FERRER C,MULLEN R T,HOFIUS D,PRITHIVIRAJ B,STONE S L. The RING-Type E3 ligase XBAT35.2 is involved in cell death induction and pathogen response[J]. Plant Physiology,2017,175(3):1469-1483.
[22]LI S C,LI W B,HUANG B,GAO X M,ZHOU X Y,YE S M,LI C B,GAO F Y,ZOU T,XIE K L,REN Y,AI P,TANG Y F,LI X M,DENG Q M,WANG S Q,ZHENG A P,ZHU J,LIU H N,WANG L X,LI P. Natural variation in PTB1 regulates rice seed setting rate by controlling pollen tube growth[J]. Nature Communications,2013,4(1):1164-1171..
[23]王剑峤.拟南芥RING-finger E3 基因SINAT1 调控花粉萌发的初步研究[D].北京:北京大学,2013.WANG Jianqiao. Preliminary study on the regulation of pollen germination by RING-finger E3 gene SINAT1 in Arabidopsis thaliana[D].Beijing:Peking University,2013.
[24]GUO L,GU H Y,LIU J J,QU L J. Four closely-related RING-type E3 ligases,APD1-4,are involved in pollen mitosis II Regulation in Arabidopsis[J]. Journal of Integrative Plant Biology,2012,54(10):814-827.
[25]PENG Y J,SHIH C F,YANG J Y,TAN C M,HSU W H,HUANG Y P,LIAO P C,YANG C H.A RING-type E3 ligase controls anther dehiscence by activating the jasmonate biosynthetic pathway gene DEFECTIVE IN ANTHER DEHISCENCE1 in Arabidopsis[J].The Plant Journal,2013,74(2):310-327.
[26]ZHOU Y,HUANG Y D,WU L,WANG G M,GU C,ZHANG S L.Phylogenetic and expression analyses of cullin family members unveil the role of PbCUL1.C1 in pollen tube growth underlying non-self S-RNase in pear[J]. Plant Molecular Biology Reporter,2020,38:601-612.
[27]XU L A,LIU T T,XIONG X P,LIU W M,YU Y J,GAO J S.Overexpression of two CCCH-type zinc-finger protein genes leads to pollen abortion in Brassica campestris ssp.chinensis[J].Genes,2020,11(11):1287.
[28]王关林,方宏筠.植物基因工程[M].2 版.北京:科学出版社,2002:344-362.WANG Guanlin,FANG Hongjun.Plant genetic engineering[M].2nd ed.Beijing:Science Press,2002:344-362.
[29]PETERSON R,SLOVIN J P,CHEN C B.A simplified method for differential staining of aborted and nonaborted pollen grains[J].International Journal of Plant Biology,2010,1(13):66-68.
[30]GONG X Q,ZHANG J Y,HU J B,WANG W,WU H,ZHANG Q H,LIU H J. FcWRKY70,a WRKY protein of Fortunella crassifolia,functions in drought tolerance and modulates putrescine synthesis by regulating arginine decarboxylase gene[J].Plant,Cell and Environment,2015,38(11):2248-2262.
[31]曹庆芹,伊华林,邓秀新.果树雄性不育研究进展[J].果树学报,2005,22(6):678-681.CAO Qingqin,YI Hualin,DENG Xiuxin.Advances in research on male sterility in fruit crops[J].Journal of Fruit Science,2005,22(6):678-681.
[32]黄建昌,潘文力,廖宗楷,林应其,周庆贤.花都无核暗柳橙的选育研究[J].中国果树,1996(3):1-3.HUANG Jianchang,PAN Wenli,LIAO Zongkai,LIN Yingqi,ZHOU Qingxian. Studies on breeding of Anliu in Huadu[J].China Fruits,1996(3):1-3.
[33]翁法令,徐建国,柯甫志.清见橘橙及其在柑橘育种中的利用[J].中国南方果树,2004,33(5):8-9.WENG Faling,XU Jianguo,KE Puzhi. Kiyomi tangor and its utilization in citrus breeding[J].South China Fruits,2004,33(5):8-9.
[34]王玉玲.无核雪柑授粉受精及种胚败育的研究[D].福州:福建农林大学,2006.WANG Yuling. Studies on biology of pollination fertilization and embryo aborted in Wuhexuegan[D]. Fuzhou:Fujian A & F University,2006.
[35]杨杰,王博,杨昌鹏,赵小龙.红马叙葡萄柚的品种特性及栽培技术[J].热带农业科学,2016,36(5):17-20.YANG Jie,WANG Bo,YANG Changpeng,ZHAO Xiaolong.Variety characteristics and cultivation techniques of Red Marsh grapefruit[J]. Chinese Journal of Tropical Agriculture,2016,36(5):17-20.
[36]RAYMOND J D,CLAUDIO A P J. RING domain E3 ubiquitin ligases[J].Annual Review of Biochemistry,2009,78:399-434.
[37]SUN J H,SUN Y H,AHMED R I,REN A Y,XIE M M. Research progress on plant RING-Finger proteins[J].Genes,2019,10(12):973.
[38]施松锋,张盛春,徐庞连,阳成伟.拟南芥SUMO E3连接酶At-MMS21参与调控花器官的发育[C]//广东省植物学会.广东省植物学会第十九期学术研讨会论文集,2010:36.SHI Songfeng,ZHANG Shengchun,XU Panglian,YANG Chengwei. SUMO E3 ligase AtMMS21 is involved in the regulation of flower organ development in Arabidopsis [C]// Guangdong Botanical Society. A collection of papers from the 19th Academic Symposium of the Botanical Society of Guangdong Province,2010:36.
[39]LIU M,SHI S F,ZHANG S C,XU P L,LAI J B,LIU Y Y,YUAN D K,WANG Y Q,DU J J,YANG C W.SUMO E3 ligase At-MMS21 is required for normal meiosis and gametophyte development in Arabidopsis[J].BMC Plant Biology,2014,14(1):153.
[40]LING Y,ZHANG C Y,CHEN T,HAO H Q,LIU P,BRESSAN R A,HASEGAWA P M,JIN J B,LIN J X. Mutation in SUMO E3 ligase,SIZ1,disrupts the mature female gametophyte in Arabidopsis[J].PLoS One,2012,7(1):e29470.
[41]张云.E3 泛素连接酶SLL8 在调控番茄花粉发育中的功能研究[D].杭州:浙江大学,2018.ZHANG Yun.The regulation of E3 ligase SLL8 on pollen development in tomato[D].Hangzhou:Zhejiang University,2018.