葡萄(Vitis vinifera L.)属于葡萄科葡萄属木质藤本植物,是世界上重要的果树之一,在世界各个国家和地区广泛种植。截止2018年,全球葡萄种植面积740 万hm2,居世界水果种植第2 位(FAO)。我国葡萄种植面积为875 khm2,占比12%,是全球第二大种植葡萄的国家[1]。葡萄广泛分布在我国许多省份,包括新疆、河北、陕西、山东和云南等地区,其中新疆种植面积最大(2018年度葡萄产业技术发展报告)。
目前,全球已经报道了71 种与葡萄有关的病害,其中46%以上由真菌和卵菌引起[2],葡萄枝干病害广泛分布于全球的葡萄种植地区,有些地区葡萄枝干病害的发病率高达100%[3]。Hofstetter 等[4]报道,每年由于枝干病害导致更换死树的损失高达15亿美元。而我国已经报道了27 种与葡萄有关的病害[5],主要包括葡萄霜霉病、葡萄白粉病、葡萄炭疽病等真菌性病害。近年来,随着葡萄树龄的增加、种植方式的改变以及环境因素的影响等原因,葡萄枝干病害发生较为普遍且个别年份发生较重。据统计,我国每年仅由于葡萄溃疡病造成3%~8%的产量损失,在一些发病严重的省份,如广西和浙江等地每年可导致10%~20%的产量损失,而在高温多雨等极端条件下可高达100%的产量损失[6],加上葡萄枝干病害的症状难以准确识别,其发病机制尚不十分清楚,田间缺乏高效的防控手段,且有加重趋势,因此,我国应该高度重视葡萄枝干病害问题。
1 国际葡萄枝干病害的分布、田间症状与病原真菌的鉴定
葡萄枝干病害是由多种病原真菌单独侵染或复合侵染引起,主要危害葡萄的多年生枝干和根部,还可危害果实、嫩梢和叶片,影响葡萄植株的树势,严重时可导致树体死亡。国际上已报道的主要葡萄枝干病害包括葡萄衰枯病(Esca disease complex)、葡萄溃疡病(Botryosphaeria dieback)、葡萄顶枯病(Eutypa dieback)、葡萄蔓枯病(Diaporthe dieback)和葡萄黑根病(Black foot disease)等5种。
1.1 葡萄衰枯病(Esca disease complex)
葡萄衰枯病于1865 年在法国首次报道[2],是葡萄重要的枝干病害之一。该病害发生危害严重,2005—2007 年,Romanazzi 等[7]对田间病害调查发现,葡萄衰枯病在意大利中东部地区的发生率已经达到32.6%。根据该病害在大多数地区的病症特点,笔者建议将其中文名定为葡萄衰枯病。
葡萄衰枯病田间症状十分复杂,一直是研究者的研究热点。田间的症状主要与葡萄品种、葡萄树龄及植株的发病部位有关。不同葡萄品种、树龄以及植株部位,葡萄衰枯病表现出不同的症状[8-9],典型症状包括:(1)叶部“虎纹斑”,在叶片上首先表现为叶片黄化,然后在叶脉之间或沿着叶的边缘扩大和变成斑点汇合,导致褪绿和出现坏死的条带,最终只有沿着中脉的狭窄绿色条纹,这种典型症状称为“虎纹斑”。如果葡萄品种为有色品种,叶部病斑则先变红,最后坏死;如果葡萄品种为非有色品种,叶部病斑则不会变红;(2)果上“黑麻疹”,症状在浆果表面布满黑色的小斑点,被称为“黑麻疹”;(3)“中风”,整株葡萄几天之内突然死亡,叶片干枯最后全部脱落,只剩下枝条,被称为“中风”;(4)葡萄衰枯病病原真菌(相关的担子菌引起)侵染葡萄的树干木质部表现为白色软腐,而对于小于8 a(年)树龄的葡萄幼树来说,在枝干和分支内部极少发现白腐症状;(5)葡萄衰枯病病原真菌(相关的子囊菌引起)侵染葡萄的树干木质部,枝干横切面为褐色至黑色小圆形病斑,枝干纵切面为褐色至黑色,长条形病斑;(6)“black goo”,患病植株在修剪口产生水滴状、黑色的渗出液,一般在苗圃可发现此现象。目前为止,人们还无法在实验室重新还原田间所有的症状,因此,该病害不排除是多种病原菌复合侵染的结果[8]。
根据树龄及表现的症状,对葡萄衰枯病在不同树龄表现出不同的症状给予不同的名字,包括Dark wood streaking、Petri disease、Grapevine leaf stripe disease、White rot和Esca proper[10]。(1)树龄在1~7 a,木质部横切面的病斑为黑色小圆点,纵切面病斑为黑色的长条纹,主要包括Dark wood streaking 和Petri disease。Dark wood streaking 一般在育苗基地发生,而Petri disease 主要在新种植园发生;(2)Grapevine leaf stripe disease一般发生在8 a左右树龄的植株,典型症状为叶片的虎纹斑;White rot一般发生超过8 a树龄的植株,典型症状为枝干木质部发生乳白色,软腐,该症状由担子菌引起;(3)Esca proper发生在8 a以上树龄的植株,典型症状包括担子菌导致枝干木质部发生乳白色,软腐,同时还可发现由子囊菌引起的木质部黑色至褐色坏死,在病株叶片表现为虎纹斑症状。
截至2020年3月,国际上报道了48种与葡萄衰枯病有关的病原真菌,主要包括Phaemoniella chlamydospora、Phaeoacremonium spp.(27种)、Cadophora spp.(5种)及一些担子菌(Coprinellus spp.、Fomiti-poria spp.、Fomitiporella spp.、Inocutis spp.、Phellinus spp.和Stereum spp.),具体的病原真菌及其分布见表1。其中,枝干内部组织腐烂、发白和变软症状主要是由担子菌引起,而Phaemoniella chlamydospore 及Phaeoacremonium spp.则引起内部枝干组织褐色坏死,纵切面枝干形成褐色长条纹。Phaeomoniella chlamydospora 和Phaeoacremonium minimum 为常见的致病菌,在全球多个国家均有报道。
表1 葡萄衰枯病相关病原真菌及报道的国家或地区
Table 1 Fungal species which have been reported associated with Esca complex disease of grapevines and their geographical distribution

?
表1 (续) Table 1(continued)
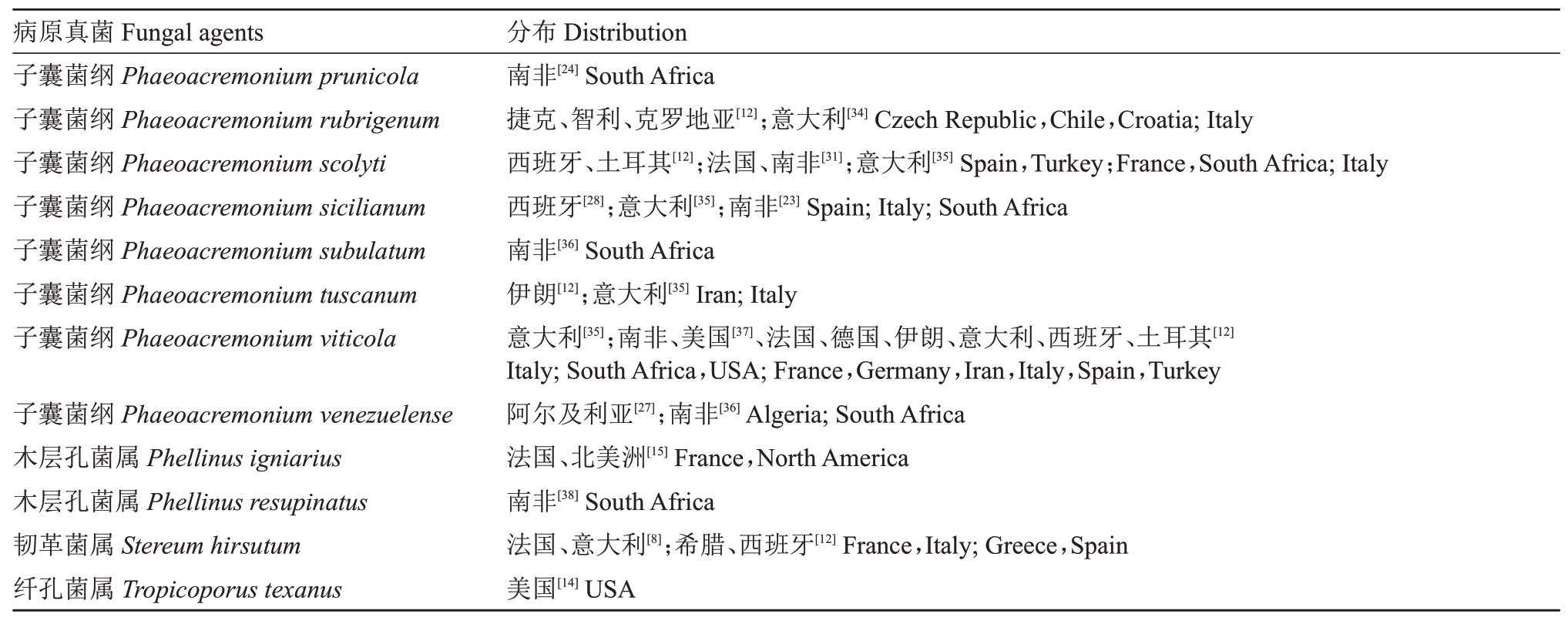
?
1.2 葡萄溃疡病(Botryosphaeria dieback)
葡萄溃疡病于1964 年在加拿大首次报道[39],是重要的枝干病害之一。葡萄溃疡病发生时典型症状包括葡萄植株的枝条或枝干顶梢枯死(dieback),在枝干内部可以观察到褐色至黑色楔形或弓形的坏死斑。此外,还可以观察到果实腐烂、芽坏死和枝条发白等田间症状。葡萄溃疡病在大部分葡萄种植区都会发生,在不同国家的发生率以及危害程度存在较大的差异。据报道,美国加州每年因葡萄枝干病害(包括葡萄溃疡病)引起的损失已超过2.6亿美元[40]。
截至2020年3月,已经报道了44种与葡萄溃疡病有关的病原真菌,主要包括Botryosphaeria、Diplodia、Dothiorella、Lasiodiplodia、Neofusicoccum、Spencermartinsia 和Sphaeropsis 这7 个属的真菌,具体的病原真菌以及其分布见表2,其中葡萄座腔菌(Botryosphaeria dothidea)、色二孢菌(Diplodia seriata)、可可毛色二孢菌(Lasiodiplodia theobromae)和小新壳梭孢(Neofusicoccum parvum)为常见的致病菌,在全球多个国家均有报道。
表2 葡萄溃疡病相关病原真菌及报道的国家或地区
Table 2 Fungal species which have been reported associated with Botryosphaeria dieback of grapevines and their geographical distribution

?
表2 (续) Table 2(continued)
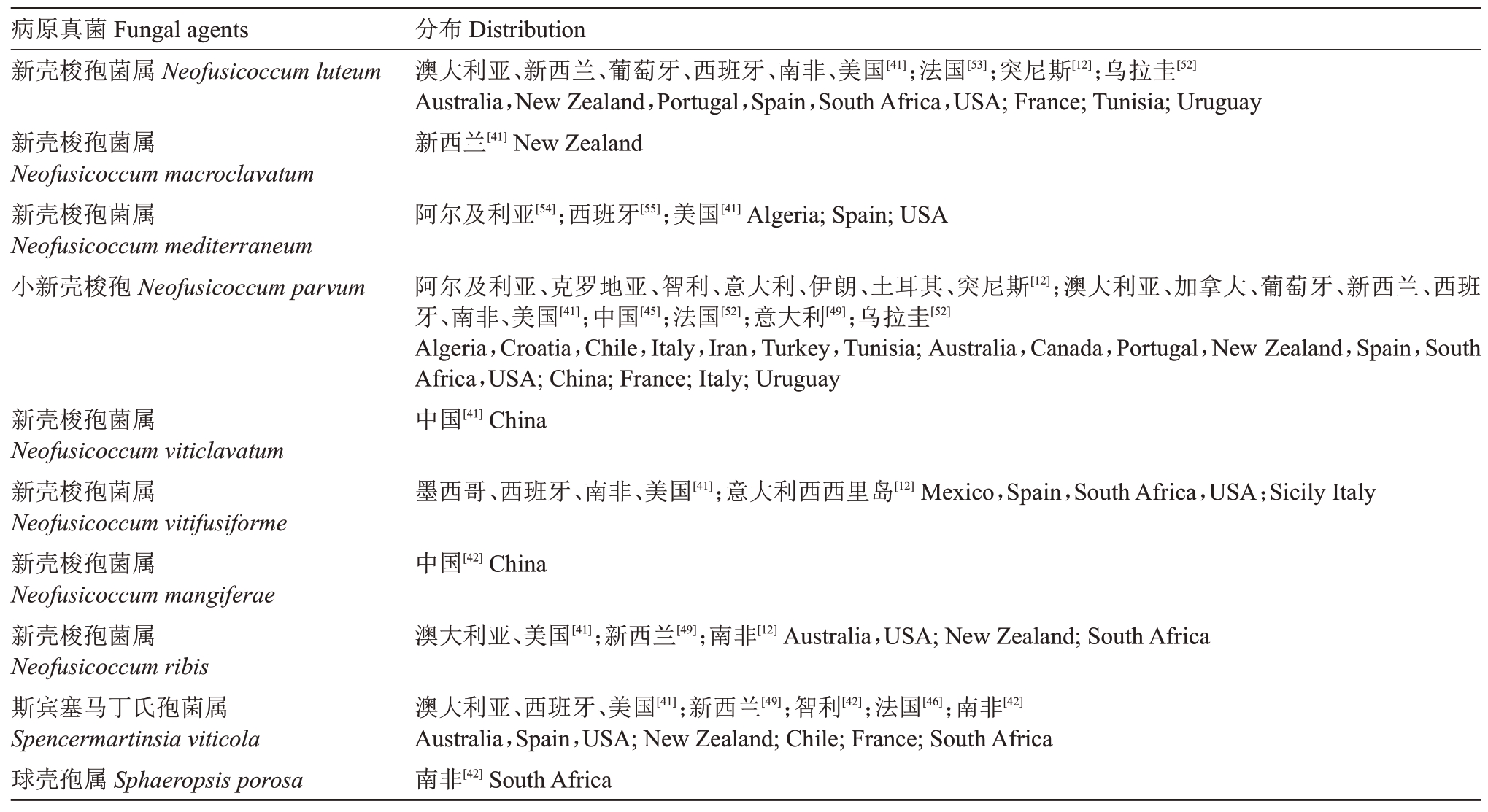
?
1.3 葡萄顶枯病(Eutypa dieback)
葡萄顶枯病最早是1973 年由澳大利亚首次报道[56],是葡萄重要的枝干病害之一。患病葡萄植株的枝条节间缩短,叶片表现为边缘坏死和叶脉间组织黄化、枯萎,形状呈杯状,最后破碎。叶片的症状一般在春天就表现出来,大多数花在未绽放之前就已经干掉,病原菌可导致果实瘦小,落果。病原菌主要通过嫁接口侵入枝干,在枝干横切面维管组织表现为楔形的棕色坏死状[57]。
截至2020年3月,已经报道了20种与葡萄顶枯病有关的病原真菌,主要包括Cryptosphaeria、Cryptovalsa、Diatrype、Diatrypella、Eutypa 和Eutypella 这6 个属,具体的病原真菌以及其分布总结见表3,其中Eutypa lata 分布广泛,是引起葡萄顶枯病主要的病原真菌,该菌在全球的11个国家均有报道。
表3 葡萄顶枯病相关病原真菌及报道的国家或地区
Table 3 Fungal species which have been reported associated with Eutypa dieback of grapevines and their geographical distribution

?
1.4 葡萄蔓枯病(Diaporthe dieback)
葡萄蔓枯病最早由1909年首次在美国发现,葡萄蔓枯病是葡萄重要的枝干病害之一,主要危害枝蔓基部和新梢,发病初期表现为红褐色梭形病斑,凹陷,后期病斑逐渐扩大,表现为黑褐色病斑,主蔓发生较严重,枝蔓越冬后将沿病斑纵向开裂,呈现出较大的裂口,最后导致整个枝蔓抽不出新梢而死亡[70]。
截至2020 年3 月,全球报道了与葡萄蔓枯病相关27 种间座壳属(Diaporthe spp.)真菌。Diaporthe ampelina 是最早报道和最常见的致病菌,常与Diaporthe amygdali 共同侵染[71-72]。引起不同国家葡萄蔓枯病的病原真菌有所不同,每个国家均超过一种致病菌。例如,在非洲主要是由Diaporthe ampelina、Diaporthe amygdali、Diaporthe ambigua、Diaporthe australafricana、Diaporthe cynaroidis、Diaporthe eres、Diaporthe foeniculina、Diaporthe kyushuensis、Diaporthe novem 和Diaporthe serafiniae 引起[71-77]。具体的病原真菌及分布总结见表4。
表4 葡萄蔓枯病相关病原真菌及报道的国家或地区
Table 4 Fungal species which have been reported associated with Diaporthe dieback of grapevines and their geographical distribution
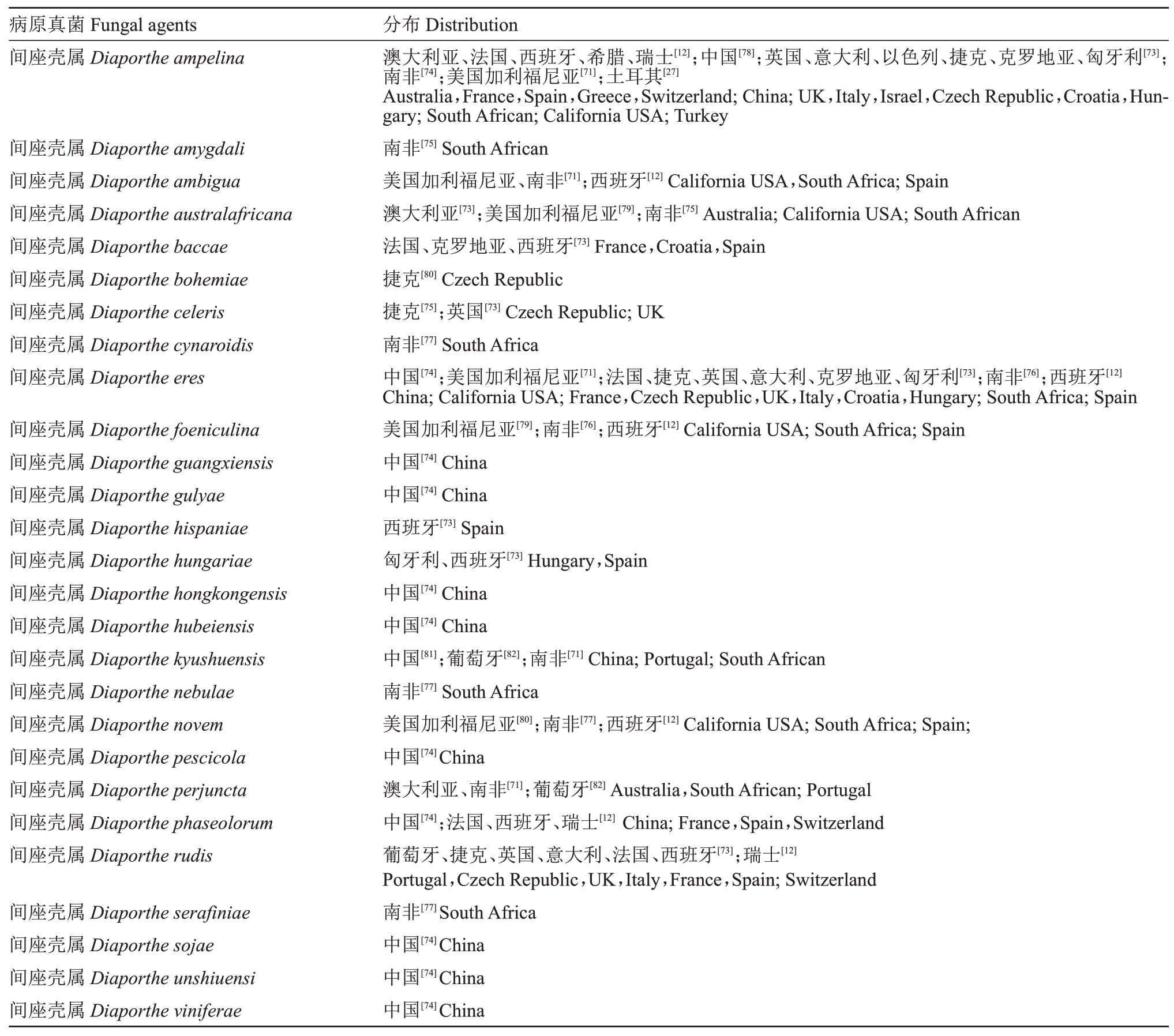
?
1.5 葡萄黑根病(Black foot disease)
葡萄黑根病于1961 年在法国首次报道[83],是主要的葡萄枝干病害之一,尤其是苗圃和新定植的葡萄园[84],由于根部形成典型棕色至黑色的坏死,因此,1969 年Badour 将该病害命名为“pied noir”。后来,Scheck 等[85]将“pied noir”的名称更改为“Black Foot Disease”。葡萄黑根病发生时,苗圃幼苗的芽延迟萌发或缺失,并且生长发育迟缓,根部为褐色至黑色坏死[86]。在新定植的葡萄园中葡萄植株表现为枝条节间缩短和叶片黄化,这与Petri disease的田间症状极为相似,一般难以区分[87]。
截止至2020年3月,已经报道32种病原菌引起葡萄黑根病,包括Campylocarpon,Cylindrocarpon、Cylindrocladiella、Dactylonectria、Ilyonectria、Neonectria、Pleiocarpon 和Thelonectria 8 个不同的属。Ilyonectria liriodendri 是首个被报道与葡萄黑根病相关的致病菌[88],目前在全球16 个国家均有报道(表5)。
表5 葡萄黑根病相关病原真菌及报道的国家或地区
Table 5 Fungal species which have been reported associated with Black foot disease of grapevines and their geographical distribution

?
2 我国葡萄枝干病害的分布、田间症状与病原真菌的鉴定
迄今为止,我国已报道的枝干病害类型包括葡萄溃疡病[96]、葡萄顶枯病[69]和葡萄蔓枯病[81],葡萄衰枯病和葡萄黑根病尚未有公开的报道。
2.1 葡萄溃疡病(Botryosphaeria dieback)
2010 年北京市农林科学院李兴红和燕继晔和中国农业科学院王忠跃等人首次在我国报道葡萄溃疡病的发生危害,该病发生时导致果梗干枯、果实干缩或掉粒、枝干溃疡、树势减弱等症状,病害发生的初期表现枝干溃疡,严重时导致树体死亡[96]。葡萄溃疡病在我国发生较普遍,在我国广大的葡萄种植区包括吉林、辽宁、河南、河北、甘肃、四川、重庆、湖北、湖南、广西和浙江等均有发生[45]。由于我国地理环境条件的多样化,葡萄溃疡病在我国不同地区的发病率不同,一般地区的发病率为3%~8%,但在严重的地区如广西和浙江等则高达10%~20%[6]。在田间发生葡萄溃疡病的品种大部分为鲜食葡萄,少部分为酿酒葡萄,包括‘巨玫瑰’(‘Muscat Kyoho’)、‘北红’(‘Beihong’)、‘红地球’(‘Red Globe’)、‘魏可’‘Wink’、‘玫瑰香’(‘Muscat Hamburg’)、‘郑州枣玉’(‘Zhengzhouzaoyu’)、‘夏黑’(‘Summer Black’)、‘Sea Red Globe’、‘紫大夫’(‘Dornkelfelder Trocken’)、毛葡萄(V. quinquangularis Rehd.)和‘赤霞珠’(‘Cabernet Sauvignon’)[45]。
截至2020 年3 月,引起我国葡萄溃疡病的病原真菌主要包括葡萄座腔菌(Botryosphaeria dothidea)、色二孢(Diplodia seriata)、可可毛色二孢(Lasiodiplodia theobromae)、小新壳梭孢(Neofusicoccum parvum)、Lasiodiplodia pseudotheobromae 和Neofusicoccum mangiferae,其中优势种群是葡萄座腔和可可毛色二孢,致病力最强的是可可毛色二孢菌[6]。可可毛色二孢和小新壳梭孢主要分布在我国亚热带季风气候区(如山西和广西等地区),色二孢主要在温带季风气候区(如山东和河北),葡萄座腔菌在全国大部分地区(如广西、湖南、山西、辽宁和吉林等地区)均有报道[45]。
2.2 葡萄顶枯病(Eutypa dieback)
2007 年由西北农林科技大学李华团队在我国陕西杨凌张家岗葡萄酒学院葡萄试验站首次报道葡萄顶枯病[81],田间发病症状表现为植株的枝条节间缩短,叶片褪色黄化,新叶明显变小,失绿,枝干内部形成楔形、灰褐色或紫褐色病斑。一般在早春可以观察到葡萄顶枯病的田间症状,但并非所有患病的植株都会表现出明显的症状,有些葡萄植株被病原菌侵染3~5 a 之后才表现出明显症状[97]。病原菌分离采用组织分离的方法,通过形态学和分子生物学(PCR、PCR- RFLP 和DNA 测序),鉴定出Eutypella vitis是引起我国葡萄顶枯病的病原[69]。
由于Eutypella vitis 的侵染发病需要较长的时间,上述论文作者在其论文中并没有获得病原菌柯赫氏法则验证的结果[97]。此外,该病害在国内的发病率尚未报道。迄今为止,葡萄顶枯病仅在陕西有一次报道,截至目前在我国其他地方再无相关报道。
2.3 葡萄蔓枯病(Diaporthe dieback)
葡萄蔓枯病发病初期主要危害葡萄的枝蔓,表现为暗紫褐色病斑,后期病斑加重,变黑、变硬,病蔓纵裂丝状,易折断。此外,发病严重时,还可危害葡萄枝干,枝干内部组织变褐色至黑色坏死,在枝干表面可发现病原菌的黑色分生孢子器[78,98]。目前,在我国北京、广西、河北、河南、山东、江苏、四川、浙江、吉林、辽宁和黑龙江等均有发生[74]。据相关文献报道,葡萄蔓枯病在我国部分地区的发病率可高达60%以上,枝蔓死亡率达10%左右,严重影响葡萄的产量和品质[99]。已经报道易感葡萄蔓枯病的品种大部分为鲜食葡萄,包括‘巨峰’(‘Kyoho’)、‘玫瑰香’(‘Muscat Hamburg’)、‘牛奶’(‘Cow’s teat’)、‘红地球’(‘Red Globe’)、‘无核白鸡心’(‘Centennial Seedless’)、‘夏黑’(‘Summer Black’)、‘亚都蜜’(‘Yotomi’)和‘醉金香’(‘Zui Jinxiang’)[74]。
截至2020 年3 月,引起我国葡萄蔓枯病的病原真菌包括Diaporthe ampelina(Phomopsis viticola)[78],Diaporthe eres、Diaporthe guangxiensis、Diaporthe gulyae、Diaporthe hongkongensis、Diaporthe hubeiensis、Diaporthe pescicola、Diaporthe phaseolorum、Diaporthe sojae、Diaporthe unshiuensis 和Diaporthe vinifera[74],北京市农林科学院的团队目前仅用离体的一年生绿枝条接种病原菌,证明了病原菌的致病性,但没有完成柯赫氏法则验证。
3 葡萄枝干病害的传播流行与防控
3.1 葡萄枝干病害的传播流行
葡萄枝干病害的侵染来源:包括患病的繁殖材料、土壤及田间患病的植株[36]。对于葡萄衰枯病,患病的母本植株、育苗过程及嫁接过程中的污染是导致繁殖材料感染的主要原因,有相关研究发现,可以通过PCR 手段在母本植株下的土壤中检测到Phaeomoniella chlamydospora,在葡萄植株的土壤及水洼处均发现有Phaeoacremonium minimum[36]。侵入:病原真菌一般从伤口(剪口、机械损伤与冻伤)侵入,在植株内定殖、生长,产生毒素导致枝干坏死,严重时可致植株死亡。如Phaeomoniella chlamydospora和Phaeoacremonium spp.主要通过伤口进行侵入,特别是剪锯口及嫁接伤口[36]。传播:病原菌的传播主要包括长距离和短距离传播,长距离传播主要通过人为的把患病繁殖材料从一个区域运输到另一个区域,这种方式是枝干病害快速在全国乃至全球范围内扩散的主要原因。在同一个葡萄园内主要进行的是短距离传播,病原菌的孢子借助雨水、风、昆虫及靠近葡萄园的多年生木本植物进行短距离扩散。有研究表明,引起葡萄顶枯病、葡萄溃疡病、葡萄蔓枯病、葡萄衰枯病的致病菌主要是孢子通过风来传播。此外,葡萄溃疡病和葡萄衰枯病致病菌也可通过侵染扦插枝条来传播,而引起葡萄黑根病的病原菌主要依靠土壤进行传播,在苗圃和土壤中常常能发现引起葡萄黑根病相关病原真菌的存在,因此,葡萄种植前土壤就有可能存在致病菌[57]。
3.2 葡萄枝干病害的防控
曾有一种化学农药用来防治葡萄枝干病害,特别对防治葡萄衰枯病和葡萄溃疡病有较好的防治效果,但由于该农药对人体以及环境危害较大,所以目前已经被禁止使用,同时,目前尚未发现高抗葡萄枝干病害的品种或遗传资源[89,100-101]。因此,葡萄枝干病害在实际生产中应采用综合性防控方法,包括但不限于化学防治、生物防治和农业防治等措施。
目前研究结果表明三唑类杀菌剂、甲氧基丙烯酸酯类杀菌剂和苯并咪唑类杀菌剂有一定的防控效果。室内生测结果表明三唑类药剂能抑制大部分枝干病害病原菌菌丝的生长,孢子萌发[57];田间伤口保护实验表明氟硅唑(Flusilazole)能够抑制Neofusicoccum luteum,Eutypa lata的侵入,保护伤口的效果明显[57];丙环唑和灭菌唑复配,苯醚甲环唑和戊唑醇复配可以有效抑制葡萄衰枯病叶部症状的出现[57]。
壳聚糖、大蒜提取物以及3种物质的复合物(壳聚糖、大蒜提取物和香草素),在室内毒力测定以及田间伤口保护实验表明,它们对两种枝干病害(葡萄衰枯病和葡萄溃疡病)的防治效果较好。此外硼酸用于田间伤口保护,效果较好[57]。
葡萄枝干病害的农业防治主要包括更新结果枝等措施。在根部和砧木还健康的条件下,剪掉患病枝干,重新培育新的结果母枝。如果根部及砧木已经出现症状,唯一的办法就是挖除病株,重新栽培新的健康植株。对于防治葡萄顶枯病以及葡萄溃疡病,可以使用更新结果枝的手段进行病害防控。
热水处理也是防控葡萄枝干病害的一种常用手段,操作一般在育苗阶段实行,热水处理的温度及时间一般为50 ℃,30 min,但是此操作对葡萄植株会造成一定的伤害,如果处理不当会导致植株质量下降,严重时可导致植株死亡。因为不同的葡萄品种及病原菌对温度的敏感性不同,所以在进行热水处理时需要综合考虑,制定出合适的温度和处理的时间。研究结果表明,‘黑比诺’(‘Pinot Noir’)、‘美乐’(‘Merlot’)、‘雷司令’(‘Riesling’)、‘Paulsen’和‘赤霞珠’(‘Cabernet Sauvignon’)对温度的敏感度依次下降[102-103]。此外,不同的病原真菌对温度的敏感性不同。研究表明,45~47 ℃处理可消除大部分Phaeomoniella chlamydospora,而对于其他的病原菌则需要51~53 ℃的高温[104]。
生物防治主要利用生防细菌及真菌对枝干病害进行防控,作用机制包括空间以及营养竞争等,目前这部分研究主要集中在室内以及田间伤口保护。室内试验及田间伤口保护试验结果表明,枯草芽孢杆菌(Bacillus subtilis)和深绿木霉(Trichoderma atroviride)、色二孢菌(Diplodia seriata)、可可毛色二孢菌(Lasiodiplodia theobromae)、Neofusicoccum austerale、小新壳梭孢(Neofusicoccum parvum)、Phaeomoniella chlamydospora和Eutypa lata的防治效果较好[57]。
4 存在的问题与展望
国际上已经报道了5 种重要的葡萄枝干病害,相对于葡萄白粉病和葡萄霜霉病等病害,葡萄枝干病害在田间发生十分复杂,有时会导致严重的损失甚至毁园,因此被认为是对全球葡萄栽培过程中具有严重威胁的病害,在国际植物病理学会下设有专门的学术组织(国际葡萄枝干病害专业委员会)协调组织该病害的研究与防控。目前,在我国已经报道了3种葡萄枝干病害(葡萄溃疡病、葡萄蔓枯病和葡萄顶枯病),并取得了一定的进展,但仍存在一些不足:葡萄衰枯病和葡萄黑根病在我国有没有发生、危害情况、典型症状等尚不清楚。目前全球已经报道了171 种与葡萄枝干病害相关的病原菌,隶属于30个属,这些致病菌通常因气候和地理区域不同而异,病原真菌可以单独侵染,也可复合侵染,然而在我国,葡萄枝干病害的致病菌优势种类有哪些?葡萄枝干病害重要病原物的致病机制、葡萄对病原物的抗性、病原物与寄主相互作用尚未十分清楚,特别是该类病害的“机会性”发生机制还需要投入更多研究力量。目前大多数的研究主要集中于新的葡萄种植园及老葡萄园,育苗基地枝干病害的发生危害情况尚未十分清楚。
因此,近期我国对葡萄枝干病害的研究重点应集中在以下几个方面:全面理清我国葡萄枝干病害的发生危害情况、田间典型症状与病原菌种类;了解全国范围内育苗基地发生病害的种类和操作过程中存在的污染环节等,制定育苗基地行业操作标准;如果有可能,应尽快建立该类病害的早期诊断和苗木无害化诊断体系,用于苗木栽培前的健康检测和病害防控;重要枝干病害病原优势菌的室内及田间防控药剂,生防菌等的筛选,以期获得高效、低毒、低残留药剂;明确葡萄枝干病害的发生与树体以及根际微生物的关系,葡萄枝干病害重要病原物的致病机制、葡萄对病原物的抗性、病原物与寄主相互作用,以深入理解该类病害的发生机制;构建适合不同气候区的葡萄枝干病害综合防控技术措施,并推广应用。
[1] Online International Organization of vine and wine Intergovernmental Organization(OIV).Statistical Report on World Vitiviniculture [EB/OL]. 2019,[2020- 06- 28]. http://www.oiv.int/en/technical-standards-and-documents/statistical-analysis/annualassessment.
[2] WILCOX W F,GUBLER W D,UYEMOTO J K. Compendium of grape diseases,disorders,and pests,second edition//Front matter[M]. America: The American Phytopathological Society,2006.
[3] VARELA C P,REDONDO V,COSTAS D,AGUIN O,MANSILLA P. Fungi associated with grapevine trunk diseases in nursery-produced Vitis vinifera plants[J].Phytopathologia Mediterranea,2019,57(3):407-424.
[4] HOFSTETTER V,BUYCK B,CROLL D,VIRET O,COULOUX A,GINDRO K. What if esca disease of grapevine were not a fungal disease?[J].Fungal Diversity,2012,54(1):51-67.
[5] 李兴红,燕继晔.图说葡萄病虫害防治关键技术[M].北京:中国农业出版社,2011.LI Xinghong,YAN Jiye. Picture shows the key technologies of grape pest control[M].Beijing:China Agricultural Press,2011.
[6] 张玮,姚晟伟,张国军,谢悦,李兴红,徐海英.中国葡萄主要品种对葡萄座腔菌的抗性评价[J].植物保护,2017,43(3):177-180.ZHANG Wei,YAO Shengwei,ZHANG Guojun,XIE Yue,LI Xinghong,XU Haiying.Resistant evaluation of main grape cultivars in China to Botryosphaeria dothidea[J]. Plant Protection,2017,43(3):177-180.
[7] ROMANAZZI G,MUROLO S,PIZZICHINI L,NARDI S. Esca in young and mature vineyards,and molecular diagnosis of the associated fungi[J]. European Journal of Plant Pathology,2009,125(2):277-290.
[8] MUGNAL L,GRANITI A,SURICO G. Esca (black measles)and brown wood-streaking: two old and elusive diseases of grapevines[J].Plant Disease,1999,83(5):404-418.
[9] SURICO G.Towards a redefinition of the diseases within the esca complex of grapevine[J]. Phytopathologia Mediterranea,2009,48(1):5-10.
[10] MONDELLO V,SONGY A,BATTISTON E,PINTO C,COPPIN C,TROTEL-AZIZ P,CLEMENT C,MUGNAI L,FLORENCE F. Grapevine trunk diseases: a review of fifteen years of trials for their control with chemicals and biocontrol agents[J].Plant Disease,2017,102(7):1189-1217.
[11] MALDONADO-GONZALEZ M M,MARTINEZ-DIZ M P,ANDRES-SODUPE M,BUJANDA R,DIAZ-LOSADA E,GRAMAJE D. Quantification of Cadophora luteo-olivacea from grapevine nursery stock and vineyard soil using droplet digital PCR[J].Plant Disease,2020,104(8):2269-2274.
[12] Online Fungal Databases.U.S.National Fungus Collections[EB/OL].[2020-10-30].https://nt.ars- grin.gov/fungaldatabases/index.cfm.
[13] TRAVADON R,LAWRENCE D P,ROONEY-LATHAM S,GUBLER W D,WILCOX W F,ROLSHAUSEN P E,BAUMGARTNER K.Cadophora species associated with wooddecay of grapevine in north America[J]. Fungal Biology,2015,119(1):53-66.
[14] BROWN A,LAWRENCE D P,BAUMGARTNER K. Role of Basidiomycete fungi in the grapevine trunk disease Esca[J].Plant Pathology,2020,69(2):205-220.
[15] CLOETE M,FISCHER M,MOSTERT L,HALLEEN F. Hymenochaetales associated with esca-related wood rots on grapevine with a special emphasis on the status of esca in South African vineyards[J]. Phytopathologia Mediterranea,2015,54(2):299-312.
[16] CLOETE M. Characterization of the Basidiomycetes associated with esca disease of South African grapevines[D]. South Africa:Stellenbosch University,2015.
[17] ANTONIO G,GIUSEPPE S,LAURA M. Esca of grapevine:A disease complex or a complex of diseases[J]. Phytopathologia Mediterranea,2000,39(1):16-20.
[18] CORREIA K C,CAMARA M P S,BARBOSA M A G,SALES R,AGUSTI-BRISACH C,GRAMAJE D,LEON M,GARCIAJIMENEZ J G,ABAD- CAMPOS P,ARMENGOL J,MICHEREFF S. Fungal trunk pathogens associated with table grape decline in Northeastern Brazil[J]. Phytopathologia Mediterranea,2013,52(2):380-387.
[19] DIAZ G A,LATORRE B A. Infection caused by Phaeomoniella chlamydospora associated with esca-like symptoms in grapevine in Chile[J].Plant Disease,2014,98(3):351-360.
[20] BARANEK M,ARMENGOL J,PECENKA J,CALZARANO F,PENAZOVA E,VACHUM M,EICHMEIER A. Incidence of symptoms and fungal pathogens associated with grapevine trunk diseases in Czech vineyards: first example from a north-eastern European grape-growing region[J]. Phytopathologia Mediterranea,2018,57(3):449-458.
[21] GRAHAM A B,JOHNSTON P R,WEIR B S.Three new Phaeoacremonium species on grapevines in New Zealand[J]. Australasian Plant Pathology,2009,38(5):505-513.
[22] ESSAKHI S,MUGNAI L,CROUS P W,GROENEWALD J Z,SURICO G. Molecular and phenotypic characterisation of novel Phaeoacremonium species isolated from esca diseased grapevines[J].Persoonia,2008,21(1):119-134.
[23] WHITE C L,HALLEEN F,FISCHER M,MOSTERT L. Characterisation of the fungi associated with esca diseased grapevines in South Africa[J].Phytopathologia Mediterranea,2011,50(4):S204-S223.
[24] SPIES C F J,MOYO P,HALLEEN F,MOSTERT L. Phaeoacremonium species diversity on woody hosts in the Western Cape Province of South Africa[J].Persoonia,2018,40(37):26-62.
[25] GRAMAJE D,ARMENGOL J,MOHAMMADI H,MOSTERT B L. Novel Phaeoacremonium species associated with petri disease and esca of grapevine in Iran and Spain[J]. Mycologia,2009,101(6):920-929.
[26] ESKALEN A,ROONEY-LATHAM S,GUBLER W D. Occurrence of Togninia fraxinopennsylvanica on esca-diseased grapevines (Vitis vinifera) and declining ash trees (Fraxinus latifolia)in California[J].Plant Disease,2006,96(6):168.
[27] BERRAF-TEBBAL A,BOUZNAD Z,SANTOS J M,COELHO M A,PEROS J-P,PHILLIPS A J L. Phaeoacremonium species associated with Eutypa dieback and esca of grapevines in Algeria[J].Phytopathologia Mediterranea,2011,50(4):S86-S97.
[28] GRAMAJE D,ARMENGOL J,COLINO M I,SANTIAGO R,MORALEJO E,OLMO D,LUQUE J,MOSTERT L. First report of Phaeoacremonium inflatipes,P.iranianum,and P.sicilianum causing Petri disease of grapevine in Spain[J]. Plant Disease,2009,93(9):964-965.
[29] CROUS P W,GAMS W,WINGFIELD M J,VAN WYK P S.Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections[J]. Mycologia,1996,88(5):786-796.
[30] ÚRBEZ-TORRES J R,HAAG P,O'GORMAN D T,BOWEN P.Grapevine trunk diseases in British Columbia: Incidence and characterization of the fungal pathogens associated with Esca and Petri diseases of grapevine[J]. Plant Disease,2014,98(4):469-482.
[31] MOSTERT L,GROENEWALD J Z,SUMMERBELL R C,ROBERT V,SUTTON D A,PADHYE A A,CROUS P W. Species of Phaeoacremonium associated with infection in humans and environmental reservoirs in infected woody plants[J]. Journal of Clinical Microbiology,2005,43(4):1752-1767.
[32] YE Q T,MANAWASINGHE I,ZHANG W,MUGNAI L,HYDE K,LI X H,YAN J Y. First Report of Phaeoacremonium minimum associated with grapevine trunk diseases in China[J].Plant Disease,2020,104(2):1259.
[33] SILVA M A,CORREIA K,ANGELICA M,BARBOSA C,PAZ M,CAMARA S,GRAMAJE D,MICHEREFF S. Characterization of Phaeoacremonium isolates associated with petri disease of table grape in northeastern Brazil,with description of Phaeoacremonium nordesticola sp. nov.[J]. European Journal of Plant Pathology,2017,149:695-709.
[34] CROUS P W,GAMS W,WINGFIELD M J,VAN WYK P S.Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections[J]. Mycologia,1996,88(5):786-796.
[35] ESSAKHI S,MUGNAI L,CROUS P W,GROENEWALD J Z,SURICO G.Molecular and phenotypic characterization of novel Phaeoacremonium species isolated from Esca diseased grapevines[J].Persoonia,2008,21(1):119-134.
[36] MOSTERT L,HALLEEN F,FOURIE P,CROUS P W. A review of Phaeoacremonium species involved in Petri disease and Esca of grapevines[J]. Phytopathologia Mediterranea,2006,45(1):S12-S29.
[37] MOSTERT L,GROENEWALD J Z,SUMMERBELL R C,GAMS W,CROUS P W. Taxonomy and pathology of Togninia(Diaporthales) and its Phaeoacremonium anamorphs[J]. Studies in Mycology,2006,54(54):1-115.
[38] CLOETE M,FISCHER M,DU PLESSIS I L,MOSTERT L,HALLEEN F.A new species of Phellinus sensu stricto associated with esca on grapevine in South Africa[J].Mycological Progress,2016,15(3):1-9.
[39] CHAMBERLAIN G C,WILLISON R S,TOWNSHEND D J I,DE RONDE J H.Two fungi associated with dead-arm disease of grapes[J].Canadian Journal of Botany,1964,42(4):351-355.
[40] SIEBERT J B. Eutypa: the economic toll on vineyards[J].Wines and Vines,2001,40:50-56.
[41] ÚRBEZ-TORRES J R.The status of Botryosphaeriaceae species infecting grapevines[J]. Phytopathologia Mediterranea,2011,50(4):5-45.
[42] DISSANAYAKE A J,PHILLIPS A J L,LI X H,HYDE K D.Botryosphaeriaceae:Current status of genera and species[J].Mycosphere,2016,7(7):1001-1073.
[43] ÚRBEZ- TORRES J R,LEAVITT G M,VOEGEL T M,GUBLER W D. Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California[J]. Plant Disease,2006,90(12):1490-1503.
[44] JAYAWARDENA R S, PURAHONG W, ZHANG W, WUBET T, LI X H, LIU M, ZHAO W, HYDE K D, LIU L H, YAN J.Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches[J]. Fungal Diversity,2018,90:1-84.
[45] YAN J Y,XIE Y,ZHANG W,WANG Y,LI X H. Species of Botryosphaeriaceae involved in grapevine dieback in China[J].Fungal Diversity,2013,61(1):221-236.
[46] COMONT G,MAYET V,CORIO-COSTET M F.First report of Lasiodiplodia viticola,Spencermartinsia viticola and Diplodia intermedia associated with Vitis vinifera grapevine decline in French vineyards[J].Plant Disease,2016,100(11):2328.
[47] LINALDEDDU B T,DEIDDA A,SCANU B,FRANCESCHINA A,SERRA S,BERRAF-TEBBAL A,ZOUAOUI BOUTITI M,BEN JAMAA M L,PHILIPS A J L. Diversity of Botryosphaeriaceae species associated with grapevine and other woody hosts in Italy,Algeria and Tunisia,with descriptions of Lasiodiplodia exigua and Lasiodiplodia mediterranea sp. nov.[J]. Fungal Diversity,2015,71(1):201-214.
[48] CARLUCCI A,CIBELLI F,LOPS F,RAIMONDO M L. Characterization of Botryosphaeriaceae species as causal agents of trunk disease on grapevines[J]. Plant Disease,2015,99(12):1678-1688.
[49] BILLONES-BAAIJENS R,SAVOCCHIA S.A review of Bryosphaeriaceae species associated with grapevine trunk diseases in Australia and New Zealand[J]. Australasian Plant Pathology,2018,48(1):1-16.
[50] BURGESS T I,TAN Y P,GARNAS J,EDWARDS J,SCARLETT K A,SHUTTLEWORTH L A,DANIEL R,DANN E K,PARKINSON L E,DINH Q,SHIVAS R G,JAMI F.Current status of the Botryosphaeriaceae in Australia[J].Australasian Plant Pathology,2019,48(1):35-44.
[51] CORREIA K C,SILVA M A,DE MORAIS M A,ARMENGOL J,PHILLIPS A J L,CAMARA M P S,MICHEREFF S J. Phylogeny,distribution and pathogenicity of Lasiodiplodia species associated with dieback of table grape in the main Brazilian exporting region[J].Plant Pathology,2016,65(1):92-103.
[52] ABREO E,MARTINEZ S,BETTUCCI L,LUPO S. Characterization of Botryosphaeriaceae species associated with grapevines in Uruguay[J].Australasian Plant Pathology,2013,42(3):241-249.
[53] PINTOS C,REDONDO V,COSTAS D,AGUIN O,MANSILLA P.Fungi associated with grapevine trunk diseases in nurseryproduced Vitis vinifera plants[J]. Phytopathologia Mediterranea,2018,57(3):407-424.
[54] BERRAF-TEBBAL A,GUEREIRO M A,PHILLIPS A J L.Phylogeny of Neofusicoccum species associated with grapevine trunk disease in Algeria,with description of Neofusicoccum algeriense sp. nov. [J]. Phytopathology Mediterranean,2014,53(53):416-427.
[55] PINTOS V C, REDONDO F V,AGUIN C O, MANSILLA V J P. First report of cankers and dieback caused by Neofusicoccum mediterraneum and Diplodia corticola on grapevine in Spain[J].Plant Disease,2011,95(10):1315.
[56] CATER M V,PRICE T V. Eutypa armeniacae associated with vascular disease in grapevine and barberry[J]. Australian Plant Pathology Society,1973,2(4):27.
[57] GRAMAJE D,ÚRBEZ-TORRES J R,SOSNOWSKI M R.Managing grapevine trunk diseases with respect to etiology and epidemiology:Current strategies and future prospects[J].Plant Disease,2018,102(1):1-28.
[58] TROUILLAS F P,ÚRBEZ-TORRES J R,GUBLER W D. Diversity of diatrypaceous fungi associated with grapevine canker diseases in California[J].Mycologia,2010,102(2):319-336.
[59] PITT W M,TROUILLAS F P,GUBLER W D,SAVOCCHIA S,SOSNOWSKI M R. Pathogenicity of diatrypaceous fungi on grapevines in Australia[J].Plant Disease,2013,97(6):749-756.
[60] MOYO P,MOSTERT L,SPIES C F J,DAMM U,HALLEEN F. Diversity of Diatrypaceae species associated with dieback of grapevines in South Africa,with the description of Eutypa cremea sp.nov.[J].Plant Disease,2018,102(1):220-230.
[61] MENDES M A S DA SILVA V L,DIANESE J C,FERREIRA M,SANTOS D,CUNHA-NETO E,URBEN A F,CASTRO J.Fungos em plants no Brasil[M]. Brasilia: Embrapa-SPI/Embrapa-Cenargen,1998:555.
[62] PEROS J P,JAMAUX-DESPREAUX I,BERGER G,GERBA D. The potential importance of diversity in Eutypa lata and cocolonizing fungi in explaining variation in development of grapevine dieback[J]. Mycological Research,1999,103(11):1385-1390.
[63] HOLEVAS C D,CHITZANIDIS A,PAPPAS A C,TZAMOS E C,ELENA K,PSALLIDAS P G,ALIVIZATOS A S,PANAGOPOULOS C G,KYRIAKOUPLOU P E,BEM F P,LASCARIS D N,VELISSARIOU D E,VLOUTOGLOU I,AMALYTIS S C,PAPLOMATAS E J,ASPROMOUGOS J S,VARVERI C.Disease agents of cultivated plants observed in Greece from 1981 to 1990[J]. Benaki Phytopathologique Institute Athens Greece,2000,19(1):1-96.
[64] LARDNER R,STUMMER B E,SOSNOWSKI M R,SCOTT E S.Molecular identification and detection of Eutypa lata in grapevine[J].Mycological Research,2005,109(7):799-808.
[65] ROLSHAUSEN P E,BAUMGARTNER K,TRAVADON R,POUZOULET J,FUJIYOSHI P,WILCOX W F. Identification of Eutypa spp. Causing Eutypa dieback of grapevine in Eastern North America[J].Plant Disease,2014,98(4):483-491.
[66] LUQUE J,MARTOS S,AROCA A,RAPOSO R,GARCIAFIGUERES F. Symptoms and fungi associated with declining mature grapevine plants in Northeast Spain[J]. Journal of Plant Pathology,2009,91(2):381-390.
[67] PAOLINELLI-ALFONSO M,SERRANO-GOMEZ C,HERNANDEZ-MARTINEZ R. Occurrence of Eutypella microtheca in grapevine cankers in Mexico[J]. Phytopathologia Mediterranea,2015,54(1):86-93.
[68] DIAZ G A,PREHN D,LATORRE B A. First report of Cryptovalsa ampelina and Eutypella leprosa associated with grapevine trunk diseases in Chile[J].Plant Disease,2011,95(4):490.
[69] 李华,李茹一,王华.酿酒葡萄新病害-葡萄顶枯病[J].酿酒科技,2007,155(5):48-50.LI Hua,LI Ruyi,WANG Hua. New disease for wine-making grape:Eutypa dieback[J]. Liquor-Making Science & Technology,2007,155(5):48-50.
[70] REDDICK.Necrosis of grapevine[M].America:Cornell University,1909,263:323-343.
[71] ÚRBEZ-TORRES J R,PEDUTO F,SMITH R J,GUBLER W D.Phomopsis dieback:a grapevine trunk disease caused by Phomopsis viticola in California[J]. Plant Disease,2013,97(12):1571-1579.
[72] VAN NIEKERK J,GROENEWALD J Z,FARR D F,FOURIE P H,HALLEER F,CROUS P W.Reassessment of Phomopsis species on grapevines[J]. Australasian Plant Pathology,2005,34(1):27-39.
[73] GUARNACCIA V,GROENEWALD J Z,WOODHALL J,ARMENGOL J,CINELLI T,EICHMEIER A,EZRA D,FONTAINE F,GRAMAJE D,GUTIERREZ-AGUIRREGABIRIA A,KALITERNA J,KISS L,LARIGNON P,LUQUE J,MUGNAI L,NAOR V,ROPOSO R,SANDOR E,VACAY K Z,CROUS P W. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe[J].Persoonia- Molecular Phylogeny and Evolution of Fungi,2018,40(1):135-153.
[74] MANAWASINGHE I S,DISSANAYAKE A J,LI X H,LIU M,WANASINGHE D N,XU J P,ZHAO W S,ZHANG W,ZHOU Y Y,HYDE K D,BROOKS S,YAN J Y.High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China[J].Frontiers in Microbiology,2019,10:1-28.
[75] MOSTERT L,CROUS P W,KANG J C,PHILLIPS A J. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa:morphological,cultural,molecular and pathological characterization[J]. Mycologia,2001,93(1):146-167.
[76] UDAYANGA D,CASTLEBURY L A,ROSSMAN A Y,CHUKEATIROTE E,HYDE K D. Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex[J].Fungal Diversity,2014,67(1):203-229.
[77] LESUTHU P,MOSTERT L,SPIES C F,MOYO P,REGNIER T,HALLEEN F. Diaporthe nebulae sp. nov. and first report of D. cynaroidis,D. novem,and D. serafiniae on grapevines in South Africa[J].Plant Disease,2019,103(5):808-817.
[78] 仇恒军. 葡萄蔓枯病的发生与防治[J]. 现代农村科技,2011(21):28.QIU Hengjun. The occurrence and control of Phomopsis viticola Redd.[J].Xiandai Nongcun Keji,2011(21):28.
[79] LAWRENCE D P,TRAVADON R,BAUMGARTNER K. Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in northern California[J]. Mycologia,2015,107(5):926-940.
[80] YANG Q,FAN X L,GUARNACCIA V,TIAN C M.High diversity of Diaporthe species associated with dieback diseases in China,with twelve new species described[J]. MycoKeys,2018,39:97-149.
[81] KUO K C,LEU L S.Phomopsis vitimegaspora:a new pathogenic Phomopsis from vines[J].Mycotaxon,1998,66:497-499.
[82] PHILLIPS A J L. The relationship between Diaporthe perjuncta and Phomopsis viticola on grapevines[J]. Mycologia,1999,91(6):1001-1007.
[83] GRASSO S,MAGNANO DI SAN LIO G.Infezioni di Cylindrocarpon obtusisporum su piante di vite in Sicilia[J].Vitis Geilweilerhof,1975,14:36-39.
[84] HALLEEN F,SCHROERS H J,GROENEWALD J Z,REGO C,OLIVEIRA H,CROUS P W.Neonectria liriodendri sp.nov.,the main causal agent of black foot disease of grapevine[J].Study in Mycology,2006,55:227-234.
[85] SCHECK H J,VASQUEZ S J,GUBLER W D. First report of black-foot disease,caused by Cylindrocarpon obtusisporum,of grapevine in California[J].Plant Disease,1998,82(4):448.
[86] AGUSTI-BRISACH C,ARMENGOL J. Black-foot disease of grapevine: an update on taxonomy,epidemiology and management strategies[J]. Phytopathologia Mediterranea,2013,52(2):245-261.
[87] GRAMAJE D,ARMENGOL P. Fungal trunk pathogens in the grapevine propagation process: potential inoculum sources,detection,identification,and management strategies[J]. Plant Disease,2011,95(9):1040-1055.
[88] MALUTA D R,LARIGNON P. Pied-noir: Mieux vaut prévenir[J].Viticulture,1991,11:71-72.
[89] SURICO G,MUGNAI L,MARCHI G. Older and more recent observations on Esca: A critical overview[J]. Phytopathologia Mediterranea,2006,45(4):S68-S86.
[90] KOIKE S T,BETTIGA L J,NGUYEN T T,GUBLER W D.First report of Cylindrocladiella lageniformis and C. peruviana as grapevine pathogens in California[J].Plant Disease,2016,100(8):1783-1784.
[91] VAN COLLER G J,DENMAN S,GROENEWALD J Z,LAMPRECHT S C,CROUS P W. Characterization and pathogenicity of Cylindrocladiella spp. associated with root and cutting rot symptoms of grapevines in nurseries[J]. Australasian Plant Pathology,2005,34(4):489-498.
[92] CARLUCCI A,LOPS F,MOSTERT L. HALLEEN F,RAIMONDO M L. Occurrence fungi causing black foot on young grapevines and nursery rootstock plants in Italy[J]. Phytopathologia Mediterranea,2017,56(1):10-39.
[93] BERLANAS C,OJEDA S,LOPEZ-MANZANARES B,ANDRES-SODUPE M,BUJANDA R,MARTINEZ-DIZ M D P,DIAZ-LOSADA E,GRAMAJE D. Occurrence and diversity of black-foot disease fungi in symptomless grapevine nursery stock in Spain[J].Plant Disease,2019,104(1):94-104.
[94] PECENKA J,EICHMEIER A,PENAZOVA E,BARANEK M,LEON M,ARMENGOL J. First report of Dactylonectria torresensis causing black-foot disease on grapevines in the Czech Republic[J].Plant Disease,2018,102(10):2038-2039.
[95] AIGOUN-MOUHOUS W,ELENA G,CABRAL A,LEON M,SABAOU N,ARMENGOL J,CHAOUIA C,MAHAMEDI A E,BERRAF-TEBBAL A. Characterization and pathogenicity of Cylindrocarpon-like asexual morphs associated with black foot disease in Algerian grapevine nurseries,with the description of Pleiocarpon algeriense sp.nov[J].European Journal of Plant Pathology,2019,154(5):887-901.
[96] LI X,YAN J,KONG F,QIAO G,ZHANG Z,WANG Z.Botryosphaeria dothidea causing canker of grapevine newly reported in China[J].Plant Pathology,2010,59(6):1170.
[97] 李茹一.葡萄顶枯病在中国的发现与鉴定[D].杨凌:西北农林科技大学,2007.LI Ruyi. Discovery and identifiction Eutypa diaback of grapevine in China[D].Yangling:Northwest A&F University,2007.
[98] 车升国,康玉柱,樊庆军,罗松,凌云.葡萄蔓枯病发病规律与防治措施分析[J].果农之友,2018(9):40-41.CHE Shengguo,KANG Yuzhu,FAN Qingjun,LUO Song,LING Yun. Analysis on the occurrence and control measures of grape vine blight[J].Fruit Growers’Friend,2018(9):40-41.
[99] 王永崇.作物病虫害分类介绍及其防治图谱:葡萄蔓割病及其防治图谱[J].农药市场信息,2016(11):66.WANG Yongcong. Introduction to the classification of crop diseases and insect pests and their control map: Grapevine cut disease and its control map[J]. Pesticide Market News,2016(11):66.
[100] WAGSCHAL I,ABOU-MANSOUR E,PETIT A N,CLEMENT C,FONTAINE F. Plant- microbe interactions[M]. India: Research Signpost,2008:367-391.
[101] LARIGNON P,FONTAINE F,FARINE S,CLEMENT C,BERTSCH C. Esca et black dead arm: deux acteurs majeurs des maladies du bois chez la vigne[J]. Comptes Rendus Biologies,2009,332(9):765-783.
[102] WAITE H,CROCKER J,FLETCHER G,WRIGHT P,DELAINE A. Hot water treatment in commercial nursery practice:an overview[J]. Australian & New Zealand Grape grower &Winemaker,2001,449:39-43.
[103] CROCKER J,WAITE H,WRIGHT P,FLETCHER G. Source area management: avoiding cutting dehydration and good nursery management may be the keys to successful hot water treatment[J].Australian & New Zealand Grape grower & Winemaker,2002,461:33-47.
[104] BERTSCH C,RAMIREZ-SUERO M,MAGNIN-ROBERT M,LARIGNON P,CHONG J,ABOU-MANSOUR E,SPAGNOLO A,CLEMENT C,FONTAINE F. Grapevine trunk diseases:complex and still poorly understood [J]. Plant Pathology,2013,62(2):243-265.