薄壳山核桃[Carya illinoinensis(Wangenh.)K.Koch]属于胡桃科(Juglandaceae)山核桃属(Carya Nutt.),别名美国山核桃[1],原产地为美国南部和墨西哥北部,是一种果材兼用的高经济价值的优良树种[2-5]。近年来,其被证明含有丰富的不饱和脂肪酸和酚类化合物[6-7],具有较高的食用价值和经济价值[8-9]。目前,国内对薄壳山核桃组织培养研究主要集中于外植体脱毒及腋芽分化[10-11]。Renukdas 等[12]以薄壳山核桃的试管无菌苗为材料诱导茎段腋芽分化,并建立了植株再生体系;严泽埔[13]通过农杆菌介导法将目的赤霉素矮化基因转入薄壳山核桃体胚内后脱菌增殖培养,以获得薄壳山核桃矮化植株。虽然都不同程度地诱导出了愈伤和体胚组织,但外植体脱毒困难,如体胚诱导发育难或再生植株难以诱导生根等,导致不能得到具有完整根系的植株,从而无法建立成熟稳定的再生遗传转化体系。
发根农杆菌是一种侵染性很强的根瘤菌科农杆菌属革兰氏阴性好氧菌,携带的Ri质粒能有效侵染众多植物,Ri质粒的T-DNA片段在植物细胞基因组中插入、整合并表达,诱导植物细胞形成转基因毛状根(hairy root)[14-15]。目前,发根农杆菌介导的植物转基因绝大多数是利用植株的无菌苗或叶片或茎段作为外植体获得转基因根。农杆菌介导植株转基因方法的应用大多集中在茄科、菊科、十字花科、旋花科、伞形科、豆科、石竹科、蓼科等草本植物中[16],而在木本植物中的应用普遍存在转化率较低的现象。郝征[17]用发根农杆菌菌株30148侵染枣的组培苗得到其毛状根诱导率为2.2%~9.4%;林彩容等[18]用菌株ATCC15834 侵染茶树成熟种子下胚轴的发状根诱导率最高为23.96%;刘雪羽等[19]采用菌株ArQual诱导光皮桦叶片产生转基因毛状根的转化率为36.4%;姚庆收等[20]将橡胶树茎段用发根农杆菌R1601 侵染后发现其毛状根诱导率达36.6%;刘思巧[21]利用发根农杆菌侵染银杏产生毛状根的诱导率达72.36%。
常规的发根农杆菌介导转基因植物的构建方法大多以无菌材料为侵染对象,需要大量的时间进行愈伤组织诱导、分化和继代,周期长,操作繁琐,效率低。而木本植物大多次生代谢物较多,组培过程中易发生褐化,严重影响外植体的脱分化和培养物的再分化进程[22],尤其是酚类化合物含量较高的经济树种,如山核桃属植物;其次木本植物组培苗时间较草本植物长,成本高,技术要求严,很难扩大生产规模[23];目前对木本植物组培技术的研究中缺乏经验理论指导,难以推广,造成在实际应用中转化率过低,效益甚微等现象,严重制约产业化发展[24]。笔者在本研究中拟利用发根农杆菌建立一种不依赖组培技术的高效诱导转化体系,在非组培条件下,通过用3 种发根农杆菌MSU440、C58C1 和K599 分别侵染薄壳山核桃种子苗的茎段,统计各自的诱导率,筛选出影响发根农杆菌转化效率的关键因素,建立并优化转化体系,最后用手持式荧光仪、荧光体式显微镜及PCR 等方法检测转化率。笔者在本研究中初步建立了转化率高且周期短的薄壳山核桃毛状根诱导体系,以期为薄壳山核桃的遗传改良和基因挖掘与功能分析奠定基础,为实现木本植物的基因功能验证提供新途径。
1 材料和方法
1.1 材料
薄壳山核桃钟山实生种子苗来自于浙江农林大学潘母港实验基地。MSU440、C58C1 和K599 菌株购自上海唯地生物技术有限公司。带绿色荧光蛋白(green fluores-cent protein,GFP)标记基因的pCAMBIA1300载体储存于浙江农林大学亚热带森林培育国家重点实验室超低温室。
1.2 方法
1.2.1 薄壳山核桃实生苗材料的准备 收获薄壳山核桃的成熟种子进行筛选,对种子表面消毒,将其种在含基质(w,70%~80%草炭土、5%~10%珍珠岩、2%~5%蛭石、5%~10%壤砂土,添加适量水并混合,并选用有机腐熟微生物菌剂进行10 d 的腐熟处理)的盆中,在催芽室(26±2)℃中进行1个月的发芽过程,后移栽至大棚生长以获得不同生长时期的薄壳山核桃幼苗。
1.2.2 发根农杆菌转化 取-80 ℃保存的K599、MSU440 和C58C1 发根农杆菌感受态于冰上融化,每100µL 感受态加入1µg 的pCAMBIA1300 质粒,用移液枪吸吹混匀,依次放于冰上静置5 min、液氮速冻5 min、37 ℃水浴5 min、冰浴5 min。冰浴中拿出放室温加入700 µL 无抗生素的TY 液体培养基(蛋白胨5 g∙L-1、酵母提取物3 g∙L-1、10 mmol∙L-1 CaCl2),28 ℃震荡培养2 h。6000 r∙min-1离心1 min收菌,留取100µL 左右上清液轻轻吹打重悬菌块,涂布于含有50 mg∙L-1硫酸卡那霉素和50 mg∙L-1链霉素的TY平板(TY液体培养基+15 g∙L-1琼脂)上,倒置放于28 ℃培养箱2~3 d。
1.2.3 发根农杆菌侵染液制备 分别挑取含有pCAMBIA1300 质粒的K599、MSU440 和C58C1 发根农杆菌单菌落,接种于1 mL 含50 mg∙L-1硫酸卡那霉素和50 mg∙L-1链霉素的TY 液体培养基中,28 ℃震荡培养12 h。随后将菌液按1:100的比例扩大培养至OD600值为0.4~1.2,6500 r∙min-1离心10 min后收集菌液,将富集的菌重悬于等体积的2-吗啉乙磺酸(MES)缓冲液(10 mmol∙L-1 MES-KOH,pH=5.2,10 mmol∙L-1 MgCl2,100 µmol∙L-1乙酰丁香酮)中,以备侵染使用。
1.2.4 转化体系的建立及不同侵染方式对毛状根的诱导 选择子叶期的薄壳山核桃幼苗进行试验,发根农杆菌菌液OD600为0.4~1.2。(1)将蘸有发根农杆菌菌液的单面刀片在薄壳山核桃近种子的胚轴或幼苗茎部0~5 cm 处轻轻水平倾斜45°,横向环割3 个刀口,每划一刀将单面刀于菌液中浸泡,以保证伤口处被菌液侵染到(图1-A);(2)1 mL注射器注射至待转化的薄壳山核桃近种子的胚轴或茎部0~5 cm 处(图1-B)。然后将侵染过的苗置于催芽室[避光,温度(26±2)℃,湿度80%~90%]中培养2 d 后,移至温室大棚[温度(23±2)℃,湿度40%~50%]培养,1个月后进行观察处理部位切面处是否出现毛状根,并用手持式荧光仪观察长出的毛状根是否有绿色荧光信号来判定是否转化成功并进行统计。

图1 侵染方式
Fig.1 Infection way
A.注射;B.环割;比例尺为5 cm。
A.Injection;B.Ring cutting;The scale is 5 cm.
1.2.5 转化体系的优化 基于前期对试验过程中可能影响毛状根诱导情况的观察,针对菌株类型、菌液浓度、处理部位及苗龄这4个因素开展3个水平的正交试验,设计L9(34)正交试验表(表1),每个处理20~25 株苗,培养1 个月后对薄壳山核桃幼苗茎部生根情况进行观测统计,以期优化现有转化体系,筛选出影响诱导薄壳山核桃茎产生毛状根的关键因素和最优条件。
表1 四因素三水平正交试验设计
Table 1 Four factors and three levels of orthogonal experimental design
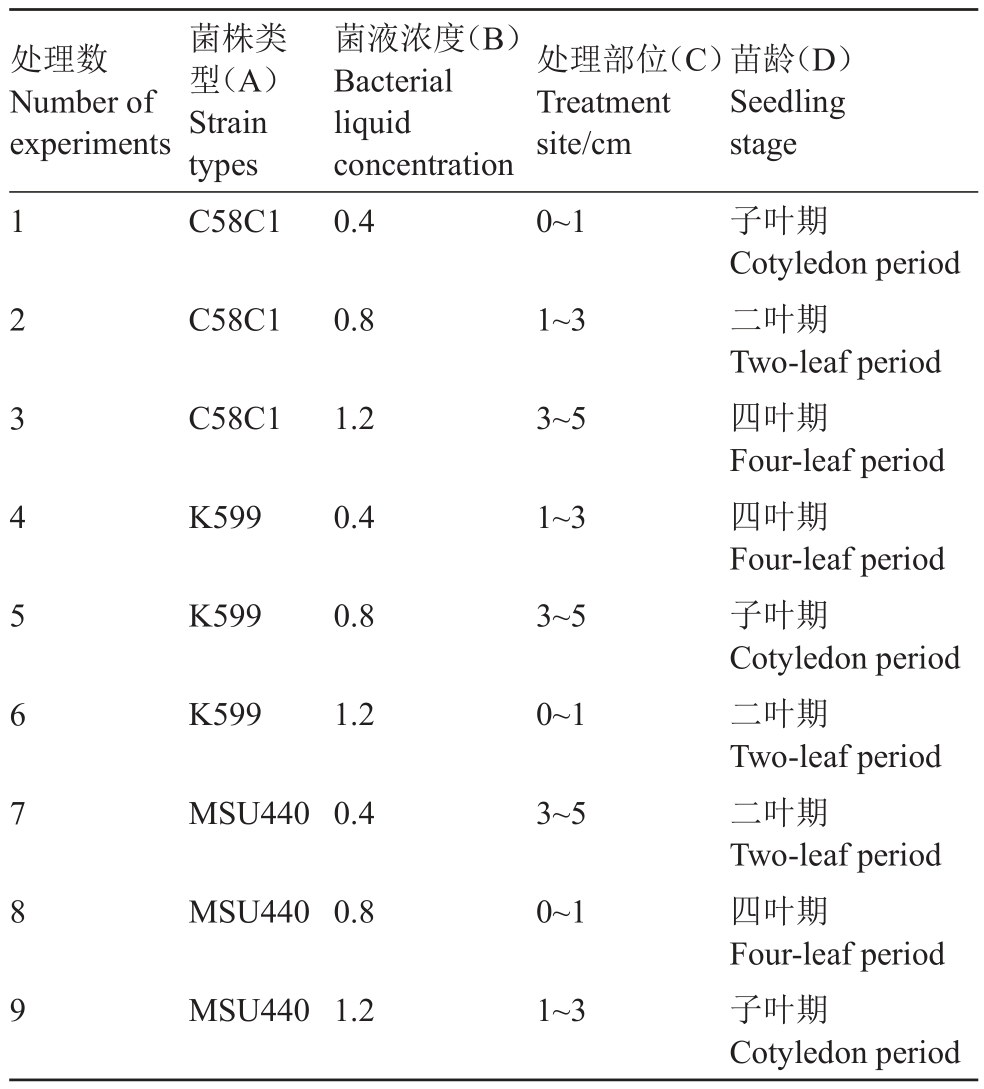
处理数Number of experiments 1 2 3 4 5 6 7 8 9菌株类型(A)Strain types C58C1菌液浓度(B)Bacterial liquid concentration 0.4处理部位(C)Treatment site/cm 0~1 C58C1 0.8 1~3 C58C1 1.2 3~5 K599 0.4 1~3 K599 0.8 3~5 K599 1.2 0~1 MSU4400.4 3~5 MSU4400.8 0~1 MSU4401.2 1~3苗龄(D)Seedling stage子叶期Cotyledon period二叶期Two-leaf period四叶期Four-leaf period四叶期Four-leaf period子叶期Cotyledon period二叶期Two-leaf period二叶期Two-leaf period四叶期Four-leaf period子叶期Cotyledon period
1.2.6 鉴定及统计分析 通过手持式荧光仪统计薄壳山核桃幼苗诱导出转化成功的毛状根的植株数目,毛状根植株诱导率/%=(具毛状根植株数/侵染植株总数)×100;毛状根植株阳性率/%=(阳性毛状根植株数/具毛状根植株数)×100;阳性毛状根植株诱导率/%=(毛状根植株诱导率×毛状根植株阳性率)×100;毛状根阳性率/%=(阳性毛状根数/毛状根总数)×100。
随机挑选20~40 条毛状根,采用改良CTAB 法提取毛状根DNA[25]。根据载体序列在上海生工合成GFP基因和Hyg基因的PCR引物,以引物对GFP-F和GFP-R(上游引物:5’-AAGGACGACGGCAACTACAA-3’;下游引物:5’-TCTGCTTGTCGGCCATGATA-3’)和引物对Hyg-F 和Hyg-R(上游引物:5’-CATATACGCCCGGAGTCGTG;下 游 引 物:5’- AGACCTGCCTGAAACCGAAC-3’)进行实时定量PCR,PCR 反应程序为95 ℃预变性5 min;95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸1 min,34个循环后再72 ℃延伸5 min,4 ℃保存。以转化空载体的毛状根为对照,鉴定阳性毛状根。PCR 扩增结束后进行2%的凝胶电泳验证,有目的条带的即为阳性根。
2 结果与分析
2.1 发根农杆菌诱导薄壳山核桃产生毛状根
通过环割和注射2 种方式进行侵染,分别统计50株(表2),发现1个月后均有幼苗在切口处长出毛状根,环割处理的毛状根植株诱导率为50%;注射处理的毛状根植株诱导率为48%。说明利用本试验方法可成功诱导薄壳山核桃子叶期幼苗的茎部产生毛状根,且表明环割与注射的侵染方式诱导毛状根的效果基本一致。因环割操作更加简便,故后续试验均采用环割的侵染方式。
表2 不同侵染方式的毛状根植株诱导率
Table 2 Induction of the incidence of hairy roots by different infection modes

侵染方式Infection way环割Blade scratch注射Needle injection诱导率Induced efficiency/%50 48
2.2 发根农杆菌诱导薄壳山核桃产生毛状根体系的优化
通过对苗龄、菌液浓度、菌种、处理部位等可能影响毛状根诱导率的因素进行探索试验,以期得到诱导薄壳山核桃幼苗产生毛状根的最佳条件组合。对统计的毛状根植株诱导率进行极差分析与多重比较,由表3 可知各因素水平的变化对薄壳山核桃生根诱导率的影响从高到低依次为:苗龄(D)>处理部位(C)>菌液浓度(B)>菌种(A)。苗龄是影响诱导率最显著的条件,且子叶期(D1)时侵染得到的生根诱导率最高;其次是侵染部位,在近种子的胚轴或幼苗茎部3~5 cm(C3)处进行环割所得到的毛状根植株诱导率最高;然后是菌液浓度,当OD600为0.8(B2)时诱导率最高;最后是菌株类型,较其他两种菌株,K599 菌株(A2)对薄壳山核桃毛状根的诱导率最高。筛选得到发根诱导率最高的组合是子叶期薄壳山核桃幼苗、环割侵染距离种子3~5 cm 处的茎部、OD600为0.8、菌株为K599,1 个月后其毛状根植株诱导率最高为56.5%。
表3 四因素三水平正交试验结果分析
Table 3 Orthogonal array of four factors and three levels and analysis of experimental results

注:k 为不同处理中各因素对应的各水平生根率的均值;R 为极差。
Note:k is the mean value of rooting rate at each level corresponding to each factor in different treatments;R is range.
处理数Number of experiments k1 B C D 1 2 3 4 5 6 7 8 9毛状根植株诱导率Induced efficiency/%15.0 28.6 28.6 33.3 56.5 19.0 19.0 38.1 33.3极差分析Range analysis k2 k3 R因素Factors A A1 A1 A1 A2 A2 A2 A3 A3 A3 0.257 0.363 0.254 0.109 B1 B2 B3 B1 B2 B3 B1 B2 B3 0.241 0.363 0.270 0.122 C1 C2 C3 C2 C3 C1 C3 C1 C2 0.210 0.318 0.347 0.138 D1 D2 D3 D3 D1 D2 D2 D3 D1 0.366 0.222 0.286 0.144
对以上正交试验的薄壳山核桃植株进行统计,得到在所有生根的植株中,毛状根植株阳性率为80%。综合可得,阳性毛状根植株诱导率最高可达45.2%。随机挑选具有毛状根的植株(表4),统计得到共具毛状根41 根,其中阳性毛状根32 根,即毛状根阳性率为78.05%。
表4 毛状根阳性率统计
Table 4 Statistics of transformation efficiency of positive hairy roots
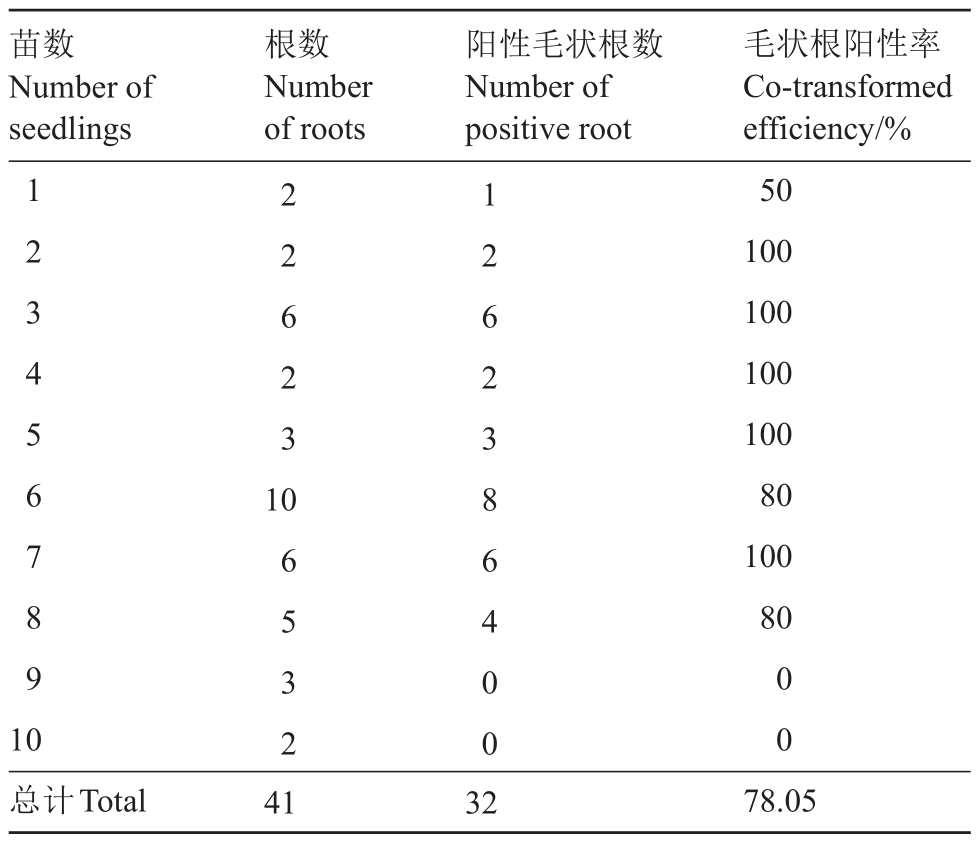
苗数Number of seedlings of roots positive rootefficiency/%1 2 1 50 2 2 2 100 3 6 6 100 4 2 2 100 5 3 3 100 6 10880 7 6 6 100 8 5 4 80 9 3 0 0 10200总计Total413278.05根数Number阳性毛状根数Number of毛状根阳性率Co-transformed
2.3 毛状根转化体系的验证
经发根农杆菌侵染薄壳山核桃后得到转基因根(图2-A~F)。为了验证以上毛状根转化体系的有效性,使用手持式荧光仪进行检测(图3-E),可以观察到阳性转基因毛状根具有绿色荧光信号。为了进一步验证,将毛状根在体式荧光显微镜下进行荧光检测。荧光显微镜观察结果表明,可以观察到具有绿色荧光信号的阳性转基因毛状根(图3-A~D)。另外,进行DNA 水平的验证,得到大小与目的基因大小一致的条带(图4)。
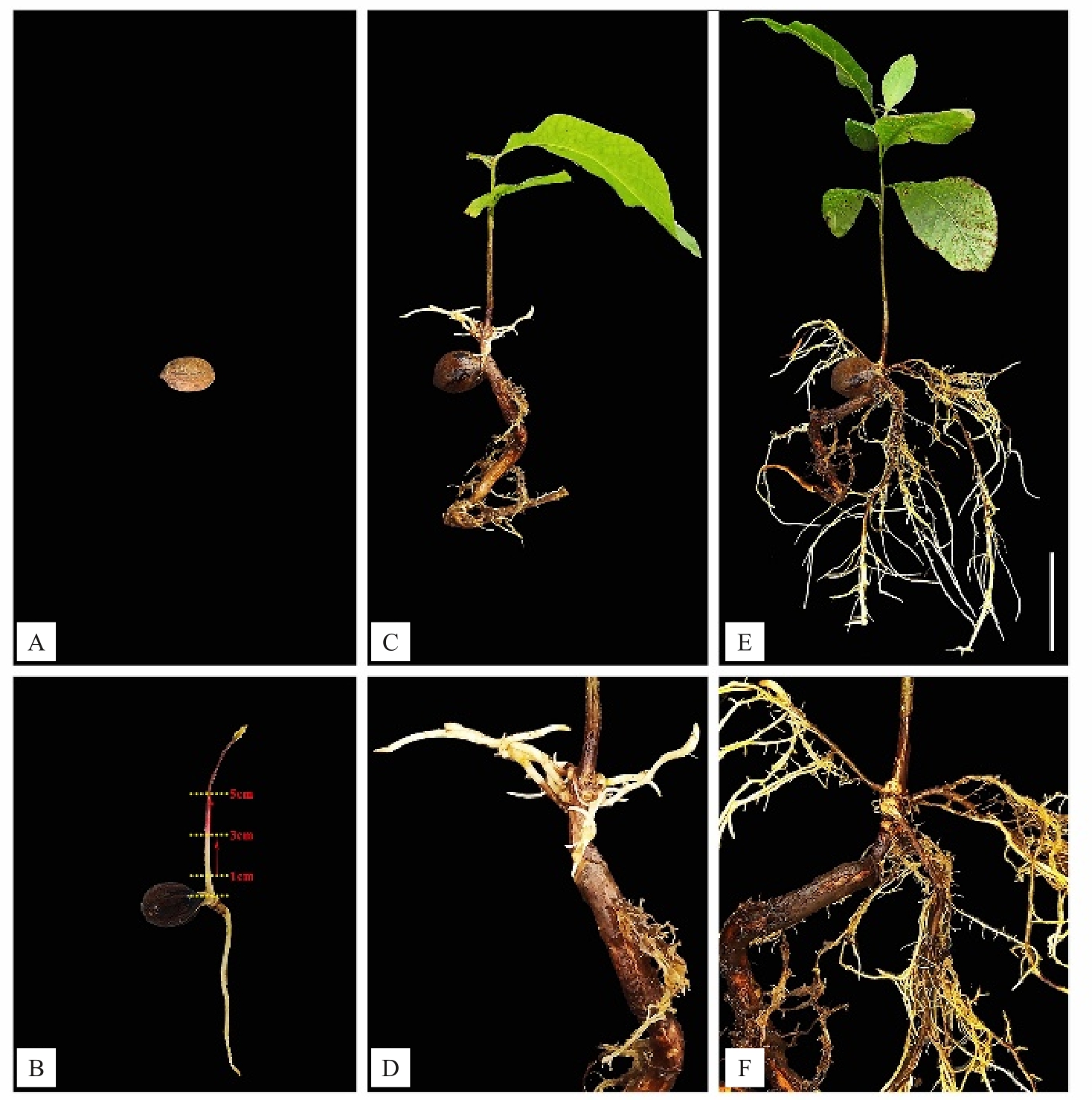
图2 侵染后薄壳山核桃毛状根的生长情况
Fig.2 Growth of hairy roots of pecan after infection
A.种子;B.子叶期幼苗:环割部位示意图;C.侵染后30 d 时的毛状根(E 为细节);D.侵染后60 d 时的毛状根(F 为细节)。比例尺为5 cm。
A.Seeds; B.Cotyledon seedlings: schematic diagram of ring cut site; C.Hairy roots at 30 days after infection (E is detail); D.Hairy roots at 60 days after infection(F is detail).The scale is 5 cm.

图3 毛状根荧光检测
Fig.3 Hairy root fluorescence detection
A 和B.明场(A)和荧光(B)下观察侵染薄壳山核桃20 d 后产生的愈伤;C 和D.明场(C)和荧光(D)下侵染薄壳山核桃30 d 后产生的的毛状根(GFP 阴性和阳性的根);E.手持式荧光仪检测毛状根的表型;标尺为1 cm。
A and B.Callus were observed under bright field(A)and fluorescence(B)after 20 days of infection.C and D.Hairy roots(GFP negative and GFP positive)were produced after 30 days of infection under bright field(C)and fluorescence(D);E.Hand-held fluorimeter was used to detect the phenotype of hairy roots;The scale is 1 cm.
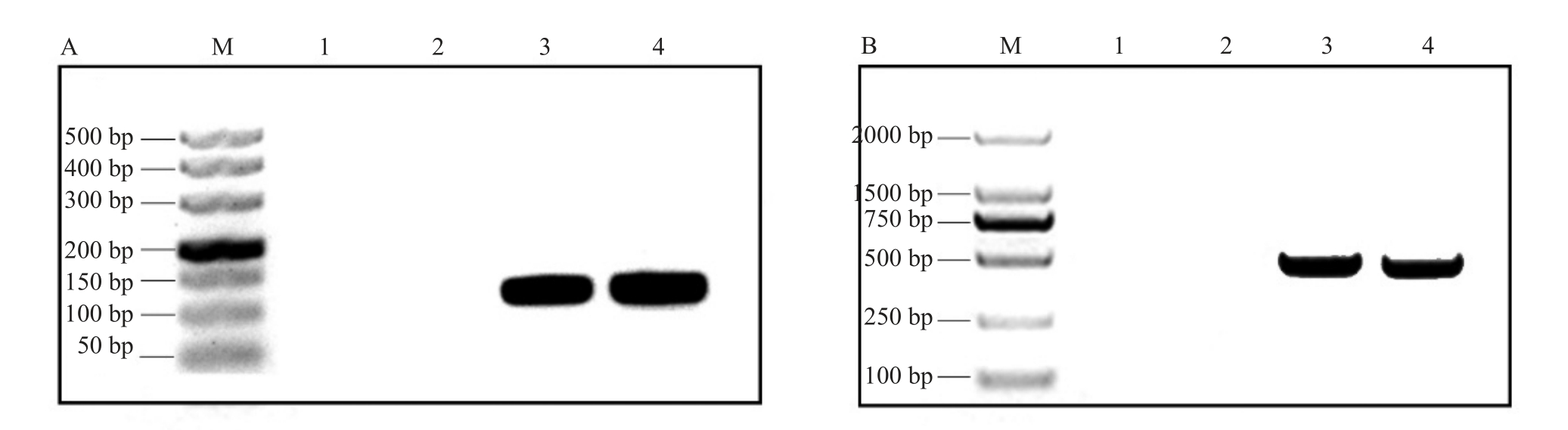
图4 阳性毛状根的DNA 分子检测
Fig.4 DNA molecule detection of positive hairy roots
A.引物为GFP;1~2.CK;3-4.阳性毛状根;M.DL500 DNA Marker。B.引物为Hyg;1~2.CK;3-4.阳性毛状根;M.DL2000 DNA Marker。
A.GFP primers;1~2.CK;3-4.Positive hairy root;M.DL500 DNA Marker.B.Hyg primers;1~2.CK;3-4.Positive hairy root;M.DL2000 DNA Marker.
3 讨 论
目前,遗传转化是研究基因功能的重要手段。在农林业上,许多重要造林树种和优质果树栽培品系扦插繁殖时生根率很低,严重制约了利用无性繁殖的方法对它们进行大面积推广的发展。而采用发根农杆菌处理木本植物茎干等繁殖材料,如枣树[17,26-27]、马褂木[28]、茶树[18]、杨树[29-30]、光皮桦[19]等,能够改善它们的生根能力,明显提高生根率,这为解决生产上林木繁殖难生根的问题提供了一条新的途径,为林木遗传改良提供了有效的技术支撑。
基因功能的研究依赖于转基因体系,因此,建立一个高效稳定的薄壳山核桃转基因体系是研究薄壳山核桃基因功能的首要任务。发根农杆菌的诱导率受到多种因素的影响,如菌株类型[19,31-34]、苗龄[35]、侵染部位[36-37]、菌液浓度[35,38-39]等,不同发根农杆菌对外植体毛状根诱导率差异较大[40]。笔者在探索体系优化条件过程中,发现含农杆碱型Ri 质粒的K599、MSU440、C58C1 菌株均能够诱导薄壳山核桃幼苗生根,其中K599菌株对薄壳山核桃的生根诱导率最高。由其他研究报道可知,K599菌株也是诱导白羽扇豆[31](Lupinus albus)、黄瓜[41-42](Cucumis sativus)、大豆[43](Glycine max)、西瓜[44](Citrullus lanatus)和木豆[26](Pigeon pea)等产生毛状根的最适菌株。外植体的不同苗龄及处理部位均会使得毛状根诱导率存在差异。笔者在本研究中发现在子叶期即2枚子叶刚刚展平,第1枚真叶还未露头的时候,靠近种子胚轴3~5 cm 处进行侵染,有利于农杆菌的顺利侵染,幼苗更容易长出毛状根。由此可见,外植体的选取是很重要的因素。大多研究报道中选择侵染的均为植株较幼嫩的组织或部位,因为这些部位的细胞处于旺盛的分裂期,更容易接受外源DNA[16]。不同农杆菌菌液浓度也是影响转化效率的重要因素之一。Meng 等[35]的研究发现木豆愈伤组织和毛状根诱导的最佳菌液浓度OD600为0.3;王天佐等[45]发现花苜蓿毛状根发生率为72%时的诱导菌液浓度OD600为0.5;在刘雪羽等[19]对光皮桦生根诱导率的研究中使用的菌液OD600为0.6~0.8;孟辉[46]的研究发现,当农杆菌LBA4404 的OD600为0.6~0.8 时,侵染冬枣茎尖获得较高的转化率为2.4%~3.1%;郝征[17]建立了发根农杆菌介导的冬枣叶片遗传转化体系,研究确定农杆菌OD600为0.8时转化效果最好。这与笔者在本研究中的结果相似,说明侵染木本植物所需的菌液OD600值可能普遍高于草本植物,一般0.8 左右侵染效率较高。由于湿度和光照对农杆菌的侵染以及转化细胞的组织分化有影响,因此需要在接菌后进行保湿和暗处理。本试验中,接种后用塑料罩子倒扣盖在薄壳山核桃幼苗上,可增加侵染部位周围的空气湿度。暗处理2 d后,用蛭石将侵染部位掩埋,可保证该部位处于黑暗条件下,加速愈伤组织的形成和分化。实验过程中发现,如果不用蛭石覆盖,则不会产生毛状根。因此,黑暗条件是薄壳山核桃毛状根产生的必要条件,这与黄瓜[47]外植体诱导毛状根的研究结果相同,其外植体接种后也需要进行暗培养。
目前,薄壳山核桃的遗传转化依然存在较多问题而导致转化效率很低。笔者在本研究中建立了一种非组织培养、简单高效的转基因体系,可以在相对较短时间内实现根的稳定转化。其中对薄壳山核桃进行侵染后将其置于温室环境中培养,该方式的主要优势在于无需无菌环境培育无菌苗获得转基因再生植株也可以实现基因功能的验证,简化了实验操作程序,降低了实验成本,并且所需设备简单,周期短、效率高,易于在生产实践中推广。目前在其他植物[48-50],如西瓜[51]、甜瓜[36]、大豆[50]等的研究中也有过报道。笔者在本研究中,实现了外源基因在薄壳山核桃的毛状根过量表达,在后续试验中可利用薄壳山核桃易根插的特性[51-52]将转基因根进行根插试验,从而获得完整转基因植株。同时利用根插扩繁进行根系组织表达、亚细胞定位、物质代谢途径等研究。这种由发根农杆菌侵染的外植体毛状根培养技术在生物技术行业中日益受到关注,并构成了一种相对较新的体外植物生物技术方法[53]。因为毛状根培养具有高生长速率、生物化学稳定性以及无需昂贵的外源激素来源等特点,故可用于深入研究植物代谢途径以及次生代谢产物和酶的产生,这对于医疗和工业应用方面具有重要价值[54]。其次,对于非模式植物中一些基因的预测多基于模式植物的同源性分析,故验证基因功能只能进行异源分析,可以借助本方法研究基因功能及其上下游调控的分子机制[35]。
4 结 论
本研究建立了非组培依赖的发根农杆菌介导的薄壳山核桃转化体系。影响发根农杆菌侵染薄壳山核桃转化效率的因素大小依次为苗龄、侵染部位、菌液OD600、菌株类型。利用本体系侵染子叶期幼苗,阳性毛状根植株诱导率高达45.2%。
[1]吕芳德,黄菁.山核桃属植物研究进展[J].经济林研究,2005,23(2):72-75.LÜ Fangde,HUANG Jing.Advances in study of Carya Nutt.[J].Non-wood Forest Research,2005,23(2):72-75.
[2]WU W,BI X L,CAO J Q,ZHANG K Q,ZHAO Y Q.New antitumor compounds from Carya cathayensis[J].Bioorganic&Medicinal Chemistry Letters,2012,22(5):1895-1898.
[3]LIANG N,SHI W Y.Composition and free radical scavenging activity of kernel oil from Torreya grandis,Carya Cathayensis,and Myrica Rubra[J].Iranian Journal of Pharmaceutical Research,2014,13(1):221-226.
[4]HUANG R M,HUANG Y J,SUN Z C,HUANG J Q,WANG Z J.Transcriptome analysis of genes involved in lipid biosynthesis in the developing embryo of Pecan(Carya illinoinensis)[J].Journal of Agricultural and Food Chemistry,2017,65(20):4223-4236.
[5]OZCARIZ- FERMOSELLE M V,FRAILE- FABERO R,GIRBÉS-JUAN T,ARCE-CERVANTES O,ORIA DE RUEDASALGUEIRO J A,AZUL A M.Use of lignocellulosic wastes of pecan (Carya illinoinensis) in the cultivation of Ganoderma lucidum[J].Revista Iberoamericana de Micologia,2018,35(2):103-109.
[6]ZHANG C C,YAO X H,REN H D,WANG K L,CHANG J.Isolation and characterization of three chalcone synthase genes in Pecan (Carya illinoinensis)[J].Biomolecules,2019,9(6):236.
[7]JIA X D,LUO H T,XU M Y,ZHAI M,GUO Z R,QIAO Y S,WANG L J.Dynamic changes in phenolics and antioxidant capacity during pecan (Carya illinoinensis) kernel ripening and its phenolics profiles[J].Molecules,2018,23(2):435.
[8]黎章矩,钱莲芳.山核桃科研成就和增产措施[J].浙江林业科技,1992,12(6):49-53.LI Zhangju,QIAN Lianfang.Achievements and measures of increase production of scientific research pecans to Carya cathayensis Sarg.[J].Journal of Zhejiang Forestry Science and Technology,1992,12(6):49-53.
[9]FENG G,MO Z H,PENG F R.The complete chloroplast genome sequence of Carya illinoinensis cv.Wichita and its phylogenetic analysis[J].Mitochondrial DNA Part B,2020,5(3):2235-2236.
[10]董筱昀,蒋泽平,蒋春,李永荣.薄壳山核桃试管离体培养中不定芽诱导及增殖技术的研究[J].江苏林业科技,2013,40(3):10-14.DONG Xiaoyun,JIANG Zeping,JIANG Chun,LI Yongrong.Adventitious bud induction and proliferation of Carya illinoensisin vitroculture[J].Journal of Jiangsu Forestry Science and Tech-nology,2013,40(3):10-14.
[11]HASSANEN S A,GABR F M. In vitro rooting of pecan [Carya illinoensis (Wangenh.) K.Koch][J].World Applied Sciences Journal,2013,21(3):315-319.
[12]RENUKDAS N N,MANOHARAN M,JR J O G.In vitro propagation of pecan [Carya illinoinensis (Wangenh) K.Koch][J].Plant Biotechnology,2010,27(2):211-215.
[13]严泽埔.基于赤霉素矮化基因诱导的薄壳山核桃砧木的培育[D].杭州:浙江农林大学,2019.YAN Zepu.Cultivation of pecan rootstocks induced by gibberellin dwarf gene[D].Hangzhou:Zhejiang Agriculture & Forestry University,2019.
[14]TEPFER D.Transformation of several species of higher plants by Agrobacterium rhizogenes:Sexual transmission of the transformed genotype and phenotype[J].Cell,1984,37(3):959-967.
[15]SEKI H,NISHIZAWA T,TANAKA N,NIWA Y,YOSHIDA S,MURANAKA T.Hairy root-activation tagging:A high-throughput system for activation tagging in transformed hairy roots[J].Plant Molecular Biology,2005,59(5):793-807.
[16]熊笙屹,厍润祥,张璐,孟令晨,于月华,倪志勇.转基因植物发根农杆菌研究的进展及应用[J].农业与技术,2017,37(16):72-74.XIONG Shengyi,SHE Runxiang,ZHANG Lu,MENG Lingchen,YU Yuehua,NI Zhiyong.Research progress and application of transgenic plant Agrobacterium rhizogenes[J].Agriculture and Technology,2017,37(16):72-74.
[17]郝征.农杆菌介导的枣树遗传转化研究[D].保定:河北农业大学,2012.HAO Zheng. Agrobacterium-mediated transformation of Chinese jujube[D].Baoding:Hebei Agricultural University,2012.
[18]林彩容,张冬敏,张文静,宋时奎,陈志丹,孙威江.3 种农杆菌对茶树发状根诱导的影响[J].西北植物学报,2021,41(3):509-516.LIN Cairong,ZHANG Dongmin,ZHANG Wenjing,SONG Shikui,CHEN Zhidan,SUN Weijiang.Induction of hairy roots of tea plant by three kinds of Agrobacterium rhizogenes[J].Acta Botanica Boreali-Occidentalia Sinica,2021,41(3):509-516.
[19]刘雪羽,杜笑雪,陈思源,胡现铬,黄华宏,童再康.发根农杆菌介导的光皮桦毛状根高频诱导体系及遗传转化[J].农业生物技术学报,2021,29(3):495-505.LIU Xueyu,DU Xiaoxue,CHEN Siyuan,HU Xiange,HUANG Huahong,TONG Zaikang. Agrobacterium rhizogenes mediated high frequency hairy rootInduction system and genetic transformation in Betula luminifera[J].Journal of Agricultural Biotechnology,2021,29(3):495-505.
[20]姚庆收,武玉永,于敏.发根农杆菌介导的橡胶树遗传转化体系的建立[J].安徽农业科学,2012,40(28):13729-13730.YAO Qingshou,WU Yuyong,YU Min.Establishment of the rubber tree genetic transformation system by Agrobacterium rhizogenes[J].Journal of Anhui Agricultural Sciences,2012,40(28):13729-13730.
[21]刘思巧.发根农杆菌介导的银杏毛状根培养及黄酮的生物合成[D].雅安:四川农业大学,2019.LIU Siqiao. Agrobacterium rhizogenes-mediated hairy root culture and flavonoid biosynthesis of Ginkgo biloba L.[D].Yaan:Sichuan Agricultural University,2019.
[22]谢志亮,吴振旺.木本植物组培褐化研究进展[J].中国南方果树,2013,42(5):42-46.XIE Zhiliang,WU Zhenwang.Research progress on tissue culture browning of woody plants[J].South China Fruits,2013,42(5):42-46.
[23]张丽丽,张明明.木本植物组织培养技术在林业科研与生产中的应用与局限[J].北京农业,2015(9):74-75.ZHANG Lili,ZHANG Mingming.Application and limitation of woody plant tissue culture technology in forestry scientific research and production[J].Beijing Agriculture,2015(9):74-75.
[24]许剑萍,林惠东.木本植物组织培养技术在林业科研与生产中的应用与局限分析[J].南方农业,2021,15(6):126-127.XU Jianping,LIN Huidong.Analysis of application and limitation of woody plant tissue culture technology in forestry research and production[J].South China Agriculture,2021,15(6):126-127.
[25]陈娟娟.山核桃生长素输出载体CcPIN 家族基因克隆及其功能初步研究[D].杭州:浙江农林大学,2020.CHEN Juanjuan.Cloning and preliminary function analysis of the auxin efflux carrier CcPIN family genes in Chinese Hickory(Carya cathayensis Sarg.)[D].Hangzhou:Zhejiang Agriculture&Forestry University,2020.
[26]张娅君.枣成熟胚离体培养及农杆菌介导叶片与胚遗传转化研究[D].郑州:河南农业大学,2012.ZHANG Yajun.Studies on mature embryos of jujube to regeneration and Agrobacterium-mediated using blades and embryos of jujube[D].Zhengzhou:Henan Agricultural University,2012.
[27]何业华,林良斌,熊兴华,何钢,林顺权,陈建华.根癌农杆菌介导的枣树遗传转化系统的建立[J].分子植物育种,2003,1(5):683-686.HE Yehua,LIN Liangbin,XIONG Xinghua,HE Gang,LIN Shunquan,CHEN Jianhua.Studies of development of efficient genetic transformation of Ziziphus jujuba[J].Molecular Plant Breeding,2003,1(5):683-686.
[28]屈林丰.生根剂与发根农杆菌对亚美马褂木扦插生根的影响[D].南宁:广西大学,2017.QU Linfeng.The influence of Liriodendron sino-americanum cuttage under treatment of rooting agent and agrobacterium rhizogenes[D].Nanning:Guangxi University,2017.
[29]丁雪.发根农杆菌介导的银中杨茎段遗传转化体系的建立及生理生化指标的测定[D].四平:吉林师范大学,2017.DING Xue.Determination of physiological and optimization on the stem of Populus alba×P.berolinensis by Agrobacterium rhizogenes[D].Siping:Jilin Normal University,2017.
[30]张淑娟.多基因转化741 杨及其基因表达的初步研究[D].保定:河北农业大学,2006.ZHANG Shujuan.Preliminary study on polygenes transformation Poplar 741 and genie expression[D].Baoding:Hebei Agricultural University,2016.
[31]詹玉洁,刘博文,张仟,许卫锋,夏天雨.发根农杆菌介导白羽扇豆转基因过表达体系的建立[J].植物生理学报,2021,57(3):655-660.ZHAN Yujie,LIU Bowen,ZHANG Qian,XU Weifeng,XIA Tianyu.Establishment of Agrobacterium rhizogenes-mediated gene overexpression system in white lupin[J].Plant Physiology Journal,2021,57(3):655-660.
[32]梅错,刘志鹏.发根农杆菌介导的箭筈豌豆毛状根遗传转化体系的建立[J].中国草地学报,2020,42(5):1-7.MEI Cuo,LIU Zhipeng. Agrobacterium rhizogenes-mediated transformation system of common vetch(Vicia sativa subsp.nigra)[J].Chinese Journal of Grassland,2020,42(5):1-7.
[33]徐悦,曹英萍,王玉,付春祥,戴绍军.发根农杆菌介导的菠菜毛状根遗传转化体系的建立[J].植物学报,2019,54(4):515-521.XU Yue,CAO Yingping,WANG Yu,FU Chunxiang,DAI Shaojun. Agrobacterium rhizogenes-mediated transformation system of Spinacia oleracea[J].Chinese Bulletin of Botany,2019,54(4):515-521.
[34]HAN K H,MEILAN R,MA C,STRAUSS S H.An Agrobacterium tumefaciens transformation protocol effective on a variety of cottonwood hybrids (genus Populus)[J].Plant Cell Reports,2000,19(3):315-320.
[35]MENG D,YANG Q,DONG B Y,SONG Z H,NIU L L,WANG L T,CAO H Y,LI H H,FU Y J.Development of an efficient root transgenic system for pigeon pea and its application to other important economically plants[J].Plant Biotechnology Journal,2019,17(9):1804-1813.
[36]王平勇,徐永阳,赵光伟,贺玉花,李明,孔维虎,张健,刘水苗,刘梦丽,徐志红.发根农杆菌介导甜瓜转基因过表达体系的建立[J].中国瓜菜,2019,32(12):15-18.WANG Pingyong,XU Yongyang,ZHAO Guangwei,HE Yuhua,LI Ming,KONG Weihu,ZHANG Jian,LIU Shuimiao,LIU Mengli,XU Zhihong.Establishment of Agrobacterium rhizogenes-mediated gene overexpression system in melon[J].China Cucurbits and Vegetables,2019,32(12):15-18.
[37]郝金凤,荆培培,张丽,哈斯阿古拉.应用农杆菌介导的生长点转化方法建立甜瓜遗传转化技术[J].华北农学报,2014,29(2):116-120.HAO Jinfeng,JING Peipei,ZHANG Li,HASI Agula.Establishment of transformation method for melon using Agrobacteriummediated plant genetic transformation via plant apical meristem[J].Acta Agriculurae Boreali-Sinica,2014,29(2):116-120.
[38]孙春玉,孙旸,刘庆忠.根癌农杆菌介导的苹果遗传转化研究进展[J].中国农学通报,2010,26(4):231-233.SUN Chunyu,SUN Yang,LIU Qingzhong.Advances in research of genetic transformation of apples with Agrobacterium[J].Chinese Agricultural Science Bulletin,2010,26(4):231-233.
[39]CASTELLANOS-AREVALO A P,ESTRADA-LUNA A A,CABRERA-PONCE J L,VALENCIA-LOZANO E,HERRERAUBALDO H,DE-FOLTER S,BLANCO-LABRA A,DELANOFRIER J P. Agrobacterium rhizogenes-mediated transformation of grain (Amaranthus hypochondriacus) and leafy (A.hybridus)amaranths[J].Plant Cell Reports,2020,39(9):1143-1160.
[40]SHARAFI A,SOHI H H,MOUSAVI A,AZADI P,RAZAVI K,NTUI V O.A reliable and efficient protocol for inducing hairy roots in Papaver bracteatum[J].Plant Cell Tissue and Organ Culture,2013,113(1):1-9.
[41]WELLER S A,STEAD D E,YOUNG J P W.Acquisition of an Agrobacterium Ri plasmid and pathogenicity by other alpha-Proteobacteria in cucumber and tomato crops affected by root mat[J].Applied and Environmental Microbiology,2004,70(5):2779-2785.
[42]柴里昂,樊怀福,刘晨,杜长霞.根癌农杆菌介导的黄瓜转基因研究进展[J].生物工程学报,2020,36(4):643-651.CHAI Li’ang,FAN Huaifu,LIU Chen,DU Changxia.Advances in Agrobacterium tumefaciens-mediated transgenic cucumber[J].Chinese Journal of Biotechnology,2020,36(4):643-651.
[43]陈安乐.大豆发根转化方法的建立及GmFRD3 在大豆耐铝性中的作用[D].长春:吉林大学,2014.CHEN Anle.Establishment of the Agrobacterium rhizogenesmediated transformation of soybean and function of GmFRD3 under Al stress in soybean[D].Changchun:Jilin University,2014.
[44]王平勇,徐永阳,赵光伟,贺玉花,孔维虎,张健,刘水苗,户克云,侯冲,王兰菊,徐志红.发根农杆菌介导西瓜转基因过表达体系的建立[J].果树学报,2019,36(12):1763-1770.WANG Pingyong,XU Yongyang,ZHAO Guangwei,HE Yuhua,KONG Weihu,ZHANG Jian,LIU Shuimiao,HU Keyun,HOU Chong,WANG Lanju,XU Zhihong.Preliminary study on Agrobacterium rhizogenes-mediated gene overexpression system in watermelon[J].Journal of Fruit Science,2019,36(12):1763-1770.
[45]王天佐,张文浩.发根农杆菌介导的花苜蓿毛状根转化体系的建立[J].草地学报,2020,28(1):268-272.WANG Tianzuo,ZHANG Wenhao. Agrobacterium rhizogenesmediated transformation of hairy root in Medicago ruthenica[J].Acta Agrestia Sinica,2020,28(1):268-272.
[46]孟辉.沾化冬枣基因工程改良的基础研究[D].济南:山东大学,2005.MENG Hui.Studies on improvement of Zhanhua winter jujube(Ziziphus Jjujuba Mill.) by genetic engineering[D].Jinan:Shandong University,2005.
[47]曹庆丰.发根农杆菌K599 侵染黄瓜形成转基因毛状根的初步研究及orf14 基因的克隆[D].杭州:杭州师范大学,2012.CAO Qingfeng.Preliminary analysis on transgeneic hairy root induced from cucumber by Agrobacterium rhizogenes K599 and cloning of orf14 gene[D].Hangzhou:Hangzhou Normal University,2012.
[48]VEENA V,CHRISTOPHER G,TAYLOR.Agrobacterium rhizogenes:recent developments and promising applications[J].Vitro Cellular&Developmental Biology Plant,2007,43(5):383-403.
[49]MEHROTRA S,RAHMAN L U,KUKREJA A K.An extensive case study of hairy-root cultures for enhanced secondary-metabolite production through metabolic-pathway engineering[J].Biotechnology and Applied Biochemistry,2011,56(4):161-72.
[50]QI X P,LI M W,XIE M,LIU X,NI M,SHAO G H,SONG C,KAY-YUEN Y A,TAO Y,WONG F L,ISOBE S,WONG C F,WONG K S,XU C Y,LI C Q,WANG Y,GUAN R,SUN F M,FAN G Y,XIAO Z X,ZHOU F,PHANG T H,LIU X,TONG S W,CHAN T F,YIU S M,TABATA S,WANG J,XU X,LAM H M.Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing[J].Nature Communications,2014,5(7):4340.
[51]王金明,张斌,王晓丽,徐小源,徐芳.薄壳山核桃根段扦插育苗试验[J].安徽农业科学,2016,44(9):189-190.WANG Jinming,ZHANG Bin,WANG Xiaoli,XU Xiaoyuan,XU Fang.Experiment on cutting propagation by root of Carya illinoensis[J].Journal of Anhui Agricultural Sciences,2016,44(9):189-190.
[52]黄有军,王正加,郑炳松,黄坚钦.山核桃根插试验[J].浙江林学院学报,2006,23(2):228-231.HUANG Youjun,WANG Zhengjia,ZHENG Bingsong,HUANG Jianqin[J].Experiment on root cutting of Carya cathayensis[J].Journal of Zhejiang Forestry College,2006,23(2):228-231.
[53]TALANO M A,OLLER A L W,GONZALEZ P S,AGOSTINI E.Hairy roots,their multiple applications and recent patents[J].Recent Patents on Biotechnology,2012,6(2):115-133.
[54]STILES A R,LIU C Z.Hairy root culture:bioreactor design and process intensification[J].Advances in Biochemical Engineering-Biotechnology,2013,134:91-114.