葡萄炭疽病(Grapevine anthracnose)是葡萄成熟期的重要病害,在葡萄产区几乎都有该病害的发生。1891 年Southworth 最先报道美国葡萄炭疽病由胶孢炭疽菌[Colletotrichum gloeosporioides(Penz.) Penz. & Sacc.]侵染,尖孢炭疽菌(C. acutatum J.H.Simmonds ex J.H.)则在澳大利亚、日本、韩国和美国葡萄上相继被报道[1]。我国葡萄炭疽病病原菌主要是胶孢炭疽菌复合种(C. gloeosporioides species complex)侵染[2-5]。Peng等[6]对云南和贵州葡萄炭疽病菌种群遗传多样性研究表明,葡萄C.gloeosporioides species complex 中,除C.gloeosporioides种外,还包含C.fructicola和C.viniferum。
当前在全国范围内,药剂防治仍是葡萄炭疽病的主要防控措施。使用的药剂类型主要有苯并咪唑氨基甲酸甲酯(Methyl Benzimidazole Carbamates,MBCs)、外醌抑制剂(Quinone outside Inhibitors,QoIs)和麦角甾醇合成抑制剂中的14α-脱甲基酶抑制剂(14α-demethylation Inhibitors,DMIs)[7]。在我国MBCs 类杀菌剂在葡萄上的应用时间最长、范围最广,该类型药剂主要有噻菌灵(Thiabendazole)、苯菌灵(Benomyl)、甲基硫菌灵(Thiophanate methyl)和多菌灵(Carbendazim)等。
MBCs类杀菌剂作用机制是通过结合β-微管蛋白亚单元(TUB2)来抑制核分裂[8]。病原菌对MBCs类药剂产生抗性,是TUB2单个有义碱基的突变,导致结合位点氨基酸发生改变,使药剂的亲合力下降或丧失,菌株表现出抗性。已报道造成MBCs 抗性的真菌TUB2 突变位点有6、50、167、198、200 和240位密码子[9]。通常在植物病原真菌中这些氨基酸突变位点发生在TUB2第198位和第200位[10-15]。作用位点突变直接参与MBCs 类药剂的抗性,已被定点突变和基因置换所证实[16]。
有关葡萄炭疽病菌对多菌灵的抗药性已有一些研究[2,7,17-19],但葡萄炭疽病菌田间种群对多菌灵的抗药性流行动态监测的研究未见报道。笔者通过连续5 a(年)监测市域葡萄炭疽病菌种群对多菌灵的敏感性,结合2 a 的田间接种防治试验结果,阐明病原菌抗药性种群的演化和流行的原因。同时通过对多菌灵不同敏感表型菌株作用靶标基因序列的比对分析,阐明抗药性分子机制。
1 材料和方法
1.1 供试培养基
马铃薯琼脂培养基,用于菌株单孢分离、保存和药剂敏感性测定[2]。
1.2 菌株采集
于2013—2017年葡萄成熟期,从江苏省句容市茅山镇、后白镇、华阳镇和白兔镇葡萄园内,采集‘巨峰’‘阳光玫瑰’和‘夏黑’葡萄果实上橘黄色炭疽病菌分生孢子堆,5 a取样共计672株,菌株背景见表1。
表1 江苏省句容市葡萄炭疽病菌取样菌株信息
Table 1 Information of Colletotrichum spp.isolates collected from field vineyard in Jurong city of Jiangsu
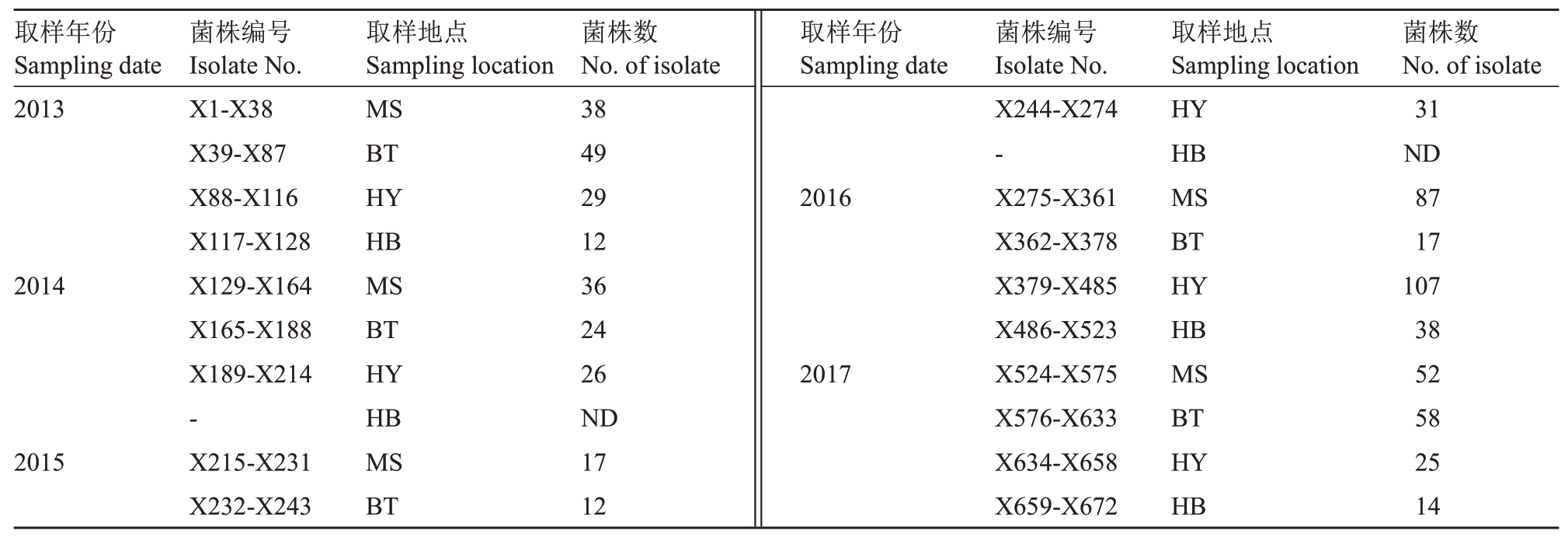
注:MS、BT、HB 和HY 分别代表句容市茅山镇、句容市白兔镇、句容市后白镇和句容市华阳镇;ND 表示未检测。
Note: MS,BT,HB and HY represent Maoshan town,Baitu town,Houbai town and Huayang town of Jurong City,respectively; ND represent not detected.
?
1.3 菌株的分离与纯化
所有菌株均经单孢纯化后编号并保存于PDA斜面上,置4 ℃冰箱保存备用。
1.4 葡萄炭疽病菌种群对多菌灵的敏感性测定
1.4.1 供试药剂 98.3%多菌灵原药(Carbendazim),江苏耕耘化学有限公司提供。将98.3%多菌灵原药用0.1 mol·L-1 盐酸溶液溶解,配制成10 000 mg·L-1的母液置于4 ℃冰箱备用。
1.4.2 葡萄炭疽病菌种群对多菌灵的敏感性测定采用菌丝生长速率法[7],随机挑选取葡萄炭疽病菌132株(2013年98株,2017年34株),在PDA培养基上25 ℃培养5 d 后,沿菌落边缘同一圆周上用直径4 mm打孔器制取菌碟,将菌碟菌丝面朝下移到含系列梯度稀释质量浓度为0、0.04、0.12、0.37、1.11、3.33、10.00 和30.00 mg·L-1多菌灵PDA平板正中央,每质量浓度3 次重复,25 ℃培养7 d,十字交叉法测量菌落直径(cm)。用菌落直径平均值计算抑制率(%),利用DPS 数据处理系统,通过浓度对数值(x)与抑制率机率值(y)之间的线性回归关系,计算出毒力回归方程和有效抑制中浓度(EC50)。
1.5 葡萄炭疽病菌种群对多菌灵的抗药性监测
采用区分剂量法[7],按上述1.4.2 方法将葡萄炭疽病菌移在10 mg·L-1的多菌灵培养基上,每菌株3个重复,设不含药剂的PDA 培养基做对照,25 ℃培养5 d后观察:能在含药平板上生长的菌株记录为抗性菌株,不能生长的菌株记录为敏感菌株,计算抗性频率(%)。
1.6 田间接种防治试验
于2017年夏黑葡萄幼果期,将整串葡萄幼穗浸于1000 mg·L-1多菌灵药液中10 s,对照用清水浸,待药液自然风干后,用喉头喷雾器分别接种C.gloeosporioides X212(抗性菌株)分生孢子悬浮液、C.gloeosporioides X309(敏感菌株)分生孢子悬浮液和混合菌株(等浓度X212 和X309 分生孢子液等体积混合)的分生孢子悬浮液。每种分生孢子悬浮液各接种10串,接种分生孢子悬浮液浓度均为1×104个·mL-1,接种量为每串2 mL,待孢子液风干后套袋,并做好试验标记。试验期间受试葡萄树不用化学杀菌剂防治,水肥管理正常进行,成熟期调查防效,整个试验2018年重复1次。
1.7 田间接种试验回分离菌株的药剂敏感性检测
从2017 年和2018 年田间试验处理葡萄串的发病果粒上回分离炭疽病菌,按1.5方法检测出回分离菌株中抗性菌株频率(%)。
1.8 炭疽病菌对多菌灵的抗药性分子机制
选取3株敏感菌株和48株抗性菌株,按DNA试剂盒(OMEGA)提取基因组DNA。靶标基因扩增引物序列为 TubF1(5’- ACTTCGTCTTCGGCCAGTCTG-3’),TubR1(5’-TTCTGGACGTTGCGCATCTG-3’)[20]。采用50 µL 反应体系,反应参数为:95 ℃预变性3 min,95 ℃变性30 s,60 ℃退火30 s,68 ℃延伸1 min,共进行35 个循环,最后68 ℃延伸4 min。PCR产物经1.2%琼脂糖凝胶电泳检测后,由南京金斯瑞生物科技有限公司进行产物纯化和序列测定。利用DNASTAR进行序列编辑,利用BLAST进行抗性菌株和敏感菌株靶标基因序列比对。
2 结果与分析
2.1 葡萄炭疽病菌种群对多菌灵的敏感性
2013 年葡萄炭疽病菌田间取样种群敏感性基线的EC50均值为0.528 5 mg·L-1,而2017年相同区域葡萄炭疽病菌田间取样种群对多菌灵EC50均值为7.787 8 mg·L-1。2017 年取样种群EC50值整体向右迁移,表明2017年田间取样种群对多菌灵的敏感性下降(图1)。
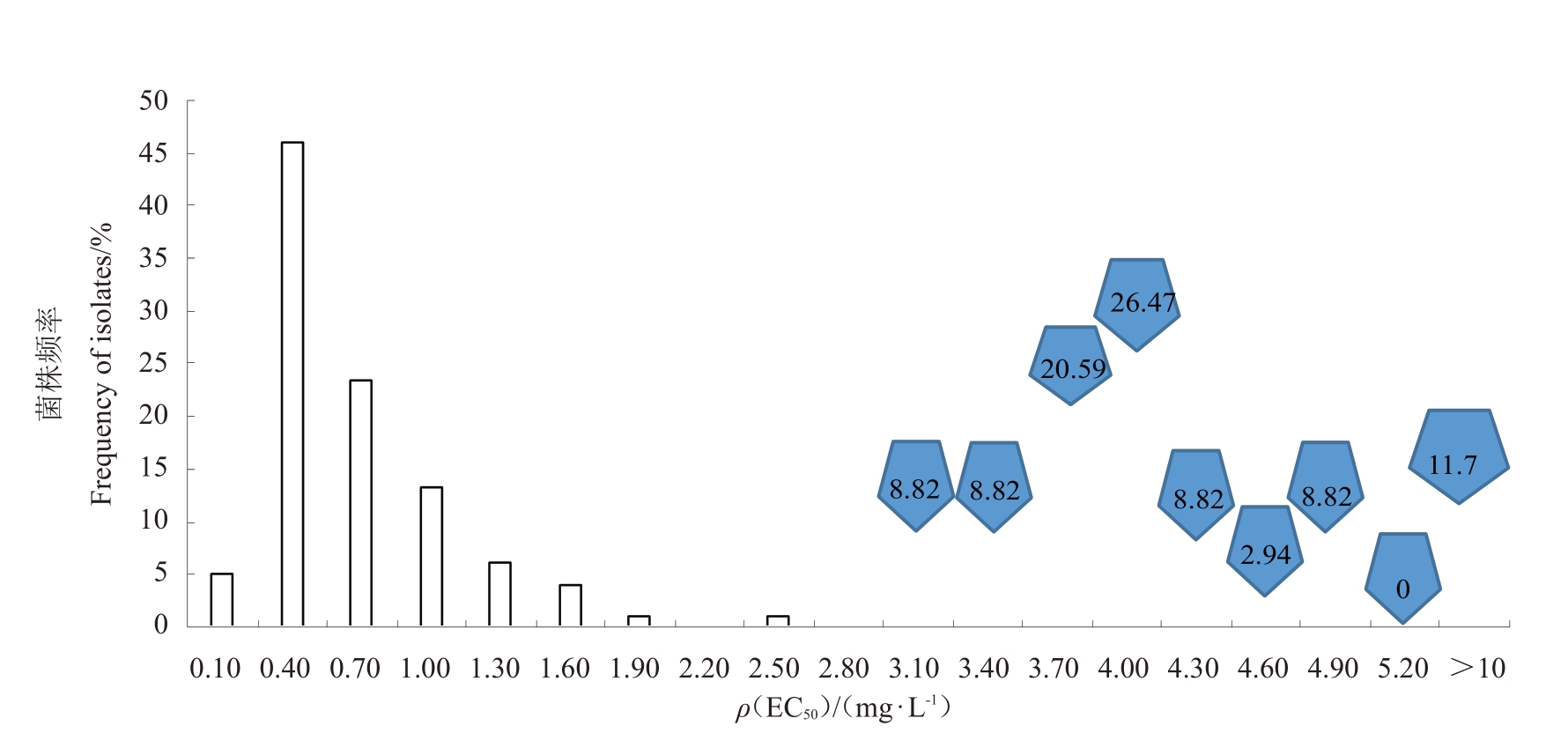
图1 2013 年(柱状图)和2017 年(方格图)葡萄炭疽病菌种群对多菌灵的敏感性分布
Fig.1 Sensitivity distribution of Colletotrichum spp.population to carbendazim in 2013(bar chat)and 2017(checkerboard)
2.2 葡萄炭疽病种群抗药性监测
采用区分剂法连续5 a 监测了取自江苏省句容市4 个葡萄种植镇的共计672 个菌株对多菌灵抗药性的流行动态。结果表明,2013—2017年取样地区中白兔镇、华阳镇和后白镇葡萄炭疽病种群的抗性菌株频率分别为4.08%~60.34%、0~24.00%和0~28.57%,3 个取样地点的抗性菌株频率在年度间有起伏,但总体呈上升趋势;而茅山镇葡萄炭疽病菌种群抗药性水平从2014 年后呈总体下降趋势(表2)。从市域范围看2013—2017 年葡萄炭疽病种群抗性水平总体呈上升趋势,总体抗性菌株频率从2013年的2.30%上升到2017年的32.21%(图2)。抗性菌株种群演化曲线呈线性上升,并已形成抗药性流行。

图2 葡萄炭疽病菌种群对多菌灵的抗药性监测
Fig.2 Resistance monitoring of Glomerella spp.population to carbendazim(2013—2017)
表2 葡萄炭疽病菌种群对多菌灵的抗药性监测
Table 2 Resistance monitoring of Colletotrichum spp.population to carbendazim
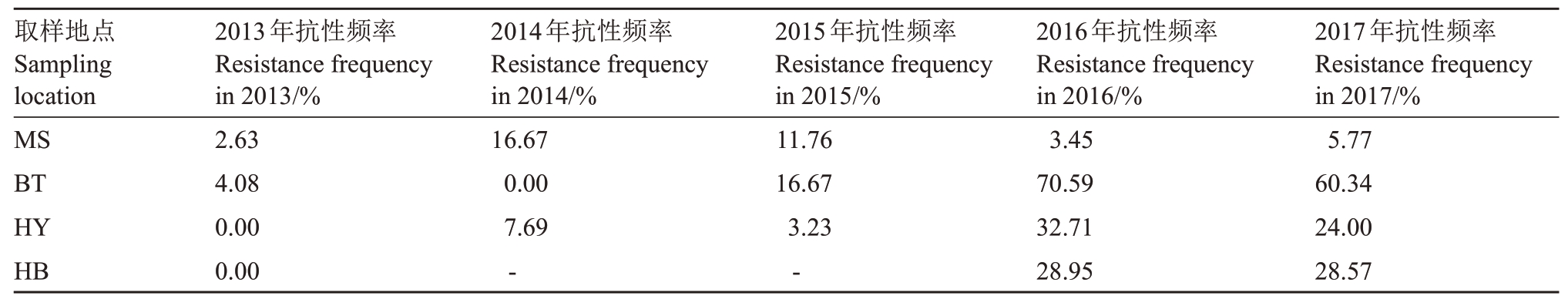
注:MS、BT、HB 和HY 分别代表句容市茅山镇、句容市白兔镇、句容市后白镇和句容市华阳镇。
Note:MS,BT,HB and HY represent Maoshan town,Baitu town,Houbai town and Huayang town of Jurong City,respectively.
?
2.3 田间接种防治试验
2017年和2018年在田间用多菌灵喷雾后,再分别用敏感菌株、抗性菌株和混合菌株分生孢子悬浮液接种的方法进行多菌灵的田间防治试验。结果表明,接种敏感菌株、抗性菌株和混合菌株分生孢子悬浮液的防治效果,2017 年分别为61.06%、0.64%和33.17%,2018 年分别为71.56%、4.18%和19.15%。两年的结果显示,多菌灵2 倍推荐用量失去了对抗性菌株接种处理的防效(表3),说明田间抗性种群的产生是药剂防效下降的主因子。
表3 多菌灵对不同敏感菌株的田间防效
Table 3 Field fruit control effects inoculated by different resistance phenotype isolates of carbendazim
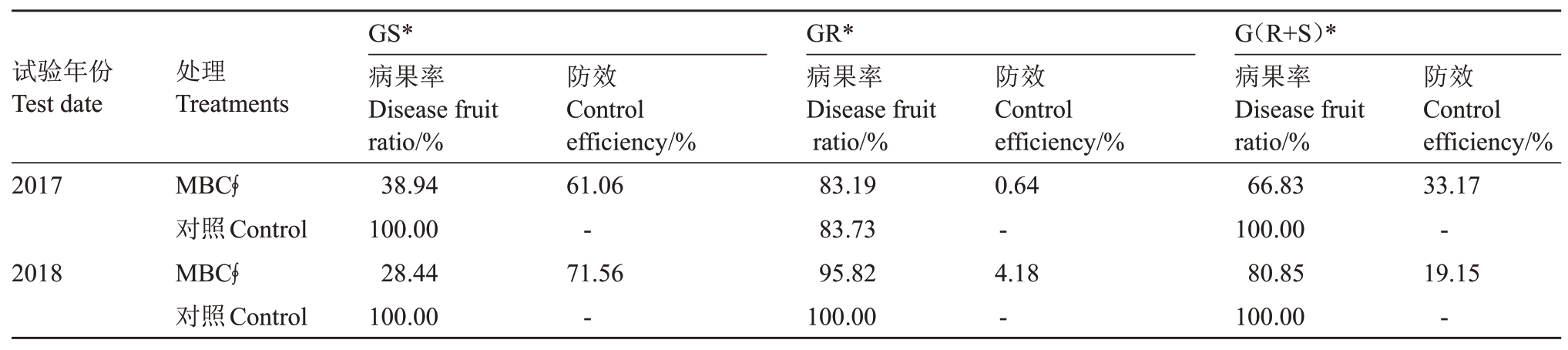
*:GS. 敏感菌株C. gloeosporioides X309 分生孢子悬浮液;GR. 抗性菌株C. gloeosporioides X212 分生孢子悬浮液;G(R+S). 等浓度C.gloeosporioides X212 菌株分生孢子悬浮液和C. gloeosporioides X309 菌株分生孢子悬浮液等体积混合;MBC ∮. 指多菌灵使用质量浓度1000 mg·L-1。接种菌株分生孢悬浮液浓度为1×104 个·mL-1。
*:GS.Inoculated by conidia suspension of sensitive isolate C.gloeosporioides X309;GR.Inoculated by conidia suspension of resistant isolate C.gloeosporioides X212;G(R+S).Inoculated by mixed conidia suspension(the same conidia suspension concentration of C.gloeosporioides X309 and C.gloeosporioides X212 were mixed in equal volume);MBC ∮.represents the concentration of carbendazim was 1000 mg·L-1.The concentration of conidia suspension was 1×104 spores·mL-1.
?
2.4 接种防治处理中回分离葡萄炭疽病菌对多菌灵的敏感性
为证明江苏丘陵地区葡萄种植区域炭疽病菌抗多菌灵种群仍呈上升趋势,并且是种群受到化学杀菌剂的选择压驱动造成,2017年和2018年进行了田间试验。在葡萄幼果期连续两年在同一田块,用多菌灵喷雾后分别接种敏感菌株、抗性菌株和混合菌株分生孢子悬浮液,再检测处理中回分离菌株对多菌灵的敏感性。结果表明,用多菌灵喷雾后再接种敏感菌株、抗性菌株和混合菌株分生孢子悬浮液的处理中,回分离菌株的抗性菌株频率,2017 年分别为0、78.38%和65.00%,2018 年分别为0、76.47%和68.18%;而用敏感菌株、抗性菌株和混合菌株分生孢子悬浮液接种后不防治的对照处理中,回分离菌株种群的抗性菌株频率,2017年分别为0、80.00%和40.00%,2018 年分别为10.00%、60.00%和30.00%(图3)。2 a的田间接种后回检测种群对药剂敏感性的结果证明,在用药剂处理后,混合接种处理的回分离菌株种群中,抗性菌株比率明显上升,说明药剂选择压是炭疽病菌种群形成抗药性流行的驱动因子。
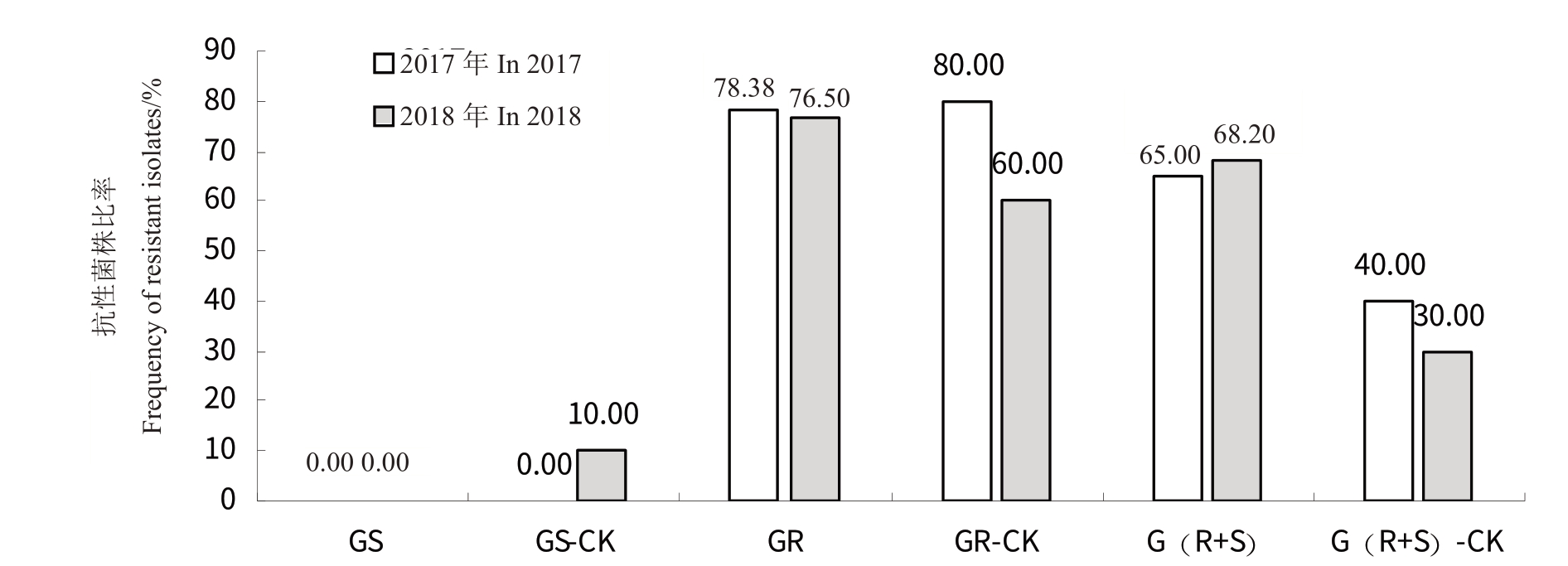
图3 回分离菌株对药剂的敏感性
Fig.3 Sensitivity of back separative isolate to carbendazim
GS.用多菌灵喷雾后接种敏感菌株C.gloeosporioides X309 分生孢子悬浮液;GS-CK. 清水喷雾后接种C.gloeosporioides X309 分生孢子悬浮液;GR.用多菌灵喷雾后接种抗性菌株C.gloeosporioides X212 分生孢子悬浮液;GR-CK.清水喷雾后接种C.gloeosporioides X212 分生孢子悬浮液;G(R+S).用多菌灵喷雾后接种混合分生孢子悬浮液(相同浓度的C.gloeosporioides X309 分生孢子悬浮液和C.gloeosporioides X212 分生孢子悬浮液等体积混合);G(R+S)-CK. 用清水喷雾后接种混合分生孢子悬浮液。分生孢悬浮液浓度为1×104 个·mL-1。多菌灵使用浓度为1000 mg·L-1。
GS. Inoculated by conidia suspension of sensitive isolate C. gloeosporioides X309 after spray with carbendazim; GS-CK. Inoculated by conidia suspension of C.gloeosporioides X309 after spray with H2O;GR.Inoculated by conidia suspension of resistant isolate C.gloeosporioides X212 after spray with carbendazim;GR-CK.Inoculated by conidia suspension of C.gloeosporioides X212 after spray with H2O;G(R+S).Inoculated by mixed conidia suspension (the same concentration of C. gloeosporioides X309 and C. gloeosporioides X212 conidia suspension were mixed in equal volume)after spray with carbendazim;G(R+S)-CK.Inoculated by mixed conidia suspension after spray with H2O.The concentration of conidia suspension was 1×104 spores·mL-1.The concentration of carbendazim was 1000 mg·L-1.
2.5 葡萄炭疽病对多菌灵的抗药性机制
对3 株敏感菌株和48 株抗性菌株靶标基因(TUB2)序列比对结果表明,江苏丘陵地区抗多菌灵葡萄炭疽病菌TUB2 突变类型有2 种,即抗性菌株TUB2第198位的谷氨酸突变成丙氨酸(E198A),或第200位的苯丙氨酸突变成络氨酸(F200Y),未发现其他突变类型。
3 讨 论
2013 年起笔者对江苏丘陵地区(句容市)主要葡萄种植镇的葡萄炭疽病种群进行了对多菌灵的抗药性检测。连续5 a的抗性监测结果表明,田间葡萄炭疽病菌种群对多菌灵的抗药性频率呈上升趋势,并已形成抗药性流行。文中5 a 取样的葡萄园都在相同乡镇,因此所得的抗药性监测数据可较为客观地反映当地葡萄炭疽病菌种群对多菌灵的抗药性形成与演化流行动态。据笔者所知,本研究是首次通过在相同区域连续多年取样监测葡萄炭疽病病原菌种群对多菌灵的抗性流行动态的研究报道。
本研究中2013 年取样菌株种群对多菌灵的敏感基线EC50均值为0.528 5 mg·L-1,与李洋等[2]报道的稍有不同。分析原因可能是源于取样种群异质性、操作水平和检测程序等的差异,影响了EC50值的测定结果。因为,只有在相同实验条件和相同操作程序下测定所建立起来的敏感基线,才能更好地符合生产实际,特别像葡萄炭疽病菌这种复合种群,寄主范围广,不同地域取样种群的异质性差异较大。因此,对其敏感性检测时除参照相关报道的EC50值外,还需取本地区种群样本进行测定,不能简单直接桥接地理差异较大地区的报道值,最好是桥接不同地域的菌株后,与在相同实验操作条件下测得值相比较,这样才更为科学。此观点仅供参考。
抗性监测中发现不同乡镇间葡萄炭疽病菌种群的抗性频率差异较大。如茅山镇2014 年葡萄炭疽病菌种群对多菌灵的抗性频率最高为16.67%,之后2015—2017 年呈下降趋势,而白兔镇、后白镇和华阳镇的葡萄炭疽病的抗性频率则呈逐年上升趋势。以上结果的原因可能是与茅山镇葡萄种植的植保合作化程度高(达80%),且该镇在2014年后合作社统一停用苯并咪唑类药剂,而其余三镇则仍然没有全面停用该类药剂相关。除药剂因子外,其他诸如取样的样本量、各地农作物种植布局、栽培模式和丘陵区域性小气候等因子是否对炭疽病菌种群的抗药性形成与流行产生影响,还需进行深入细致的研究。
敏感性检测中发现葡萄炭疽病菌抗性菌株的区分剂量用5 mg·L-1也可以区分出敏感和抗性种群,大量检测数据表明,分别用5 mg·L-1或10 mg·L-1做区分剂量的检测,结果的相似率在95%以上。笔者在监测中所用的多菌灵对葡萄炭疽病菌抗药性检测的区分剂量是10 mg·L-1,较叶佳等[19]的报道相比偏高,但较好地排除了自然种群中天然存在的低敏感菌株(异质菌株)对检测结果的干扰。
连续2 a田间防效试验结果表明,多菌灵2倍推荐剂量失去对抗性菌株接种处理的防效,充分证明多菌灵抗性突变类型属质量抗性类型。该类型抗性突变的抗性治理,不能采用加大施用剂量(饱和治理)的措施。因此最好停止此措施,让自然敏感种群稀释,使得抗性种群的比率下降,使其不能形成抗药性流行。
为证明药剂的定向选择作用是病原菌产生抗药性的主要因子,本文首次进行了喷雾防治后再接种不同敏感表型菌株的田间试验。2 a 的田间接种试验结果表明,在不用药剂的情况下,自然种群菌株中敏感菌株会稀释种群中的抗性种群,表现在抗性菌株接种后不防治的处理中,回分离种群的抗性菌株占比下降;而在药剂选择压的作用下,混合菌株接种处理后回分离菌株中的抗性菌株与敏感菌株的比率显著高于初始接种比率50%,说明药剂选择是葡萄炭疽病菌抗性种群形成与流行的驱动因子。
MBCs类杀菌剂抗药性作用机制是单基因控制的点突变型,靶标基因单碱基的有义突变造成编码氨基酸的改变,进而改变靶标蛋白的结构,使得药剂对靶标失去亲合,病原菌表现出对药剂不敏感。本研究抗性菌株TUB2 突变位点的测序结果表明,抗性菌株TUB2 第198 位的谷氨酸突变成丙氨酸(E198A),或第200 位的苯丙氨酸突变成络氨酸(F200Y),是江苏丘陵地区葡萄炭疽病种群对多菌灵产生抗性的分子机制。本研究结果与Nalumpang等[21]、李河等[22]和Chung等[23-24]报道TUB2基因E198A位点突变能造成C.gloeosporioides 表现出对MBCs的高水平抗性相一致。Chen 等[15]报道Monilinia fructicola TUB2 不同密码子的突变导致对MBCs 类杀菌剂的不同抗性水平,E198A 通常比F200Y 具有更高的抗性水平。本研究没有对抗多菌灵取样测序菌株的抗性水平进行详细区分,因此有关葡萄炭疽病菌TUB2基因E198A和F200Y两种突变类型是否分别控制着不同水平的抗药性表型,还需要进一步研究。有关江苏省域葡萄炭疽病菌复合种群遗传多样性、复合种群内不同种类菌株对苯并咪唑类杀菌剂抗药性差异等方面的研究还在进行中。
[1] SOUTHWORTH E A. Ripe rot of grapes and apples[J]. Journal of Mycology,1891,6(4):164-173.
[2] 李洋,刘长远,陈秀蓉,赵奎华,苗则彦,梁春浩,王辉.辽宁省葡萄炭疽菌鉴定及对多菌灵敏感性研究[J].植物保护,2009,35(4):74-77.LI Yang,LIU Changyuan,CHEN Xiurong,ZHAO Kuihua,MIAO Zeyan,LIANG Chunhao,WANG Hui. Identification of the grape anthracnose and its sensitivity to Carbendazim in Liaoning[J].Plant Protection,2009,35(4):74-77.
[3] 邓维萍,杜飞,杨敏,杨积忠,何霞红,王海宁,李成云,朱书生.云南葡萄炭疽病菌遗传多样性分析[J].云南农业大学学报,2015,30(2):173-184.DENG Weiping,DU Fei,YANG Min,YANG Jizhong,HE Xiahong,WANG Haining,LI Chengyun,ZHU Shusheng. The genetic diversity of Colletotrichum spp. population isolated from grapes in Yunnan province[J]. Journal of Yunnan Agricultural University,2015,30(2):173-184.
[4] 邓维萍,杨敏,杜飞,杨积忠,朱书生.云南葡萄产区葡萄炭疽病病原鉴定及致病力分析[J].植物保护学报,2013,40(1):61-67.DENG Weiping,YANG Min,DU Fei,YANG Jizhong,ZHU Shusheng. Identification of the pathogen causing grape anthracnose in Yunnan and its pathogenicity[J].Acta Phytophylacia Sinica,2013,40(1):61-67.
[5] 雷龑,林雄杰,陈婷,刘鑫铭,蔡盛华,范国成.福建葡萄炭疽病病原鉴定及致病性分析[J]. 果树学报,2014,31(6):1123-1127.LEI Yan,LIN Xiongjie,CHEN Ting,LIU Xinming,CAI Shenghua,FAN Guocheng. Pathogen identification and pathogenicity analysis of grape ripe rot in Fu-jian[J].Journal of Fruit Science,2014,31(6):1123-1127.
[6] PENG L J,SUN T,YANG Y L,CAI L,HYDE K D,BAHKALI A H,LIU Z Y. Colletotrichum species on grape in Guizhou and Yunnan provinces,China[J].Mycoscience,2013,54:29-41.
[7] 杨敬辉,陈宏州,肖婷,张文文,庄义庆.葡萄炭疽病菌对多菌灵的抗药性检测[J].江西农业学报,2015,27(1):32-35.YANG Jinghui,CHEN Hongzhou,XIAO Ting,ZHANG Wenwen,ZHUANG Yiqing. Resistance detection of grape anthracnose to Carbendazim[J].Acta Agricultural Jiangxi,2015,27 (1):32-35.
[8] DAVIDSE L C. Benzimidazole fungicides: mechanism of action and biological impact[J]. Annual Review of Phytopathology,1986,24:43-65.
[9] MA Z H,MICHAILIDES T J.Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi[J]. Crop Protection,2005,24:853-863.
[10] LUCK J E,GILLINGS M R.Rapid identification of benomyl resistant strains of Botrytis cinerea using the polymerase chain reaction[J].Mycological Research,1995,99(12):1483-1488.
[11] KOENRAADT H,SOMERVILLE S C,JONES A L. Characterization of mutations in the beta-tubulin gene of benomyl-resistant field strains of Venturia inaequalis and other plant pathogenic fungi[J].Phytopathology,1992,82:1348-1354.
[12] ALBERTINI C,GREDT M,LEROUX P.Mutations of the β-Tubulin gene associated with different phenotypes of benzimidazole resistance in the cereal eyespot fungi Tapesia yallundae and Tapesia acuformis[J]. Pesticide Biochemistry Physiology,1999,64:17-31.
[13] BARALDI E,MARI M,CHIERICI E,PONDRELLI M,BERTOLINI P,PRATELLA G C. Studies on thiabendazole resistance of Penicillium expansum of pears: pathogenic fitness and genetic characterization[J].Plant Pathology,2003,52:362-370.
[14] BANNO S,FUKUMORI F,ICHIISHI A,OKADA K,UEKUSA H,KIMURA M,FUJIMURA M. Genotyping of benzimidazoleresistant and dicarboximide-resistant mutations in Botrytis cinerea using real-time polymerase chain reaction assays[J]. Phytopathology,2008,98(4):397-404.
[15] CHEN F,LIU X,SCHNABEL G. Field strains of Monilinia fructicola resistant to both MBC and DMI Fungicides isolated from stone fruit orchards in the Eastern United States[J]. Plant Disease,2013,97:1063-1068.
[16] LI J,KATIYAR S K,EDLIND T D. Site-directed mutagenesis of Saccharomyces cerevisiae β-tubulin:interaction between residue 167 and benzimidazole compounds[J]. FEBS Letters,1996,385:7-10.
[17] 陈聃,时浩杰,吴慧明,徐志宏,张传清.浙江省葡萄炭疽菌对甲基硫菌灵和戊唑醇的抗药性研究[J].果树学报,2013,30(4):665-668.CHEN Dan,SHI Haojie,WU Huiming,XU Zhihong,ZHANG Chuanqing. Resistance of Colletotrichum gloeosporioides causing grape ripe rot to Thiophanate-methyl and Tebuconazole in Zhejiang[J].Journal of Fruit Science,2013,30(4):665-668.
[18] 王昭.辽宁省葡萄园5 种主要病害的化学防治方法[J].果树医院,2013(6):37-38.WANG Zhao. Chemical control methods of five major diseases in vineyards of Liaoning Province[J]. Fruit Hospital,2013(6):37-38.
[19] 叶佳,张传清.葡萄炭疽病菌对甲基硫菌灵、戊唑醇和醚菌酯的敏感性检测[J].农药学学报,2012,14(1):111-114.YE Jia,ZHANG Chuanqing. Detection of sensitivity of grape anthracnose to thiophanate methyl,tebuconazole and kresoximmethyl[J]. Chinese Journal of Pesticide Sciences,2012,14 (1):111-114.
[20] HU M J,GRABKE A,DOWLING M E,HOLSTEIN H J,SCHNABEL G. Resistance in Colletotrichum siamense from peach and blueberry to Thiophanate-methyl and Azoxystrobin[J].Plant Disease,2015,99(6):806-814.
[21]NALUMPANG S,MIYAMOTO Y,MIYAKE C,IZUMI Y,AKITMITSU K,KONGTRAGOUL P. Point mutations in the beta-tubulin gene conferred carbendazim-resistant phenotypes of Colletotrichum gloeosporioides causing‘Nam Dok Mai’mango anthracnose[J]. Journal of Agricultural Technology,2010,6 (2):365-378.
[22] 李河,李司政,王悦辰,刘君昂,徐建平,周国英.油茶苗圃炭疽病原菌鉴定及抗药性[J].林业科学,2019,55(5):85-94.LI He,LI Sizheng,WANG Yuechen,LIU Jun’ang,XU Jianping,ZHOU Guoying. Identification of the pathogens causing anthracnose of Camellia oleifera in nursery and their resistence to fungicides[J].Scientia Silvae Sinicae,2019,55(5):85-94.
[23] CHUNG W H,ISHII H,NISHIMURA K,FUKAYA M,YANO K,KAJITANI Y.Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan[J].Plant Disease,2006,90(4):506-512.
[24] CHUNG W H,CHUNG W C,PENG M T,YANG H R,HUANG J W. Specific detection of benzimidazole resistance in Colletotrichum gloeosporioides from fruit crops by PCR-RFLP[J].New Biotechnology,2010,27(1):17-24.