火龙果(Hylocereus undatus)是仙人掌科,量天尺属,量天尺的果实。螺螨酯化学名称为3-(2,4-二氯苯基)-2-氧代-1-氧杂螺[4.5]-癸-3-烯-4-基-2,2-二甲基丁酸酯,英文通用名为spirodiclofen,分子式为C21H24Cl2O4。新型季酮酸杀螨剂,具有高效低毒的特点,主要防治螨虫,如全爪螨属、锈螨属、短须螨属、刺绣螨属和叶螨属,可用于柑橘、梨果、核果、葡萄、草莓、啤酒花和坚果等作物。作用机制独特,抑制螨虫体内的脂肪合成,破坏能量代谢活动,从而最终杀死螨虫[1]。
国内外文献关于螺螨酯的研究主要包括螺螨酯新的合成工艺[2],喷雾助剂对螺螨酯的增效作用[3],对红蜘蛛种群增长影响[4],对螨虫的防效[5]和二斑叶螨实验种群的亚致死效应[6-7],山楂叶螨[8-9]和二斑叶螨[10-12]对该药的抗性,对土壤微生物及土壤酶的毒性效应[13],在植株和动物中的代谢[14],柑橘类水果[15-18]和苹果[19]中的消解动态和残留量分析,香橙和香橙茶饮料中的风险监测中的风险监测[20],李子到梅干加工方式对螺螨酯残留量的影响[21],生姜种植土壤中螺螨酯降解菌的筛选与鉴定[22]。Ge等[23]报道了螺螨酯在烟草中的加速溶剂萃取,硅胶净化,高效液相色谱二极管阵列检测器检测的残留检测方法。赵亚洲等[24]报道了螺螨酯在土壤中乙腈-水(体积比4∶1)混合振荡提取,静置后过膜,高效液相色谱二极管阵列检测器检测的残留检测方法。Sun 等[25]报道了螺螨酯在柑橘中的乙腈提取,N-丙基乙二胺固相吸附剂(PSA)净化,上清液过滤膜后超高效液相色谱三重四极杆串联质谱检测的残留检测方法。刘济宁等[26]报道了柑橘及土壤中螺螨酯的混合提取剂(V 乙腈∶V 水=4∶1)提取、Florisil固相萃取小柱净化,气相色谱电子捕获检测器测定的残留检测方法。白芸等[27]报道了苹果及土壤中螺螨酯乙腈提取,Florisil 固相萃取小柱净化,气相色谱电子捕获检测器测定的残留检测方法。谢莉等[17]报道了柑橘和土壤中螺螨酯的丙酮提取,二氯甲烷萃取,气相色谱电子捕获检测器测定的残留检测方法。李东等[28]报道了柑橘中螺螨酯的乙腈提取,高速离心后氮吹浓缩定容,气相色谱电子捕获检测器测定的残留检测方法。许鹏军等[29]报道9 种果品中螺螨酯的乙腈涡旋提取,混合使用乙二胺-N-丙基硅烷(PSA)和ODS-C18两种基质分散萃取净化,气相色谱四极杆质谱联用测定的残留检测方法。赵亚洲等[30]报道了螺螨酯在3种土壤中的淋溶特性,发现一般情况下,螺螨酯淋溶作用弱,造成地下水污染的可能性较小,容易被土壤吸附,对土壤环境可能存在一定的风险性。我国果品中规定了264种农药的2 046项农药最大残留限量[31],但未制定螺螨酯在红龙果中的最大残留限量。国内外关于火龙果全果和果肉中螺螨酯的残留检测方法、残留安全性评价及残留膳食摄入评估未见报道。笔者建立了超高效液相色谱三重四极杆串联质谱检测火龙果全果和果肉中螺螨酯的残留检测方法对其残留量进行膳食摄入风险评估,为螺螨酯在火龙果上的残留安全提供数据支持。
1 材料和方法
1.1 材料
供试品为24%螺螨酯悬浮剂,由天津市汉邦植物保护剂有限责任公司提供;供试作物品种:海南为‘金都1 号’,浙江为‘蜜宝’红心火龙果,云南为‘紫红龙’,贵州为‘紫红龙’,广西为‘金都1号’,广东为‘金都1号’。
1.2 方法
1.2.1 田间试验方法 田间试验时间为2018年,地点分别为海南省儋州市中国热带科学院试验场,浙江省绍兴市滨海新城,云南省玉溪市元江县,贵州省黔南州罗甸县龙坪镇,广西南宁市隆安县那桐镇大藤村,广东省高要市白储镇高岗围村。施药剂量均按有效成分(mg·kg-1,w,后同)进行计算。每个处理3个重复,每个重复小区面积不小于30 m2,3个重复小区不需随机排列,每株树用水量为100 mL,均不套袋。螺螨酯最终残留量试验按剂量80 mg·kg-1和120 mg·kg-1施药2次和3次,最后1次施药后7、14和21 d在最终残留试验小区内随机采集火龙果样品,每小区不少于12 个果实。在对照小区内采集火龙果的对照样品。田间采集的火龙果样本切去果柄,将火龙果沿纵向均匀地切成4~8 瓣,取不相邻的果瓣,分为两组,第一组用于制备全果样品,第二组用于制备果肉样品。样品加干冰粉碎后,-20 ℃保存待测。
1.2.2 检测方法(1)试剂与仪器。甲酸(色谱纯),CNW Technologies;乙腈(色谱纯),默克股份两合公司;PSA 吸附剂,天津博纳艾杰尔科技有限公司;GCB 吸附剂,天津博纳艾杰尔科技有限公司;螺螨酯标准物质(99.0%),德国Dr.Ehrenstorfer。高效液相色谱仪(LC-30AD),日本岛津仪器公司;质谱仪(LCMS-8050),日本岛津仪器公司;食品切碎搅拌机(R10 V.V.),法国Robot Coupe公司。
(2)提取及净化。准确称取10 g 样品(精确至0.01 g)于100 mL 离心管中,加入5 mL 水,20 mL 乙腈,2.5 g 氯化钠,放置多管涡旋振荡器中涡旋10 min,4 000 r·min-1离心5 min,移取2 mL上清液至含有0.05 g PSA和0.02 g GCB的2 mL离心管中,涡旋1 min,4 000 r·min-1离心5 min,经0.22µm针孔滤膜过滤于进样小瓶中,待LC/MS/MS分析。
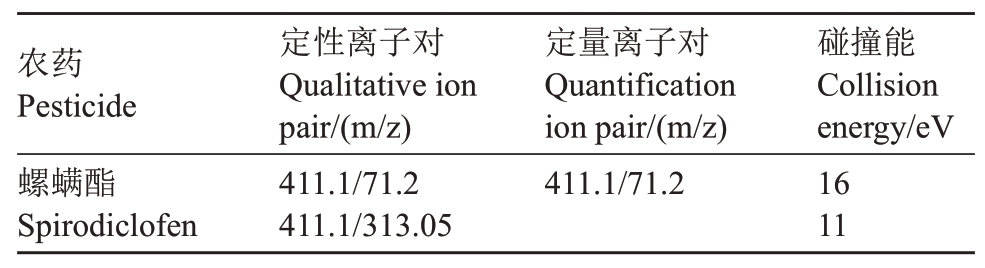
(3)仪器条件。液相色谱柱:Shim-pack GISS(2.1 mm ×50 mm,1.9 µm);柱温:40 ℃;流速:0.3 mL·min-1;流动相:0.1%甲酸水溶液-乙腈(V∶V=20∶80);电离方式:ESI+;CID 气:270 kpa;雾化气流量:3.0 L·min-1;加热气流量:10 L·min-1;接口温度:300 ℃;DL 温度:250 ℃;加热块温度:400 ℃;保留时间:1.4 min;进样量:2µL;接口电压:4.0 kV;运行时间:6 min。检测方式为多重反应监测(MRM)(表1)。
表1 MRM 模式下螺螨酯的监测离子和碰撞电压
Table 1 MRM parameters selected forspirodiclofen analysis
?
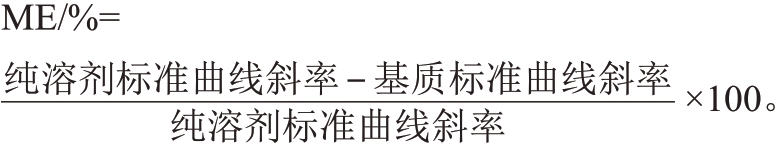
(4)基质效应评价。对纯溶剂与基质配制标准曲线的斜率进行比较,若基质效应(Matrix effect,ME)≥±20%,则采用基质配制标准溶液制作标准曲线,ME<±20%则采用纯溶剂配制标准溶液制作标准曲线。基质效应(ME)计算公式:
1.3 长期膳食摄入评估
依据国家卫生行政部门发布的中国居民营养与健康状况监测调查[32],结合螺螨酯残留化学评估推荐的规范残留试验中值和已制定的螺螨酯最大残留限量(MRLs),计算螺螨酯的国家估算每日摄入量(NEDI),计算公式见参考文献[33-34]。
2 结果与分析
2.1 检测方法
2.1.1 标准曲线 将螺螨酯标准品用乙腈溶解定容制成1 000 mg·L-1的母液,再用空白基质溶液逐级稀释为0.002、0.005、0.01、0.02、0.05、0.1、0.2、0.5 mg·L-1系列标准溶液,在1.2.2(3)条件下进行检测,横坐标为质量浓度(mg·L-1),纵坐标为峰面积(A),标准曲线y=28 570 214.614 9x+9 992.204 6,r2=0.998 9。
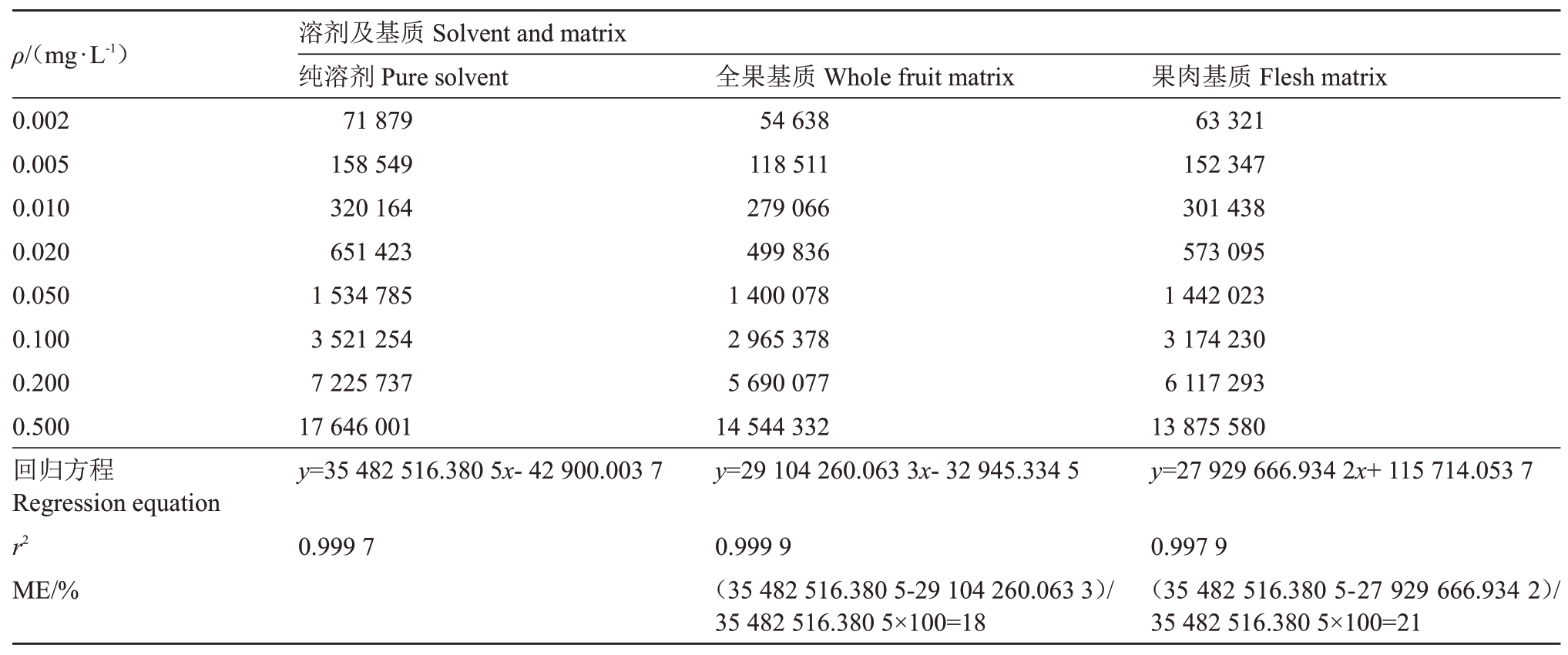
2.1.2 基质效应评价 从表2 可以看出,螺螨酯在全果中基质效应ME 值为18%,在果肉中基质效应ME值为21%,表明螺螨酯基质效应较强,为最大程度减小基质效应的干扰,故本试验采用全果和果肉空白基质配制标准曲线。
表2 螺螨酯在全果和果肉中基质效应评价(峰面积)
Table 2 Evaluation table of matrix effect of spirodiclofen in the whole fruit and flesh of dragon(Peak area)
?
2.1.3 添加回收率 将不同浓度的螺螨酯标液分别加入火龙果全果和果肉空白对照样品中,混合均匀,静置2 h,按1.2.2(2)进行样品的提取和净化(表3)。当添加水平是0.01、0.1 和5 mg·kg-1时,螺螨酯在火龙果全果中的平均回收率为87%~105%,相对标准偏差为4.6%~13%;当添加水平是0.01、0.1 和1 mg·kg-1 时,火龙果果肉中的平均回收率为81%~90%,相对标准偏差为6.6%~8.1%。农业行业标准《农作物中农药残留试验准则》规定添加浓度大于1 mg·kg-1时,回收率应在70%~110%,相对标准偏差应≤10%;添加浓度大于0.01 mg·kg-1 而小于等于0.1 mg·kg-1时,回收率应在70%~120%,相对标准偏差应≤20%;添加浓度大于0.001 mg·kg-1而小于等于0.01 mg·kg-1时,回收率应在60%~120%,相对标准偏差应≤30%。本试验检测结果的精密度、重现性、准确度均符合标准的规定。螺螨酯在火龙果全果和果肉中的最低检测浓度均为0.01 mg·kg-1。
表3 火龙果全果和果肉中螺螨酯的添加回收率
Table 3 The fortified recovery of spirodiclofen in the whole fruit and flesh of dragon %
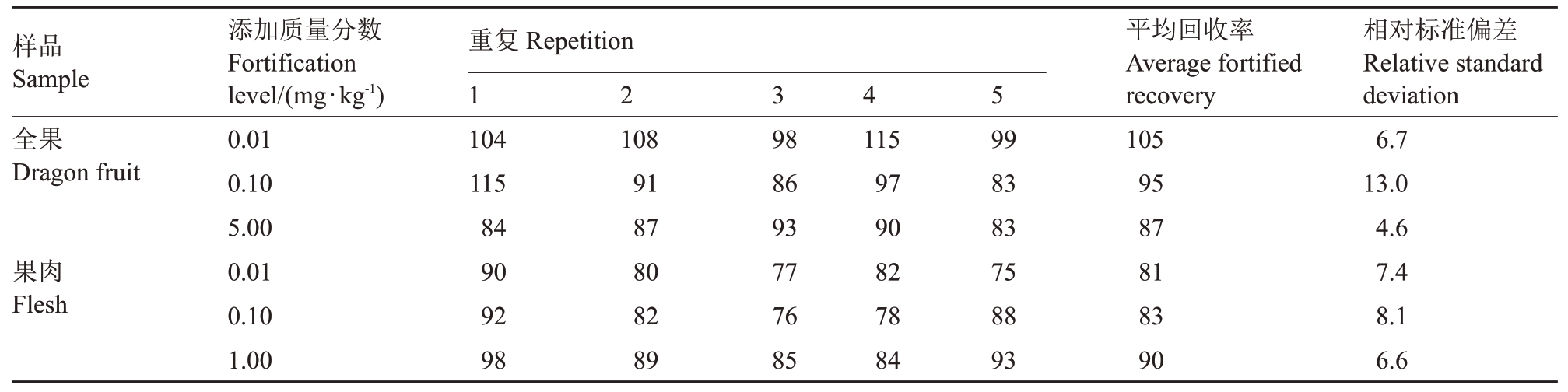
注:1~5 为5 个重复。Note:1-5 mean five repeats.
?
2.2 火龙果全果和果肉中螺螨酯最终残留量
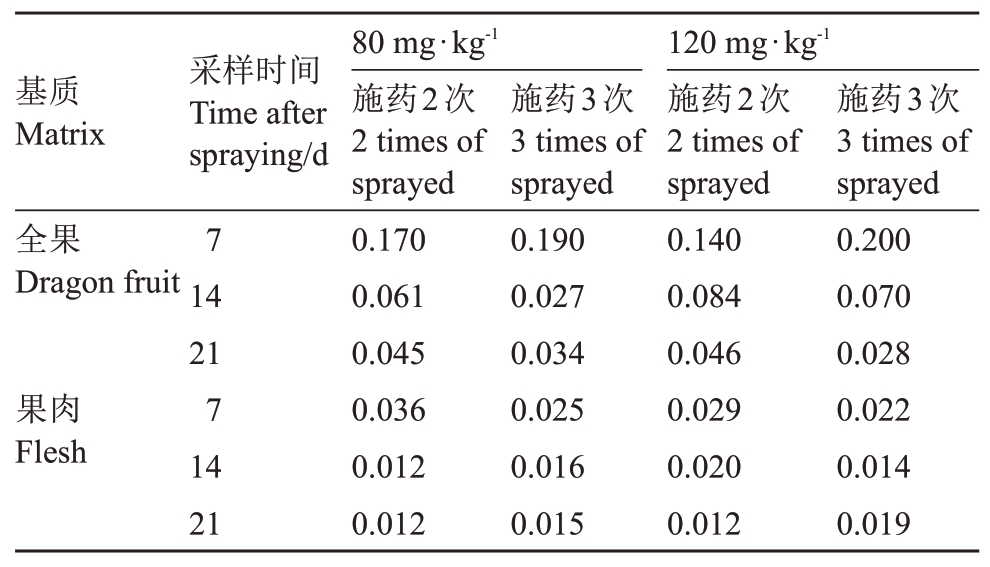
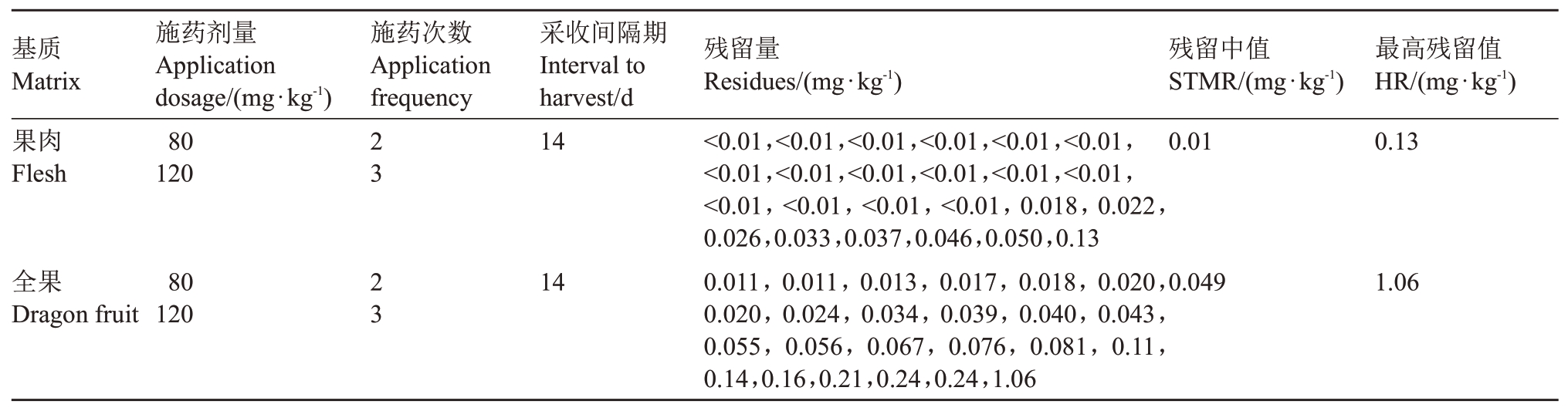
2018 年海南省、浙江省、云南省、贵州省、广东省、广西自治区六地不同施药剂量、施药间隔和采收间隔期最终残留量检测结果见表4。火龙果全果中螺螨酯最终残留量为,采收间隔7 d时,以低剂量施药2次时残留量为≤0.71 mg·kg-1,施药3次时残留量为≤0.61 mg·kg-1;以高剂量施药2 次时残留量为≤0.27 mg·kg-1,施药3 次时残留量为≤0.45 mg·kg-1。采收间隔14 d 时,以低剂量施药2 次时残留量为≤0.17 mg·kg-1,施药3 次时残留量为≤0.061 mg·kg-1;以高剂量施药2次时残留量为≤0.23 mg·kg-1,施药3次时残留量为≤0.19 mg·kg-1。采收间隔21 d 时,以低剂量施药2次时残留量为≤0.16 mg·kg-1,施药3次时残留量为≤0.68 mg·kg-1;以高剂量施药2 次时残留量为≤0.089 mg·kg-1,施药3次时残留量为≤0.055 mg·kg-1。火龙果果肉中螺螨酯最终残留量如下,采收间隔7 d 时,以低剂量施药2 次时残留量为≤0.14 mg·kg-1,施药3 次时残留量为≤0.066 mg·kg-1;以高剂量施药2 次时残留量为≤0.093 mg·kg-1,施药3 次时残留量为≤0.054 mg·kg-1。采收间隔14 d时,以低剂量施药2 次时残留量为≤0.018 mg·kg-1,施药3 次时残留量为≤0.034 mg·kg-1;以高剂量施药2次时残留量为≤0.059 mg·kg-1,施药3次时残留量为≤0.023 mg·kg-1。采收间隔21 d 时,以低剂量施药2 次时残留量为≤0.016 mg·kg-1,施药3次时残留量为≤0.032 mg·kg-1;以高剂量施药2次时残留量为≤0.020 mg·kg-1,施药3次时残留量为≤0.041 mg·kg-1。不同基质、施药剂量、施药次数和采样间隔螺螨酯残留量对比见表5,残留中值和残留最大值见表6。
表4 火龙果全果和果肉中螺螨酯最终残留量测定(n=3)
Table 4 The final residues of spirodicl of enindragonfruit andflesh(n=3)(mg·kg-1)
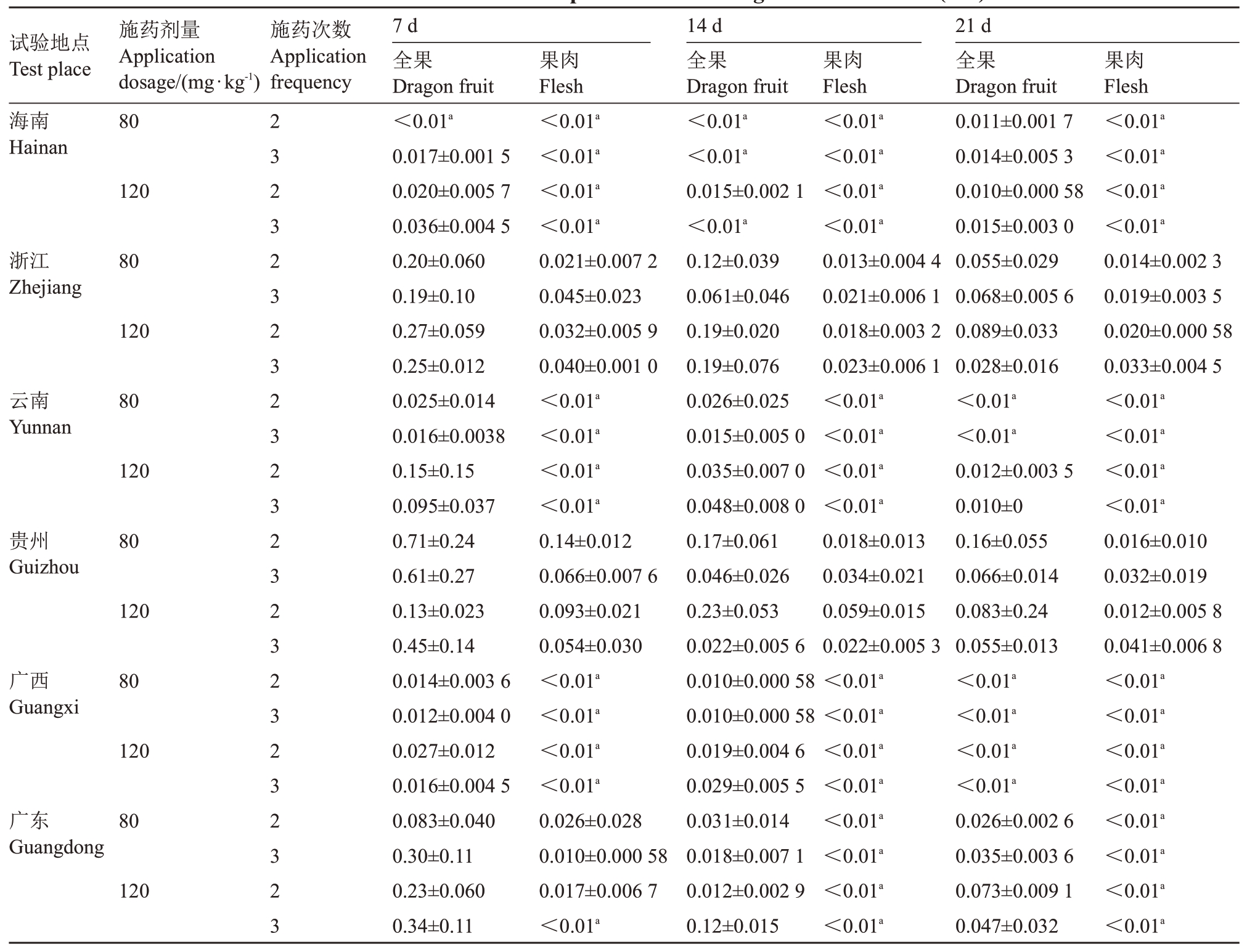
注:a 表示低于最低检测浓度。
Note:a mean the limit of quantification(LOQ).
?
表5 不同基质、施药剂量、施药次数和采样间隔螺螨酯残留量对比
Table 5 Comparison of spirodiclofen residues in different matrixes,dosages,frequencies and intervals to harvest(g a.i.·hm-2)
?
表6 不同基质、施药剂量、施药次数和采样间隔螺螨酯残留中值和残留最大值
Table 6 STMR and HR of spirodiclofen residues in different matrix,dosage,frequency and interval to harvest
?
2.3 长期膳食摄入评估
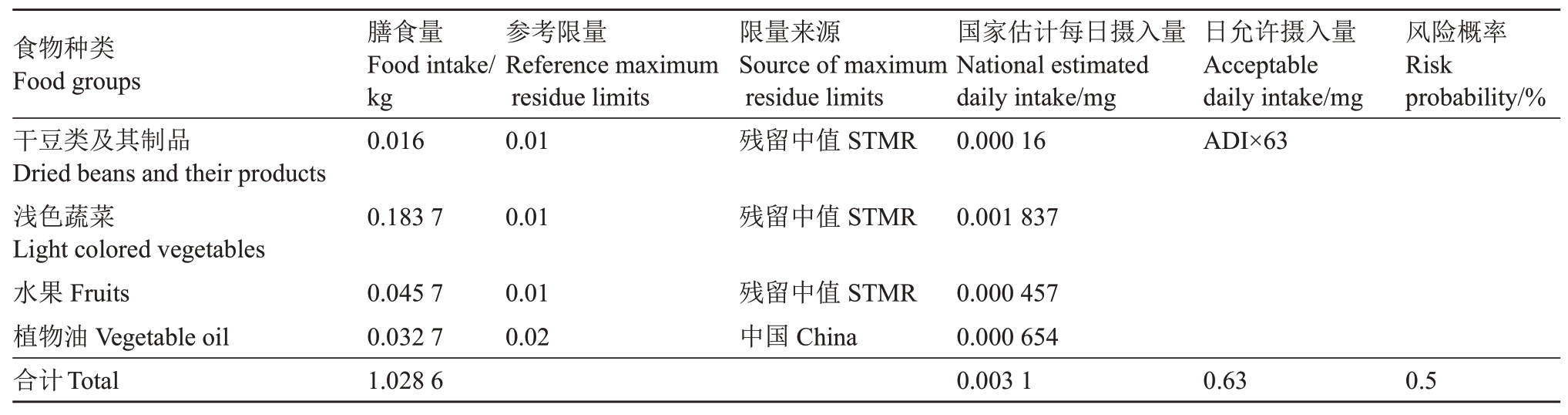
目前,螺螨酯登记农作物有冬枣、柑橘、棉花、苹果、樱桃。本实验室对绿豆、黄秋葵、猕猴桃和火龙果进行了残留试验,绿豆(干豆类及其制品)、黄秋葵(浅色蔬菜)、猕猴桃(水果)、火龙果全果(水果)、火龙果果肉(水果)的残留中值分别为0.01、0.026 5、0.18 mg·kg-1、0.01 和0.049 mg·kg-1。我国城乡居民的干豆类及其制品、浅色蔬菜、水果和植物油的每日食物摄入量分别为0.016 kg、0.183 7 kg、0.045 7 kg和0.032 7 kg(表7)。我国已规定棉籽(植物油)中螺螨酯最大残留限量为0.02 mg·kg-1 [35]。我国规定螺螨酯的ADI值为0.01 mg·kg-1,我国居民的平均体重为63 kg。因火龙果的主要食用部位是果肉,因此仅对果肉中的残留量进行膳食评估。根据1.3 中公式进行计算,螺螨酯普通人群的国家估计每日摄入量是0.003 1 mg,占日允许摄入量的0.5%左右。
表7 螺螨酯风险评估计算表
Table 7 Risk assessment for dietary residue intake of spirodiclofen
?
3 讨 论
本试验前处理方法有机提取溶剂为市场上易购买的乙腈,且用量较少,有利于节省试验成本,减少试剂对环境的污染。前处理方法提取10 min,离心5 min,涡旋1 min,再次离心5 min,用时较短,有利于提高试验效率。当施药次数和施药剂量相同时,火龙果全果和果肉中螺螨酯残留量均随着采收间隔期的增加在减少,说明采收间隔期与最终残留量存在负相关。可能是因为随着采收时间的延长,螺螨酯在消解;同时火龙果没有停止生长,其生长的稀释作用进一步降低螺螨酯最终残留量。当采收间隔期和施药次数相同时,两个施药剂量之间的螺螨酯残留量没有明显规律,可能是因为作物生长的稀释作用和生长环境是农药消解的主要因素。在剂量增加1.5倍后最终残留量并没有增加相应的倍数,说明在施药剂量增加1.5倍的范围内,施药剂量和最终残留量相关性不大。当施药剂量和采收间隔期相同时,两个施药次数之间的螺螨酯残留量没有明显规律,可能是因为第一次施药时距采收时间较长,第一次施用的螺螨酯在采收时消解较多,对最终残留量贡献不大。一年六地火龙果全果和果肉中螺螨酯残留量检测结果可以看出,当施药剂量、施药次数和采样间隔相同时,螺螨酯在全果中的残留量大于果肉中的残留量,原因可能是在施药时主要着药部位是火龙果果皮,火龙果果肉被果皮包被没有直接着药,只有少量传导到果肉中。螺螨酯内吸性较差,但部分果肉中也有检出,原因可能是因为该试验所用剂型为悬浮剂,黏附力度强,能够长时间附着在火龙果表面[36]。
我国和国际食品法典委员会均未制定火龙果中螺螨酯的最大残留限量,无法通过检测结果直接判定按本试验的施药方法施药后的火龙果残留是否合格,因此需要对按照本试验的施药次数、施药剂量和采收间隔期使用后的火龙果进行膳食摄入评估。24%螺螨酯悬浮剂,用于防治火龙果上红蜘蛛,用药量3 000~4 000 倍液(60~80 mg·kg-1),施药1~2 次,安全间隔期为14 d 时,螺螨酯普通人群的国家估计每日摄入量是0.003 1 mg,风险概率为0.5%,认为螺螨酯在火龙果中的残留对一般人群健康的影响是在一个可接受的风险水平。本试验火龙果的最高残留值为1.06 mg·kg-1,按照食品中农药最大残留限量制定指南推荐最大残留限量为3 mg·kg-1。
4 结 论
研究结果表明,24%螺螨酯悬浮剂在火龙果上使用时,用药量120 mg·kg-1,施药2~3 次,安全间隔期21 d,对一般人群健康不会产生不可接受的风险。
[1] 胡笑形.农药手册[M].北京:化学工业出版社,2015:934.HU Xiaoxing. Pesticide handbook[M]. Beijing: Chemical Industry Press,2015:934.
[2] 周勇.新型杀螨剂螺螨酯的合成工艺及其衍生物的合成与生物活性研究[D].杭州:浙江工业大学,2010.ZHOU Yong. Studies on the synthesis technology of spirodiclofen and the synthesis,bioactivity evaluation of the derivatives[D].Hangzhou:Zhejiang University of Technology,2010.
[3] 杨石有,张蕊,张贺,王红刚,王洪星,陈银华.五种喷雾助剂对240 g/L 螺螨酯悬浮剂和15%哒螨灵乳油防治木瓜秀粉蚧的增效作用[J].农药学学报,2019,21(4):531-537.YANG Shiyou,ZHANG Rui,ZHANG He,WANG Honggang,WANG Hongxing,CHEN Yinhua. Synergistic effect of five spray adjuvants on spirodiclofen 240 g/L suspension concentrate and pyridaben 15% emulsifiable concentrate for the control of Paracoccus marginatus[J].Chinese Journal of Pesticide Science,2019,21(4):531-537.
[4] LIN T,YOU Y,ZENG Z H,CHEN Y X,CHI H,XIA J M,ZHAO J W,CHEN Y,TIAN H J,WEI H. Effects of spirodiclofen on life history traits and population growth of a spider mite predator Oligota flavicornis (Coleoptera: Staphyllinidae)based on the age-stage two-sex life table theory[J].Pest Management Science,2019,75(3):639-647.
[5] VECHIA J F D,FERREIRA M C,ANDRADE D J. Interaction of spirodiclofen with insecticides for the control of Brevipalpus yothersi in citrus[J]. Pest Management Science,2018,74(11):2438-2443.
[6] SABER M,AHMADI Z,MAHDAVINIA G.Sublethal effects of spirodiclofen,abamectin and pyridaben on life-history traits and life-table parameters of two-spotted spider mite,Tetranychus urticae (Acari: Tetranychidae) [J]. Experimental and Applied Acarology,2018,75(1):55-67.
[7] 张寿芳,沈修婧,蔡瑜如,付麟云,沈慧敏.甲氰菊酯和螺螨酯对二斑叶螨实验种群的亚致死效应[J]. 植物保护,2012,38(5):68-72.ZHANG Shoufang,SHEN Xiujing,CAI Yuru,FU Linyun,SHEN Huimin. Sublethal effects of fenpropathrin and spirodiclofen on Tetranychus urticae[J]. Plant Protection,2012,38(5):68-72.
[8] 封云涛,郭晓君,庾琴,张润祥,张苗,史高川,范仁俊.山楂叶螨对螺螨酯的抗药性及对七种杀螨剂的交互抗性[J].应用昆虫学报,2018,55(3):497-502.FENG Yuntao,GUO Xiaojun,YU Qin,ZHANG Runxiang,ZHANG Miao,SHI Gaochuan,FAN Renjun. Selection of Amphitetranychus viennensis for resistance to spirodiclofen and cross-resistance to seven other acaricides[J]. Chinese Journal of Applied Entomology,2018,55(3):497-502.
[9] 封云涛,郭晓君,庾琴,张润祥.山楂叶螨对螺螨酯的抗药性选育及现实遗传力[J].农药,2017,56(2):148-150.FENG Yuntao,GUO Xiaojun,YU Qin,ZHANG Runxiang. Selection of Amphitetranychus viennensis for resistance to spirodiclofen and cross-resistance to seven other acaricides[J]. Agrochemicals,2017,56(2):148-150.
[10] 李忠洲,周玉书,朴静子,田如海,高萍.二斑叶螨对螺螨酯的抗性选育及其解毒酶活性测定[J]. 应用昆虫学报,2013,50(2):454-459.LI Zhongzhou,ZHOU Yushu,PIAO Jingzi,TIAN Ruhai,GAO Ping. Determination of selection for resistance to spirodiclofen and its effect on detoxification enzymes in Tetranychus urticae[J]. Chinese Journal of Applied Entomology,2013,50(2): 454-459.
[11] 田如海,周玉书,李忠洲,朴静子,高萍.二斑叶螨对螺螨酯的抗性选育及现实遗传力[J]. 植物保护学报,2012,39(3):287-288.TIAN Ruhai,ZHOU Yushu,LI Zhongzhou,PIAO Jingzi,GAO Ping. Realized heritability and laboratory resistance selection of two-spotted spider mite to spirodiclofen[J].Acta Phytophylacica Sinica,2012,39(3):287-288.
[12] 吕娟娟,王进军,张寿芳,沈慧敏. 二斑叶螨抗螺螨酯品系GST 基因的克隆与表达分析[J]. 昆虫学报,2013,56(4):438-445.LÜ Juanjuan,WANG Jinjun,ZHANG Shoufang,SHEN Huimin. Cloning and expression profiling of glutathione S-transferase genes in the spirodiclofen-resistant strain of Tetranychus urticae Koch (Acari: Tetranychidae) [J].Acta Entomologica Sinica,2013,56(4):438-445.
[13] 张霞,赵海燕,谢峰,袁旭杰,王飞菲,张清明.螺螨酯对土壤微生物及土壤酶的毒性效应[J]. 环境科学与技术,2015,38(9):18-23.ZHANG Xia,ZHAO Haiyan,XIE Feng,YUAN Xujie,WANG Feifei,ZHANG Qingming. Toxicological effects of spirodiclofen on soil microbial population and enzyme activities[J].Environmental Science&Technology,2015,38(9):18-23.
[14] KOESTER J. Metabolism of spirodiclofen (BAJ 2740) in animals and plants[J]. Pflanzenschutz Nachrichten Bayer,2002,55(2/3):237-254.
[15] 欧英娟,彭晓春,吴彦瑜,廖树妹,吴胤.螺螨酯和联苯肼酯在柑橘中的残留降解及其膳食风险评估[J].广东化工,2016,43(8):39-41.OU Yingjuan,PENG Xiaochun,WU Yanyu,LIAO Shumei,WU Yin. Degradation of spirodiclofen and bifenazate residue in citrus and its risk assessment on the dietary exposure[J]. Guangdong Chemical,2016,43(8):39-41.
[16] 谢莉,杨仁斌,傅强,肖少怀,柳王荣.螺螨酯在柑橘和土壤中残留及消解动态[J].中国农学通报,2012,28(31):271-276.XIE Li,YANG Renbin,FU Qiang,XIAO Shaohuai,LIU Wangrong. Residue and dissipation dynamics of spirodiclofen in orange and soil[J].Chinese Agricultural Science Bulletin,2012,28(31):271-276.
[17] 谢莉,杨仁斌,傅强,肖少怀,柳王荣.气相色谱法测定柑橘和土壤中螺螨酯残留[J].环境监测管理与技术,2012,24(2):40-44.XIE Li,YANG Renbin,FU Qiang,XIAO Shaohuai,LIU Wangrong. Determination for spirodiclofen residues in orange and soils by GC[J]. Environmental Monitoring Management and Technology,2012,24(2):40-44.
[18] SUN H B,LIU C Y,WANG S W,LIU Y P,LIU M J. Dissipation,residues,and risk assessment of spirodiclofen in citrus[J].Environmental Monitoring and Assessment,2013,185(12):10473-10477.
[19] 袁龙飞,李薇,李莉,许瑞翔,石凯威.螺螨酯在苹果和土壤中的残留分析及消解动态研究[J]. 食品安全质量检测学报,2016,7(9):3449-3454.YUAN Longfei,LI Wei,LI Li,XU Ruixiang,SHI Kaiwei.Degradation dynamics and residues analysis of spirodiclofen in apple and soil[J]. Journal of Food Safety and Quality,2016,7(9):3449-3454.
[20] LEE K G,LEE S K.Monitoring and risk assessment of pesticide residues in yuza fruits (Citrus junos Sieb. ex Tanaka) and yuza tea samples produced in Korea[J]. Food Chemistry,2012,135(4):2930-2933.
[21] ALISTER C,ARAYA M,BECERRA K,VOLOSKY C,SAAVEDRA J,KOGAN M. Industrial prune processing and its effect on pesticide residue concentrations[J]. Food Chemistry,2018,268:264-270.
[22] 贾海飞,朱永哲.生姜种植土壤中螺螨酯降解菌Enterobacter sp.QD26-6 的筛选与鉴定[J].农药,2015,54(5):327-329.JIA Haifei,ZHU Yongzhe. Screening and identification of spirodiclofen-degrading bacteria Enterobacter sp. QD26-6 for soil under ginger cultivation[J]. Agrochemicals,2015,54(5): 327-329.
[23] GE X S,WU X Q,QI H J,QIN X L,SUN H W.A simple and effective method for the determination of chlorantraniliprole and spirodiclofen in tobacco by liquid chromatography after accelerated solvent extraction and silica gel clean-up[J].Journal of Liquid Chromatography and Related Technologies,2014,37(2):145-154.
[24] 赵亚洲,谭红,席陪宇,李景壮,段亚玲,何锦林.螺螨酯在土壤中的残留分析[J].农药,2014,53(7):509-511.ZHAO Yazhou,TAN Hong,XI Peiyu,LI Jingzhuang,DUAN Yaling,HE Jinlin. Residue analysis of spirodiclofen in soil[J].Agrochemicals,2014,53(7):509-511.
[25] SUN D L,ZHU Y M,PANG J X,ZHOU Z Q,JIAO B N. Residue level,persistence and safety of spirodiclofen-pyridaben mixture in citrus fruits[J].Food Chemistry,2016,194:805-810.
[26] 刘济宁,韩志华,吴冠群,石利利,单正军.柑桔与土壤中螺螨酯农药残留量分析[J]. 农业环境科学学报,2007,26(S):647-650.LIU Jining,HAN Zhihua,WU Guanqun,SHI Lili,SHAN Zhengjun. Gas chromatographic determination of spirodiclofen residues in orange and soil[J].Journal of Agro-Environment Science,2007,26(S):647-650.
[27] 白芸,许鹏军,高晓莎,孙微,张红艳.苹果及土壤中的螺螨酯残留分析方法[J].分析科学学报,2009,25(2):229-231.BAI Yun,XU Pengjun,GAO Xiaosha,SUN Wei,ZHANG Hongyan.Determination of residual spirodiclofen in apple and soil[J].Journal of Analytical Science,2009,25(2):229-231.
[28] 李东,李卫兵,祝子铜,余琪,徐佳文,章应俊,彭芳.气相色谱法测定柑橘中螺螨酯残留量[J]. 食品安全质量检测学报,2014,5(8):2496-2499.LI Dong,LI Weibing,ZHU Zitong,YU Qi,XU Jiawen,ZHANG Yingjun,PENG Fang. Determination of spirodiclofen in oranges by gas chromatography[J]. Journal of Food Safety and Quality,2014,5(8):2496-2499.
[29] 许鹏军,高晓莎,陶晡,张红艳,江树人.分散固相萃取-气相色谱四极杆质谱联用测定9 种果品中的螺螨酯残留[J].分析化学,2008,36(11):1515-1520.XU Pengjun,GAO Xiaosha,TAO Bu,ZHANG Hongyan,JIANG Shuren. Determination of spirodiclofen residue in nine fruits by gas chromatography- quadrupole mass spectrometry with dispersive solid phase extraction[J].Chinese Journal of Analytical Chemistry,2008,36(11):1515-1520.
[30] 赵亚洲,李景壮,席培宇,段亚玲,陈恺,谭红,何锦林.螺螨酯在3 种土壤中的淋溶特性[J].江苏农业科学,2015,43(1):359-361.ZHAO Yazhou,LI Jingzhuang,XI Peiyu,DUAN Yaling,CHEN Kai,TAN Hong,HE Jinlin. Leaching characteristics of spirodiclofen in three soils[J]. Journal of Henan Agricultural Sciences,2015,43(1):359-361.
[31] 庞荣丽,王瑞萍,郭琳琳,乔成奎,罗静,田发军,王彩霞,李君,谢汉忠.我国果品中农药残留限量标准现状分析[J].果树学报,2020,37(8):1236-1246.PANG Rongli,WANG Ruiping,GUO Linlin,QIAO Chengkui,LUO Jing,TIAN Fajun,WANG Caixia,LI Jun,XIE Hanzhong.Evaluation of the current standard for pesticide residue limits in fruits in China[J]. Journal of Fruit Science,2020,37(8): 1236-1246.
[32] 农业农村部. 中华人民共和国农业部公告第2308 号[EB/OL]. (2015-10-08)[2002-08-09]. http://www.moa.gov.cn/nybgb/2015/shiqi/201712/t20171219_6103890.htm.
[33] 张志恒,汤涛,徐浩,李振,杨桂玲,王强.果蔬中氯吡脲残留的膳食摄入风险评估[J]. 中国农业科学,2012,45(10):1982-1991.ZHANG Zhiheng,TANG Tao,XU Hao,LI Zhen,YANG Guiling,WANG Qiang. Dietary intake risk assessment of forchlorfenuron residue in fruits and vegetables[J]. Scientia Agricultura Sinica,2012,45(10):1982-1991.
[34] WANG L,ZHAO P Y,ZHANG F Z,LI Y J,DU F P,PAN C P.Dissipation and residue behavior of emamectin benzoate on apple and cabbage field application[J].Ecotoxicology and Environmental Safety,2012,78:260-264.
[35] 中华人民共和国卫生和计划生育委员会,中华人民共和国农业农村部,国家市场监督管理总局.食品安全国家标准食品中农药最大残留限量:GB 2763-2019[S].北京:中国农业出版社,2019.The Ministry of Health of the People's Republic of China,The Ministry of Agriculture of the People's Republic of China,State Administration of market supervision.National food safety standard maximum residue limits for pesticides in food: GB 2763-2019[S].Beijing:China Agriculture Press,2019.
[36] 李伟雄. 农药剂型悬浮剂及其应用前景[J]. 广东农业科学,2007(6):63-65.LI Weixiong.Application prospects of pesticide suspension concentrates[J]. Journal of Guangdong Agricultural Science,2007(6):63-65.