火龙果(Hylocereus spp.)又名红龙果、仙蜜果等,属于仙人掌科(Cactaceae)量天尺属和蛇鞭柱属的多年生攀援性多肉植物。因其拥有较高的经济价值,目前被世界多国引入进行栽培种植[1]。我国主要种植地区有海南、广东、广西、贵州和云南等[2]。火龙果果实不仅外观独特且营养价值丰富,具有促进肠胃消化、降血糖血脂、血压及美容护肤等多种功效,可谓是集食用、药用和观赏于一身的重要热带经济水果[3]。
由新暗色柱节孢菌(Neoscytalidium dimidiatum)引起的火龙果溃疡病是一种严重威胁火龙果生产的重要病害,已在多个火龙果种植国家和地区报道过[4-6]。随着火龙果在海南省种植面积的扩大和种植年限的增长,火龙果溃疡病在产区发生情况呈连年递增的趋势,已是影响海南省火龙果产业的第一大病害,不仅影响植株生长和果实外观,而且给生产造成巨大损失。该病原菌主要危害火龙果的茎部和果实[7-10],典型发病症状为:病部初期出现褪绿轻微凹陷的圆形小斑点,中期逐渐变为橘黄色,严重时病斑连成一片并逐渐硬化,后期病部中央凹陷,周围木栓化,呈灰白色或灰褐色隆起,似溃疡。
吡唑醚菌酯(pyraclostrobin)是一种广谱甲氧基丙烯酸酯类(QoI类)杀菌剂,其通过在细胞色素b和c1间的电子转移抑制线粒体呼吸,进而抑制真菌孢子萌发和菌丝生长,具有广谱、高效、毒性低、提高对氮的吸收和产量等特点[11-12]。吡唑醚菌酯作为一种广泛使用的杀菌剂,常期使用可能导致植物病原菌对其敏感度下降或产生抗药性:贺瑞等[13]报道海南杧果蒂腐病菌对吡唑醚菌酯产生严重抗药性;贾俊超等[14]报道,田间已发现25种病原菌在14种寄主作物上表现出对QoI类杀菌剂的抗药性。通过前期对海南省火龙果园进行实地田间调查发现,果园常使用有效成分中含有吡唑醚菌酯的农药来防治火龙果病害。然而,海南省内各地的火龙果溃疡病菌对吡唑醚菌酯药剂敏感性水平,尚未见研究报道。本研究中,笔者2019—2020年从海南省火龙果种植产区采集火龙果溃疡病的典型病样标本,经分离、纯化和鉴定,并采用菌丝生长速率法测定了分离菌株对吡唑醚菌酯的敏感性,旨在进一步明确火龙果溃疡病菌对吡唑醚菌酯的敏感性水平,为海南火龙果溃疡病的科学防治提供理论依据。
1 材料和方法
1.1 供试菌株
供试菌株为本实验室于2019—2020 年从海南省各火龙果种植市(县)采集、分离、纯化、鉴定和保存的59株火龙果溃疡病菌(N.dimidiatum)菌株。
1.2 供试菌株的分离与纯化
对从海南省海口、儋州、琼海、昌江、白沙、澄迈、乐东和东方8个火龙果种植市(县)采回的发病植株样本,采用组织分离法分离病原菌:切取病果和病茎圆形病斑(5 mm×5 mm)若干,75%(φ)乙醇消毒35 s,5%次氯酸钠消毒40 s,无菌水清洗3次后,用无菌纸吸干组织块表面水分至于PDA 平板上,培养箱28 ℃培养2~3 d,待长出菌丝,取边缘菌丝转移到新PDA平板上,28 ℃培养5 d产孢,镜检初步确认病原菌。再采用单孢分离法获得病原菌纯菌落:将菌丝放于无菌水中轻轻涮洗,显微镜镜检(孢子悬浮液稀释到在10×下每个视野约观察到1~5个孢子时)进行涂板培养24 h,挑取单条菌丝进行转板,获得病原菌纯菌落。培养7 d后通过形态学观察鉴定、分子鉴定及柯赫氏法则致病性测定后将其转移至PDA 斜面试管中保存备用[15]。
1.3 供试药剂
98%吡唑醚菌酯原药(湖北猫尔沃生物医药有限公司),用丙酮为溶剂配制质量浓度为10 mg·mL-1的母液,保存于4 ℃冰箱备用。
1.4 含药培养基制备
采用系列稀释法,经初筛确定有效浓度范围后,将吡唑醚菌酯母液用丙酮稀释为系列梯度浓度,取1 mL 稀释药剂加入49 mL 融化的PDA 培养基中混匀倒板,分别配成终质量浓度为6、4、2、0.5 和0.2 μg·mL-1的含药培养基,并以不含药的PDA 培养基加入1 mL 丙酮作为溶剂对照,加入1 mL 无菌水的PDA培养基作为空白对照,所用供试培养基均加入终质量浓度为50 mg·L-1的水杨肟酸,以降低旁呼吸通路对药剂的影响。
1.5 火龙果溃疡病菌对吡唑醚菌酯的敏感性测定
采用菌丝生长速率法[16]:供试菌株在PDA 培养基上培养3 d 后沿菌落边缘用5 mm 直径打孔器取菌饼。将菌饼菌丝面朝上分别接种到5 个不同浓度的含药培养基和对照培养基上,每浓度设置3 个重复,置于28 ℃培养箱中培养,48 h 后采用十字交叉法测量菌落直径,每菌株3次重复试验。
1.6 数据处理
根据公式计算菌丝生长抑制率:菌丝生长抑制率/%= [(对照菌落生长直径-处理菌落生长直径)/对照菌落生长直径-0.5]×100;采用Microsoft Excel 2016软件计算各菌株的毒力回归方程和相关系数,根据回归方程计算EC50。
针对59株供试菌株EC50值,采用R语言对试验数据进行正态性、方差齐性检验并以采样市(县)为因素进行单因素方差分析[17],将其中符合连续性正态分布的供试菌株EC50均值作为海南省火龙果溃疡病菌对吡唑醚菌酯的敏感基线[18-20]。抗性水平以火龙果溃疡病菌对吡唑醚菌酯敏感基线为参照,抗性水平(RF)=供试菌株EC50值/敏感基线,当EC50值小于敏感基线5倍时,为敏感菌株;当EC50值为敏感基线的5~10 倍时,为低抗菌株;当EC50值为敏感基线的10~40倍时,为中抗菌株;当EC50值大于敏感基线40倍时,为高抗菌株[21]。
2 结果与分析
2.1 病原菌分离、纯化及保存
于2019—2020 年从海南省海口市、儋州市、乐东县和东方市等火龙果种植市(县)采集火龙果溃疡病不同发病情况的果实和茎部样本。挑选具有火龙果溃疡病典型症状的火龙果果实和茎部样本经分离纯化获得59株火龙果溃疡病菌,其中海口8株,儋州14株,琼海3株、昌江3株、白沙3株、澄迈3株、东方15株和乐东10株。
2.2 海南省火龙果溃疡病菌对吡唑醚菌酯的敏感基线
将59 株火龙果溃疡病菌对吡唑醚菌酯的EC50值使用R 语言的shapiro.test 函数命令进行正态性检验(W 检验),W= 0.964 3,p= 0.080 9(p > 0.05);结果表明海南省59 株火龙果溃疡病菌对吡唑醚菌酯的敏感性呈正态分布,EC50值为0.254 0~2.223 3 μg·mL-1,平均值为0.984 1 μg·mL-1,其中敏感性最弱菌株EC50值是敏感性最强菌株的8.75 倍,表明火龙果溃疡病菌对吡唑醚菌酯的敏感性存在差异(表1)。
表1 海南省火龙果溃疡病菌对吡唑醚菌酯的敏感性
Table 1 Sensitivity of Neoscytalidium dimidiatum to pyraclostrobin in Hainan
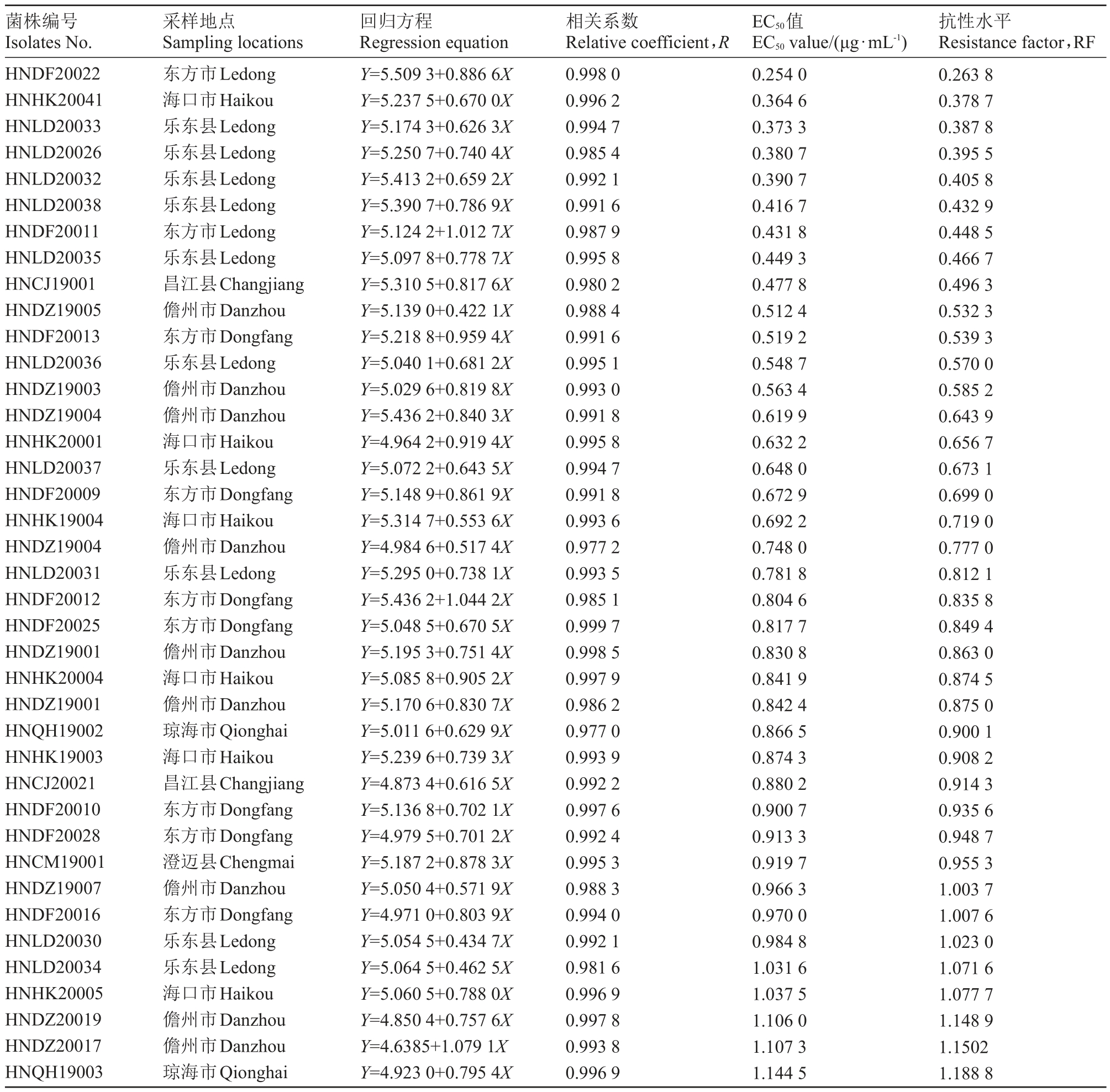
菌株编号Isolates No.采样地点Sampling locations回归方程Regression equation相关系数Relative coefficient,R EC50值EC50 value/(μg·mL-1)抗性水平Resistance factor,RF HNDF20022 HNHK20041 HNLD20033 HNLD20026 HNLD20032 HNLD20038 HNDF20011 HNLD20035 HNCJ19001 HNDZ19005 HNDF20013 HNLD20036 HNDZ19003 HNDZ19004 HNHK20001 HNLD20037 HNDF20009 HNHK19004 HNDZ19004 HNLD20031 HNDF20012 HNDF20025 HNDZ19001 HNHK20004 HNDZ19001 HNQH19002 HNHK19003 HNCJ20021 HNDF20010 HNDF20028 HNCM19001 HNDZ19007 HNDF20016 HNLD20030 HNLD20034 HNHK20005 HNDZ20019 HNDZ20017 HNQH19003东方市Ledong海口市Haikou乐东县Ledong乐东县Ledong乐东县Ledong乐东县Ledong东方市Ledong乐东县Ledong昌江县Changjiang儋州市Danzhou东方市Dongfang乐东县Ledong儋州市Danzhou儋州市Danzhou海口市Haikou乐东县Ledong东方市Dongfang海口市Haikou儋州市Danzhou乐东县Ledong东方市Dongfang东方市Dongfang儋州市Danzhou海口市Haikou儋州市Danzhou琼海市Qionghai海口市Haikou昌江县Changjiang东方市Dongfang东方市Dongfang澄迈县Chengmai儋州市Danzhou东方市Dongfang乐东县Ledong乐东县Ledong海口市Haikou儋州市Danzhou儋州市Danzhou琼海市Qionghai Y=5.509 3+0.886 6X Y=5.237 5+0.670 0X Y=5.174 3+0.626 3X Y=5.250 7+0.740 4X Y=5.413 2+0.659 2X Y=5.390 7+0.786 9X Y=5.124 2+1.012 7X Y=5.097 8+0.778 7X Y=5.310 5+0.817 6X Y=5.139 0+0.422 1X Y=5.218 8+0.959 4X Y=5.040 1+0.681 2X Y=5.029 6+0.819 8X Y=5.436 2+0.840 3X Y=4.964 2+0.919 4X Y=5.072 2+0.643 5X Y=5.148 9+0.861 9X Y=5.314 7+0.553 6X Y=4.984 6+0.517 4X Y=5.295 0+0.738 1X Y=5.436 2+1.044 2X Y=5.048 5+0.670 5X Y=5.195 3+0.751 4X Y=5.085 8+0.905 2X Y=5.170 6+0.830 7X Y=5.011 6+0.629 9X Y=5.239 6+0.739 3X Y=4.873 4+0.616 5X Y=5.136 8+0.702 1X Y=4.979 5+0.701 2X Y=5.187 2+0.878 3X Y=5.050 4+0.571 9X Y=4.971 0+0.803 9X Y=5.054 5+0.434 7X Y=5.064 5+0.462 5X Y=5.060 5+0.788 0X Y=4.850 4+0.757 6X Y=4.6385+1.079 1X Y=4.923 0+0.795 4X 0.998 0 0.996 2 0.994 7 0.985 4 0.992 1 0.991 6 0.987 9 0.995 8 0.980 2 0.988 4 0.991 6 0.995 1 0.993 0 0.991 8 0.995 8 0.994 7 0.991 8 0.993 6 0.977 2 0.993 5 0.985 1 0.999 7 0.998 5 0.997 9 0.986 2 0.977 0 0.993 9 0.992 2 0.997 6 0.992 4 0.995 3 0.988 3 0.994 0 0.992 1 0.981 6 0.996 9 0.997 8 0.993 8 0.996 9 0.254 0 0.364 6 0.373 3 0.380 7 0.390 7 0.416 7 0.431 8 0.449 3 0.477 8 0.512 4 0.519 2 0.548 7 0.563 4 0.619 9 0.632 2 0.648 0 0.672 9 0.692 2 0.748 0 0.781 8 0.804 6 0.817 7 0.830 8 0.841 9 0.842 4 0.866 5 0.874 3 0.880 2 0.900 7 0.913 3 0.919 7 0.966 3 0.970 0 0.984 8 1.031 6 1.037 5 1.106 0 1.107 3 1.144 5 0.263 8 0.378 7 0.387 8 0.395 5 0.405 8 0.432 9 0.448 5 0.466 7 0.496 3 0.532 3 0.539 3 0.570 0 0.585 2 0.643 9 0.656 7 0.673 1 0.699 0 0.719 0 0.777 0 0.812 1 0.835 8 0.849 4 0.863 0 0.874 5 0.875 0 0.900 1 0.908 2 0.914 3 0.935 6 0.948 7 0.955 3 1.003 7 1.007 6 1.023 0 1.071 6 1.077 7 1.148 9 1.1502 1.188 8
续表Continued Table
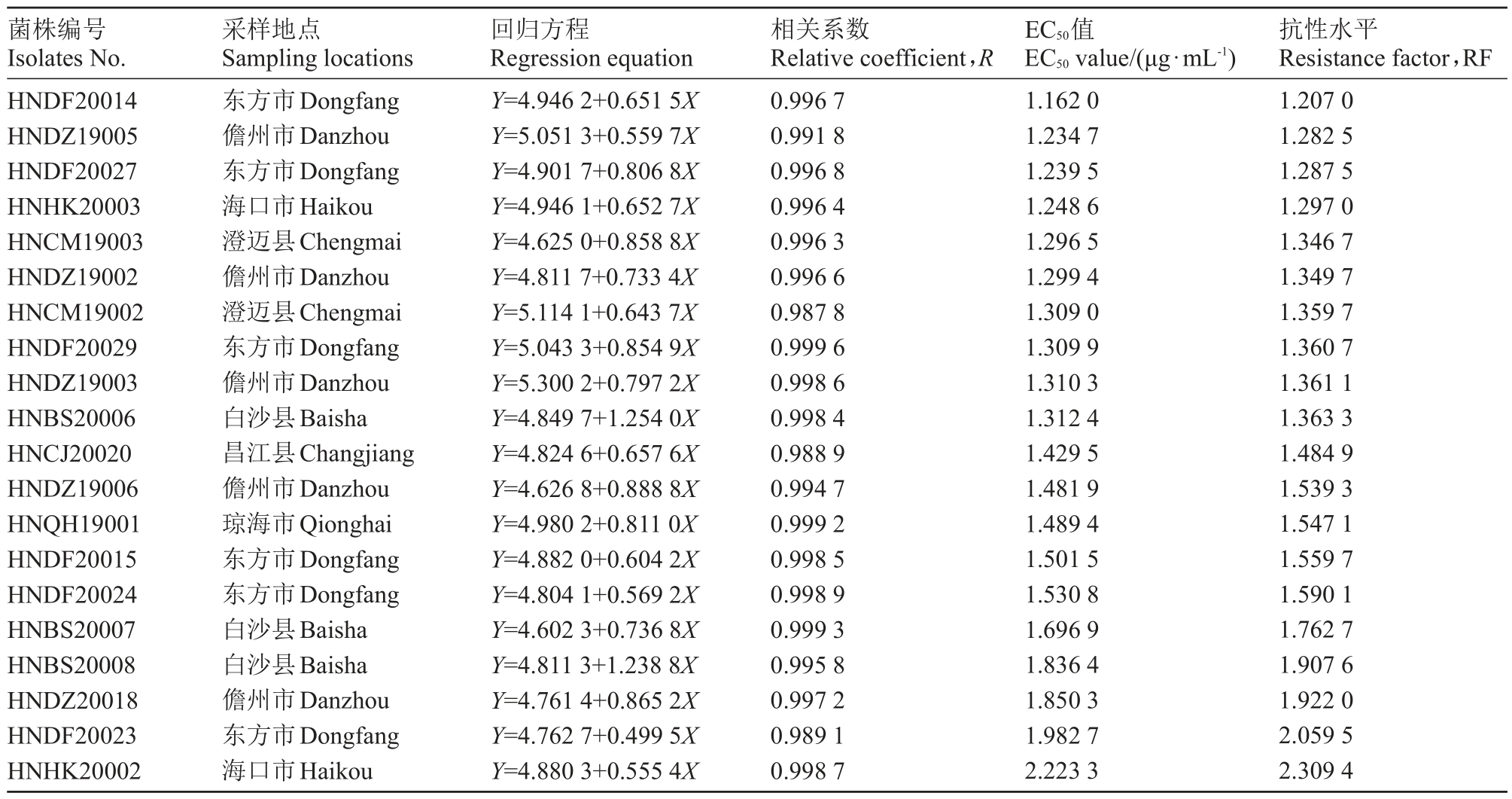
菌株编号Isolates No.采样地点Sampling locations回归方程Regression equation相关系数Relative coefficient,R EC50值EC50 value/(μg·mL-1)抗性水平Resistance factor,RF HNDF20014 HNDZ19005 HNDF20027 HNHK20003 HNCM19003 HNDZ19002 HNCM19002 HNDF20029 HNDZ19003 HNBS20006 HNCJ20020 HNDZ19006 HNQH19001 HNDF20015 HNDF20024 HNBS20007 HNBS20008 HNDZ20018 HNDF20023 HNHK20002东方市Dongfang儋州市Danzhou东方市Dongfang海口市Haikou澄迈县Chengmai儋州市Danzhou澄迈县Chengmai东方市Dongfang儋州市Danzhou白沙县Baisha昌江县Changjiang儋州市Danzhou琼海市Qionghai东方市Dongfang东方市Dongfang白沙县Baisha白沙县Baisha儋州市Danzhou东方市Dongfang海口市Haikou Y=4.946 2+0.651 5X Y=5.051 3+0.559 7X Y=4.901 7+0.806 8X Y=4.946 1+0.652 7X Y=4.625 0+0.858 8X Y=4.811 7+0.733 4X Y=5.114 1+0.643 7X Y=5.043 3+0.854 9X Y=5.300 2+0.797 2X Y=4.849 7+1.254 0X Y=4.824 6+0.657 6X Y=4.626 8+0.888 8X Y=4.980 2+0.811 0X Y=4.882 0+0.604 2X Y=4.804 1+0.569 2X Y=4.602 3+0.736 8X Y=4.811 3+1.238 8X Y=4.761 4+0.865 2X Y=4.762 7+0.499 5X Y=4.880 3+0.555 4X 0.996 7 0.991 8 0.996 8 0.996 4 0.996 3 0.996 6 0.987 8 0.999 6 0.998 6 0.998 4 0.988 9 0.994 7 0.999 2 0.998 5 0.998 9 0.999 3 0.995 8 0.997 2 0.989 1 0.998 7 1.162 0 1.234 7 1.239 5 1.248 6 1.296 5 1.299 4 1.309 0 1.309 9 1.310 3 1.312 4 1.429 5 1.481 9 1.489 4 1.501 5 1.530 8 1.696 9 1.836 4 1.850 3 1.982 7 2.223 3 1.207 0 1.282 5 1.287 5 1.297 0 1.346 7 1.349 7 1.359 7 1.360 7 1.361 1 1.363 3 1.484 9 1.539 3 1.547 1 1.559 7 1.590 1 1.762 7 1.907 6 1.922 0 2.059 5 2.309 4
去除1株特异性较强的菌株HNHK20002后,将58株火龙果溃疡病菌对吡唑醚菌酯的EC50值使用R语言的Shapiro test 函数命令进行正态性检验(W 检验),W=0.969 4,p=0.150 2(p>0.05);结果表明58株供试菌株对吡唑醚菌酯的EC50分布符合正态分布,以EC50为横坐标,以菌株分布频率为纵坐标,绘制频数分布直方图(图1),其EC50分布符合连续性正态分布,EC50均值0.962 7 μg·mL-1即为海南省火龙果溃疡病菌对吡唑醚菌酯的敏感基线。
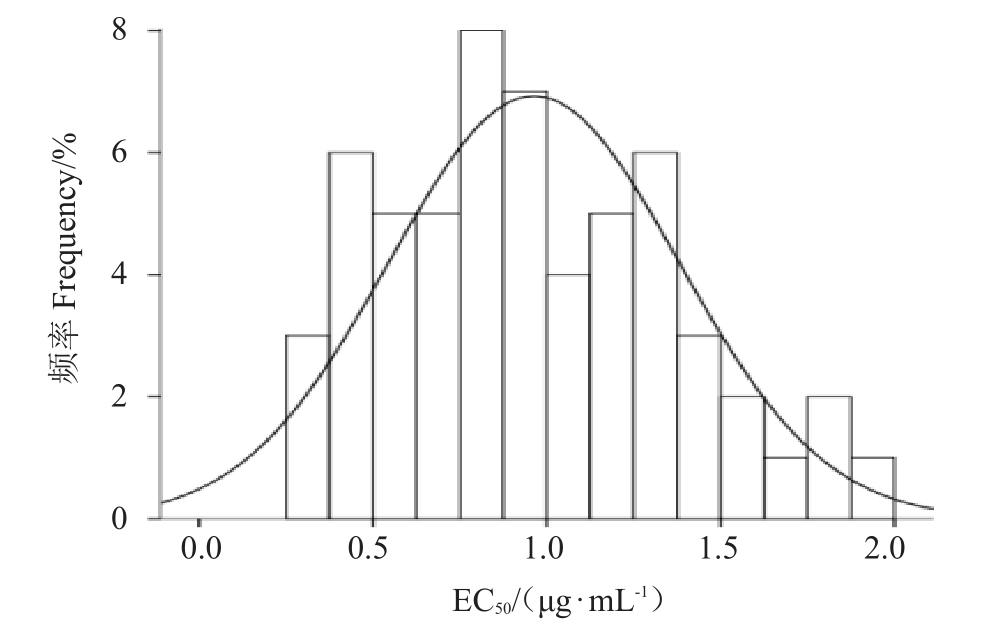
图1 58 株菌株EC50连续性正态分布
Fig.1 Continuous normal distribution for EC50 of 58 strains to pyraclostrobin
2.3 不同地区火龙果溃疡病菌对吡唑醚菌酯的敏感性
从海南省8个火龙果种植市(县)采集的59株火龙果溃疡病菌对吡唑醚菌酯的敏感性存在一定的差异。最敏感菌株为东方HNDF20022,EC50 值为0.254 0 μg·mL-1,最不敏感菌株为海口HNHK20002,EC50 值为2.223 3 μg·mL-1。使用R 语言的Bartlett test 在α=0.05 水平下进行方差齐性检验p=0.462 2(p > 0.05),结果表明,数据在不同水平下为等方差。对种植市(县)间进一步使用TukeyHSD 测试,结果表明,海南8 个地区除白沙和乐东的菌株在α=0.05 水平上差异显著外,其余地区间对吡唑醚菌酯的敏感性差异均不显著。根据敏感基线0.962 7 μg·mL-1,海南省火龙果溃疡病菌群体目前对吡唑醚菌酯仍均较为敏感,59 株病菌的平均抗性为1.02,最高抗性为2.31,均小于敏感基线5 倍范围。经各火龙果种植市(县)间EC50均值比较,白沙>澄迈>琼海>儋州>东方>海口>昌江>乐东(图2),其中乐东县分离的病原菌对吡唑醚菌酯的敏感性最高,EC50平均值为0.600 6 μg·mL-1,平均抗性为0.62,最高抗性为1.07;白沙县分离的病原菌对吡唑醚菌酯相对敏感性较低,EC50平均值为1.615 2 μg·mL-1,平均抗性为1.68,最高抗性为1.91;白沙县的EC50均值是乐东县的2.69倍(表2)。根据敏感基线白沙县产生抗药风险的可能性最高。2019和2020年2 a间火龙果溃疡病菌菌株对吡唑醚菌酯的EC50分别为0.974 0 μg·mL-1和0.989 3 μg·mL-1,下降了0.01 μg·mL-1,说明2019和2020年2 a间火龙果溃疡病菌菌株之间敏感性差异不明显。
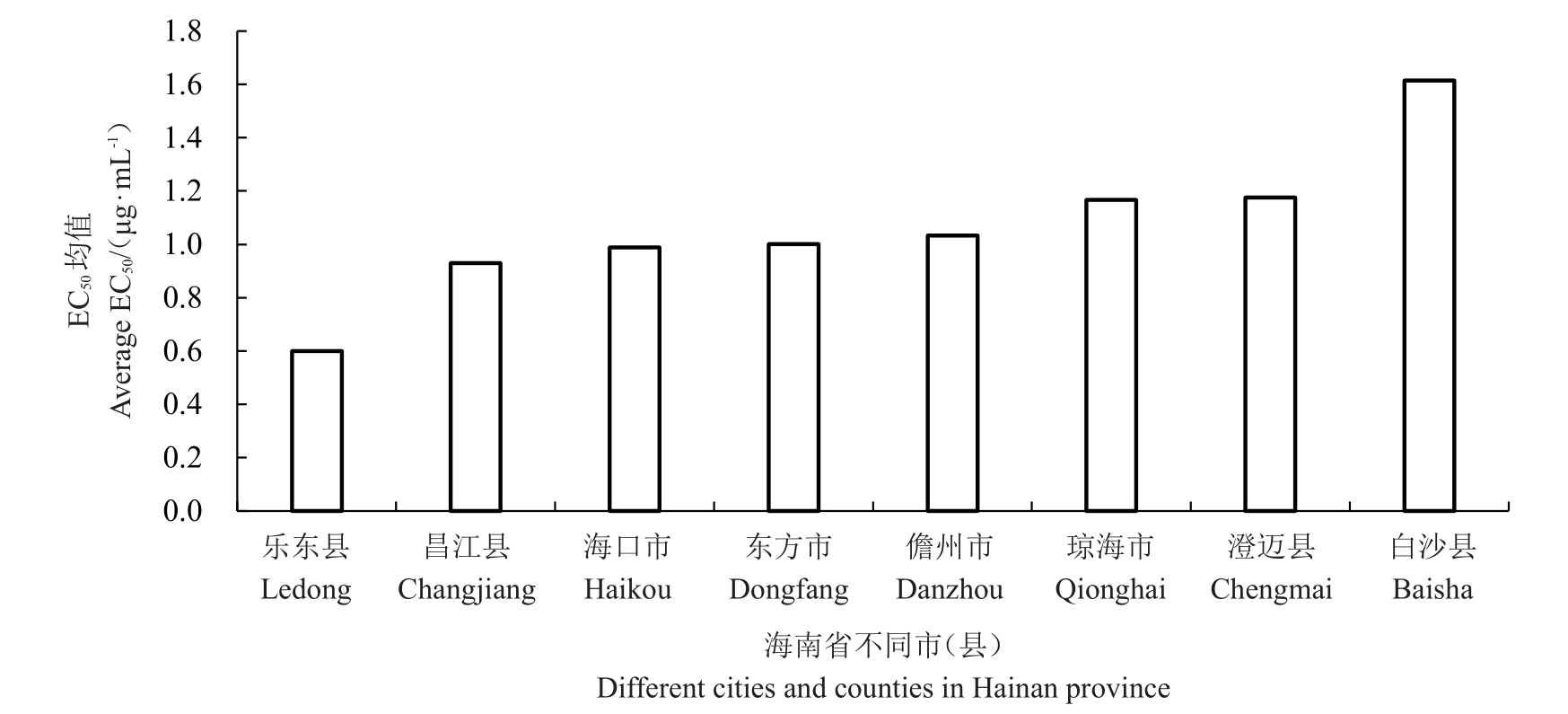
图2 不同市(县)火龙果溃疡病菌对吡唑醚菌酯的敏感性比较
Fig.2 Sensitivity of Neoscytalidium dimidiatum to pyraclostrobin in different cities and counties
表2 海南省各地火龙果溃疡病菌对吡唑醚菌酯的抗性水平
Table 2 Resistance factor for Neoscytalidium dimidiatum to pyraclostrobin in different areas in Hainan
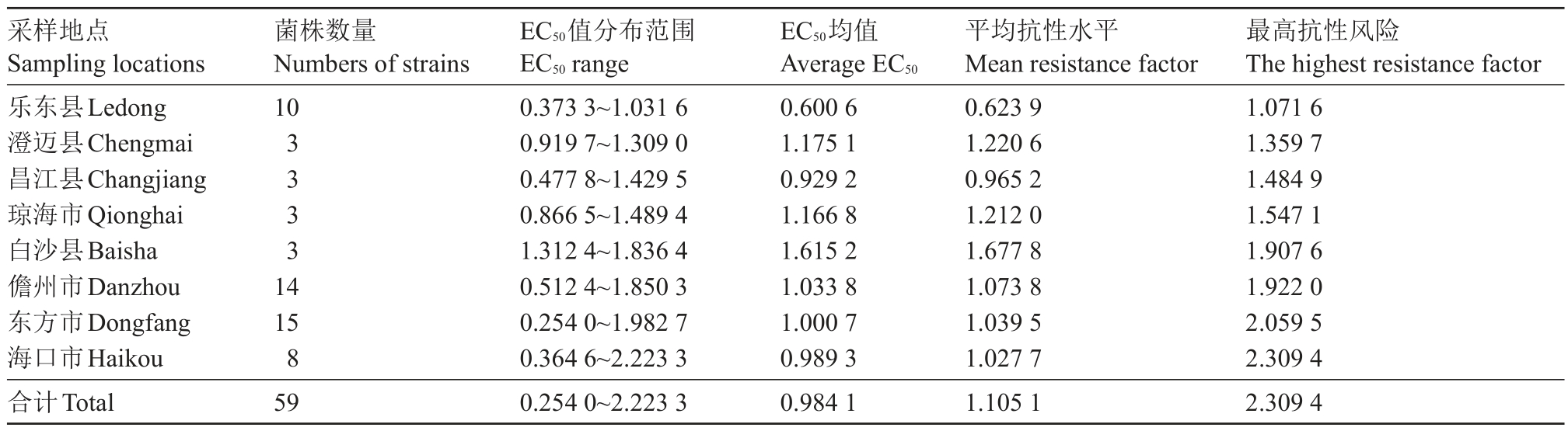
采样地点Sampling locations乐东县Ledong澄迈县Chengmai昌江县Changjiang琼海市Qionghai白沙县Baisha儋州市Danzhou东方市Dongfang海口市Haikou合计Total菌株数量Numbers of strains 10 3 3 3 3 14 15 8 59 EC50值分布范围EC50 range 0.373 3~1.031 6 0.919 7~1.309 0 0.477 8~1.429 5 0.866 5~1.489 4 1.312 4~1.836 4 0.512 4~1.850 3 0.254 0~1.982 7 0.364 6~2.223 3 0.254 0~2.223 3 EC50均值Average EC50 0.600 6 1.175 1 0.929 2 1.166 8 1.615 2 1.033 8 1.000 7 0.989 3 0.984 1平均抗性水平Mean resistance factor 0.623 9 1.220 6 0.965 2 1.212 0 1.677 8 1.073 8 1.039 5 1.027 7 1.105 1最高抗性风险The highest resistance factor 1.071 6 1.359 7 1.484 9 1.547 1 1.907 6 1.922 0 2.059 5 2.309 4 2.309 4
3 讨 论
海南省地处热带、亚热带地区,具有典型的热带季风气候,是火龙果的优质产区,但由于高温高湿常有台风等自然条件的影响,火龙果种植区易有病虫害发生。火龙果溃疡病是制约火龙果产业发展的重要病害之一,除了危害茎部,影响火龙果植株的生长外,还危害果实,进而影响产量及质量。目前,火龙果溃疡病菌的防治主要依赖于化学药剂。吡唑醚菌酯作为一种广谱性杀菌剂,具有高效、低毒、低残留等诸多优点,在火龙果种植地区被作为推荐药剂,大范围地用于田间病害防治,但由于其本身作用位点单一,病原菌很容易因产生变异,而使吡唑醚菌酯作用效果降低[12,22],这对病害的防控极为不利。目前已有许多病原菌建立了对吡唑醚菌酯的敏感基线,如海南省杧果蒂腐病菌[13]、水稻稻瘟病菌[23]、苹果腐烂病菌[24]和山东葡萄白腐病菌[25]等。到目前为止,还没有火龙果溃疡病相关药剂敏感性的监测分析报道,因此非常有必要建立火龙果溃疡病菌对吡唑醚菌酯的敏感基线,用于监测海南地区火龙果溃疡病菌对吡唑醚菌酯的敏感性发展情况。由于海南省内几乎不可能采集到未用药前的野生菌株,因此笔者采用火龙果溃疡病菌对吡唑醚菌酯的EC50值进行检测分析,当EC50值符合连续正态分布时,EC50均值作为敏感基线,结果表明供试菌株EC50值间符合连续性正态分布,EC50均值0.962 7 μg·mL-1作为海南火龙果溃疡病菌对吡唑醚菌酯的敏感基线。根据前人的研究,王会会等[9]使用25%吡唑醚菌酯乳油对海南省琼海市火龙果溃疡病菌进行室内药剂筛选,设置质量浓度为4、2、1、0.5、0.25 μg·mL-1,得到EC50结果为1.029 1 μg·mL-1;林珊宇等[26]使用42.4%吡唑醚菌酯·氟唑菌酰胺悬浮剂对广西火龙果溃疡病菌进行室内药剂筛选,得到EC50结果为0.057 5 μg·mL-1;贤小勇等[27]使用96%吡唑醚菌酯原药对广西火龙果溃疡病菌进行室内药剂筛选,设置质量浓度为4、2、1、0.5、0.25 μg·mL-1,得到EC50结果为1.857 2 μg·mL-1,前人研究与本研究获得的EC50均值均较相近,广西壮族自治区和海南省火龙果溃疡病菌对吡唑醚菌酯的敏感性较相近。
笔者实验室于2019—2020年间对海南8个火龙果种植市(县)进行了实地调查,其中乐东和昌江作为新开发的火龙果产区,种植年限相对较短,用药量相对于其他地区较少,而白沙和琼海为火龙果种植的传统产区,火龙果种植年限相对较长,且用药量也相对较高,其中白沙主要由企业承包种植,所以用药量相对更大。笔者测定了从海南省海口、乐东、东方、儋州、白沙、昌江、澄迈和琼海8个地区分离的59个菌株对吡唑醚菌酯的敏感性,表明海南59株火龙果溃疡病菌对吡唑醚菌酯的敏感性差异性较小,EC50为0.254 0~2.223 3 μg·mL-1。其中,乐东县菌株对吡唑醚菌酯十分敏感,而白沙县菌株对吡唑醚菌酯敏感性相对较低,地区间以白沙和乐东之间EC50的差异最为显著,白沙县EC50均值为乐东县的2.69倍,平均抗性的2.71 倍,这与笔者实验室的田间调查结果相吻合。从研究的结果来看,目前海南地区火龙果溃疡病菌对吡唑醚菌酯仍较敏感,吡唑醚菌酯仍可作为防治火龙果溃疡病的杀菌剂使用。但是随着种植年限的增长,药剂不断累积使用,不排除存在产生抗药性的风险。海南省火龙果溃疡病菌对吡唑醚菌酯药剂敏感性水平目前尚未见报道,笔者在本研究中采用菌丝生长速率法测定了溃疡病菌菌株对吡唑醚菌酯的敏感性,进一步明确了近年内海南省火龙果溃疡病菌对吡唑醚菌酯的敏感性水平。对某种杀菌剂的敏感性监测是一个长期的工作,本研究结果为海南省火龙果溃疡病菌对常用杀菌剂吡唑醚菌酯的敏感性长期监测奠定了基础,为优化杀菌剂使用策略和科学防治火龙果溃疡病等工作提供了一定的科学依据。
4 结 论
海南省火龙果产区火龙果溃疡病菌对吡唑醚菌酯目前未产生抗药性,但不同种植市(县)的火龙果溃疡病菌对吡唑醚菌酯的敏感性存在差异,因此各火龙果种植地区使用吡唑醚菌酯时应根据其敏感性情况对施药比率与频率进行调整。其中白沙地区建议与其他相关药剂交替使用,减缓耐药性的发生,避免产生抗药性菌株。
[1]FABRICE L B,FABRICE V,ERIC I.Pitahaya (Hylocereus spp.):A new fruit crop,a market with a future[J].Fruits,2006,61(4):237-250.
[2]易润华,甘罗军,晏冬华,吴泽菁,童依婷,吴凤发.火龙果溃疡病病原菌鉴定及生物学特性[J].植物保护学报,2013,40(2):102-108.YI Runhua,GAN Luojun,YAN Donghua,WU Zejing,TONG Yiting,WU Fengfa.Identification and biological characteristics of Neoscytalidium dimidiatum causing pitaya canker[J].Acta Phytophylacica Sinica,2013,40(2):102-108.
[3]赵湖冰,姚媛媛,杨乐乐,田野,田孟伦,刘晓柱.火龙果研究现状与开发前景[J].山东化工,2018,47(14):52-54.ZHAO Hubing,YAO Yuanyuan,YANG Lele,TIAN Ye,TIAN Menglun,LIU Xiaozhu.Pitaya research status and its development prospect[J].Shandong Chemical Industry,2018,47(14):52-54.
[4]MASRATUL H M,BAHARUDDIN S,LATIFFAH Z.Identification and molecular characterizations of Neoscytalidium dimidiatum causing stem canker of red-fleshed dragon fruit(Hylocereus polyrhizus)in Malaysia[J].Journal of Phytopathology,2013,161(11/12):841-849.
[5]EZRA D,LIARZI O,Hershcovich M.First report of internal black rot caused by Neoscytalidium dimidiatum on Hylocereus undatus(pitahaya)fruit in Israel[J].Plant Disease,2013,97(11):1513.
[6]CHUANG M F,NI H F,YANG H R,SHU S L,LAI S Y.First report of stem canker disease of pitaya (Hylocereus undatus and H.polyrhizus)caused by Neoscytalidium dimidiatum in Taiwan[J].Plant Disease,2012,96(6):906-907.
[7]张荣,刘爱媛,白成艳,刘成明,罗笑容,姜峰,梁瑞伟,郑红莉.火龙果溃疡病的症状观察和病原菌鉴定[J].果树学报,2013,30(5):854-856.ZHANG Rong,LIU Aiyuan,BAI Chengyan,LIU Chengming,LUO Xiaorong,JIANG Feng,LIANG Ruiwei,ZHENG Hongli.Symptom observation and pathogen identification on canker disease of pitaya[J].Journal of Fruit Science,2013,30(5):854-856.
[8]戴俊,王会会,符碧海,王萌,陶挺燕,谢昌平,朱朝华.火龙果溃疡病和茎腐病病原菌的生物学特性测定[J].中国南方果树,2017,46(1):78-82.DAI Jun,WANG Huihui,FU Bihai,WANG Meng,TAO Tingyan,XIE Changping,ZHU Chaohua.Determination of biological characteristics of Neoscytalidium dimidiatum and Fusicoccum sp.on pitaya[J].South China Fruits,2017,46(1):78-82.
[9]王会会,符碧海,戴俊,徐倩,王萌,陶挺燕,谢昌平,朱朝华.火龙果溃疡病菌的鉴定及室内药剂筛选[J].中国南方果树,2016,45(1):8-12.WANG Huihui,FU Bihai,DAI Jun,XU Qian,WANG Meng,TAO Tingyan,XIE Changping,ZHU Chaohua.Identification of dragonfruitcankerpathogenandindoorscreeningoffungicides[J].South China Fruits,2016,45(1):8-12.
[10]XU M,PENG Y,QI Z,YAN Z,YANG L,HE M D,LI Q X,LIU C L,RUAN Y Z,WEI S S,XIE J,XIA Y Q,TANG H.Identification of Neoscytalidium dimidiatum causing canker disease of pitaya in Hainan,China[J].Australasian Plant Pathology,2018,47(5):547-553.
[11]杨丽娟,柏亚罗.甲氧基丙烯酸酯类杀菌剂:吡唑醚菌酯[J].现代农药,2012,11(4):46-56.YANG Lijuan,BAI Yaluo.Strobilurin fungicide:Pyraclostrobin [J].Modern Agrochemicals,2012,11(4):46-56.
[12]杜宜新,阮宏椿,石妞妞,甘林,杨秀娟,陈福如.福建玉米小斑病菌对戊唑醇、吡唑醚菌酯和硝苯菌酯的敏感性[J].西北农林科技大学学报(自然科学版),2017,45(8):69-75.DU Yixin,RUAN Hongchun,SHI Niuniu,GAN Lin,YANG Xiujuan,CHEN Furu.Sensitivity of Bipolaris in Fujian to tebuconazole,pyraclostrobin and meptyldinocap[J].Journal of Northwest A&F University (Nature Science Edition),2017,45(8):69-75.
[13]贺瑞,赵磊,符瑞,陈芷岑,林晓翠,杨叶.海南芒果蒂腐病菌对吡唑醚菌酯的抗药性测定[J].植物保护,2018,44(4):188-193.HE Rui,ZHAO Lei,FU Rui,CHEN Zhicen,LIN Xiaocui,YANG Ye.Resistance of Botryodiplodia theobromae caused mango stem end rot to pyraclostrobin in Hainan[J].Plant Protection,2018,44(4):188-193.
[14]贾俊超,马琳,范志金,夏倩,刘秀峰.病原菌对Strobilurin 类杀菌剂抗药性机理的研究进展[J].农药学学报,2008,10(1):1-9.JIA Junchao,MA Lin,FAN Zhijin,XIA Qian,LIU Xiufeng.Progress on study of resistance mechanism of strobilurin fungicides[J].Chinese Journal of Pesticide Science,2008,10(1):1-9.
[15]方中达.植病研究方法[M].3 版.北京:中国农业出版社,1998.FANG Zhongda.Research method of plant pathology[M].3 ed.Beijing:China Agricultural Press,1998.
[16]杨凤珍,郭建国,杜蕙,王春明,郭成,金社林,王生荣.甘肃玉米大斑病菌对吡唑醚菌酯的敏感性监测分析[J].西北农业学报,2016,25(10):1548-1553.YANG Fengzhen,GUO Jianguo,DU Hui,WANG Chunming,GUO Cheng,JIN Shelin,WANG Shengrong.Monitoring and analyzing sensitivity of Setosphaeria turcica to pyraclostrobin in Gansu[J].Acta Agricultural Boreali-Occidentalis Sinica,2016,25(10):1548-1553.
[17]ARABIAT S,KHAN M F R,BOLTON M,SECOR G.Stability of tetraconazole-resistant isolates of Cercospora beticola after exposure to different temperature and time treatments[J].Journal of Plant Pathology,2017,99(1):177-184.
[18]杨敬辉,许媛,肖婷,褚姝频,芮东明,姚克兵.葡萄炭疽病菌(Colletotrichum spp.)种群对多菌灵的抗药性监测[J].果树学报,2021,38(2):242-249.YANG Jinghui,XU Yuan,XIAO Ting,CHU Shupin,RUI Dongming,YAO Kebing.Resistance monitoring of Colletotrichum spp.population to carbendazim in grape vineyards[J].Journal of Fruit Science,2021,38(2):242-249.
[19]徐杰,冀志蕊,王娜,张俊祥,林云光,周宗山.葡萄炭疽病菌对4 种杀菌剂的敏感性分析[J].果树学报,2020,37(6):882-890.XU Jie,JI Zhirui,WANG Na,ZHANG Junxiang,LIN Yunguang,ZHOU Zongshan.Sensitivity analysis of Colletotrichum gloeosporioides to four fungicides[J].Journal of Fruit Science,2020,37(6):882-890.
[20]顾乃图,范昆,成世杰,李本杰,付丽,夏晓明.苹果轮纹病菌对腐霉利的敏感性检测[J].果树学报,2016,33(10):1277-1285.GU Naitu,FAN Kun,CHENG Shijie,LI Benjie,FU Li,XIA Xiaoming.Detection of the sensitivity of Botryosphaeria dothidea to procymidone[J].Journal of Fruit Science,2016,33(10):1277-1285.
[21]刘世江,丁怡,赵琪君,李明,文小东,宋星陈,李荣玉.贵州省水稻纹枯病菌对丙环唑和吡唑醚菌酯的敏感性[J].福建农业学报,2019,34(11):1294-1301.LIU Shijiang,DING Yi,ZHAO Qijun,LI Ming,WEN Xiaodong,SONG Xingchen,LI Rongyu.Sensitivites of Rhizoctonia solani to propiconazole and pyraclostrobin on rice plants in Guizhou province[J].Fujian Journal of Agricultural Sciences,2019,34(11):1294-1301.
[22]王丽,石延霞,李宝聚,刘长令,向文胜.甲氧基丙烯酸酯类杀菌剂研究进展[J].农药科学与管理,2008,29(9):24-27.WANG Li,SHI Yanxia,LI Baoju,LIU Changling,XIANG Wensheng.The progresses of research on strobilurin fungicides[J].Pesticide Science and Administration,2008,29(9):24-27.
[23]梁梦琦.长江中下游稻区稻瘟病菌对稻瘟灵和吡唑醚菌酯的抗性监测[D].北京:中国农业科学院,2018.LIANG Mengqi.Monitoring sensitivity of Magnaporthe oryzae to isoprothiolane and Pyraclostrobin in middle and lower reaches of Yangtze River[D].Beijing: Chinese Academy of Agricultural Sciences,2018.
[24]王帅.苹果树腐烂病菌对吡唑醚菌酯的抗药性风险评估及生物源杀菌剂的室内活性评价[D].杨凌:西北农林科技大学,2018.WANG Shuai.Resistance risk assessment of Valsa mali to pyraclostrobin and antifungal activity of biological fungicides[D].Yangling:Northwest A&F University,2018.
[25]李宝燕,栾炳辉,石洁,汪少丽,田园园,聂乐兴,王英姿.胶东地区葡萄白腐病菌对吡唑醚菌酯的敏感性及与其他4 种药剂的敏感性比较[J].农药学学报,2020,22(6):959-966.LI Baoyan,LUAN Binghui,SHI Jie,WANG Shaoli,TIAN Yuanyuan,NIE Lexing,WANG Yingzi.Sensitivity of Coniella diplodiella to pyraclostrobin in Jiaodong Area and comparison with four other fungicides[J].Chinese Journal of Pesticide Science,2020,22(6):959-966.
[26]林珊宇,贤小勇,朱桂宁,韦小妹,钟有超,黄黎芳.防治火龙果溃疡病的药剂筛选及田间应用[J].农药,2018,57(12):921-924.LIN Shanyu,XIAN Xiaoyong,ZHU Guining,WEI Xiaomei,ZHONG Youchao,HUANG Lifang.Effective fungicides screening and field application effects against pitaya canker[J].Agrochemicals,2018,57(12):921-924.
[27]贤小勇,林珊宇,朱桂宁,韦小妹,覃武,梁桂东,钟有超.杀菌剂对火龙果溃疡病的室内毒力和田间防效[J].南方农业学报,2018,49(7):1338-1345.XIAN Xiaoyong,LIN Shanyu,ZHU Guining,WEI Xiaomei,QIN Wu,LIANG Guidong,ZHONG Youchao.Indoor virulence and field effects of fungicides on pitaya canker[J].Journal of Southern Agriculture,2018,49(7):1338-1345.