猕猴桃(Actinidia chinensis)是一种多年生雌雄异株落叶藤本植物[1]。果实中含有丰富的维生素C、可溶性膳食纤维、矿物质元素、原花青素、黄酮等功效物质[2-3],具有较高的食用价值和一定的保健效果,尤其是在降血压血脂、降胆固醇、生津润燥、美容养颜、安神益智等方面具有较好的作用[4-5],素来备受广大消费者的喜爱[6]。我国猕猴桃种植面积约25万hm2、产量约120 万t,位居世界第1 位。猕猴桃是我国农业发展的主要果树之一[7],但果实不耐贮藏严重影响了其产业发展,因此果实采收后应找到合适的方法尽快贮藏[8]。由于化学防腐保鲜剂存在诸多弊端,而植物提取物取材容易、经济实惠、杀菌效果好,对人体和环境无危害,因此利用天然提取物作为防腐保鲜剂已成为当今热点[9-10]。黄芩、丁香、肉桂、皂角刺、广藿香、菖蒲和油茶粕等植物中含有丰富的黄酮类物质、多酚类物质、挥发油类物质和皂苷类物质,这些物质对真菌具有较好的杀菌抑菌效果,同时皂苷类物质还是天然的表面活性剂,其植物提取物常被用于果蔬的防腐保鲜,且多种提取物混用具有一定的协同作用,但复配提取物在猕猴桃鲜果协同防腐保鲜上的应用较少。笔者前期采用响应面法对经过抑菌植物筛选后所得的7种植物提取物[11]进行协同抑菌试验,得到7 种植物提取物最佳抑菌的复配比,即黄芩、丁香、肉桂、皂角刺、广藿香、菖蒲和油茶粕的质量配方比为1.375∶1.125∶0.45∶0.5∶1.35∶1.25∶2.8;0.8 mg·mL-1复配物处理时,对病原菌的抑制率可达85.28%。笔者旨在利用前期研究中所得中药提取复配物产生的天然抗菌物质,代替化学防腐保鲜剂,开发适合猕猴桃果实贮藏特性的植物源防腐保鲜剂。以‘红阳’猕猴桃果实为试材,采用中药提取物分别处理好果和病果(接种软腐病原菌孢子),贮藏在(4±1)℃冷库中,每隔一段时间测定一次相关指标,测定果实贮藏过程中不同时间段的各种生理生化指标、相关耐贮性指标与转录水平联合分析,同时对实验结果进一步采用主成分分析,分析中药提取物对猕猴桃果实耐贮藏的影响[12],分析中药提取物对提高猕猴桃果实贮藏性能的机制,继而为猕猴桃鲜果的绿色保藏提供一定理论基础。
1 材料和方法
1.1 材料与试剂
以5~8年生‘红阳’猕猴桃植株的成熟果实作为实验材料,果实采自湖南省长沙宁乡猕猴桃种植基地。实验所选果实大小适中、饱满圆润、无干枯、外观有光泽、无病虫害以及任何人为机械性损伤。供试病原菌为葡萄座腔菌和间壳座腔菌,由实验室(植物病虫害生物学与防控湖南省重点实验室课题组)自行分离鉴定所得。供试药剂为下列植物提取物,参考原有研究进行提取物制备[13],采用乙醇作为提取溶剂,850 W 超声提取2 次,每次提取2 h,减压蒸馏浓缩至20~30 mL后,提取物用30 mL三氯甲烷洗涤2~3次,再将其浓缩至浸膏状后干燥,4 ℃条件下保存,不同植物略有修改。药剂采用黄芩、丁香、肉桂、皂角刺、广藿香、菖蒲和油茶粕的提取混合物,质量配方比为1.375∶1.125∶0.45∶0.5∶1.35∶1.25∶2.8,提取物处理的终质量浓度为0.8 mg·mL-1(干药量)。同时参考李荣宇等[14](黄酮、多糖、皂苷)、赵永钦等[15](生物碱)、王萍等[16](多酚)、韩璐等[17](有机酸)、卢丽娜等[18](油酸)的方法对复配物中化学成分进行测定,通过液质联用技术测定提取物中主要抑菌化学物质的相对含量,测定结果如表1。
表1 提取复配物中部分化学成分的含量
Table 1 Contents of part chemical components in extracted compound
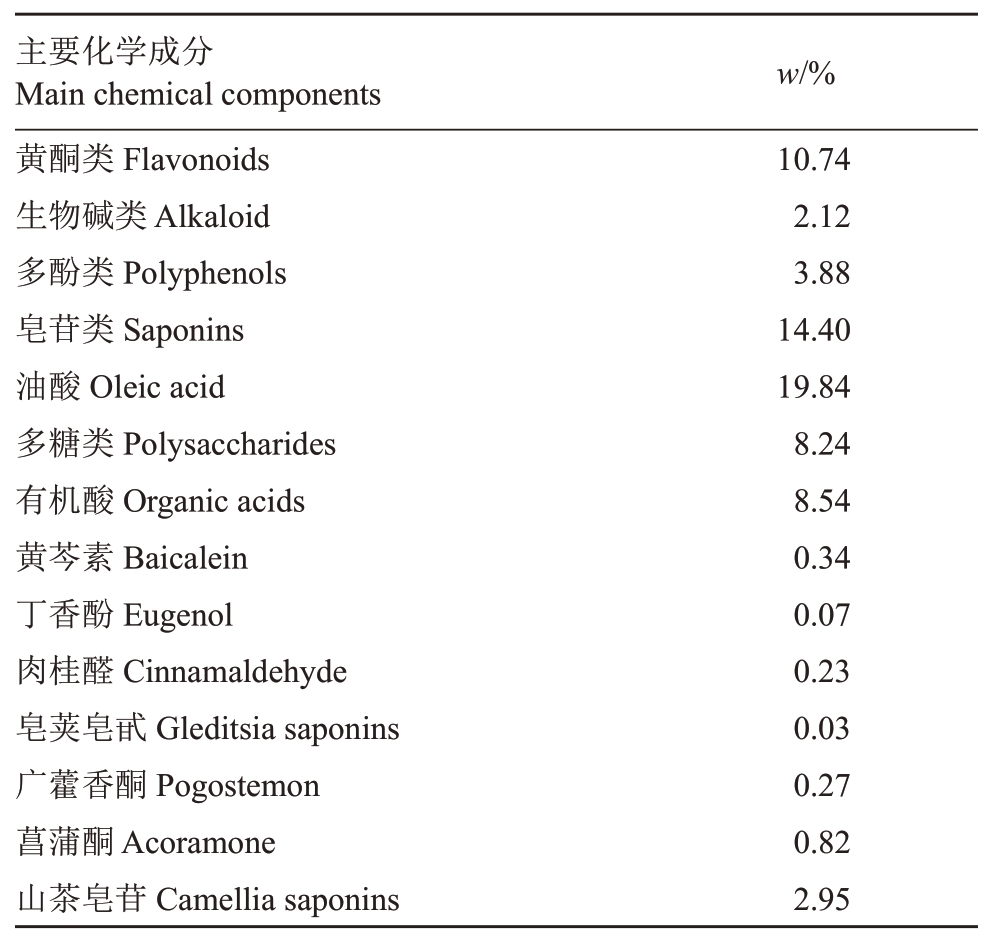
?
氢氧化钠、酚酞、草酸、淀粉、碘、碘化钾、茚三酮、甘氨酸、硝酸钙、咔唑、硫酸、半乳糖醛酸、乙醇、3,5-二硝基水杨酸、石英砂、乙酸、乙酸钠、高锰酸钾、H2O2、磷酸缓冲液、EDTA-Na2、核黄素、甲硫氨酸、氮蓝四唑,化学试剂来自国药集团化学试剂有限公司,均为分析纯。ETH ELISA kit 乙烯测定试剂盒、纤维素酶活性测定试剂盒、POD 酶活性测定试剂盒、蛋白质含量测定试剂盒、可溶性糖含量测定试剂盒均购置于索莱宝科技有限公司。
1.2 仪器与设备
ZW1105051705 紫外可见分光光度计(上海光谱仪器有限公司);AUW220D 电子天平(日本shimadzu公司);SC-10色差仪(上海人和科学仪器有限公司);GY-3果实硬度计(深圳蔚仪金相试验仪器有限公司);DK-98-IIA 电热恒温水浴锅(天津市泰斯特仪器有限公司);LY-TGL16MD 台式高速冷冻离心机(湘仪集团);QILINBEIER QB8002 振荡器、QILINBEIER KB3 涡旋混合器、QILINBEIER LX200 离心机、RAININ A1045549E 多道移液器(日本SANYO公司)等。
1.3 方法
1.3.1 实验设计与处理(1)果实采摘。晴天下午18:00—19:00 开始采摘,采摘后立即将猕猴桃果实分批放入已经预冷的塑料筐中(25 cm×38 cm×18 cm),运回实验冷库。
(2)果实前处理。先用自来水冲洗干净,然后先放在15 ℃冷库中自然晾干。
(3)果实处理。选取硬度大小、果形相似,成熟度一致的果实进行实验,菌处理为接种病原混合孢子悬浮液(1×105~5×105 cfu·mL-1),喷施菌孢子悬浮液后在(27±1)℃温度下贮藏48 h。再分别对好果和病果(菌处理)进行药剂处理。每个处理100个果实,3 次重复。用喷雾器把药剂均匀地喷在好果和病果的表面,然后用小毛刷涂抹均匀,所有果实均放在(4±1)℃、RH=90%~92%的冷库进行贮藏。
(4)实验分组及测定。实验共分为4个组,分别为对照组、菌处理组、药剂处理组和菌+药剂处理组。每个处理每隔10 d 取样1 次,每次随机取5~15个果实进行相关指标的测定,试验设3次重复。
1.3.2 果实外观品质的测定 (1)果实好果率测定。以好果实数占果实总数的百分比表示。好果率/%=好果数/果实总数×100。每10 d测定1次。
(2)果实失重率。采用称重法测定。失重率/%=(贮藏前质量-贮藏后质量)/贮藏前质量×100。
(3)果实硬度的测定。采用GY-3果肉硬度计测定。猕猴桃从冷库取出来后刮掉果实赤道面果皮,面积约为0.25 cm2,将探头插入果肉,读取硬度计上显示的数据,3次重复,单位为kg·cm-2。
(4)果实果心颜色的测定。采用SC-10 色差仪测定。猕猴桃从冷库取出来后,将果实从赤道面切开,测定果心处颜色。其中a*正值表示果面红色程度,a*负值表示果面绿色程度。
1.3.3 果实内在品质指标的测定 参考Brummell等[19]的方法对果实淀粉和果胶含量进行测定,参考曾邹林[20]的方法对果实可滴定酸、维生素C、可溶性固形物、氨基酸含量进行测定,果实可溶性糖和蛋白质含量采用相关试剂盒说明方法进行测定。
1.3.4 果实内在酶活性的测定 参考Famiani 等[21]的方法对果实果胶酶活性进行测定,参考黎桂坤[22]的方法对果实淀粉酶活性、果实CAT酶活性和果实SOD酶活性进行测定,果实POD酶活性和果实纤维素酶活性采用相关试剂盒说明方法进行测定。
1.3.5 果实呼吸、乙烯释放量的测定 参考Jhalegar等[23]的方法对果实呼吸强度进行测定,果实乙烯释放量采用相关试剂盒说明方法进行测定。
1.3.6 果实转录组分析 取每个处理贮藏28 d时果实皮下果肉,每个处理取10个果实果肉混匀,设置3个生物学重复。数据分析由上海美吉生物医药科技有限公司完成(基于Illumina Novaseq 6000 测序平台,对猕猴桃果肉转录出来的所有mRNA 进行测序,采用Illumina TruseqTM RNA sample prep Kit 方法进行文库构建)。
1.3.7 数据处理 采用SPSS 16.0 软件对数据进行主成分分析[24],采用Origin 2019和WPS 2019软件作图,采用Statistix 8.0软件进行数据显著性分析,图中不同大写字母表示同一时期不同处理间的极显著性差异(p <0.01)。
2 结果与分析
2.1 中草药处理对果实的保鲜效果
猕猴桃果实贮藏期间外在品质变化见图1。由图1-a可知,随着果实贮藏时间的增加,失重率一直增加,菌处理组相对于药剂组失重率更快,在贮藏80 d 时,菌处理组失重率达11.93%,相对于药剂组高70.21%,说明菌在快速消耗果实有机质。由图1-b可知,药剂组果实在50 d 内好果率均为100%,菌处理组此时仅为84%,在贮藏80 d 时药剂组好果率仍为86%,菌处理组仅为6%,此时几乎全部坏掉,而菌+药组好果率为70%,较对照组(65%)高,说明药剂可杀菌,同时也有较好防腐耐贮作用。由图1-c可知,硬度值在刚采摘时较高,为15.03 kg·cm-2,后期逐步下降,在20 d 时菌处理组果实硬度下降至6.03 kg·cm-2,此时果实已有一定软化,在贮藏40 d 时已可食用,硬度仅为2.83 kg·cm-2,而此时药剂处理组硬度仍为7.50 kg·cm-2,说明药剂可延缓果实软化,提高耐贮性。由图1-c可知,果实在刚开始贮藏时,果心的a 值较小(1.73),说明果心此时显现浅红色,随着果实贮藏期的延长,色度a值逐渐增大,说明果实果心红色逐渐增加明显,在果实贮藏20 d内,菌处理组和菌+药处理组有较明显的增加,剩余2组增加不明显,但在40 d 后各组果实红色均明显增加。综上,药剂可延缓果实的失重、果实硬度的降低及颜色的加深,提高果实的贮藏期。
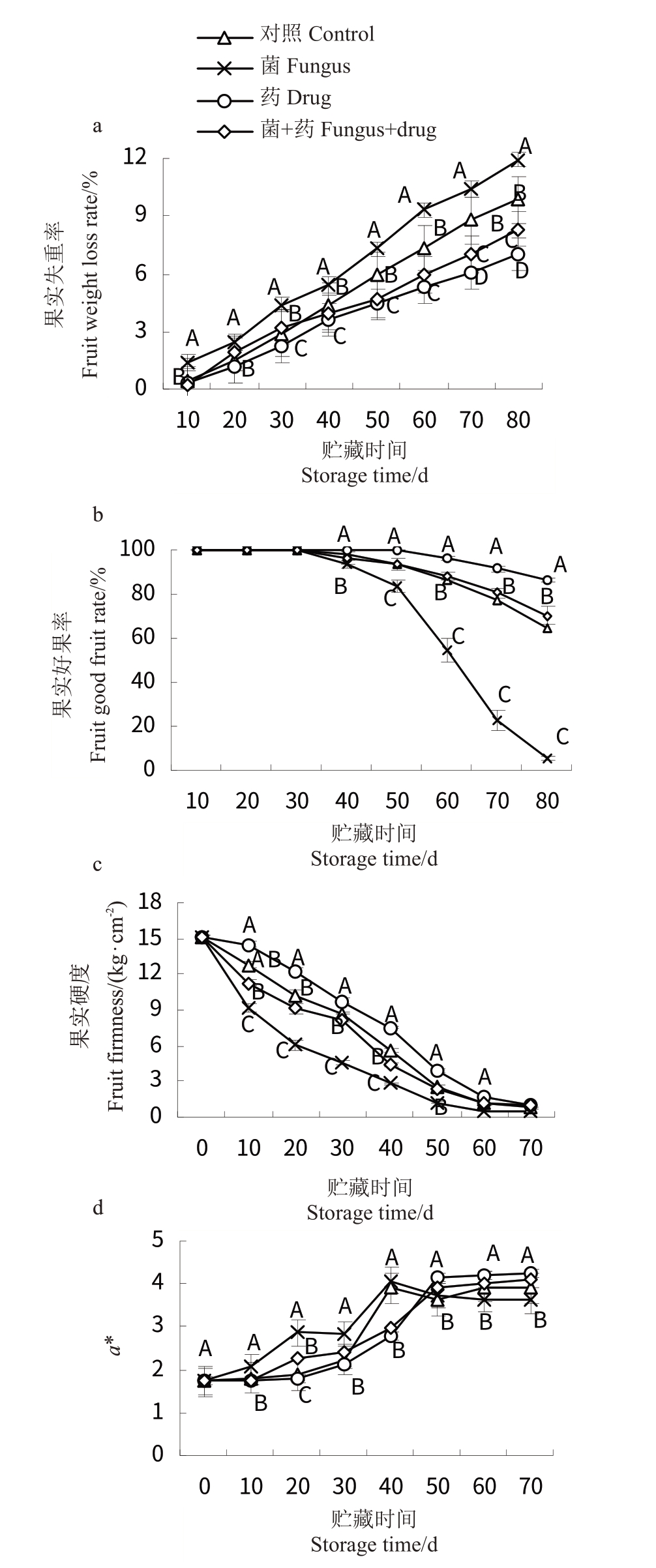
图1 果实外观品质分析
Fig.1 Analysis of fruit appearance quality
2.2 中草药处理对果实营养物质含量的影响
猕猴桃果实贮藏期间内在品质变化见图2。由图2-a可知,果实在贮藏20 d时,菌处理组果实可溶性固形物含量急速上升至16.8%,随后缓慢下降至13%左右,药剂、对照和菌+药剂组也有较大程度上的增加,但较菌处理组缓慢,峰值出现在贮藏40~50 d。由图2-b 可知,果实的酸度值持续下降,在贮藏40 d左右时,各处理组果实酸度值均呈现很大程度的降低,虽然药剂组可有效延缓果实酸值的降低,但在40 d 时相对于采摘时的1.189%下降了58.56%。由图2-c可知,果实中维生素C含量的变化趋势和酸值基本一致,随着贮藏期的延长,果实维生素C含量逐渐降低,贮藏50 d左右时,果实维生素C含量降低至趋于平缓,药剂组下降相对较慢,在贮藏10 d时,药剂组维生素C 含量较菌处理组高44.11%。由图2-d可知,糖含量与可溶性固形物含量的变化趋势大致一致,菌处理组果实在贮藏30 d 时糖含量达到了峰值(13.01%),而药剂组可延缓至贮藏50 d出现峰值(14.20%)。综上,药剂可延缓糖、可溶性固形物、维生素C 含量和酸度在贮藏后期的降低,亦可延缓糖和可溶性固形物含量在贮藏前期的增加;但菌处理则与药处理组表现出相反的效果,说明药剂可增加果实的耐贮性。
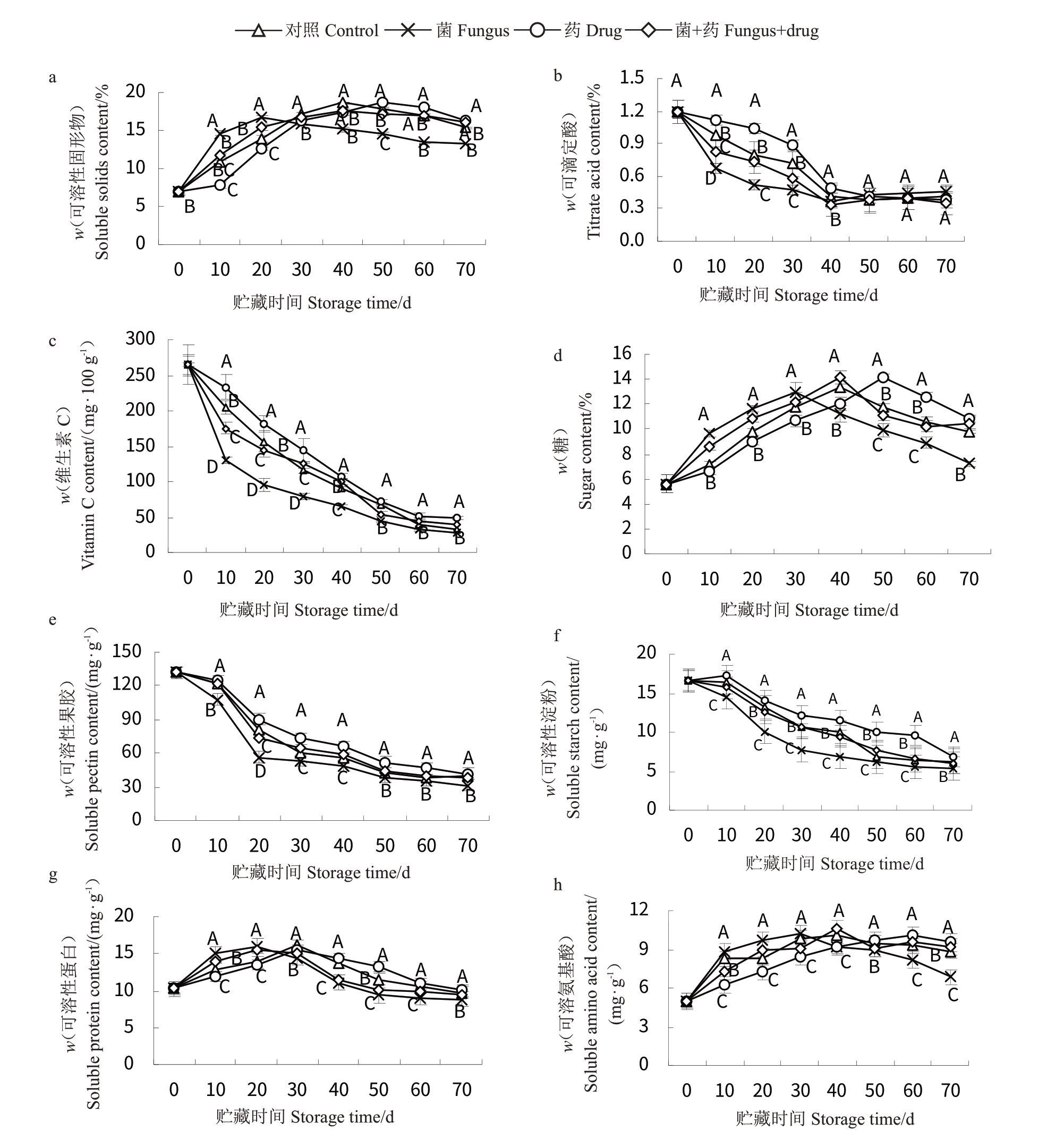
图2 果实内在品质测定
Fig.2 Fruit intrinsic quality measurement
2.3 中草药处理对果实营养物质含量的影响
由图2-e 可知,果实果胶含量呈逐步下降的趋势,且前20 d内各处理下降均较快,但药剂处理组可一定程度上延缓果胶含量的降低,在贮藏20 d 时较菌处理组高59.56%,在贮藏末期各组果胶含量已经非常低,仅为刚采摘时的25%左右。由图2-f 可知,果实可溶性淀粉含量的变化趋势和果胶较为相似,除了药剂处理组在10 d 有略微的升高外,其余各组随着贮藏时间的推移,淀粉含量持续降低,但药剂组降低较为缓慢,与其他处理组均表现出极显著性差异(p <0.01),例如在40 d 时,相对于菌处理组(6.838 mg·g-1),药处理组的含量高67.57%。由图2-g可知,菌、菌+药组可溶性蛋白质含量在贮藏20 d时出现了峰值,分别为16.02%、15.42%,对照与药剂组在贮藏30 d时出现峰值,分别为16.14%、15.56%,在贮藏70 d时可溶蛋白含量下降至平缓。由图2-h可知,氨基酸的变化趋势和蛋白质相似,在贮藏30 d时,菌处理组氨基酸含量达到了峰值(10.21 mg·g-1),然后随着贮藏时间的推移快速下降,而药处理组相对于其他3 组均表现为前期增加量、后期降低量相对缓慢。综上,淀粉和果胶含量的变化趋势较为一致,持续降低,蛋白与氨基酸含量的变化趋势较为一致,先升后降,药处理组相较于菌组,可降低或者延缓大分子的降解,提高果实的耐贮性。
2.4 中草药处理对果实贮藏期活性氧清除酶和几种水解酶活性的影响
贮藏期间各组果实内酶活性强弱如图3所示,6种酶均表现为先升后降的变化趋势。图3-a~c 为抗氧化(自由基清除)酶,菌处理果实SOD活性持续下降,中草药处理使果实SOD 前30 d 内出现了上升,之后缓慢下降,各时间段SOD活性与好果对照组差异不显著;中草药处理进一步提高了好果的SOD活性,各时间段与其对照的差异均极显著(p <0.01),在贮藏20 d 时,相对于菌剂处理组,酶活性提高了51.77%;POD 活性与SOD 活性表现出了相似的规律;中草药处理对CAT 的促进效应有所减弱,但0~50 d内,好果处理与其对照、病果处理与其对照间差异依然极显著(p <0.01)。表明中草药处理普遍提高了果实活性氧清除酶活性,并以对O2-清除酶(SOD和POD)的作用为主。图3-d~f属于大分子糖分解酶,淀粉酶、纤维素酶和果胶酶活性总体上均表现出贮藏20~30 d 内出现了上升,之后呈缓慢下降的趋势,菌处理组酶活性上升较为明显,各个时间段较其余3组几乎均具有极显著差异(p <0.01),药剂可有效抑制酶活性的升高,以及推迟酶活性峰值出现的时间,3 种酶在菌处理组贮藏20 d 时出现酶活性峰值,药处理组则在30 d时出现峰值,且远低于菌处理组的峰值,表明中草药处理普遍降低了果实多糖分解酶活性,并以对淀粉酶的抑制作用为主。综合6个酶活性可以看出,猕猴桃经过药剂处理后,抗自由基酶活性有所提高,对大分子酶活有一定的抑制作用,菌处理果实后效果相反,果实抗自由基酶活性得到抑制,对大分子酶活性出现了促进作用。采用药剂处理病果,其酶活性效果与对照组基本一致或略好。
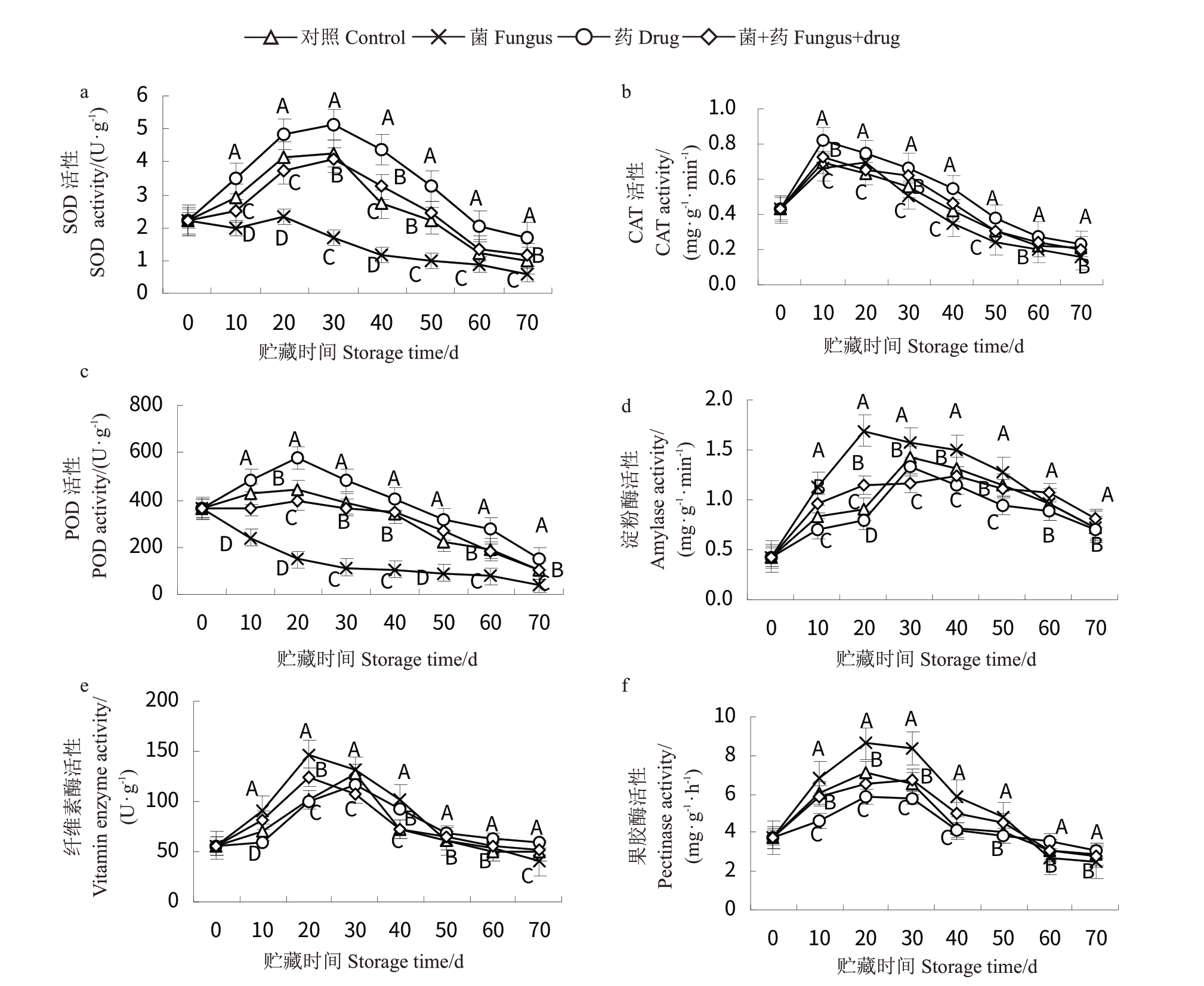
图3 果实酶活性变化
Fig.3 Changes of fruit enzyme activity
2.5 中草药处理对果实呼吸、乙烯释放量的影响
猕猴桃贮藏期间呼吸作用强度如图4所示。由图4-a可知,不同贮藏时间猕猴桃果实的呼吸强度具有较大的变化,总体表现为先升后降。除药剂处理组猕猴桃在30 d 时出现最大呼吸强度外,其余3 个处理组猕猴桃果实均在贮藏20 d时出现最大呼吸强度,说明猕猴桃属于呼吸跃变型,同时中药复配物可延缓以及降低呼吸强度。例如在贮藏10 d 时,药剂处理组较其他处理组均极显著降低(p <0.01),在贮藏10 d 时,药剂处理组相对于菌处理组降低了29.14%。果实乙烯释放量与呼吸强度的变化趋势大致一致,为先升后降的趋势(图4-b),菌处理组在贮藏20 d 时,乙烯释放量率先达到峰值(2 440.36 nmol·kg-1·h-1),与其他处理具有极显著差异(p <0.01),其他处理组在贮藏30 d 时达到峰值,在贮藏40 d后,各处理乙烯释放量开始急速下降,此时果实已较为软化。因此,可说明中药复配物有降低呼吸强度、乙烯释放量的作用。
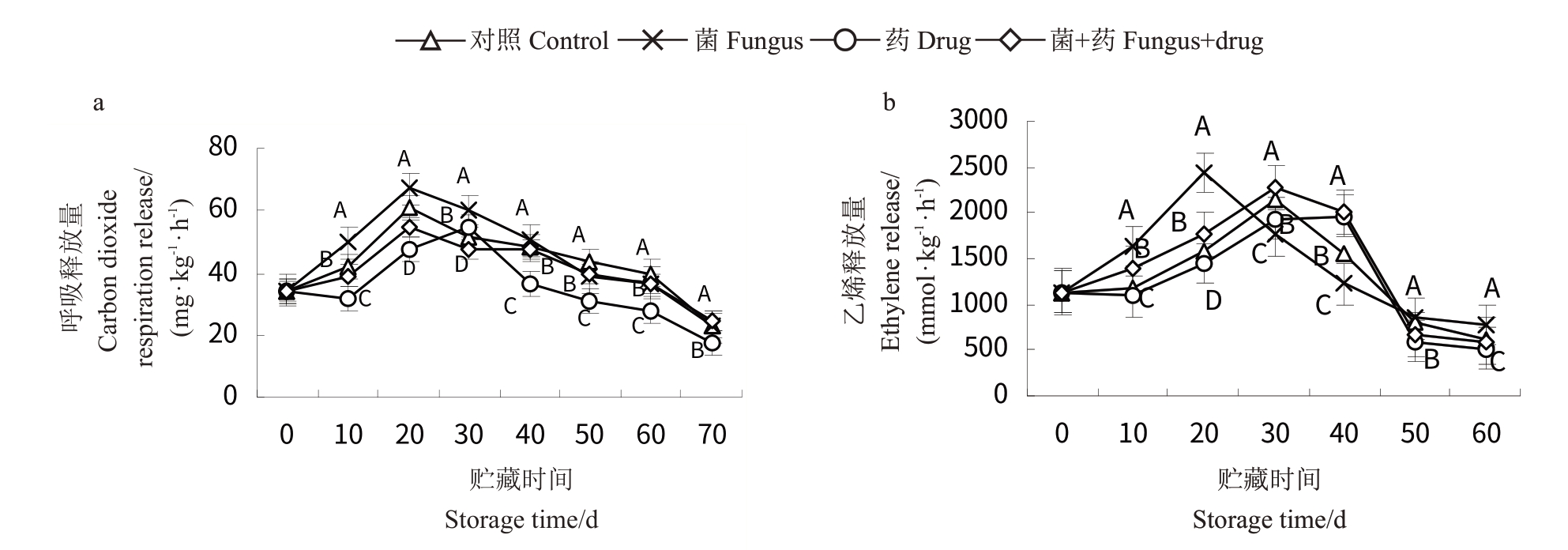
图4 果实呼吸强度、乙烯释放量变化
Fig.4 Changes of fruit respiration and ethylene release
2.6 中草药处理对果实贮藏期关键基因表达水平的影响
贮藏过程中影响果实衰老软化的因素有很多,其中主要有抗氧化酶代谢过程、乙烯代谢过程、能量代谢过程和多糖代谢过程等,它们分别与果实抗氧化酶活性、维生素C含量、有机酸含量,乙烯含量,呼吸作用,淀粉酶、果胶酶、纤维素酶活性、可溶性固形物、可溶性糖含量、硬度等有着密切的关系。4个处理组果实贮藏28 d 时,不同代谢过程的转录水平列见表2,各种抗氧化酶基因转录水平总体上表现为菌处理组最低,对照、菌+药剂处理组一般,药剂处理组最高,列如超氧化物歧化酶Achn319351 在药剂、对照、菌+药剂、菌处理组四组中的转录水平分别为192.33、142.46、166.7、23.27。乙烯代谢基因在菌处理组中表现出最高的转录水平,对照和药剂处理组表现出较低的转录水平,菌+药剂处理组表现出一般的转录水平,列如ACC 酶Achn326461 在药剂、对照、菌+药剂、菌处理组四组中的转录水平分别为721.2、880.0、5004、15 881。能量代谢基因总体上菌处理、菌+药剂处理组转录水平较高,对照、药剂处理组表现出较低的转录水平,如磷酸烯醇式丙酮酸羧激酶Achn041831 在药剂、对照、菌+药剂、菌处理组四组中的转录水平分别为76.56、109.2、154.4、164.1。4组果实多糖代谢基因转录水平与其能量代谢基因转录水平相似,如淀粉酶Achn217211在药剂、对照、菌+药剂、菌处理组四组中的转录水平分别为146.7、166.3、179.1、225.4。各组果实基因转录水平与果实各种相应酶活性的强弱基本一致。
表2 果实采后部分关键基因转录水平
Table 2 Transcription level of part key genes in postharvest fruits
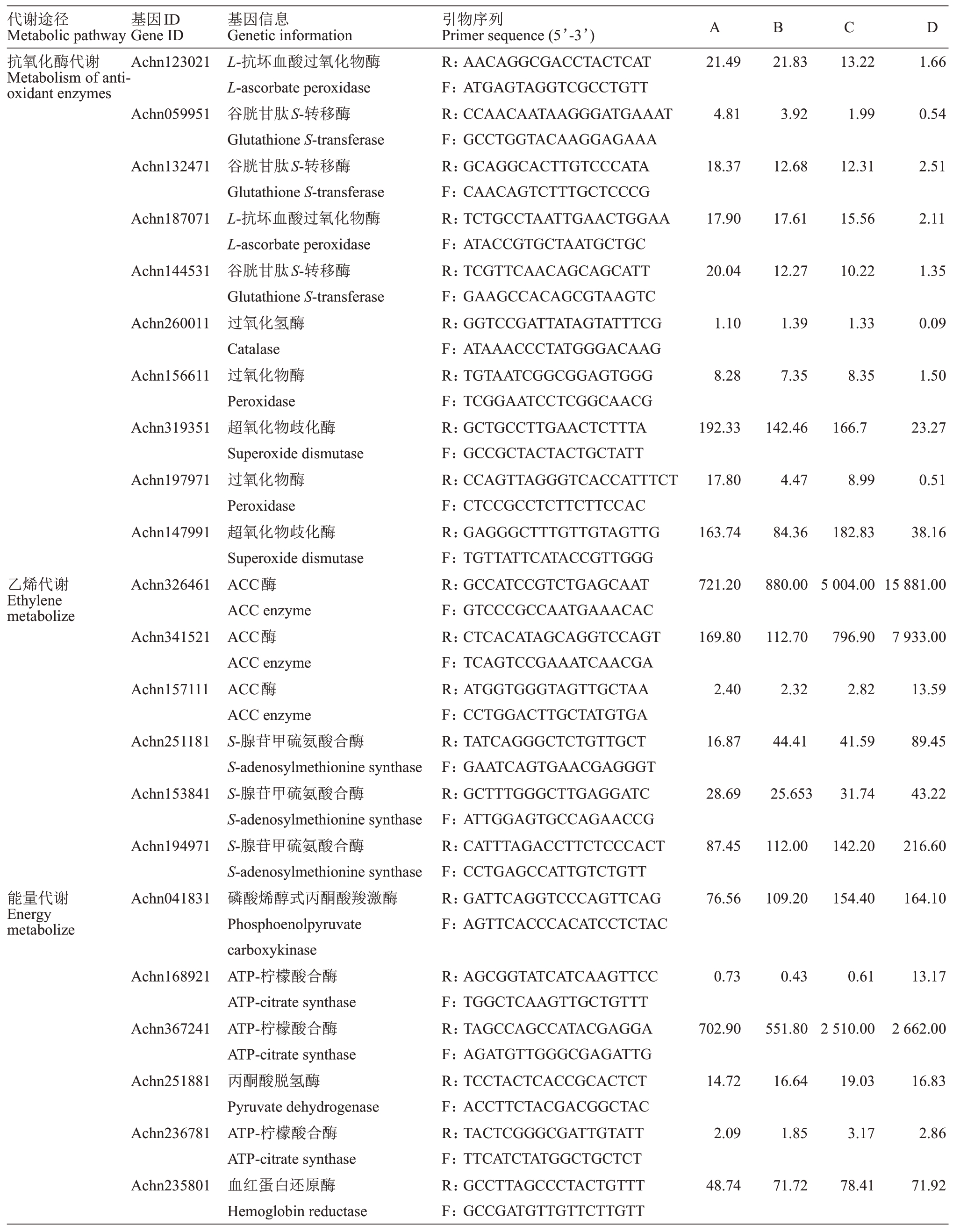
注:A.药剂处理组;B.对照组;C.菌+药剂处理组;D.菌处理组。“-”表示未测出。
Note:A.Drug treatment group;B.Control group;C.Fungus+drug group;D.Fungus group.“-”indicates not detected.
?
表2 (续) Table 2(continued)
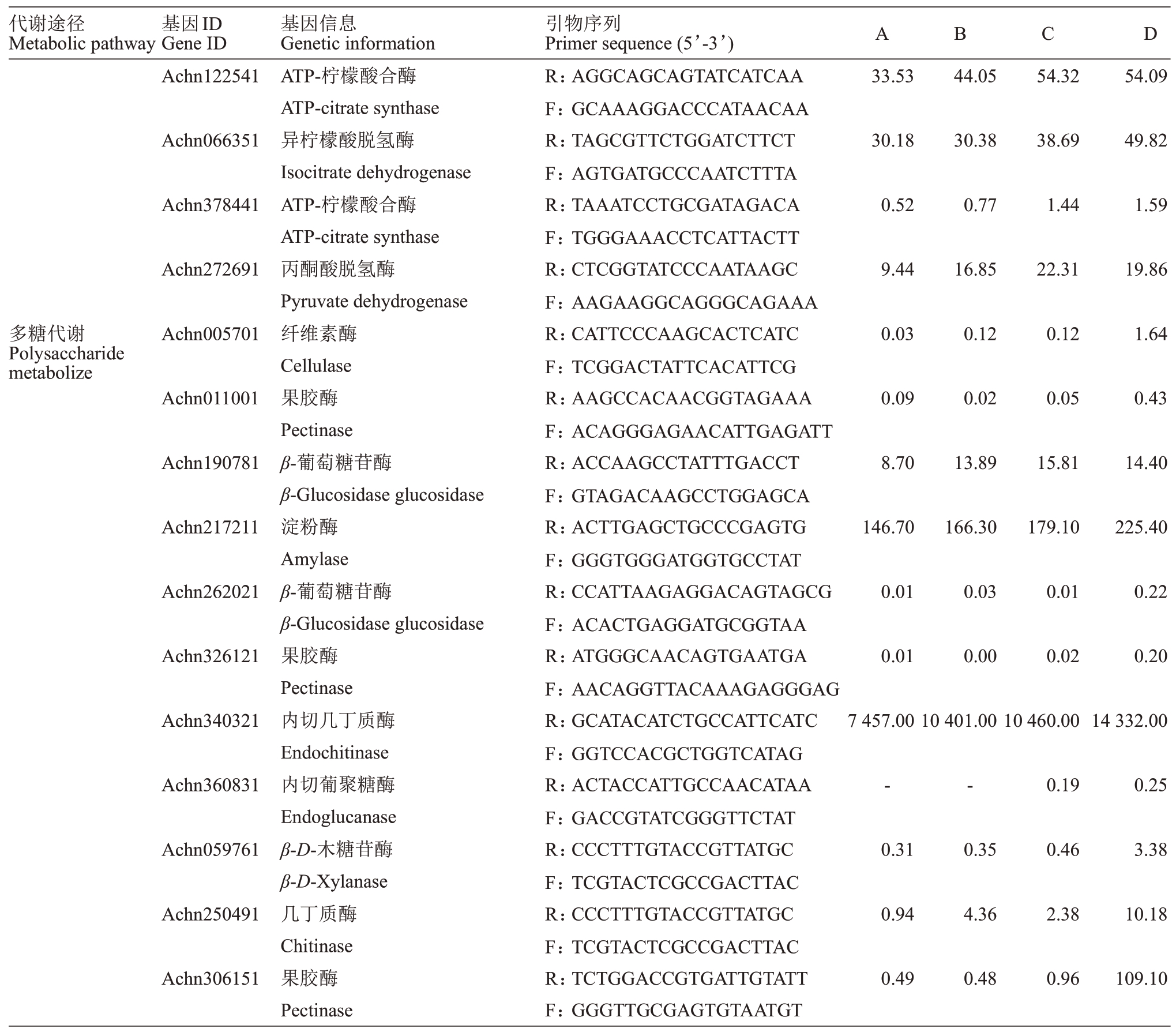
?
2.7 主成分分析
2.7.1 主成分个数的确定 由图5 可知,药剂处理组第一个主成分的贡献率为58.23%,第二个主成分的贡献率为36.12%;对照组第一个主成分的贡献率为55.52%,第二个主成分的贡献率为38.13%;菌+药剂处理组第一个主成分的贡献率为54.18%,第二个主成分的贡献率为39.54%;菌处理组第一个主成分的贡献率为50.83%,第二个主成分的贡献率为45.39%。4 个处理组,前2 项的累积贡献率均大于93.65%,基本包含了原来变量的大部分信息,所以选取前两个主要成分完全可行。4 个处理,其中可溶性固形物、糖、氨基酸含量、淀粉酶活性等均在主成分1 的左侧,说明这些指标对第一成分的贡献率比较小,这些指标在果实贮藏期有上升的趋势。而硬度、酸度、维生素C、淀粉、果胶含量、POD 酶活性等均在主成分1 的右侧,说明它们对第一成分的贡献率比较高,这些指标有一个共同的特点那就是在果实贮藏初期,各指标的值都是较高的正值,然后随着贮藏时间的延长直线下降,说明果实正在加速软化和腐化,第一个主成分可解释果实软化、腐败的趋势。其中可溶性固形物、糖、氨基酸含量、呼吸强度、蛋白、氨基酸含量、淀粉酶、果胶酶、纤维素酶活性等在主成分2 的上侧,同时这些指标也有一个同样的特征,呈现“由升到降”的趋势,这说明果实大分子物质正逐步转向小分子物质,以及到最后小分子物质的逐步消耗,同样也解释果实软化、腐败的过程。
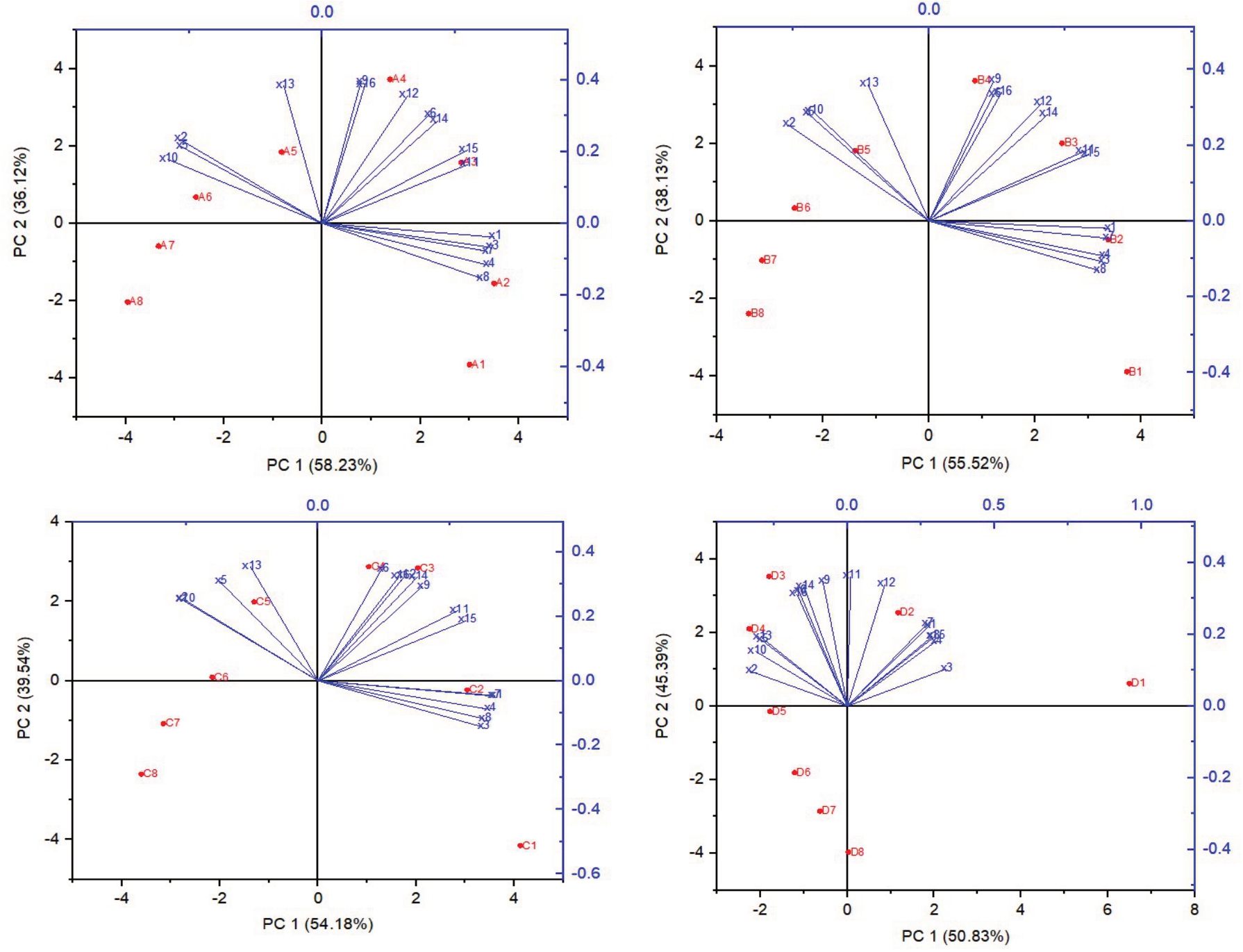
图5 各处理主成分分析
Fig.5 Principal component analysis of each treatment
X1~X16 分别代表果实硬度、可溶性固形物含量、酸含量、维生素C 含量、糖含量、呼吸强度、淀粉含量、果胶含量、蛋白含量、氨基酸含量、CAT 酶、SOD 酶、淀粉酶、果胶酶、POD 酶和纤维素酶活性。A1~A8 代表药剂处理组的8 个时期,B1~B8 代表对照组的8 个时期,C1~C8 代表菌+药剂组的8 个时期,D1~D8 代表菌处理组的8 个时期。
X1-X16 represent fruit firmness,soluble solid content,acid content,vitamin C content,sugar content,respiratory strength,starch content,pectin content,protein content,amino acid content,CAT enzyme,SOD enzyme,amylase,pectinase,POD enzyme and cellulase activity.A1-A8 represent 8 periods of the drug treatment group,B1-B8 represent 8 periods of the control group,C1-C8 represent 8 periods of the fungus+drug group,and D1-D8 represent 8 periods of the fungus treatment group.
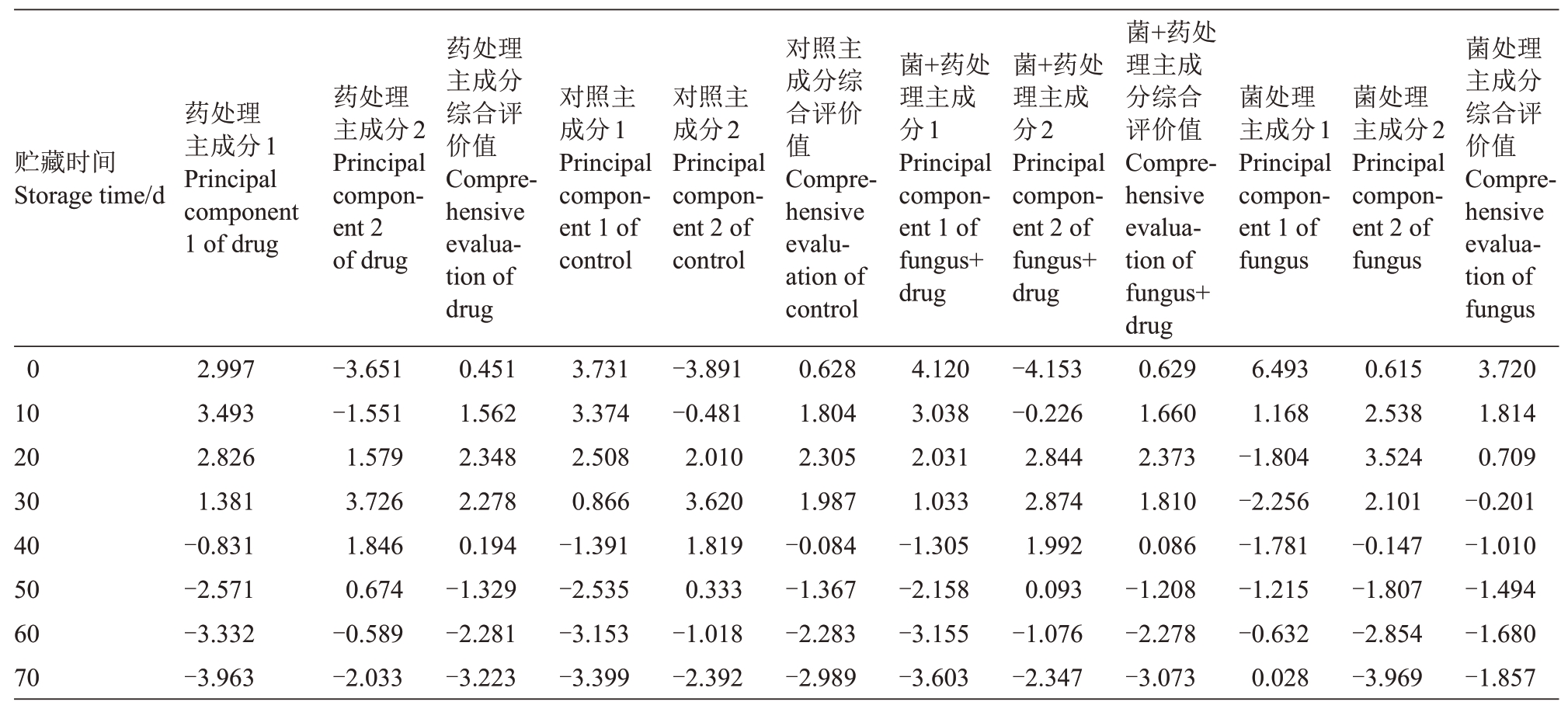
2.7.2 不同贮藏期间各处理猕猴桃理化指标的综合评价 各处理猕猴桃果实贮藏期间综合评价变量及F值结果见如表3。由表3可知,各处理第一个主成分值的变化趋势和图5 中右侧指标一致,总体上是从高到低一直下降的趋势。各处理第2个主成分值的主要表现为由“低-高-低”的趋势,图5中上侧指标一致。各处理综合评价值是对2个主成分的总结性归纳,除了菌处理组各处理综合评价值一直从大向小变化外(品质总体上一直下降),其余各组均表现出了“低-高-低”的变化趋势,说明果实在贮藏过程中综合指标表现出“低-高-低”的变化趋势,到中后期则与第1 主成分指标同步,表现出逐步下降。各处理综合评价值在第30天时便出现了负值,说明第30天时便开始有果实品质下降的现象,这与好果率在第40 天时便出现坏果一致。药剂处理组效果最好,贮藏50 d 时才出现品质下降,60 d 时仅出现了4%的坏果。同时菌+药组果实略好于对照组。
表3 猕猴桃贮藏期间综合评价变量及F 值
Table 3 Comprehensive evaluation variables and F values of kiwifruit during storage
?
3 讨 论
采用中药提取物对猕猴桃好果及猕猴桃病果进行果实耐贮性实验。经过提取物处理后,可延缓果实软化、降低果实失水率,果实好果率有明显的增加。可能是提取物中含有的黄芩素、丁香酚、广藿香酮、菖蒲酮、油茶皂素和肉桂醛等活性物质可杀灭病果病原真菌[25-26],在对病原菌进行有效杀灭的同时提取物中的有机酸、活性多糖、黄酮、多酚、油酸等物质可有效降低果实水分、有机物的丢失,抑制果实大分子分解酶的活性[27];郑亦游等[28]采用3 g·L-1茶树精油处理猕猴桃果实,可有效延缓猕猴桃成熟衰老、降低果实的腐烂率、延缓果实的软化。提取物处理可延缓果实维生素C、可滴定酸含量的降低和还原糖、可溶性固形物、可溶性蛋白、可溶性果胶、可溶性淀粉在贮藏前期的增加,从而降低或者延缓大分子的降解,促进果实的耐贮性。延缓大分子的降解可有效增加果实硬度,这与硬度的变化规律十分吻合。导致大分子物质的延缓降解,可能是提取物抑制了这些大分子酶活性的缘故,贾德翠等[29]采用肉桂提取物处理猕猴桃,有助于果实淀粉酶活性和果实果胶酶活性的降低及推迟最大值出现的时间,且与空白组的测定结果有极显著差异(p <0.01)。
提取物处理可提高氧自由基清除酶SOD 酶、CAT 酶和POD 酶的活性,抑制或降低淀粉酶、果胶酶和纤维素酶的活性;氧自由基酶活性的提高可有效消除果实体内氧自由基,延缓果实衰老腐败,大分子降解酶活性的降低可有效延缓果实淀粉、果胶等的降解,提高果实硬度。主要是由于提取物中的酚类和黄酮类物质本身具有一定清除氧自由基的能力,同时复配药剂中的油酸和多糖类物质可起到保护膜的作用,可阻断空气及外界病原菌与果实的接触,从而降低呼吸、减少自由基的生成。同时相关酶活性的升高和降低可能与相关控制酶基因的表达有关,李明霞等[30]采用微波处理猕猴桃,发现果实软化主要与PG、PME、Cx和β-Gal酶基因的变化有关,其酶活性与果实硬度呈负相关,通过对猕猴桃相关淀粉酶、果胶酶基因的转录水平结果分析,刚好也验证了其酶活性的变化规律结果。同时提取物处理可延缓呼吸和乙烯最大强度出现时间,以及降低其呼吸、乙烯释放量,从而降低果实生理代谢、延缓果实的软化,可能是相关代谢酶活性及基因表达降低所致[31]。苏苗[32]采用O3处理猕猴桃时,相关乙烯代谢酶ACS、ACO 活性均有明显的降低。同时能量、乙烯相关转录水平结果刚好也验证了二者在果实贮藏期间的变化规律结果。
影响猕猴桃耐贮性的生理生化、酶指标非常多,但不能从宏观上或统计学上说明其与果实贮藏性能变化趋势的联系,通过对各项指标进行主成分分析,果实生理指标总体上表现出“低-高-低”的变化趋势,中药提取物可延缓果实贮藏前期生理生化指标的升高,和后期的降低。进一步说明中药提取物可抑制果实贮藏期间的生理活性,提高果实耐贮性。
4 结 论
采用0.8 mg·mL-1中药提取物处理猕猴桃可以较好地保持果实的好果率、品质和硬度,降低果实乙烯、呼吸代谢、提高抗氧化酶活性、降低大分子糖分解酶活性等。采用转录组技术测定基因转录水平,其基因转录水平与相关代谢水平表现出基本一致性;采用主成分分析法分析猕猴桃耐贮性,其分析结果的变化规律与相关代谢测定结果的变化规律一致,进一步说明果实在贮藏过程中的理化变化趋势,从统计学角度说明了中药提取物对猕猴桃鲜果具有很好的防腐保鲜作用。在今后的研究中可对转录水平的基因进行Q-PCR验证,进一步从基因表达层面解释中药提取物对猕猴桃耐贮性的机制。
[1] 赵洋,穆雪,李春艳,汪卫星.猕猴桃属(Actinidia Lindl.)植物亲缘关系研究进展[J].果树学报,2019,36(9):1214-1228.ZHAO Yang,MU Xue,LI Chunyan,WANG Weixing. Research advances on the genetic relationships of kiwifruit (Actinidia Lindl.)[J].Journal of Fruit Science,2019,36(9):1214-1228.
[2] PARKAR S G,ROSENDALE D,PATURI G,HERATH T D,STOKLOSINSKI H,PHIPPS J E,HEDDERLEY D,ANSELL J. In vitro utilization of gold and green kiwifruit oligosaccharides by human gut microbial populations[J]. Plant Foods for Human Nutrition,2012,67(3):200-207.
[3] LATOCHA P. The nutritional and health benefits of kiwiberry(Actinidia arguta): A review[J]. Plant Foods for Human Nutrition,2017,72(4):325-334.
[4] BOZONET S M,CARR A C,PULLAR J M,VTSSERS M C.Enhanced human neutrophil vitamin C status,chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit[J]. Nutrients,2015,7(4): 2574-2588.
[5] GAMMON C S,KRUGER R,BROWN S J,CONLON C A,VON HURST P R,STONEHOUSE W.Daily kiwifruit consumption did not improve blood pressure and markers of cardiovascular function in men with hypercholesterolemia[J]. Nutrition Rresearch,2014,34(3):235-240.
[6] LIN H H,TSAI P S,FANG S C,LIU J F.Effect of kiwifruit consumption on sleep quality in adults with sleep problems[J].Asia Pacific Journal of Clinical Nutrition,2011,20(2):169-174.
[7] 方金豹,钟彩虹.新中国果树科学研究70 年:猕猴桃[J].果树学报,2019,36(10):1352-1359.FANG Jinbao,ZHONG Caihong. Fruit scientific research in New China in the past 70 years: Kiwifruit[J]. Journal of Fruit Science,2019,36(10):1352-1359.
[8] 黄文俊,钟彩虹.猕猴桃果实采后生理研究进展[J].植物科学学报,2017,35(4):622-630.HUANG Wenjun,ZHONG Caihong. Research advances in the postharvest physiology of kiwifruit[J]. Plant Science Journal,2017,35(4):622-630.
[9] ZHANG M,XU L,ZHANG L,ZHANG L Y,GUO Y H.Effects of quercetin on postharvest blue mold control in kiwifruit[J].Scientia Horticulturae,2018,228:18-25.
[10] HE J,WU D,ZHANG Q,CHEN H,LI H,HAN Q,LAI X,WANG H,WU Y,YUAN J,DONG H,QIN W. Efficacy and mechanism of cinnamon essential qil on inhibition of Colletotrichum acutatum isolated from‘Hongyang’kiwifruit[J]. Frontiers in Microbiology,2018,9:1288-1231.
[11] 石浩,王仁才,王琰,何小娥,周倩,卜范文.猕猴桃采后病害植物源杀菌剂的筛选及其抑菌效果分析[J]. 经济林研究,2020,38(1):75-82.SHI Hao,WANG Rencai,WANG Yan,HE Xiaoe,ZHOU Qian,BU Fanwen. Screening of plant-derived fungicides for postharvest diseases in Actinidia chinensis and analysis on their antibacterial effects[J].Non-wood Forest Research,2020,38(1):75-82.
[12] 马立平.由多指标向少数几个综合指标的转化-主成分分析法[J].数据,2000,26(8):35-37.MA Liping.Transformation from multiple indexes to a few comprehensive indexes principal component analysis[J].Data,2000,26(8):35-37.
[13] 石浩,王仁才,王琰,庞立,卜范文,何小娥,周倩.黄芩抑菌物的提取工艺及抑菌稳定性[J].湖南农业大学学报(自然科学版),2019,45(2):199-204.SHI Hao,WANG Rencai,WANG Yan,PANG Li,BU Fanwen,HE Xiaoe,ZHOU Qian. Extraction of antibacterial active substances from Scutellaria baicalensis and antimicrobial stability of the extract[J]. Journal of Hunan University of Science &Technology(Natural Science Edition),2019,45(2):199-204.
[14] 李荣宇,林少玲,骆月姬,苏靖雯.不同产地牛大力水提物中总黄酮、总皂苷及总多糖的含量测定[J].中国药师,2020,23(6):1208-1210.LI Rongyu,LIN Shaoling,LUO Yueji,SU Jingwen. Content determination of total flavonoids,saponins and polysaccharides in Millettia speciosa champ.from different producing areas[J].China Pharmacist,2020,23(6):1208-1210.
[15] 赵永钦,成余勤,岑巧梦,邵敬宝,马美兰,石森林.延胡索叶不同发育期总生物碱含量测定[J].浙江农业科学,2020,61(6):1057-1059.ZHAO Yongqin,CHENG Yuqin,CEN Qiaomeng,SHAO Jingbao,MA Meilan,SHI Senlin.Determination of total alkaloids in leaves of Corydalis yanhusuo at different developmental stages[J].Acta Agriculturae Zhejiangensis,2020,61(6):1057-1059.
[16] 王萍,王宇鹤,李辉,赖普辉,高寒.枇杷核不同极性萃取物总黄酮、总多酚含量与其抗氧化活性的相关性[J]. 化学试剂,2020,42(9):1067-1072.WANG Ping,WANG Yuhe,LI Hui,LAI Puhui,GAO han. Correlation between the content total flavonoids and total polyphenols in loquat nuclear with different extracts and their antioxidant activities[J].Chemical Reagents,2020,42(9):1067-1072.
[17] 韩璐,黄薏霏,那袭雪,张文涛,宋丹,杨宏,宁小清.白花九里明提取物中3 种有机酸及总黄酮、总有机酸的含量测定[J].化学与生物工程,2020,37(8):63-67.HAN Lu,HUANG Yifei,NA Xixue,ZHANG Wentao,SONG Dan,YANG Hong,NING Xiaoqing. Content determination of three organic acids,total flavonoids,and total organic acid in Blumea megacephala extract[J]. Chemistry & Bioengineering,2020,37(8):63-67.
[18] 卢丽娜,孙利芹,田焕玲,任蒙,王长海.32 株海洋微藻总脂含量及其脂肪酸组成的研究[J].中国油脂,2009,34(11):68-73.LU Lina,SUN Liqin,TIAN Huanling,REN Meng,WANG Changhai. Total lipid content and fatty acid composition of 32 strains of marine microalgae [J]. China Oils and Fats,2009,34(11):68-73.
[19] BRUMMELL D A,DAL CIN V,LURIE S,CRISOSTO C H,LABAVITCH J M. Cell wall metabolism during the development of chilling injury in cold-stored peach fruit: association of mealiness with arrested disassembly of cell wall pectins[J].Journal of Experimental Botany,2004,55(405):2041-2052.
[20] 曾邹林.不同处理对软枣猕猴桃耐贮性的影响研究[D].长沙:湖南农业大学,2014.ZENG Zoulin. Effects of different treatments on the storability of actinidia mollissima [D]. Changsha: Hunan Agricultural University,2014.
[21] FAMIANI F,BALDICCHI A,FARINELLI D,CRUZ-CASTILLO J G,MAROCCHI F,MASTROLEO M,MOSCATELLO S,PROIETTI S,BATTISTELLI A. Yield affects qualitative kiwifruit characteristics and dry matter content may be an indicator of both quality and storability[J]. Scientia Horticulturae,2012,146:124-130.
[22] 黎桂坤.不同处理对南方冬枣耐贮性影响研究[D].长沙:湖南农业大学,2014.LI Guikun.Study on the effect of different treatments on the storability of southern winter jujube [D]. Changsha: Hunan Agricultural University,2014.
[23] JHALEGAR M J,SHARMA R R,PAL R K,VISHAL R. Effect of postharvest treatments with polyamines on physiological and biochemical attributes of kiwifruit (Actinidia deliciosa) cv.Allison[J].Fruits,2012,67(1):13-22.
[24] LIU R X,KUANG J,GONG Q,HOU X L.Principal component regression analysis with SPSS[J]. Comput Methods Programs Biomed,2003,71(2):141-147.
[25] ZHANG J,SI C,ZOU J,FAN R,GUO A,WEI X.Antimicrobial effects of silver nanoparticles synthesized by Fatsia japonica leaf extracts for preservation of citrus fruits[J]. Journal of Food Science,2017,82(7/9):1861-1866.
[26] RIBERA A,COTORAS M,ZIGA G E. Effect of extracts from in vitro-grown shoots of Quillaja saponaria Mol.on Botrytis cinerea Pers.[J]. World Journal of Microbiology & Biotechnology,2008,24(9):1803-1811.
[27] 李元政,胡文忠,萨仁高娃,龙娅,老莹,杨香艳,廖嘉.天然植物提取物的抑菌机制及其在果蔬保鲜中的应用[J].食品与发酵工业,2019,45(14):239-244.LI Yuanzheng,HU Wenzhong,SA Rengaowa,LONG Ya,LAO Ying,YANG Xiangyan,LIAO Jia. Antimicrobial mechanisms of natural plant extracts and applications in preserving fruits and vegetables[J]. Food and Fermentation Industries,2019,45(14):239-244.
[28] 郑亦游,顾鑫怡,吴莎萍,沈蓥颖,孙功成,付长春.茶树精油缓释剂对采后猕猴桃贮藏品质的影响[J].浙江树人大学学报(自然科学版),2019,19(1):32-37.ZHENG Yiyou,GU Xinyi,WU Shaping,SHEN Yingying,SUN Gongcheng,FU Changchun. Effect of tea tree oil corrosion inhibitor on storage quality during kiwifruit ripening[J].Journal of Zhejiang Shuren University (Natural Science Edition),2019,19(1):32-37.
[29] 贾德翠,涂洪强,肖志伟,石浩.肉桂提取物处理对‘红阳’猕猴桃果实耐贮性的影响[J]. 安徽农业大学学报,2018,45(3):562-568.JIA Decui,TU Hongqiang,XIAO Zhiwei,SHI Hao. Effects of cinnamon extracts on storability of‘Hongyang’kiwifruit[J].Journal of Anhui Agricultural University,2018,45(3):562-568.
[30] 李明霞,韩建群,王琦,何雨婷,郭艳明,董明.低强度微波处理对猕猴桃细胞壁降解酶活性的影响[J].食品与发酵工业,2015,41(11):52-58.LI Mingxia,HAN Jianqun,WANG Qi,HE Yuting,GUO Yanming,DONG Ming. Effect of low intensity microwave on cell wall degradation enzymes activity of kiwifruit[J]. Food and Fermention Industries,2015,41(11):52-58.
[31] WANG Z Y,MACRAE E A,WRIGHT M A,BOLITHO K M,ROSS G S,ATKINSON R G. Polygalacturonase gene expression in kiwifruit:relationship to fruit softening and ethylene production[J].Plant Molecular Biology,2000,42(2):317-328.
[32] 苏苗.采后O3 处理对猕猴桃品质、乙烯代谢及其抗性酶活性的影响[D].杨凌:西北农林科技大学,2018.SU Miao. Effects of postharvest O3 treatment on quality,ethylene metabolism and resistance enzyme activity of kiwifruit[D].Yangling:Northwest Agriculture and Forestry University,2018.