果实无核是柑橘育种目标之一,而无核育种也是实现我国柑橘果实品质提升的重要手段。我国柑橘资源丰富,地方品种繁多,但多数品种果实有核甚至多核,是导致近年来其市场竞争力降低和种植面积骤减的主要原因[1]。三倍体一般雌雄配子不育,有性杂交难以形成种子,是天然的无核类型。实践证明,二倍体与四倍体倍性杂交创制三倍体是培育无核柑橘的最有效途径[2]。但柑橘四倍体资源缺乏,极大限制了倍性杂交在柑橘三倍体育种中的应用。
除了柚、枸橼和少数宽皮橘外,柑橘多数品种具有珠心多胚现象[3]。自然条件下,多胚种子珠心细胞存在一定概率的基因组加倍现象,种子萌发后可产生四倍体植株(也称为双二倍体),为发掘柑橘四倍体提供了一条便捷途径。Aleza 等[4]通过实生播种和流式细胞仪对实生苗倍性分析,从30余个多胚性柑橘品种的4442 株实生幼苗筛中选获得80 株四倍体。但直接利用流式细胞仪对实生幼苗进行倍性检测筛选四倍体,工作量大,效率低。针对上述问题,梁武军等[5]基于多倍体具备的独特形态学特征,提出了一种利用植株形态特征发掘柑橘四倍体的方法,即通过观察植株叶片形态特征先筛选疑似多倍体,再用流式细胞仪对其倍性快速鉴定,大大节约了直接对所有幼苗进行倍性分析的时间和劳动成本。在此基础上,周锐等[6]通过优化播种过程和形态初选方法,将柑橘四倍体发掘平均准确率由35%提高至85%以上,过程缩短至40 d以内。利用该方法,目前已成功获得红橘[5]、金柑[6]、甜橙[6-7]、枳橙[8]等四倍体植株。
常山胡柚、温岭高橙、新会橙是我国地方特色品种,栽培历史悠久,风味独特,但种子较多;橘血橙杂种是克里曼丁橘与血橙的有性杂交后代优良单株,种子多胚,果实花青素含量极高;衢州香橙、酸橙是我国柑橘栽培的重要砧木类型。发掘上述品种资源的四倍体,可以为培育柑橘三倍体无核新品种提供优良的四倍体亲本,以及为矮化、适应性强的四倍体砧木培育提供珍贵的种质材料,具有重要的研究意义和实践价值。
1 材料和方法
1.1 实验材料
常山胡柚(Citrus aurantium)、温岭高橙(C.grandis×C.sinensis.)、新会橙(C.sinensis)和衢州香橙(C.junos Sieb.ex Tan.)成熟果实2020年秋季分别采自浙江省常山县、浙江省温岭市、广东省肇庆市德庆县柑橘研究所、陕西省陕南柑橘综合试验站;橘血橙杂种(C.clementina×C.sinensis)和酸橙(C.aurantium)成熟果实2020 年秋季采自华中农业大学柑橘种质资源圃。
1.2 种子催芽播种和实生幼苗形态初选
种子催芽播种、实生幼苗形态学初选参考周锐等[6]的方法。种子剥出并用清水洗净后,将其置于1 mol·L-1 NaOH溶液浸泡15 min去除果胶。人工剥去外种皮后,将其均匀铺于垫有湿滤纸的培养皿,置于28 ℃恒温箱(暗培养)催芽。胚根萌发后,将其带至生长室播种于营养钵(约100 粒/钵)。幼苗长至有2~3 枚真叶时,利用“观根辨叶看油胞”法筛选疑似四倍体。生长室培养条件:温度(25±1)℃;光照16 h。
种子胚数统计:剥去种子内外种皮,在Olympus SZX7体视显微镜下统计胚数并拍照,每个品种至少统计10粒种子。
1.3 倍性鉴定
用流式细胞仪和根尖染色体计数2种方法检测植株倍性。流式细胞仪(Cyflow space, Sysmex, Japan)倍性检测参考解凯东等[9]的方法。根尖染色体计数参考夏强明[10]的方法并适当修改。取四倍体及其二倍体亲本生长旺盛的根尖,饱和对二氯苯溶液室温下预处理3 h 后,用新鲜的卡诺固定液(V 乙醇∶V 乙酸=3∶1)固定24 h,最后转移至75%(φ)乙醇溶液4 ℃保存备用。制片前,根尖用酶液(1%果胶酶(Sigma-aldrich)、2%纤维素酶‘Onozuka’(R-10,Yakult)和1%果胶酶(Y-23,Yakult))37 ℃水浴处理90 min后,用火焰干燥法进行染色体制片,并用荧光显微镜(Imager.M2,Zeiss,Germany)镜检,染色体图像用ZEN软件采集。
四倍体群体发生频率/%=四倍体植株数/群体植株总数×100。
1.4 四倍体幼苗根、茎和叶等形态指标测定
以二倍体植株为对照,测量相同发育时期(约播种后30~50 d)四倍体实生幼苗形态指标,包括主根长、根粗、须根数目;茎粗;叶长、叶宽及叶片厚度。主根长用直尺测量;主根粗(根尖1 cm处)、茎粗(根颈1 cm 处)、叶长、叶宽及叶片厚度用游标卡尺测量。每个品种的二倍体实生苗测量10株,对应四倍体除温岭高橙、新会橙、橘血橙杂种测量所有株数外,其余品种均测量6株以上。
1.5 植株移栽
形态指标测定结束后,将四倍体植株及其二倍体分别独立移栽至装有营养土(V 进口土∶V 基质土∶V 蛭石∶V 珍珠岩=4∶4∶1∶1)的营养钵,继续置于生长室培养。待幼苗长至7~8枚真叶时,将其转移至温室,期间保证正常肥水管理。
1.6 SSR分子鉴定
基因组DNA 提取和SSR 分子标记分别参考Cheng 等[11]和周锐等[6]的方法。筛选的11 对多态性SSR引物(表1)由生工生物工程(上海)股份有限公司合成。PCR 反应体系10 μL:2× PCR mix 5 μL,正、反向引物各0.25 μL(10 μmol·L-1),DNA模板1 μL,无菌水3.5 μL。PCR 反应在ProFlex PCR 仪(ABI,USA)进行,扩增程序:95 ℃预变性5 min,95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸10 s,35 个循环,72 ℃延伸5 min,4 ℃保存。PCR产物由全自动毛细管电泳系统(QI Axcel Advanced,QIAGEN)电泳分离。
表1 SSR 引物序列
Table 1 Sequence of SSR primers

引物名称Primer name MEST256 MEST131 MEST86 MEST88 MEST56 mCrCI03D12a mCrCIR03G05 mCrCIR07D06 mCrCIR01F08a mCrCI02D04b Ma2_1480引物序列Primer Sequence F:CATTAAAATATCCGTGCCGC;R:GAGCAAGTGCGTTGTTGTGT F:TACCTCCACGTGTCAAACCA;R:GCTGTCACGTTGGGTGTATG F:CCTCTCTGGCTTCTGGATTG;R:CCAACTGACACTAATCCTCTTCC F:GCCTGTTTGCTTTCTCTTTCTC;R:ATGAGAGCCAAGAGCACGAT F:AGTCCGCCTTTGCTTTTTCT;R:GGTGCAAAAGAGAGCGAGAG F:GCCATAAGCCCTTTCT;R:CCCACAACCATCACC F:CCACACAGGCAGACA;R:CCTTGGAGGAGCTTTAC F:CCTTTTCACAGTTTGCTAT;R:TCAATTCCTCTAGTGTGTGT F:ATGAGCTAAAGAGAAGAGG;R:GGACTCAACACAACACAA F:AGCAAACCCCACAAC;R:CTCTCTTTCCCCATTAGA F:CAATCACAGGAGCGACTTCA;R:CTCAATTCAGCAAACCGACA文献Reference[12][12][12][12][4][4][13][13][14][15][16]
2 结果与分析
2.1 常山胡柚等6个品种的种子均为多胚性种子
从常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙、酸橙的80、20、25、55、5、136个成熟果实中,剥取获得736、62、328、452、164和1273粒种子,平均单果种子分别为9.2、3.1、13.1、8.2、32.8、9.4 粒。为调查6 个品种种子的胚性及其多胚程度,取每个品种10粒种子统计胚数。结果显示(图1),常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙、酸橙平均每粒种子包含1.4、3.4、8.0、7.1、5.6、5.8 个胚,表明6个品种均为多胚类型且多胚性程度依次为:新会橙>橘血橙杂种>酸橙>衢州香橙>温岭高橙>常山胡柚。
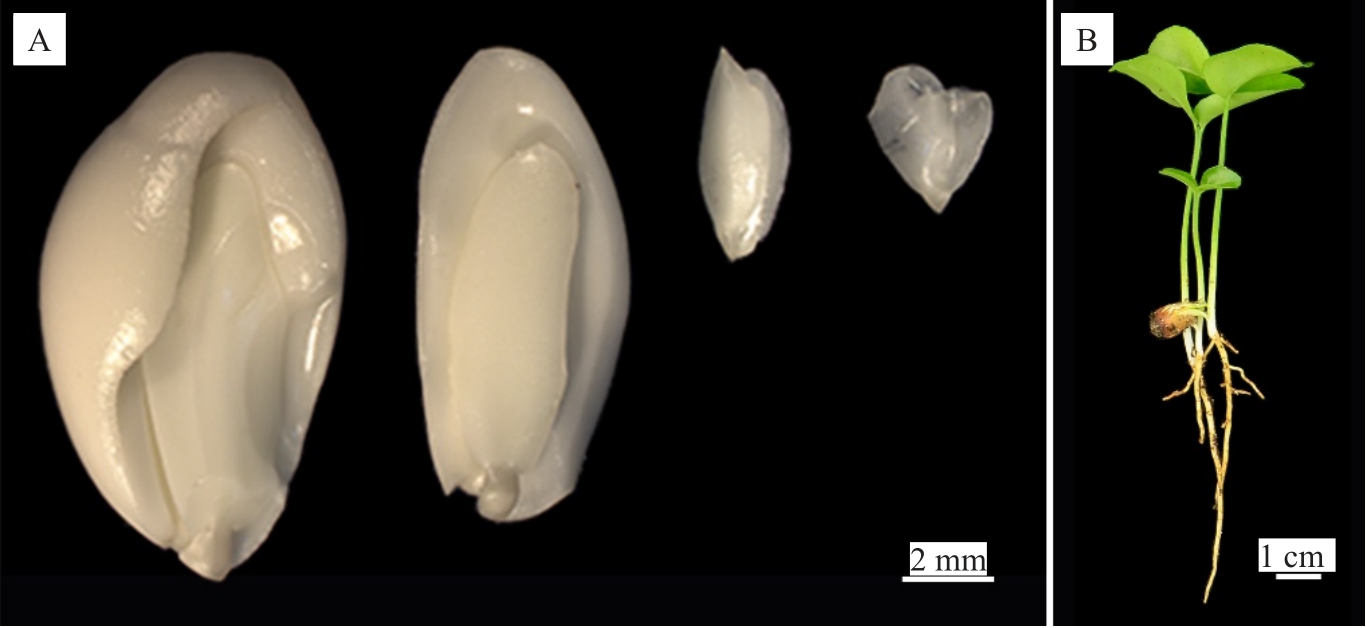
图1 酸橙种子的多胚现象
Fig.1 Polyembryony in Sour orange seeds
A.酸橙多胚性种子;B.酸橙一粒种子长出3 株幼苗。
A.Polyembryonic seeds of Sour orange;B.Three seedlings generated from one seed of Sour orange.
2.2 形态学初选结合倍性分析发掘四倍体幼苗50余株
将常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙和酸橙的种子通过催芽播种,分别获得890、72、373、709、303 和1992 株实生幼苗。依据多倍体形态特征(根粗、须根少;叶片厚、颜色深、叶形指数小;油胞密度低等),从常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙和酸橙分别筛选获得24、4、7、11、10 和88 株疑似多倍体(表2)。用流式细胞仪和根尖染色体计数对疑似多倍体进行倍性鉴定(图2),获得常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙和酸橙四倍体14株、2株、4株、3株、6株和24 株。常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙和酸橙的形态初选准确率分别为58.3%、50.0%、57.1%、27.3%、60.0%和27.3%;四倍体群体发生频率分别为1.57%、2.78%、1.07%、0.42%、1.98%和1.20%(表2)。
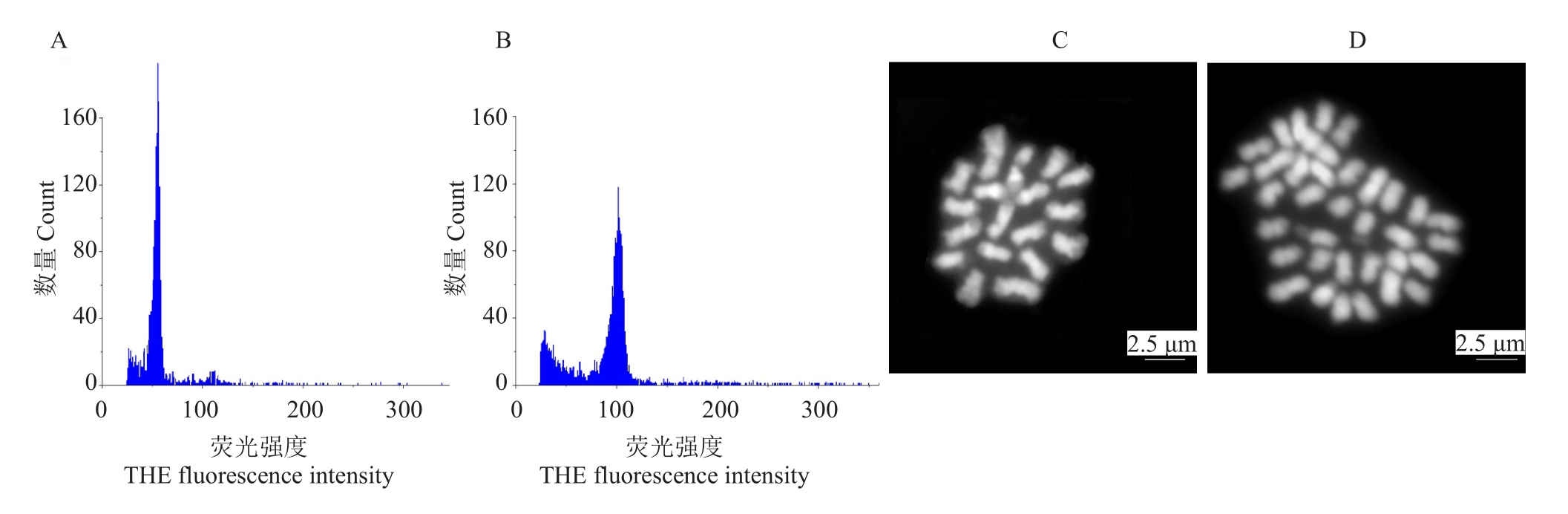
图2 流式细胞仪和根尖染色体压片鉴定酸橙疑似四倍体植株倍性
Fig.2 Ploidy analysis of putative tetraploids screened from Sour orange using flow cytometry and shoot tip chromosome counting technique
A-B.流式细胞仪倍性鉴定(A.二倍体;B.四倍体);C-D.根尖染色体计数(C.二倍体,2n=2x=18;D.四倍体,2n=4x=36)。
A-B.Ploidy histograms of the diploid and tetraploid plant determined by flow cytometry(A.Diploid;B.Tetraploid);C-D.Shoot tip chromosome counting(C.Diploid,2n=2x=18;D.Tetraploid,2n=4x=36).
表2 常山胡柚等6 个地方品种的四倍体发掘情况
Table 2 The tetraploid seedlings identified from six citrus polyembryonic genotypes
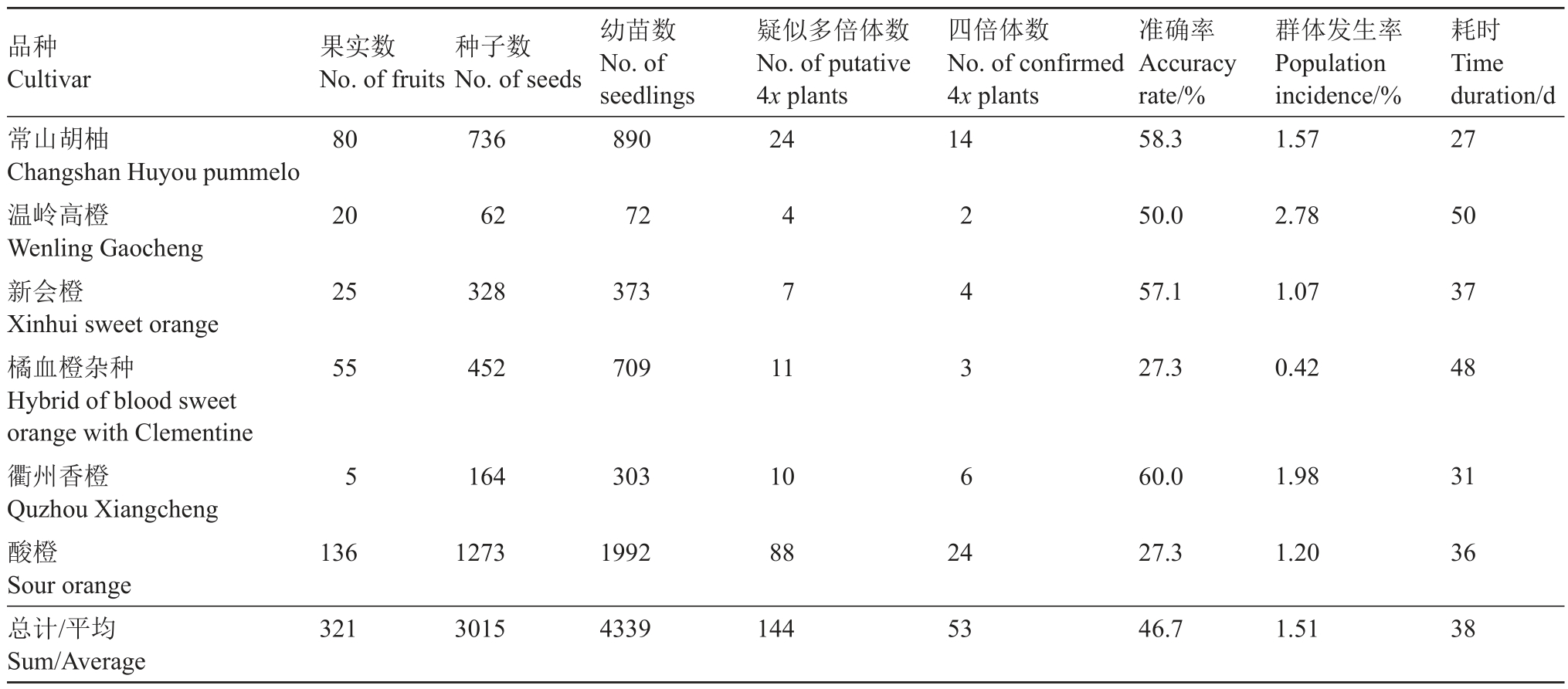
品种Cultivar常山胡柚Changshan Huyou pummelo温岭高橙Wenling Gaocheng新会橙Xinhui sweet orange橘血橙杂种Hybrid of blood sweet orange with Clementine衢州香橙Quzhou Xiangcheng酸橙Sour orange总计/平均Sum/Average果实数No.of fruits 80种子数No.of seeds 736幼苗数No.of seedlings 890疑似多倍体数No.of putative 4x plants 24四倍体数No.of confirmed 4x plants 14准确率Accuracy rate/%58.3群体发生率Population incidence/%1.57耗时Time duration/d 27 20 62 72 50.0 2.78 50 25 328 373 57.1 1.07 37 55 452 709 4 7 11 27.3 0.42 48 5 164 303 10 2 4 3 6 60.0 1.98 31 136 1273 1992 88 24 27.3 1.20 36 321 3015 4339 144 53 46.7 1.51 38
2.3 四倍体与二倍体幼苗根、茎和叶片形态差异明显
对获得的四倍体及相应二倍体实生幼苗的根、茎和叶片形态指标进行测量,结果表明,四倍体植株的主根长、侧根数均显著低于相应的二倍体(图3);四倍体根粗、叶片厚度显著高于二倍体;对于茎粗,温岭高橙和新会橙的四倍体植株显著低于二倍体,其余4 个品种的四倍体与二倍体之间差异不显著;对于叶形指数,温岭高橙的四倍体显著大于二倍体,橘血橙杂种的四倍体显著小于二倍体,其余4 个品种的四倍体与二倍体之间差异不显著(表3)。
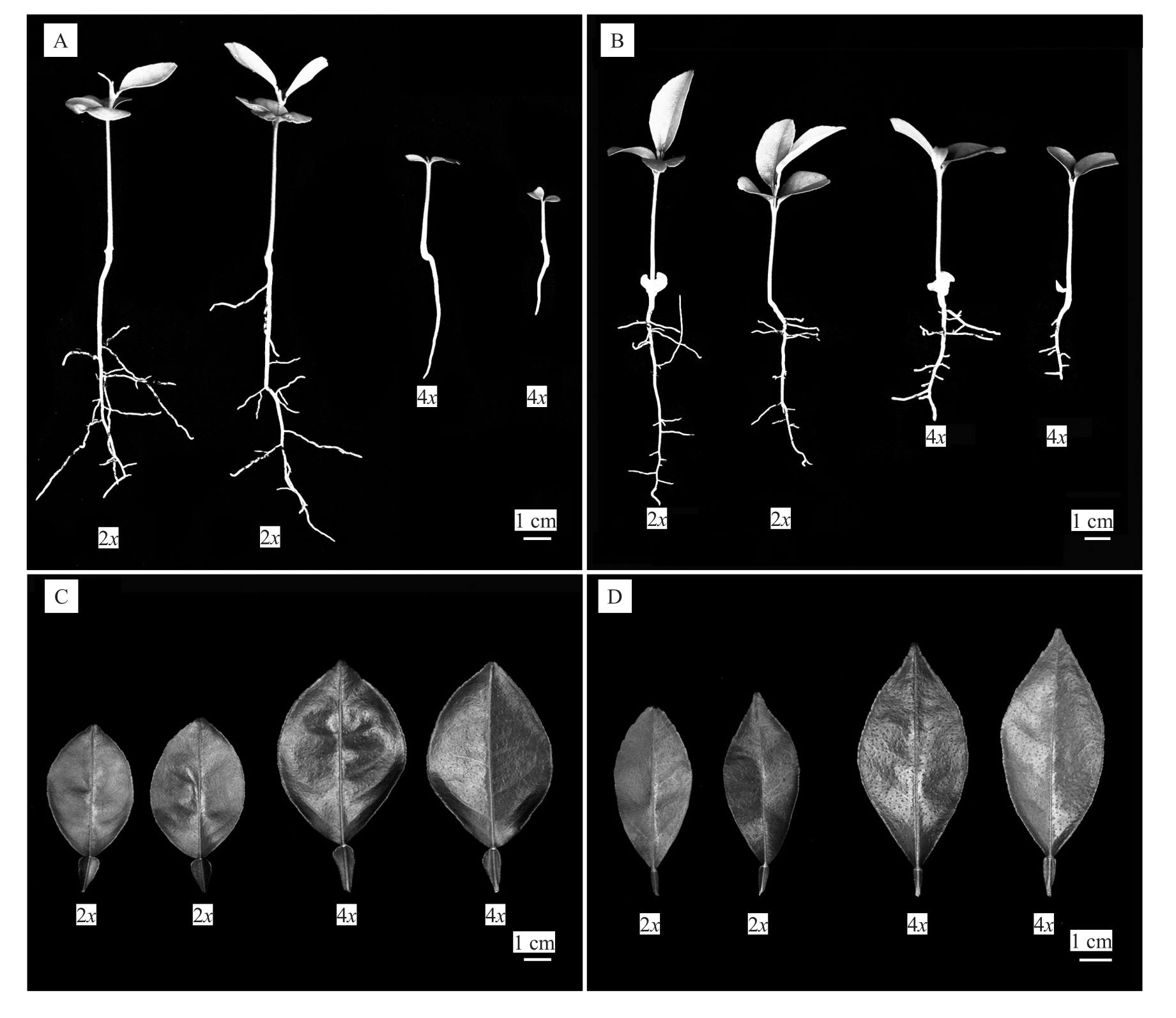
图3 温岭高橙和橘血橙杂种四倍体及其相应二倍体实生幼苗形态差异
Fig.3 Morphological difference between tetraploids and the corresponding diploids seedling of Wenling Gaocheng and Hybrid of blood sweet orange with Clementine
A.温岭高橙幼苗形态;B.橘血橙杂种幼苗形态;C.温岭高橙叶片形态;D.橘血橙杂种叶片形态。
A.Morphological difference between the diploid and tetraploid seedlings of Wenling Gaocheng; B.Hybrid of blood sweet orange with Clementine;C.Leaf morphological difference between the diploid and tetraploid seedlings of Wenling Gaocheng;D.Hybrid of blood sweet orange with Clementine.
表3 四倍体与二倍体的根、茎和叶片形态特征比较
Table 3 Root,stem and leaf morphological comparison between tetraploid and their corresponding diploid seedlings
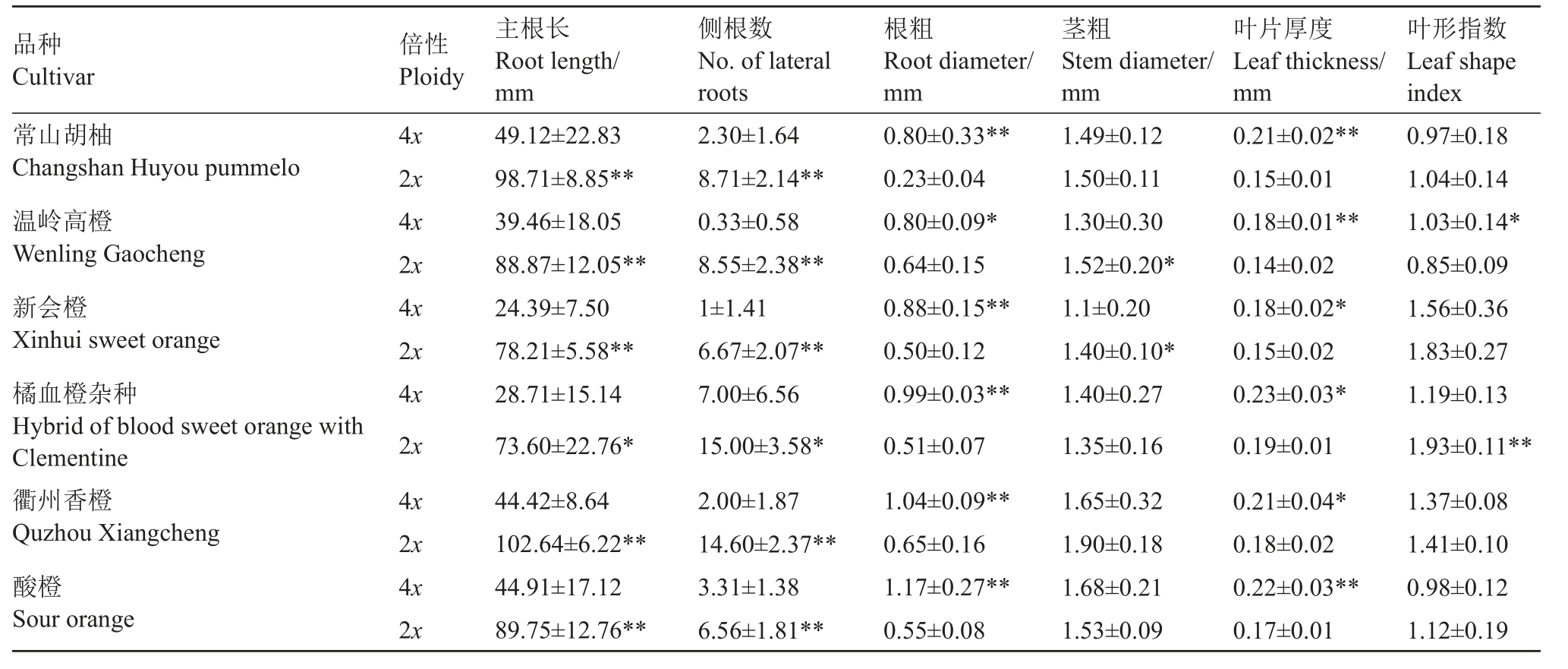
注:表中数据(平均值±标准差)后面*表示二倍体和四倍体之间的显著差异(*p <0.05;**p <0.01)。
Note:The values(mean±standard deviation)with*indicate significant difference between diploid and tetraploid(*p <0.05;**p <0.01).
品种Cultivar叶形指数Leaf shape index倍性Ploidy主根长Root length/mm侧根数No.of lateral roots根粗Root diameter/mm茎粗Stem diameter/mm叶片厚度Leaf thickness/mm常山胡柚Changshan Huyou pummelo温岭高橙Wenling Gaocheng新会橙Xinhui sweet orange橘血橙杂种Hybrid of blood sweet orange with Clementine衢州香橙Quzhou Xiangcheng酸橙Sour orange 0.97±0.18 1.04±0.14 1.03±0.14*0.85±0.09 1.56±0.36 1.83±0.27 1.19±0.13 1.93±0.11**1.37±0.08 1.41±0.10 0.98±0.12 2x 89.75±12.76**6.56±1.81**0.55±0.081.53±0.090.17±0.011.12±0.19 4x 2x 4x 2x 4x 2x 4x 2x 4x 2x 4x 49.12±22.83 98.71±8.85**39.46±18.05 88.87±12.05**24.39±7.50 78.21±5.58**28.71±15.14 73.60±22.76*44.42±8.64 102.64±6.22**44.91±17.12 2.30±1.64 8.71±2.14**0.33±0.58 8.55±2.38**1±1.41 6.67±2.07**7.00±6.56 15.00±3.58*2.00±1.87 14.60±2.37**3.31±1.38 0.80±0.33**0.23±0.04 0.80±0.09*0.64±0.15 0.88±0.15**0.50±0.12 0.99±0.03**0.51±0.07 1.04±0.09**0.65±0.16 1.17±0.27**1.49±0.12 1.50±0.11 1.30±0.30 1.52±0.20*1.1±0.20 1.40±0.10*1.40±0.27 1.35±0.16 1.65±0.32 1.90±0.18 1.68±0.21 0.21±0.02**0.15±0.01 0.18±0.01**0.14±0.02 0.18±0.02*0.15±0.02 0.23±0.03*0.19±0.01 0.21±0.04*0.18±0.02 0.22±0.03**
2.4 SSR分子鉴定
从筛选获得的11个多态性SSR标记中,每个品种至少筛选3个扩增效果好的SSR标记对发掘的四倍体植株进行分子鉴定(图4)。结果显示,温岭高橙、新会橙、橘血橙杂种、衢州香橙、酸橙所有四倍体的带型与其二倍体亲本完全一致,表现出高度一致的遗传背景,表明这些四倍体可能来自二倍体亲本珠心细胞自然加倍形成的双二倍体。而常山胡柚的14株四倍体中,12株四倍体的条带与其二倍体亲本完全一致,其余2 株四倍体均扩增出了其二倍体亲本没有的条带,表明胡柚的14株四倍体有2个来源,推测其分别来自珠心细胞和合子细胞(胡柚与附近其他未知柑橘类型天然杂交)加倍形成的双二倍体。
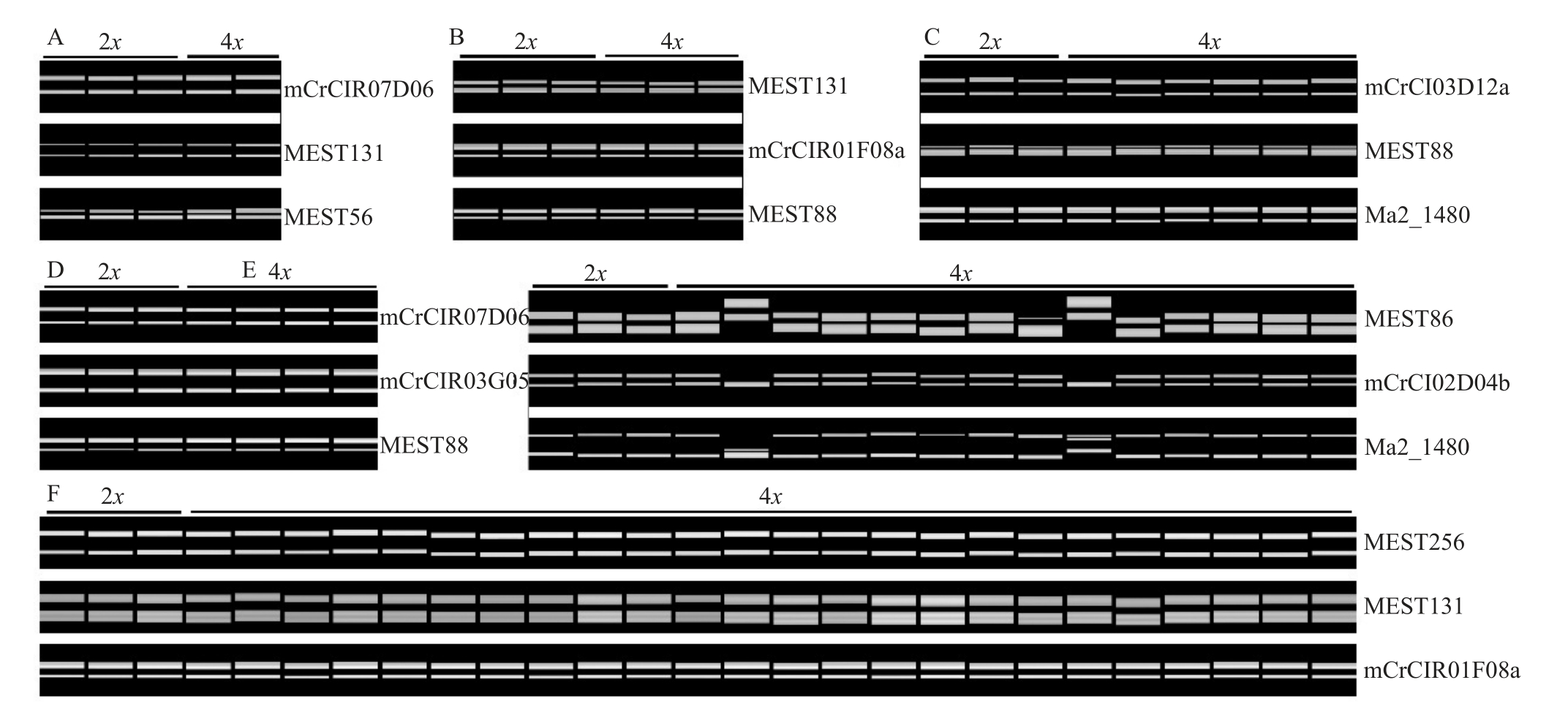
图4 柑橘四倍体植株的SSR 分子鉴定
Fig.4 SSR profiles of the tetraploids with their corresponding diploid parents
A.温岭高橙;B.橘血橙杂种;C.衢州香橙;D.新会橙;E.常山胡柚;F.酸橙。
A.Wenling Gaocheng;B.Hybrid of blood sweet orange with Clementine;C.Quzhou Xiangcheng;D.Xinhui sweet orange;E.Changshan Huyou pummelo;F.Sour orange.
3 讨 论
笔者在本研究中以柑橘6 个多胚品种常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙、酸橙为材料,基于幼苗形态初选结合倍性快速鉴定,从实生后代群体发掘出53 株四倍体后代。虽然衢州香橙四倍体发掘前人已有报道[17],即从400 株实生群体发掘出3 株四倍体;但本文报道的四倍体发掘效率更高,即从164粒种子发出的303株小苗中发掘出四倍体6 株。衢州香橙、酸橙四倍体可用于培育柑橘矮化、多抗的新型砧木;常山胡柚、温岭高橙、新会橙和橘血橙杂种的四倍体可作为亲本与二倍体倍性杂交创制三倍体无核新种质。
笔者用11 个SSR 标记对53 株四倍体进行遗传鉴定,51 株(96.2%)四倍体的带型与其二倍体亲本完全一致,表明多胚性柑橘种子中形成的四倍体更多偏向来自珠心细胞染色体自然加倍,这与前人报道的基本一致[4,7]。但不同的是,常山胡柚中,还产生了2株遗传背景与二倍体亲本不一致的四倍体后代,且这2 株四倍体均扩增出二倍体亲本没有的条带,表明其可能来自有性杂交。据报道,柑橘部分品种容易产生2n卵细胞[4,18-19],2n卵细胞与二倍体花粉受精可产生四倍体后代[20],但本文中所用胡柚母本树附近没有种植四倍体柑橘类型,可以排除其由2n卵细胞和四倍体植株产生的二倍体花粉受精形成的可能性。除2n 卵细胞外,柑橘也可以形成2n 花粉[21],2n 卵细胞和2n 花粉受精可以形成四倍体,但柑橘2n 花粉发生频率一般较低[4,22-23];且与n 花粉相比,2n 花粉在柱头上萌发所需时间较长,同等条件下,与卵细胞受精的竞争力没有n花粉强[24]。因此,基本上也可以排除上述2株四倍体由未减数2n卵细胞与外源2n 花粉受精形成。种子珠心细胞染色体自然条件下能够加倍,而合子细胞与珠心细胞所处环境基本一致,推测合子细胞的染色体也能像珠心细胞一样,可以自发加倍形成双二倍体。前人未报道合子细胞染色体自然加倍的情况,可能原因是所选材料多胚程度较高,合子胚在种子发育后期已败育,即使发生了染色体加倍,也无法获得合子细胞加倍形成的四倍体。本文中的温岭高橙、新会橙、橘血橙杂种、衢州香橙和酸橙,种子多胚程度较高(分别为3.4、8.0、7.1、5.6、5.8个胚/种子),可能是其实生后代没有获得由合子细胞加倍形成四倍体的重要原因。而常山胡柚由于多胚程度较低(1.4 个胚/种子),珠心胚干扰程度轻,使得部分合子细胞在加倍后得以存活。基于以上分析,推测发掘的与亲本遗传背景有差异的2株胡柚四倍体可能是胡柚与其附近的未知柑橘类型的有性杂交后合子细胞经染色体自然加倍而形成的。
致谢:广东省肇庆市德庆县柑橘研究所胡益波提供了新会橙果实,陕西省陕南柑橘综合试验站丁德宽提供了衢州香橙果实。
[1]XIE K D,YUAN D Y,WANG W,XIA Q M,WU X M,CHEN C W,CHEN C L,GROSSER J W,GUO W W.Citrus triploid recovery based on 2x×4x crosses via an optimized embryo rescue approach[J].Scientia Horticulturae,2019,252:104-109.
[2]OLLITRAULT P,DAMBIER D,LURO F,FROELICHER Y.Ploidy manipulation for breeding seedless triploid citrus[J].Plant Breeding Reviews,2008,30:323-352.
[3]张斯淇,徐强,邓秀新.无融合生殖与柑橘多胚现象的研究进展[J].植物科学学报,2014,32(1):88-96.ZHANG Siqi,XU Qiang,DENG Xiuxin.Advances in apomixis and polyembryony research in Citrus plants[J].Plant Science Journal,2014,32(1):88-96.
[4]ALEZA P,FROELICHER Y,SCHWARZ S,AGUSTÍ M,HERNÁNDEZ M,JUÁREZ J,LURO F,MORILLON R,NAVARRO L,OLLITRAULT P.Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment[J].Annals of Botany,2011,108(1):37-50.
[5]梁武军,解凯东,郭大勇,谢宗周,伊华林,郭文武.10 个柑橘砧木类型同源四倍体的发掘与SSR 鉴定[J].果树学报,2014,31(1):1-6.LIANG Wujun,XIE Kaidong,GUO Dayong,XIE Zongzhou,YI Hualin,GUO Wenwu.Spontaneous generation and SSR molecular characterization of autotetraploids in ten citrus rootstocks[J].Journal of Fruit Science,2014,31(1):1-6.
[6]周锐,解凯东,王伟,彭珺,谢善鹏,胡益波,伍小萌,郭文武.依据多倍体形态特征快速高效发掘柑橘四倍体[J].园艺学报,2020,47(12):2451-2458.ZHOU Rui,XIE Kaidong,WANG Wei,PENG Jun,XIE Shanpeng,HU Yibo,WU Xiaomeng,GUO Wenwu.Efficient identification of tetraploid plants from seedling populations of apomictic citrus genotypes based on morphological characteristics[J].Acta Horticulturae Sinica,2020,47(12):2451-2458.
[7]梁武军,解凯东,郭大勇,谢宗周,徐强,伊华林,郭文武.柑橘10 个品种实生后代多倍体的发掘及SSR 鉴定[J].园艺学报,2014,41(3):409-416.LIANG Wujun,XIE Kaidong,GUO Dayong,XIE Zongzhou,XU Qiang,YI Hualin,GUO Wenwu.Spontaneous generation and SSR characterization of polyploids from ten citrus cultivars[J].Acta Horticulturae Sinica,2014,41(3):409-416.
[8]彭滢,李晓妍,肖璇.柑橘多胚性砧木枳橙同源四倍体的发掘与SSR 鉴定[J].分子植物育种,2020,18(4):1211-1215.PENG Ying,LI Xiaoyan,XIAO Xuan.Excavation and SSR identification of autotetraploids in citrus polyembryonic rootstock citrange[J].Molecular Plant Breeding,2020,18(4):1211-1215.
[9]解凯东,彭珺,袁东亚,强瑞瑞,谢善鹏,周锐,夏强明,伍小萌,柯甫志,刘高平,GROSSER J W,郭文武.以本地早橘和槾橘为母本倍性杂交创制柑橘三倍体[J].中国农业科学,2020,53(23):4961-4968.XIE Kaidong,PENG Jun,YUAN Dongya,QIANG Ruirui,XIE Shanpeng,ZHOU Rui,XIA Qiangming,WU Xiaomeng,KE Fuzhi,LIU Gaoping,GROSSER J W,GUO Wenwu.Production of citrus triploids based on interploidy crossing with Bendizao and Man tangerines as female parents[J].Scientia Agricultura Sinica,2020,53(23):4961-4968.
[10]夏强明.基于2n 雌配子有性群体定位柑橘着丝粒及其序列特征分析[D].武汉:华中农业大学,2020.XIA Qiangming.Localization and sequence analysis of citrus centromeres based on a sexual population derived from 2n megagametophytes [D].Wuhan:Huazhong Agricultural University,2020.
[11]CHENG Y J,GUO W W,YI H L,PANG X M,DENG X X.An efficient protocol for genomic DNA extraction from Citrus species[J].Plant Molecular Biology Reporter,2003,21(2):177-178.
[12]GARCÍA-LOR A,LURO F,NAVARRO L,OLLITRAULT P.Comparative use of InDel and SSR markers in deciphering the interspecific structure of cultivated citrus genetic diversity:a perspective for genetic association studies[J].Molecular Genetics and Genomics,2012,287(1):77-94.
[13]CUENCA J,FROELICHER Y,ALEZA P,JUÁREZ J,NAVARRO L,OLLITRAULT P.Multilocus half-tetrad analysis and centromere mapping in citrus:evidence of SDR mechanism for 2n megagametophyte production and partial chiasma interference in mandarin cv.‘Fortune’[J].Heredity,2011,107(5):462-470.
[14]SNOUSSI H,DUVAL M F,GARCIA-LOR A,BELFALAH Z,FROELICHER Y,RISTERUCCI A M,PERRIER X,JACQUEMOUD-COLLET J P,NAVARRO L,HARRABI M,OLLITRAULT P.Assessment of the genetic diversity of the Tunisian citrus rootstock germplasm[J].BMC Genetics,2012,13:16.
[15]KAMIRI M,STIFT M,SRAIRI I,COSTANTINO G,EL MOUSSADIK A,HMYENE A,BAKRY F,OLLITRAULT P,FROELICHER Y.Evidence for non-disomic inheritance in a Citrus interspecific tetraploid somatic hybrid between C.reticulata and C.limon using SSR markers and cytogenetic analysis[J].Plant Cell Reports,2011,30(8):1415-1425.
[16]XU Q,CHEN L L,RUAN X A,CHEN D J,ZHU A D,CHEN C L,BERTRAND D,JIAO W B,HAO B H,LYON M P,CHEN J J,GAO S,XING F,LAN H,CHANG J W,GE X H,LEI Y,HU Q,MIAO Y,WANG L,XIAO S X,BISWAS M K,ZENG W F,GUO F,CAO H B,YANG X M,XU X W,CHENG Y J,XU J,LIU J H,LUO O J,TANG Z H,GUO W W,KUANG H H,ZHANG H Y,ROOSE M L,NAGARAJAN N,DENG X X,RUAN Y J.The draft genome of sweet orange (Citrus sinensis)[J].Nature Genetics,2013,45(1):59-66.
[17]蒋景龙,阳妮,李丽,秦公伟,邓家锐,赵桦,任可心,丁德宽.衢州香橙四倍体种质发掘及形态特征性评价[J].果树学报,2021,38(5):655-663.JIANG Jinglong,YANG Ni,LI Li,QIN Gongwei,DENG Jiarui,ZHAO Hua,REN Kexin,DING Dekuan.Identification and characterization of tetraploids from seedlings of Citrus junos‘Quzhou xiangcheng’[J].Journal of Fruit Science,2021,38(5):655-663.
[18]ESEN A,SOOST R K.Unexpected triploids in Citrus:their origin,identification,and possible use[J].Journal of Heredity,1971,62:329-333.
[19]ALEZA P,CUENCA J,HERNÁNDEZ M,JUÁREZ J,NAVARRO L,OLLITRAULT P.Genetic mapping of centromeres of the nine Citrus clementina chromosomes using half-tetrad analysis and recombination patterns in unreduced and haploid gametes[J].BMC Plant Biology,2015,15:80.
[20]解凯东,王惠芹,王晓培,梁武军,谢宗周,伊华林,邓秀新,GROSSER J W,郭文武.单胚性二倍体为母本与异源四倍体杂交大规模创制柑橘三倍体[J].中国农业科学,2013,46(21):4550-4557.XIE Kaidong,WANG Huiqin,WANG Xiaopei,LIANG Wujun,XIE Zongzhou,YI Hualin,DENG Xiuxin,GROSSER J W,GUO Wenwu.Extensive citrus triploid breeding by crossing monoembryonic diploid females with allotetraploid male parents[J].Scientia Agricultura Sinica,2013,46(21):4550-4557.
[21]ROUISS H,CUENCA J,NAVARRO L,OLLITRAULT P,ALEZA P.Tetraploid citrus progenies arising from FDR and SDR unreduced pollen in 4x×2x hybridizations[J].Tree Genetics&Genomes,2017,13(1):10.
[22]LURO F,MADDY F,JACQUEMOND C,FROELICHER Y,MORILLON R,RIST D,OLLITRAULT P.Identification and evaluation of diplogyny in clementine (Citrus clementina) for use in breeding[J].Acta Horticulturae,2004,663(663):841-848.
[23]向素琼,龚桂枝,郭启高,汪卫星,李春艳,李晓林,梁国鲁.柑橘属2n 花粉自然发生与沙田柚2n 花粉诱导研究[J].西南农业大学学报(自然科学版),2005,27(5):51-55.XIANG Suqiong,GONG Guizhi,GUO Qigao,WANG Weixing,LI Chunyan,LI Xiaolin,LIANG Guolu.Spontaneous generation of 2n pollen in citrus and induction of 2n pollen in Citrus grandis[J].Journal of Southwest Agricultural University (Natural Science),2005,27(5):51-55.
[24]ZHAO C G,TIAN M D,LI Y J,ZHANG P D.Slow-growing pollen-tube of colchicine-induced 2n pollen responsible for low triploid production rate in Populus[J].Euphytica,2017,213(4):94.