中国是梨生产大国,梨栽培面积和总产量稳居世界第一,据联合国粮农组织最新数据,2019 年中国梨栽培面积约95.1万hm2,总产量约1 700.0万t[1]。黄河故道地区作为我国梨重要产区之一,其主栽品种砀山酥梨叶片黄化现象较为普遍,严重时造成新梢顶叶脱落,导致叶片光合能力下降,光合同化产物减少,降低了梨产量及质量[2],严重制约了当地梨产业的发展。
叶片黄化主要表现为叶绿素含量显著减少,而叶绿素作为光合作用的决定因素,影响着叶内N 代谢和光合同化产物蔗糖、葡萄糖等的积累[3]。研究表明,水分胁迫下的玉米叶内叶绿素含量显著下降,光合速率降低[4]。高温胁迫加快了番茄叶内叶绿素的降解,抑制了植株光合速率[5],说明叶绿素代谢与光合代谢相关。对水稻和马铃薯的研究表明,叶内或果皮内叶绿素含量的减少,导致其光合强度降低,并伴随着可溶性糖含量的降低[6-7]。因此,叶片等组织内叶绿素积累可增强其光合作用,并影响光合同化产物的代谢。而植物光合作用的强弱可由叶绿素荧光特性直接体现[8]。
梨的主要同化产物为山梨醇和蔗糖[9]。山梨醇合成起始于3–磷酸甘油醛(Glyceraldehyde 3-phosphate,G3P),G3P 经多种酶催化形成6-磷酸葡萄糖(Glucose 6-phosphate,G6P),G6P 进一步在6-磷酸山梨醇脱氢酶(Sorbitol 6-phosphate dehydrogenase,S6PDH)的催化下生成6-磷酸梨醇(Sorbitol 6-phosphate,S6P),并最终在6-磷酸山梨醇磷酸酯酶(Sorbitol 6-phosphate phosphatase,S6PPP)的催化作用下生成山梨醇[10],合成的山梨醇则在山梨醇转运蛋白(Sorbitol transporter,SOT)作用下,完成由“源”到“库”的转移[11];就其代谢途径而言,山梨醇可在NADP+型山梨醇脱氢酶(NADP+-SDH)的作用下主要分解成葡萄糖,在NAD+型山梨醇脱氢酶(NAD+-SDH)作用下完成与果糖之间的相互转化[12-13]。而蔗糖的合成能力取决于细胞质内的磷酸蔗糖合成酶(Sucrose phosphate synthase,SPS)、蔗糖合成酶(Sucrose synthase,SS)的相对活性[14],其跨膜运输和在植物中的分配则依赖于蔗糖转运蛋白(Sucrose transporters,SUTs)[15],而其贮藏功能很大程度上受泡膜单糖转运蛋白(Tonoplast monosaccharide transporter,TMT)在内的膜转运特性的影响[16]。
前期研究表明,叶面喷施浓度(w)为0.2%的Fe-SO4溶液可有效诱导砀山酥梨黄化叶复绿[17]。迄今,国内外关于外源FeSO4处理与叶片光合特性和糖代谢之间的关系鲜见系统性研究。笔者在本研究中以砀山酥梨正常、黄化植株为试材,以清水处理正常叶片、黄化叶片为对照,经黄化叶片叶面喷施0.2%Fe-SO4,测定对照组叶片内及FeSO4处理后不同时期黄化叶片内叶绿素含量,并通过测定各时期黄化叶片和对照组叶片内山梨醇、蔗糖、葡萄糖和果糖含量以及叶绿素荧光参数,分析其光合代谢、糖代谢相关基因的表达量,以揭示外源FeSO4对砀山酥梨黄化叶片中叶绿素积累、叶片光合能力和糖合成代谢的影响。
1 材料和方法
1.1 试验材料
试验于2020 年5 月进行,以安徽省砀山县园艺场15年生砀山酥梨黄化植株与正常植株为试材,栽植密度为5 m×6 m,树势中庸,树形为开心形,立地水平与管理水平一致。于黄化梨树新梢生长期全树喷施0.2% FeSO4溶液,单株喷施用量为10 L,对照为喷施清水的正常植株叶片和黄化植株叶片,计3个处理,每个处理以3株树为1次重复,3次重复,共计27 株。每个处理之间设置无任何处理的梨树隔离行。分别于FeSO4处理前取对照组正常叶片和黄化叶片叶样,处理后3、6、9和12 d取处理叶片样品,并于-80 ℃保存备用。
1.2 试验方法
1.2.1 叶绿素含量的测定 采用丙酮提取法提取各处理功能叶片的叶绿素,以分光光度计法测定其总叶绿素含量,具体实施方案和计算方法参考张以顺等[18]的方法。
1.2.2 叶片中叶绿素荧光的测定 选取位于树体中部东、西两侧的新梢中段内大小、生理状况基本一致的对照组内正常叶片、黄化叶片以及FeSO4处理的黄化叶片各30枚(即单次处理的每株树选取10枚叶样,总计90枚叶样),并挂牌标记[19]。以FMS-2便携脉冲调制式荧光仪于晴天上午9:00—11:00 测定各叶片叶绿素荧光参数。具体实施方案参照徐新翔等[20]的方法。
1.2.3 光合参数的测定 以便携式光合仪(Li-6400,USA)于晴天中午11:00—13:00测定叶片净光合速率(Net photosynthetic rate,Pn)、气孔导度(Stomatal conductivity,Gs)、蒸腾速率(Transpiration rate,Tr)和胞间CO2浓度(Intercellular CO2 concentration,Ci),具体参数设置参考程贝等[21]的方法。叶片水分利用效率(Water use efficiency,WUE)和气孔限制值(Somatal limitation value,Ls)根据公式:WUE=Pn/Tr,Ls=1-Ci/Ca(Ca为空气中CO2浓度),计算得出。
1.2.4 糖含量的测定 每个样随机取20~30 枚叶样,混匀后称取0.5 g样品,3次重复。将样品放入研钵中,加入10 mL双蒸水充分研磨后,将匀浆转移至50 mL 离心管内,置于80 ℃水浴锅中水浴15 min,待其冷却后,于12 000 r∙min-1离心10 min,以1次性注射器抽取上清,用0.22 μm 水溶性滤膜抽滤待用。取10 μL滤液,用高效液相色谱法(High Performance Liquid Chromatoghraphy,HPLC)测定其山梨醇、蔗糖、葡萄糖和果糖含量。色谱柱采用Rezex RCM-Monosaccharede Ca2+(8%),规格为150 mm×7.8 mm。工作条件:柱温35 ℃,流动相为V 乙腈∶V 水=7.5∶2.5,LC-6A 示差检测器,泵为LC-6A,流速为1 mL∙min-1。
1.2.5 总RNA和实时荧光定量PCR 以广州Omega 生物科技有限公司植物复杂样本总RNA 提取试剂盒(E •Z •N •A.®Plant RNA Kit)提 取 叶 片总RNA,并按北京艾德莱生物科技有限公司的荧光反转录试剂盒(PC18-TRUEscript 1st Strand cDNA Synthesis Kit)操作说明合成cDNA。
从梨基因组数据库(http://peargenome.njau.edu.cn/)中以及NCBI(https://www.ncbi.nlm.nih.gov/)查询、克隆获得了砀山酥梨幼果中光合及山梨醇、蔗糖代谢相关基因的全长序列,并根据获得的序列,使用Premier 5.0 软件设计实时荧光定量PCR(Quantitative real-time PCR,qRT-PCR)扩增引物(引物序列见表1、表2、表3)。扩增反应于ABI STEP-ONE 荧光定量PCR仪上进行,使用北京启迪生物公司提供的SYBR® Green I 染料,反应总体系20 µL,其中,SYBR Plus qPCR Master Mix M(2×)10 µL,SYBR ROX High(50×)0.4µL,1µL模板cDNA、正向引物和反向引物各0.6µL、DEPC水7.4µL。反应程序为95 ℃预变性5 min,95 ℃变性10 s,60 ℃退火20 s,72 ℃延伸30 s,共40个循环,每个样品3次重复。以梨GAPDH基因作为内参,使用2-△△CT法求待测样品相对表达量。
表1 光合作用相关基因荧光PCR 引物序列
Table 1 Primer sequences of genes related to photosynthesis for qRT-PCR
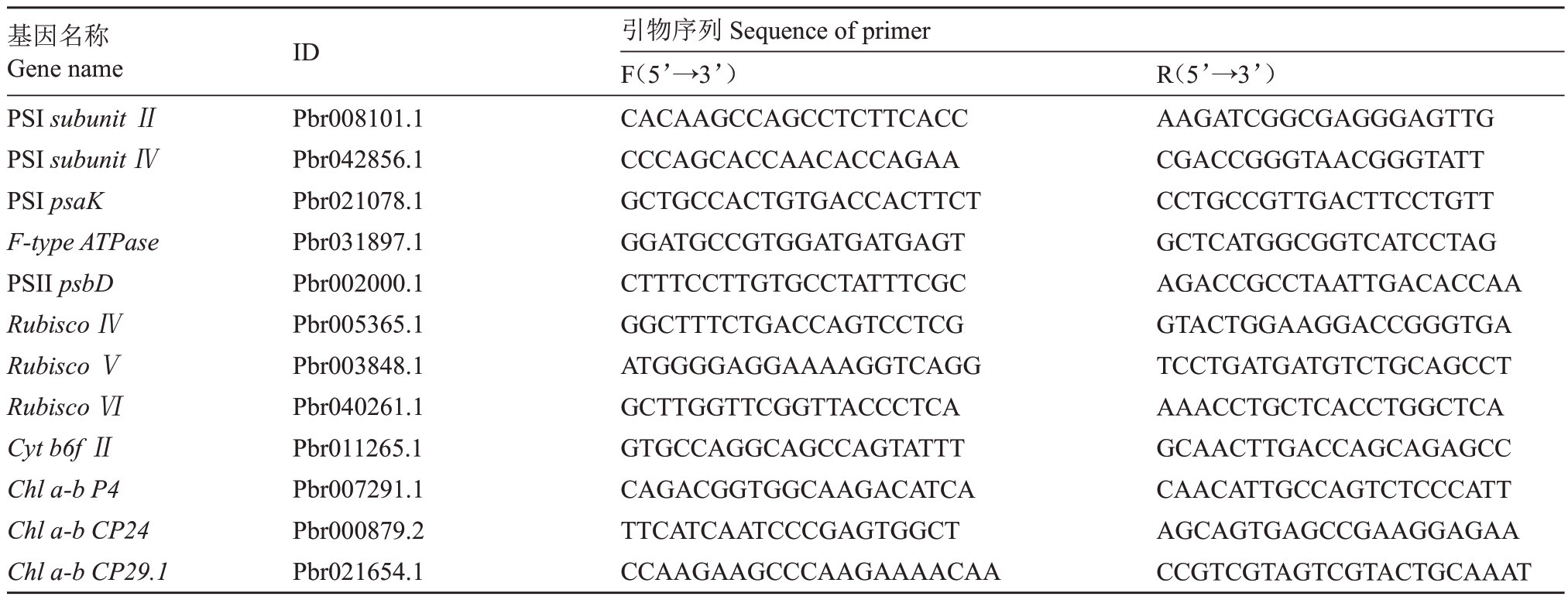
基因名称Gene name PSI subunit ⅡPSI subunit ⅣPSI psaK F-type ATPase PSII psbD Rubisco ⅣRubisco ⅤRubisco ⅥCyt b6f ⅡChl a-b P4 Chl a-b CP24 Chl a-b CP29.1 ID Pbr008101.1 Pbr042856.1 Pbr021078.1 Pbr031897.1 Pbr002000.1 Pbr005365.1 Pbr003848.1 Pbr040261.1 Pbr011265.1 Pbr007291.1 Pbr000879.2 Pbr021654.1引物序列Sequence of primer F(5’→3’)CACAAGCCAGCCTCTTCACC CCCAGCACCAACACCAGAA GCTGCCACTGTGACCACTTCT GGATGCCGTGGATGATGAGT CTTTCCTTGTGCCTATTTCGC GGCTTTCTGACCAGTCCTCG ATGGGGAGGAAAAGGTCAGG GCTTGGTTCGGTTACCCTCA GTGCCAGGCAGCCAGTATTT CAGACGGTGGCAAGACATCA TTCATCAATCCCGAGTGGCT CCAAGAAGCCCAAGAAAACAA R(5’→3’)AAGATCGGCGAGGGAGTTG CGACCGGGTAACGGGTATT CCTGCCGTTGACTTCCTGTT GCTCATGGCGGTCATCCTAG AGACCGCCTAATTGACACCAA GTACTGGAAGGACCGGGTGA TCCTGATGATGTCTGCAGCCT AAACCTGCTCACCTGGCTCA GCAACTTGACCAGCAGAGCC CAACATTGCCAGTCTCCCATT AGCAGTGAGCCGAAGGAGAA CCGTCGTAGTCGTACTGCAAAT
表2 山梨醇代谢相关基因荧光PCR 引物序列
Table 2 Primer sequences of genes related to sorbitol metabolism for qRT-PCR
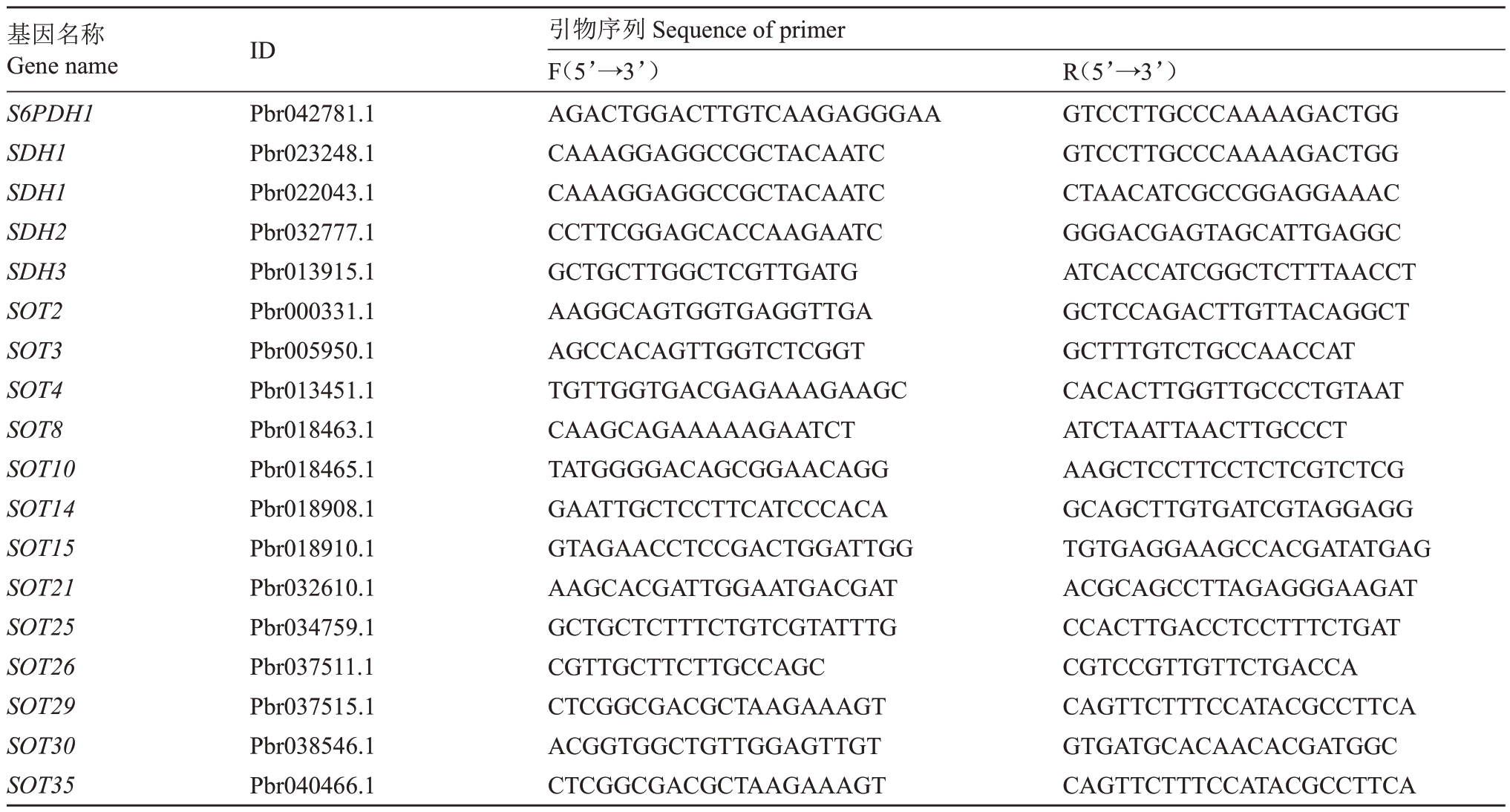
基因名称Gene name S6PDH1 SDH1 SDH1 SDH2 SDH3 SOT2 SOT3 SOT4 SOT8 SOT10 SOT14 SOT15 SOT21 SOT25 SOT26 SOT29 SOT30 SOT35 ID Pbr042781.1 Pbr023248.1 Pbr022043.1 Pbr032777.1 Pbr013915.1 Pbr000331.1 Pbr005950.1 Pbr013451.1 Pbr018463.1 Pbr018465.1 Pbr018908.1 Pbr018910.1 Pbr032610.1 Pbr034759.1 Pbr037511.1 Pbr037515.1 Pbr038546.1 Pbr040466.1引物序列Sequence of primer F(5’→3’)AGACTGGACTTGTCAAGAGGGAA CAAAGGAGGCCGCTACAATC CAAAGGAGGCCGCTACAATC CCTTCGGAGCACCAAGAATC GCTGCTTGGCTCGTTGATG AAGGCAGTGGTGAGGTTGA AGCCACAGTTGGTCTCGGT TGTTGGTGACGAGAAAGAAGC CAAGCAGAAAAAGAATCT TATGGGGACAGCGGAACAGG GAATTGCTCCTTCATCCCACA GTAGAACCTCCGACTGGATTGG AAGCACGATTGGAATGACGAT GCTGCTCTTTCTGTCGTATTTG CGTTGCTTCTTGCCAGC CTCGGCGACGCTAAGAAAGT ACGGTGGCTGTTGGAGTTGT CTCGGCGACGCTAAGAAAGT R(5’→3’)GTCCTTGCCCAAAAGACTGG GTCCTTGCCCAAAAGACTGG CTAACATCGCCGGAGGAAAC GGGACGAGTAGCATTGAGGC ATCACCATCGGCTCTTTAACCT GCTCCAGACTTGTTACAGGCT GCTTTGTCTGCCAACCAT CACACTTGGTTGCCCTGTAAT ATCTAATTAACTTGCCCT AAGCTCCTTCCTCTCGTCTCG GCAGCTTGTGATCGTAGGAGG TGTGAGGAAGCCACGATATGAG ACGCAGCCTTAGAGGGAAGAT CCACTTGACCTCCTTTCTGAT CGTCCGTTGTTCTGACCA CAGTTCTTTCCATACGCCTTCA GTGATGCACAACACGATGGC CAGTTCTTTCCATACGCCTTCA
表3 蔗糖代谢相关基因荧光PCR 引物序列
Table 3 Primer sequences of genes related to sucrose metabolism for qRT-PCR
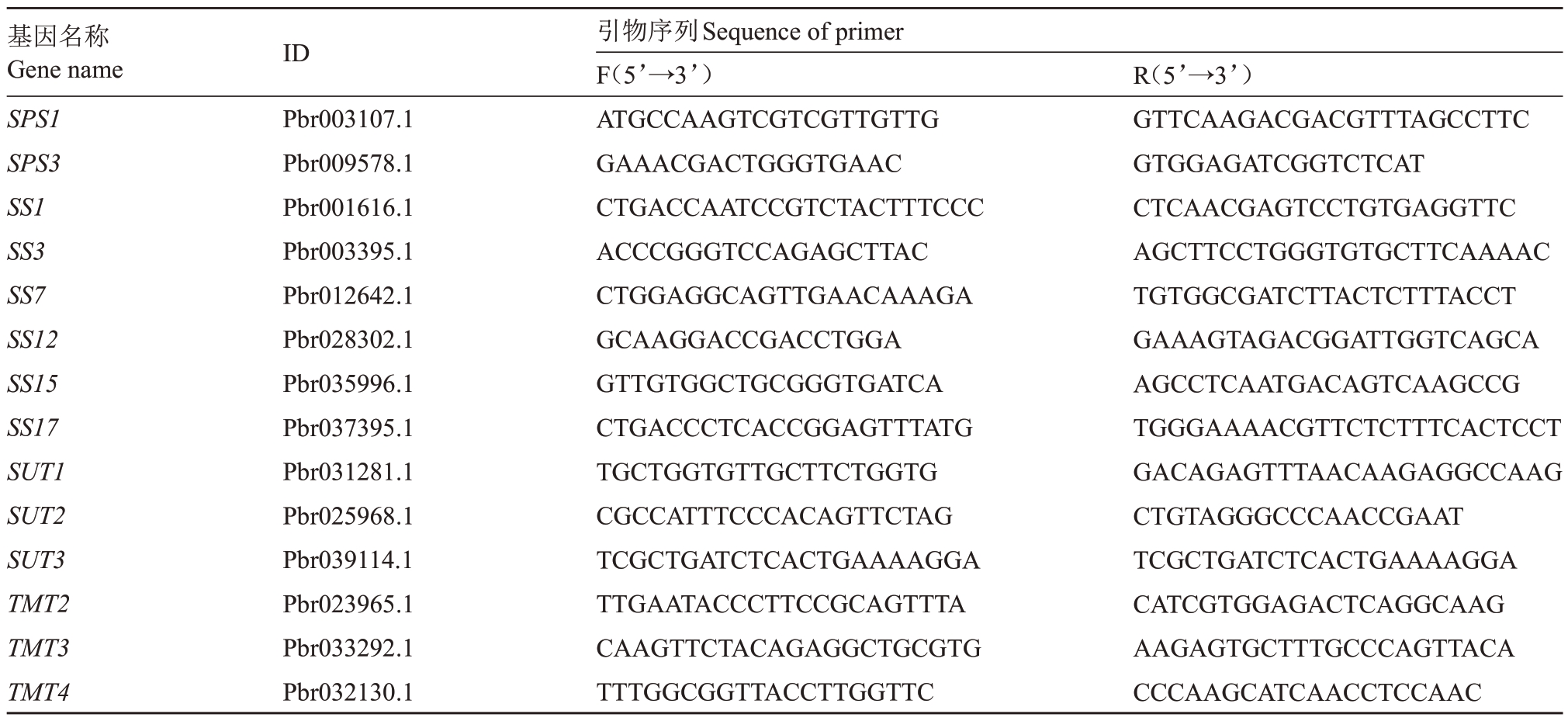
基因名称Gene name SPS1 SPS3 SS1 SS3 SS7 SS12 SS15 SS17 SUT1 SUT2 SUT3 TMT2 TMT3 TMT4 ID Pbr003107.1 Pbr009578.1 Pbr001616.1 Pbr003395.1 Pbr012642.1 Pbr028302.1 Pbr035996.1 Pbr037395.1 Pbr031281.1 Pbr025968.1 Pbr039114.1 Pbr023965.1 Pbr033292.1 Pbr032130.1引物序列Sequence of primer F(5’→3’)ATGCCAAGTCGTCGTTGTTG GAAACGACTGGGTGAAC CTGACCAATCCGTCTACTTTCCC ACCCGGGTCCAGAGCTTAC CTGGAGGCAGTTGAACAAAGA GCAAGGACCGACCTGGA GTTGTGGCTGCGGGTGATCA CTGACCCTCACCGGAGTTTATG TGCTGGTGTTGCTTCTGGTG CGCCATTTCCCACAGTTCTAG TCGCTGATCTCACTGAAAAGGA TTGAATACCCTTCCGCAGTTTA CAAGTTCTACAGAGGCTGCGTG TTTGGCGGTTACCTTGGTTC R(5’→3’)GTTCAAGACGACGTTTAGCCTTC GTGGAGATCGGTCTCAT CTCAACGAGTCCTGTGAGGTTC AGCTTCCTGGGTGTGCTTCAAAAC TGTGGCGATCTTACTCTTTACCT GAAAGTAGACGGATTGGTCAGCA AGCCTCAATGACAGTCAAGCCG TGGGAAAACGTTCTCTTTCACTCCT GACAGAGTTTAACAAGAGGCCAAG CTGTAGGGCCCAACCGAAT TCGCTGATCTCACTGAAAAGGA CATCGTGGAGACTCAGGCAAG AAGAGTGCTTTGCCCAGTTACA CCCAAGCATCAACCTCCAAC
1.3 数据统计分析
数据运用单因素方差分析(ANOVA)的方法,以Duncan多重极差法进行各处理的平均值检验,并以Excel 2019、GraphPad Prism 8 和SPSS 23(SPSS Inc., Chicago, IL, USA)软件,在p <0.05 的水平上计算最小显著性差异(LSD)值,分析各处理间的差异显著性。
2 结果与分析
2.1 FeSO4处理对砀山酥梨黄化叶内叶绿素含量的影响
如图1所示,自然条件下,清水处理砀山酥梨正常叶内(CN)叶绿素含量显著高于清水处理黄化叶(CC)。FeSO4处理黄化叶后3、6、9 和12 d(T3 d、T6 d、T9 d、T12 d),其叶内叶绿素含量显著高于CC,且T12 d>T9 d>T6 d>T3 d。因此,外源FeSO4处理促进了砀山酥梨黄化植株叶内叶绿素含量的积累。

图1 砀山酥梨正常叶、黄化叶与FeSO4处理不同时期黄化叶内叶绿素含量
Fig.1 Chlorophyll content of normal leaves,chlorotic leaves and chlorotic leaves in different periods with FeSO4 application for Dangshan Suli pear
CN 和CC 分别表示叶面喷施清水的对照组内正常叶和黄化叶;T3 d、T6 d、T9 d、T12 d 分别表示叶面喷施0.2%FeSO4 后第3、6、9 和12 d 的黄化叶;下同。
CN and CC indicate normal and chlorotic leaves treated with distilled water in control group,respectively;T3 d,T6 d,T9 d and T12 d indicate chlorotic leaves at 3rd,6th,9th and 12th day after spraying 0.2%FeSO4,respectively;the same below.
2.2 FeSO4处理对砀山酥梨黄化叶片后叶绿素荧光的影响
如图2 所示,CN叶内叶绿素荧光参数Fm、Fv/Fm、Y(II)、Fv/F0、ETR、qP 及Fm′值均显著高于CC。其中,T6 d~T12 d叶内Fm、Fv/Fm、Y(II)、Fv/F0及ETR值均较CC显著 增 加,T9 d~T12 d 叶 内Fm′值 也 显 著 高 于CC。但T3 d~T9 d叶内qP值较CC显著下降。
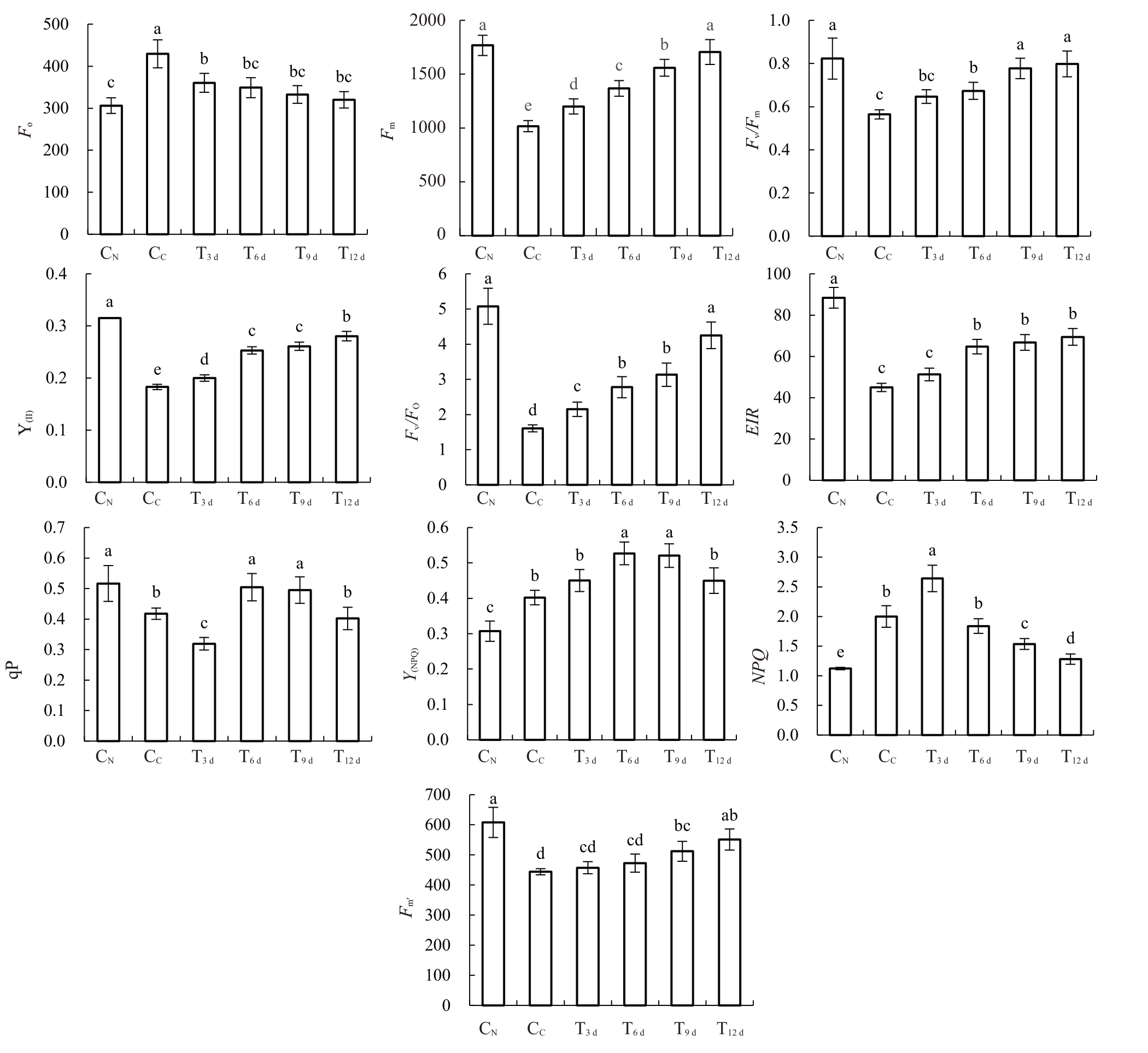
图2 砀山酥梨正常叶、黄化叶与FeSO4处理不同时期黄化叶叶绿素荧光参数值
Fig.2 Chlorophyll fluorescence values of normal leaves,chlorotic leaves and chlorotic leaves in different periods with FeSO4 application for Dangshan Suli pear
另一方面,CN叶内叶绿素荧光参数F0、NPQ 及Y(NPQ)均显著低于CC,而T3 d~T12 d叶内F0值较CC显著下降,T6 d~T12 d叶内NPQ 值也显著低于CC。但Y(NPQ)值在T6 d~T9 d叶内显著上升,其他时期则与CC无异。
因此,FeSO4处理促进了叶片内叶绿素的合成,并在显著提升叶绿素F0、Fv/Fm、Y(II)、Fv/F0及ETR 值的同时,显著降低了Fm值。
2.3 FeSO4处理对砀山酥梨黄化叶光合特性的影响
如图3所示,CN叶内Pn、Gs、Tr、WUE及Ls值均显著高于CC处理,T3 d~T12 d叶内该5个指标值均在处理后逐步升高而显著高于CC;而叶内Ci值则表现为CN显著低于CC,T6 d~T12 d叶内Ci值显著低于CC,且彼此之间无显著性差异。

图3 砀山酥梨正常叶、黄化叶与FeSO4处理不同时期黄化叶光合特性
Fig.3 Photosynthetic properties of normal leaves,chlorotic leaves and chlorotic leaves in different periods with FeSO4 application for Dangshan Suli pear
因此,FeSO4处理显著提升了黄化叶片Pn、Gs、Tr、WUE及Ls值,不同程度降低了其Ci含量,反映了叶内光合能力的增强。
2.4 FeSO4处理对砀山酥梨黄化叶内糖含量的影响
如图4所示,CN叶内果糖、山梨醇含量均显著高于CC,T3 d~T12 d叶内果糖含量均较CC显著上升,山梨醇含量仅在T6 d、T9 d叶内显著高于CC,且两者均在T6 d时达到最大。另一方面,CN与CC的葡萄糖、蔗糖含量无显著性差异,且T6 d~T12 d叶内蔗糖含量也无显著变化,但T6 d~T12 d叶内葡萄糖含量均较CC显著增加。
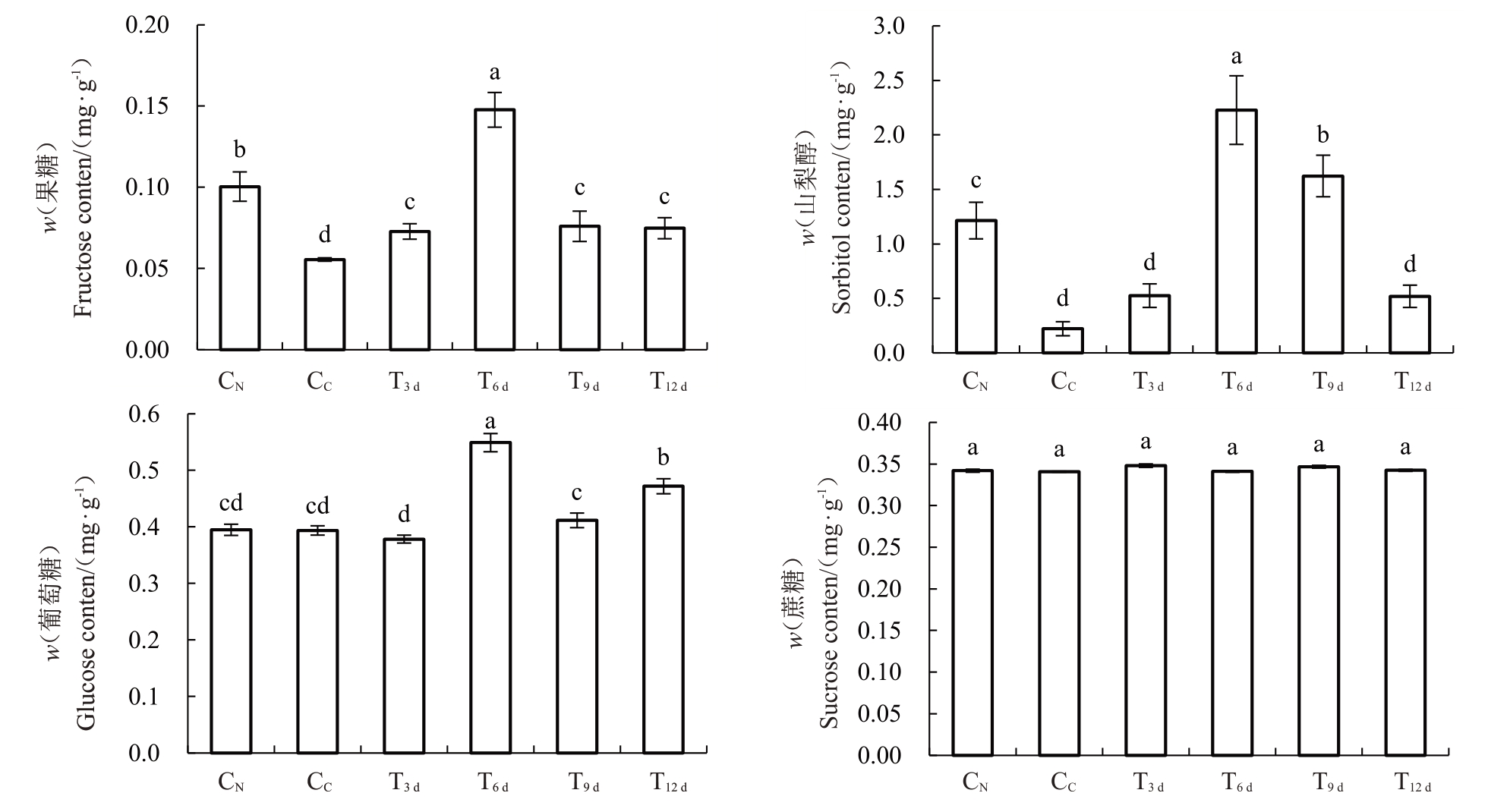
图4 砀山酥梨正常叶、黄化叶与FeSO4处理不同时期黄化叶内糖含量
Fig.4 Sugar products content of normal leaves,chlorotic leaves and chlorotic leaves in different periods with FeSO4 application for Dangshan Suli pear
因此,FeSO4处理促进了黄化叶内光合产物的代谢。
2.5 FeSO4处理对砀山酥梨黄化叶内光合相关基因表达量的影响
如图5 所示,CN叶内光系统Ⅰ蛋白亚基编码基因PSⅠsubunit Ⅱ/Ⅳ、PSⅠpsaK、光系统II 蛋白亚基编码基因PSⅡpsbD 和F 型ATP 酶编码基因FType ATPase Ⅳ表达量均显著高于CC,T3 d~T12 d叶内该4个基因表达量则均较CC显著增加。

图5 砀山酥梨正常叶、黄化叶与FeSO4处理不同时期黄化叶内光合基因相对表达量
Fig.5 Relative expression of photosynthetic genes in normal leaves,chlorotic leaves and chlorotic leaves in different periods with FeSO4 application for Dangshan Suli pear
其次,CN叶内Rubisco 活化酶基因Rubisco activase Ⅳ/Ⅴ表达量显著高于CC,而Rubisco activase VI 无显著性差异。T3 d叶内Rubisco activase Ⅳ表达量较CC无显著差异,在T6 d~T9 d叶内,其表达量上升而显著高于CC。Rubisco activase Ⅴ则在T3 d~T12 d的表达量均显著高于CC。Rubisco activase VI 则仅在T9 d时升高而显著高于CN,其他时期表达量均与CC无显著差异。
另一方面,CN叶内光合膜蛋白基因Cytb6f II、采光复合物I 叶绿素a-b 结合蛋白Chl a-b P4/CP24/29.1的表达量也均显著高于CC。且该3个基因表达量在T6 d~T12 d叶内均显著高于CC,而后下降。
因此,FeSO4处理组叶内显著增加的光合作用及糖类物质的积累可能与其PSⅠsubunit Ⅱ/Ⅳ、FType ATPase Ⅳ、Rubisco activase Ⅳ、Chl a-b P4/CP24等相关基因表达量显著上升有关。
2.6 FeSO4处理对砀山酥梨黄化叶内山梨醇代谢相关基因表达量的影响
如图6 所示,就山梨醇合成途径而言,CN叶内S6PDH1、SDH2/3 均与CC无显著性差异,而CN叶内SDH1显著低于CC。其中,较CC而言,S6PDH1在T3 d和T12 d叶内表达量显著上升,而SDH1、SDH2表达量分别于T6 d、T9 d叶内显著增加,SDH3 则在T6 d和T9 d显著增加,其他时期各基因表达量均与CC无显著差异。
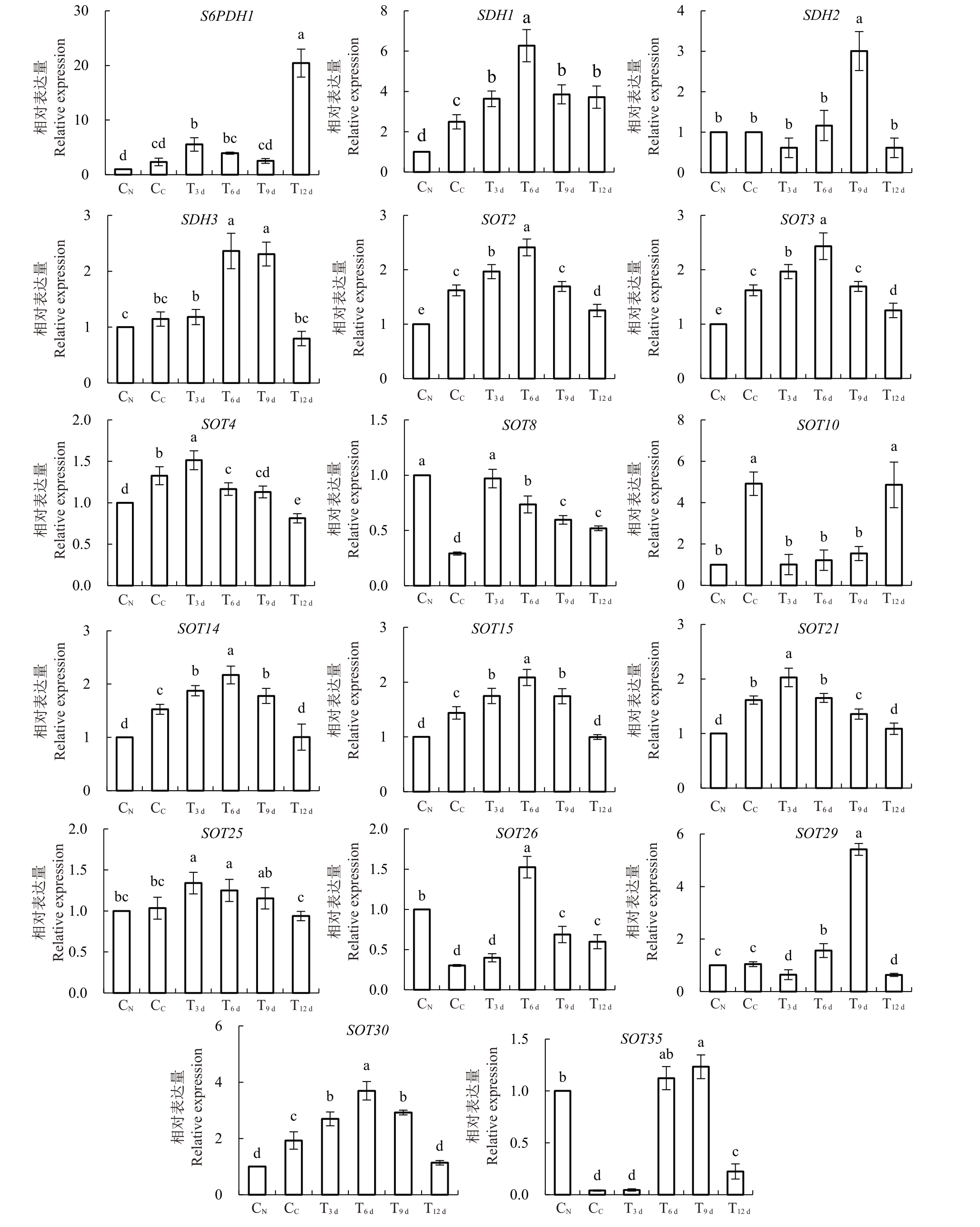
图6 砀山酥梨正常叶、黄化叶与FeSO4处理不同时期黄化叶内山梨醇代谢基因相对表达量
Fig.6 Relative expression of sorbitol metabolic genes in normal leaves,chlorotic leaves and chlorotic leaves in different periods with FeSO4 application for Dangshan Suli pear
就山梨醇转运而言,CN叶内SOT8/26/35相对表达量均显著高于CC,T3 d~T12 d 叶内SOT8 显著高于CC,而SOT26/35 表达量除在T3 d叶内与CC无显著差异外,其T9 d~T12 d叶内表达量也均较CC显著上升;其次,CN 叶内SOT2/3/4/10/14/15/21/30 基因相对表达量均显著低于CC,其中SOT14/15/30 在T3 d~T9 d叶内表达量逐步升高而显著高于CC;SOT2/3表达量则在T3 d~T6 d叶内显著高于CC,SOT4/21表达量则仅在T3 d叶内较CC显著增加,其他时期各基因表达量均显著低于CC 或与之无显著差异;此外,虽然CN 叶内SOT25/29 表达量与CC无显著性差异,但T3 d~T6 d叶内SOT25 表达量与T6 d~T9 d叶内SOT29 表达量均较CC显著上调。
因此,FeSO4处理T6 d~T9 d的叶内显著增加的山梨醇含量主要与FeSO4处理后前期(T3 d~T6 d)内上调的S6PDH1、SDH2/3山梨醇合成基因表达量相关,且FeSO4处理通过促进SOT8/14/15/26/29/30 基因的表达可能增强了山梨醇的转运,促进了叶内山梨醇代谢。
2.7 FeSO4处理对砀山酥梨黄化叶内蔗糖代谢相关基因表达量的影响
如图7 所示,就蔗糖合成基因而言,CN 叶内SPS1 表达量显著高于CC,FeSO4处理后,其T3 d~T12 d叶内表达量均显著高于CC。虽然CN和CC叶内SPS3表达量无显著差异,但其在T6 d、T9 d均显著上升;另一方面,CN叶内SUS1/3/7/12/15/17 相对表达量均显著低于CC。其中,T3 d~T12 d叶内SUS1/3/7/12 表达量均较CC显著降低,T3 d~T9 d叶内SUS15表达量则无显著变化,但在T12 d时期较CC显著上调。SUS17 表达量则表现为先增加后降低,在T9 d中最高,且除T12 d叶内表达量与CC无显著差异外,T3 d~T9 d叶内表达量均显著高于CC。
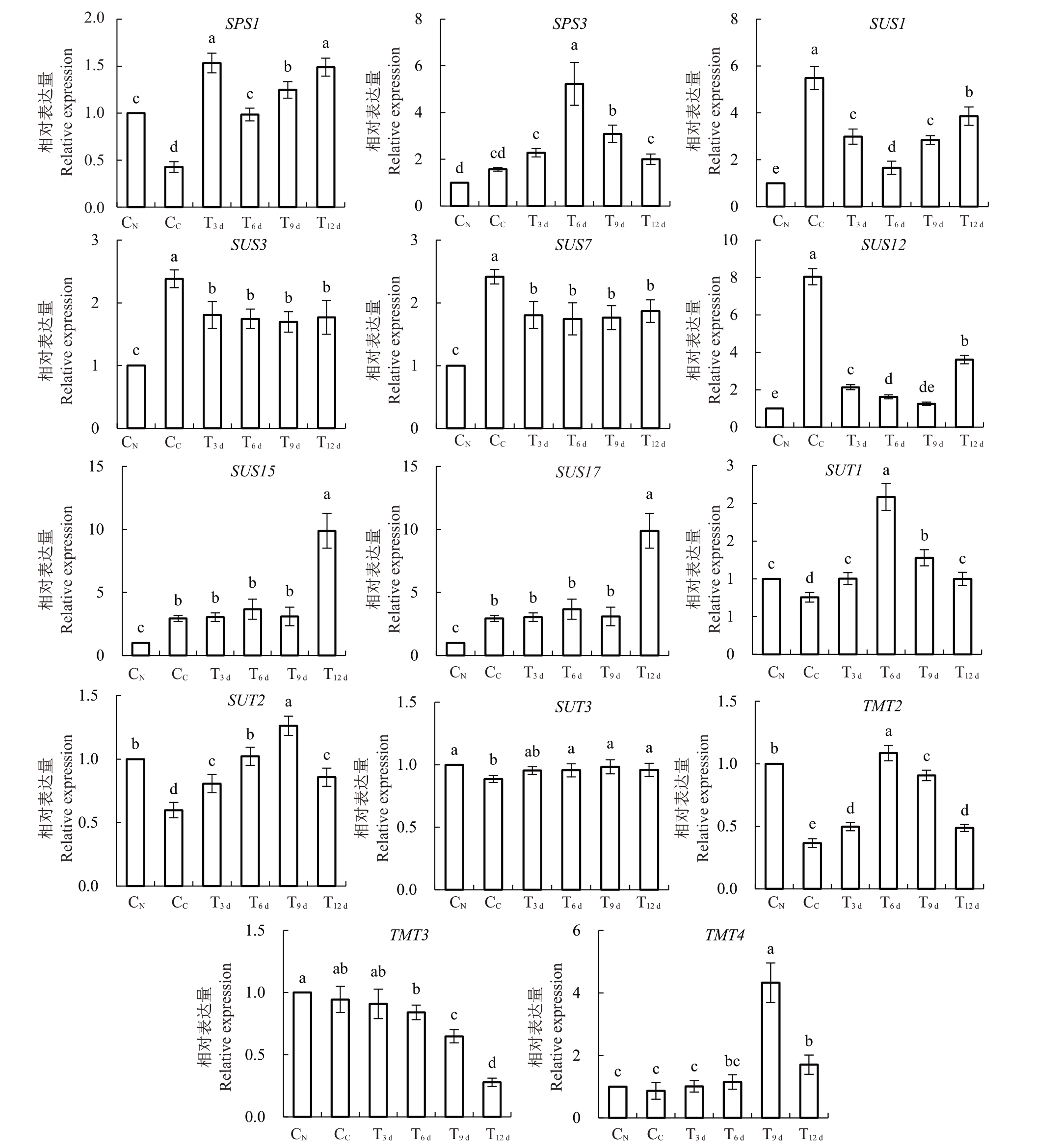
图7 砀山酥梨正常叶、黄化叶与FeSO4处理不同时期黄化叶内蔗糖代谢基因相对表达量
Fig.7 Relative expression of sucrose metabolic genes in normal leaves,chlorotic leaves and chlorotic leaves in different periods with FeSO4 application for Dangshan Suli pear
其次,就蔗糖转运基因而言,CN叶内SUT1/2/3表达量均显著均低于CC。其中SUT1/2 表达量先升后降,分别于T6 d、T9 d达到最高,其各时期表达量均较CC显著升高。而T3 d叶内SUT3 表达量与CC无显著差异,但T3 d~T9 d叶内表达量也显著高于CC。
再者,就液泡膜单糖转运蛋白基因而言,CN叶内TMT2表达量显著高于CC,TMT3/4表达量在两者之间无显著差异。T3 d~T12 d叶内TMT2 表达量均较CC显著上升而表现为先升后降,并于T3 d叶内达到最高;TMT3 表现为T9 d、T12 d叶内表达量较CC显著下降,TMT4则表现为T9 d、T12 d叶内表达量较CC显著增加,其他时期各基因表达量虽有变化,但均与CC无显著性差异。
因此,虽然FeSO4组(T3 d~T12 d)蔗糖含量与CC无显著性差异,但FeSO4处理后显著上升的SPS1/3 说明了该过程中蔗糖合成过程的加剧,因此,在该过程中,SUS1/3/7/12 可能发挥着催化蔗糖分解的功能,且在SPS 和SUS 的共同作用下,叶内蔗糖含量处于相对稳定状态,且该时期内显著增加的果糖含量也与之相符。与此同时,显著增加的蔗糖转运蛋白SUT1/2/3、液泡膜单糖转运蛋白TMT2/4也可能说明蔗糖向其他生长点的运输。
3 讨 论
Fe2+参与卟啉合成,并影响叶绿素代谢[22]。研究表明,叶面喷施铁肥可缓解柑橘幼叶失绿[23],外源Fe2+处理也可显著提高砂梨幼叶叶绿素含量[24]。说明Fe2+有利于叶绿素积累。本试验中,T3 d~T12 d叶内叶绿素含量均显著高于CC,说明Fe2+促进了其叶绿素合成,与前人研究结果相符。
叶绿素荧光参数可反映梨树的光合状况。其F0、Fv/Fm、Y(Ⅱ)、qP 和Fv/F0、Fm、NPQ 和Y(NPQ)值分别反映了PSⅡ反应中心的受损程度、植物潜在的最大光能转换率、实际光能转换效率、PSⅡ光化学反应中心活性、光抑制和植物光保护能力[25-26]。本研究中,CC叶内F0值显著高于CN,说明黄化叶内PSⅡ反应中心受损。而T3 d~T12 d叶内F0值较CC显著下降,说明FeSO4处理修复了PSⅡ反应中心;CC叶内显著低于CN的Fv/Fm、Y(Ⅱ)值说明黄化叶的光能转换效率较低,而T6 d~T9 d叶内Fv/Fm、Y(Ⅱ)值均较CC显著升高,说明FeSO4处理提升了其光能转换率。其次,CN叶内qP、Fv/F0显著高于CC,而Fm值显著高于CC,说明黄化叶化学反应中心活性低,光抑制现象严重。而FeSO4处理后,其qP、Fv/F0整体显著增加,Fm值显著降低,说明FeSO4处理解除了光抑制,并增强了其PSⅡ光化学反应中心活性和碳同化能力;而CC叶内显著高于CN的NPQ、Y(NPQ)值可能是叶片为保护逆境胁迫下PSⅡ光化学反应中心进一步受损的应激生理所致。本试验中,T3 d叶内NPQ值较CC显著增加,并于T6 d~T12 d显著下降至CN水平,而Y(NPQ)也于T6 d~T9 d叶内显著增加,并于T12 d显著下降。说明FeSO4增强了黄化叶内转换耗散过剩光能为热的光保护作用,同时也减弱了逆境胁迫信号。与此同时,T6 d~T12 d叶内ETR的上升可能与该时期的活跃的PSⅠ电子传递和qP活性的增强相关。该研究结果与赵志军等[27]对缺铁苹果黄化叶片复绿光合特性的研究结果相一致。
光合特性体现了叶片光合作用的强弱[28]。研究表明,叶绿素含量低的茶树叶内,其Pn、Gs和Tr值较低,Ci值较高[29],与本研究中叶绿素含量较低的CC叶内各指标变化相符,由此说明黄化叶内气体交换、暗反应减缓,光合作用受阻。而FeSO4处理后黄化叶内显著升高的Pn、Gs、Ts、WUE 和Ls值,反映了FeSO4可促进黄化叶的气孔开放,加速叶片的蒸腾,促进水分利用而增强CO2的交换和同化能力,降低其Ci值,从而提高净光合速率,增强光合作用。
光合作用影响着光合同化产物的积累[30]。水稻叶内叶绿素含量的提升,显著增加了其蔗糖、总糖和淀粉含量[31]。本试验中,CN叶内果糖、山梨醇含量显著高于CC,外源FeSO4处理T3 d~T12 d果糖与T6 d~T9 d叶内山梨醇含量均显著增加,说明该时期叶片的同化能力不断增强。同时,试验结果显示T6 d~T9 d叶内蔗糖含量无显著性差异,可能与增强的蔗糖降解过程相关。因此,为进一步明确黄化叶片光合作用增强的具体机制以及探讨叶内山梨醇、蔗糖分配调控叶内光合同化产物积累的模式,本研究分析了相关途径中相关基因的表达。
研究表明,小麦叶绿素缺失突变体mt135 内光合基因psb、psa表达量显著降低,其光合功能完全丧失[32],而Rubisco活化酶活性及其编码基因的表达均正向调控黄化叶内光合作用[33],说明光合基因相对表达量影响着植物的光合能力。本试验中,CN叶内PS Ⅰsubunit Ⅱ/Ⅳ/psaK、PS ⅡpsbD 和F- Type ATPase Ⅳ均显著高于CC,而T3 d~T9 d叶内该基因表达量均较CC显著升高。其Rubisco activase Ⅳ/Ⅴ、Cytb6f II 和Chl a-b P4/CP24/CP 29.1 表达量也均显著高于CC,而T6 d~T12 d叶内的该基因表达量也显著增加。说明CN叶内高光合性能与其光合基因高表达相关,与现有研究报道相符。而FeSO4处理通过促进黄化叶PSⅠsubunit Ⅱ/Ⅳ/psaK基因的表达,形成了更多色素蛋白复合物,加速催化了叶内电子的传递。而其升高的Rubisco activaseⅣ/Ⅴ、Cytb6f Ⅱ、Chl a-b P4/CP24/CP 29.1 表达则分别增强了其CO2固定能力和类囊体膜中电子和质子的转移能力,从而提升了整叶的光合能力。除此之外,Rubisco活化酶还具有ATP 水解酶活性[34],因此其还可能与F-Type ATPase Ⅳ编码蛋白共同作用,为叶内光合代谢持续供能,从而维持光合性能。
研究认为,S6PDH 负责催化6-磷酸山梨醇和6-磷酸葡萄糖的可逆反应,且更倾向于山梨醇的积累,而SDH 可催化山梨醇和果糖、葡萄糖之间相互转化[10],其转运基因SOT 的表达也可促进果实山梨醇的积累[35]。本试验中,CN叶内S6PDH与CC无显著变化,说明CN与CC之间山梨醇的初始合成无显著差异。但CC叶内较CN显著增加的SDH1表达量,可能说明了山梨醇向葡萄糖的转化,这与CC内低水平的山梨醇含量和与CN 无显著差异的葡萄糖水平相符。而FeSO4处理后,S6PDH的显著上升,说明山梨醇合成的增强,而该处理内SDH 基因表达量的增加,加速了山梨醇向果糖、葡萄糖的合成,造成果糖、葡萄糖的积累;其次,CC叶内显著上升的SOT2/4/10/15/等基因的表达,可能说明了山梨醇向果实及其他生长点的运输,以保证生长点的正常发育,继而导致其山梨醇含量降低。而各基因表达量随着FeSO4处理时间延长先升后降,表明山梨醇由“源”到“库”的转运活性减弱,以确保叶内充足的同化产物,为增强的光合作用供能。
研究表明SPS促进组织内蔗糖的合成反应属于不可逆反应,而SUS 既可以合成蔗糖,也可以分解蔗糖[14]。本试验中,增强的光合作用、增加的山梨醇含量和SPS1/3 基因表达量的显著增加均说明了蔗糖合成能力的增强,因此,SUS在蔗糖合成过程中可能主要负责催化蔗糖的分解。加之CC叶内高表达的蔗糖转运基因SUT1/2/3、TMT2/4,说明更多的蔗糖经其作用而转运至其他生长点。最终,在SPS、SS、SUT 以及TMT 的共同作用下,叶内蔗糖含量处于一个动态平衡过程中。
4 结 论
FeSO4处理可促进砀山酥梨黄化叶内叶绿素的合成,促进其光合基因PSⅠsubunit Ⅱ/Ⅳ、F-Type ATPase Ⅳ、Rubisco activase Ⅳ、Chl a-b P4/CP2等的表达,进而整体提升其叶片光合参数Pn、Gs、Tr、WUE及Ls值和叶绿素荧光特性F0、Fv/Fm、Y(II)、Fv/F0及ETR值,减轻PSⅡ反应中心的受损程度,在提高其光能转换效率以及光保护能力的同时,缓解了光抑制,使植物吸收的光能最大程度地用于光合电子传递,提升其CO2 同化能力,并通过促进山梨醇代谢基因S6PDH1、SDH2/3、SOT8/14/15/26/30 和蔗糖代谢基因SPS1/3、SUS、SUT1/2/3、TMT2 的表达,而增强山梨醇合成、转运,促进蔗糖合成、降解与转运,整体提升叶内山梨醇和果糖的含量。
[1]联合国粮农组织数据库[OL].2019.https://www.fao.org/faostat/en/#data/QCL.Food and Agriculture Organization of the United Nations (FAOSTAT)[OL].2019.https://www.fao.org/faostat/en/#data/QCL.
[2]曹晓艳,谭博,苏玉芳,沈成山,徐宫善.果树黄化病研究进展[J].北方果树,2014(2):1-3.CAO Xiaoyan,TAN Bo,SU Yufang,SHEN Chengshan,XU Gongshan.Research advances on yellow leaf disease of fruit trees[J].Northern Fruits,2014(2):1-3.
[3]LI J,CAO X M,JIA X C,LIU L Y,CAO H W,QIN W Q,LI M.Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L.[J].Frontiers in Plant Science,2021,12:710093.
[4]赵天宏,沈秀瑛,杨德光,马秀芳.水分胁迫及复水对玉米叶片叶绿素含量和光合作用的影响[J].杂粮作物,2003,23(1):33-35.ZHAO Tianhong,SHEN Xiuying,YANG Deguang,MA Xiufang.Effects on chlorophyll content and photosynthetic rate of maize leaves under water stress and rewatering[J].Rain Fed Crops,2003,23(1):33-35.
[5]AHAMMED G J,XU W,LIU A R,CHEN S C.COMT1 Silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato[J].Frontiers in Plant Science,2018,9:998.
[6]赵海新.碱胁迫对水稻叶绿素及叶片脯氨酸和可溶性糖含量的影响[J].作物杂志,2020(1):98-102.ZHAO Haixin.Effects of alkali stress on chlorophyll and the contents of proline and soluble sugar in rice[J].Crops,2020(1):98-102.
[7]YUAN Y J,MEI L H,WU M B,WEI W,SHAN W,GONG Z H,ZHANG Q,YANG F Q,YAN F,ZHANG Q,LUO Y Q,XU X,ZHANG W F,MIAO M J,LU W J,LI Z G,DENG W.SlARF10, an auxin response factor, is involved in chlorophyll and sugar accumulation during tomato fruit development[J].Journal of Experimental Botany,2018,69(22):5507-5518.
[8]林琭,汤昀,闫万丽,张纪涛,籍增顺.不同水势对温室黄瓜蒸腾及室内湿度的影响[J].山西农业科学,2015,43(8):953-958.LIN Jue,TANG Yun,YAN Wanli,ZHANG Jitao,JI Zengshun.Effects of different water potential on transpiration of cucumber and humidity in greenhouse[J].Journal of Shanxi Agricultural Sciences,2015,43(8):953-958.
[9]SUZUE Y,TSULUDA M,HATANO S,KANANYAMA Y,YAMADA K,SHIRATAKE K,YAMAKI S.Changes in the activity and gene expression of sorbitol-and sucrose-related enzymes associated with leaf development of‘La France’pear[J].Journal of the Japanese Society for Horticultural Science,2006,75(1):45-50.
[10]刘政,安莉园,林世华,秦仲麒,伍涛,李先明,涂俊凡,杨夫臣,朱红艳,杨立.梨树山梨醇代谢及其调控因子研究进展[J].中国南方果树,2018,47(4):165-168.LIU Zheng,AN Liyuan,LIN Shihua,QIN Zhongqi,WU Tao,LI Xianming,TU Junfan,YANG Fuchen,ZHU Hongyan,YANG Li.Research progress on sorbitol metabolism and its regulatory factors in pear trees[J].South China Fruits,2018,47(4):165-168.
[11]YAMAKI S.Metabolism and accumulation of sugars translocated to fruit and their regulation[J].Journal of the Japanese Society for Horticultural Science,2010,79(1):1-15.
[12]IIDA M,BANTOG N A,YAMADA K,SHIRATAKE K,YAMAKI S.Sorbitol and other sugar-induced expressions of the NAD+-dependent sorbitol dehydrogenase gene in Japanese pear fruit[J].Journal of the American Society for Horticultural Science,2004,129(6):870-875.
[13]WANG X X,FU X L,CHEN M,HUAN L,LIU W H,GAO Y G,XIAO W,CHEN X D,LI L,GAO D S.Sugar metabolism changes in response to the ultraviolet B irradiation of peach(Prunus persica L.)[J].BioRxiv,2017,1(4):145870.
[14]SUZUKI A,KANAYAMA Y,YAMAKI S.Occurrence of two sucrose synthase isozymes during maturation of japanese pear fruit[J].Journal of the American Society for Horticultural Science,1996,121(5):943-947.
[15]白雪梅,张立军,吴晓丹,胡凯,阮燕晔.植物蔗糖转运蛋白[J].植物生理学通讯,2006,42(6):1195-1202.BAI Xuemei,ZHANG Lijun,WUXiaodan,HU Kai,RUAN Yanye.Sucrose transporters in plants[J].Plant Physiology Communications,2006,42(6):1195-1202.
[16]MARTINOIA E,MEYER S,DE ANGELI A,NAGY R.Vacuolar transporters in their physiological context[J].Annual Review of Plant Biology,2012,63(1):183-213.
[17]贾兵,郭国凌,余桃,王友煜,叶振风,刘莉,刘普,衡伟.‘砀山酥梨’黄化叶复绿过程中铁和氮的增益作用[J].果树学报,2020,37(11):1676-1686.JIA Bing,GUO Guoling,YU Tao,WANG Youyu,YE Zhenfeng,LIU Li,LIU Pu,HENG Wei.Reinforced effect of iron and nitrogen in the process of chlorotic leaves regreening of‘Dangshansuli’pear[J].Journal of Fruit Science,2020,37(11):1676-1686.
[18]张义顺,黄霞,陈云凤.植物生理学实验教程[J].北京:高等教育出版社,2009,139-140.ZHANG Yishun,HUANG Xia,CHEN Yunfeng.Experimental tutorial of plant physiology[J].Beijing:Higher Education Press,2009,139-140.
[19]谢鹏,蔚露,牛自勉,韩苹苹,李志强.间伐果园梨树叶片叶绿素荧光的部位间差异[J].山西农业科学,2015,43(6):682-685.XIE Peng,YU Lu,NIU Zimian,HAN Pingping,LI Zhiqiang.The difference of chlorophyll fluorescence in leaves of different positions of pear tree in tree-thinning orchard[J].Journal of ShanxiAgricultural Sciences,2015,43(6):682-685.
[20]徐新翔,侯昕,王芬,贾志航,葛顺峰,姜远茂.低钾胁迫对苹果砧木M9T337 幼苗光合荧光特性及13C 吸收分配的影响[J].园艺学报,2020,47(3):529-540.XU Xinxiang,HOU Xin,WANG Fen,JIA Zhihang,GE Shunfeng,JIANG Yuanmao.Effects of low potassium stress on photosynthetic fluorescence characteristics and13C absorption and distribution of M9T337 seedlings[J].Acta Horticulturae Sinica,2020,47(3):529-540.
[21]程贝,王卫华,吴忠东.非均匀盐胁迫对番茄幼苗耐盐性的影响[J].园艺学报,2018,45(5):887-896.CHENG Bei,WANG Weihua,WU Zhongdong.Effects of nonuniform salt stress on salt tolerance of tomato seedlings[J].Acta Horticulturae Sinica,2018,45(5):887-896.
[22]KOBAYASHI T,NISHIZAWA N K.Iron uptake, translocation and regulation in higher plants[J].Annual Review of Plant Biology,2012,63(1):131-152.
[23]马晓丽,刘雪峰,袁项成,向苹苇,曾德刚.树干输液和叶面喷施铁肥对缺铁黄化柑橘的矫正效果[J].安徽农业科学,2018,46(10):115-117.MA Xiaoli,LIU Xuefeng,YUAN Xiangcheng,XIANG Pingwei,ZENG Degang.Correction effect of iron fertilizer application by spraying on the leaf and trunk-injection on iron deficiency chlorosis in citrus trees[J].Journal of Anhui Agricultural Sciences,2018,46(10):115-117.
[24]李含芬,马春晖.缺铁胁迫对沙梨叶片黄化、再复绿和根系中Fe(Ⅲ)还原酶活性的影响[J].青岛农业大学学报(自然科学版),2009,26(3):203-206.LI Hanfen,MA Chunhui.Effect of Fe-deficiency stress on the leaf chlorosis,regreening and root ferric-chelate reductase activity of Pyrus pyrifolia Nakai[J].Journal of Qingdao Agricultural University(Natural Science),2009,26(3):203-206.
[25]DEMMIG B,WINTER K,KRÜGER A,CZYGAN F C.Photoinhibition and zeaxanthin formation in intact leaves:A possible role of the xanthophy II cycle in the dissipation of excess light energy[J].Plant Physiology,1987,84(2):218-224.
[26]MAXWELL K,JOHNSON G N.Chlorophyll fluorescence-a practical guide[J].Journal of Experimental Botany,2000,51(345):659-668.
[27]赵志军,刘子英,高一宁,崔美香,刘贵巧,薛进军.铁肥虹吸输液对缺铁失绿苹果叶片光合生理指标和荧光参数的影响[J].植物营养与肥料学报,2013,19(4):878-884.ZHAO Zhijun,LIU Ziying,GAO Yining,CUI Meixiang,LIU Guiqiao,XUE Jinjun.Effect of iron fertilizer siphon transfusion on photosynthetic physiological indices and chlorophyll fluorescence parameters of iron deficiency chlorosis in apple trees[J].Journal of Plant Nutrition and Fertilizer,2013,19(4):878-884.
[28]周葱.缺铁黄化对‘砀山酥梨’叶片光合特性、氮代谢相关酶活性及基因表达的影响[D].合肥:安徽农业大学,2015.ZHOU Cong.Effects of iron stress on photosynthetic characters,related enzyme activity and genes expression of nitrogen metabolism in‘Dangshansuli’leaves[D].Hefei:Anhui Agricultural University,2015.
[29]吴焕焕,张虹,任志红,肖文敏,孙海伟,窦玲.叶面喷硒对茶树叶片光合特性及产量的影响[J].山东农业科学,2021,53(6):64-68.WU Huanhuan,ZHANG Hong,REN Zhihong,XIAO Wenmin,SUN Haiwei,DOU Ling.Effects of foliar application of nanoselenium on photosynthetic characteristics and yield of tea[J].Shandong Agricultural Sciences,2021,53(6):64-68.
[30]李卫东.桃库源关系中源叶光合作用及其碳水化合物代谢的研究[D].北京:中国农业大学,2005.LI Weidong.Photosynthesis and carbohydrate metabolism in source leaves in response to sink-source manipulations in peach Prunus persica(L.)Batsch)[D].Beijing:China Agricultural University,2005.
[31]吴殿星,舒庆尧,夏英武,夏建峰.水稻转绿型白化突变系W25返白复绿过程中叶片的生理变化[J].浙江农业大学学报,1999,25(1):1-4.WU Dianxing,SHU Qingyao,XIA Yingwu,XIA Jianfeng.Study on physiological changes in leaves of a rice greenable albino mutant line W25 during albinism-greenism process in late growth period[J].Journal of Zhejiang Agricultural University,1999,25(1):1-4.
[32]夏家平,郭会君,谢永盾,赵林姝,古佳玉,赵世荣,李军辉,刘录祥.小麦叶绿素缺失突变体Mt135 的叶绿体基因差异表达分析[J].作物学报,2012,38(11):2122-2130.XIA Jiaping,GUO Huijun,XIE Yongdun,ZHAO Linshu,GU Jiayu,ZHAO Shirong,LI Junhui,LIU Luxiang.Differential expression of chloroplast genes in chlorophyll-deficient wheat mutant Mt135 derived from space mutagenesis[J].Acta Agronomica Sinica,2012,38(11):2122-2130.
[33]潘璐,刘杰才,李晓静,宋阳,张之为,马立国,崔世茂.高温和加富CO2 温室中黄瓜Rubisco 活化酶与光合作用的关系[J].园艺学报,2014,41(8):1591-1600.PAN Lu,LIU Jiecai,LI Xiaojing,SONG Yang,ZHANG Zhiwei,MA Liguo,CUI Shimao.Correlation between rubisco activase and photosynthesis of cucumber in greenhouse under high temperature and elevated CO2[J].Acta Horticulturae Sinica,2014,41(8):1591-1600.
[34]KALLIS R P,EWY R G,PORTIS JR A R.Alteration of the adenine nucleotide response and increased Rubisco activation activity of Arabidopsis Rubisco activase by sited-directed mutagenesis[J].Plant Physiology,2000,123(3):1077-1086.
[35]戴美松,徐飞,施泽彬,徐昌杰.砂梨山梨醇转运蛋白(SOT)基因家族成员表达特性及在果实糖积累中的作用初探[J].园艺学报,2015,42(8):1457-1466.DAI Meisong,XU Fei,SHI Zebin,XU Changjie.Preliminary study on expression characteristics of sorbitol transporter (SOT)gene family and the role in sugar accumulation in Pyrus pyrifolia fruits[J].Acta Horticulturae Sinica,2015,42(8):1457-1466.