桃(Prunus persica L.)是世界第六大水果,也是我国第四大果品。近年来,我国桃树栽培面积迅速扩大,但相关的技术普及滞后,导致其果实外观整齐度差、着色差、风味淡等问题,进而影响出口[1-2]。目前,各地区桃果实的成熟期分布不均衡,总体上早熟和中熟品种居多。生产上早熟桃品种占70%以上,而且这些早熟桃一般果个较小,品质较差,肉质软,不耐贮运,而优质、硬溶、耐贮运的中、晚熟品种所占比例甚小[3-4],因此,如何提高桃果实的产量与品质备受关注。
喷施植物生长调节剂,如茉莉酸甲酯和二氢茉莉酸丙酯[5]、油菜素内酯[6]、一氧化氮[7]等是提高桃果实品质的常用手段,但这些药剂可能存在残留的问题。褪黑素(Melatonin)是一种具有广谱性和突破性效果的高能植物生长调节剂,在很多水果(如苹果、香蕉、草莓和菠萝)中都天然地存在一定的量[8]。褪黑素本身为生物体内源代谢产物,能够被生物降解,对人体无毒性、安全、无残留[9]。作为多调节分子的褪黑素,能够对植物的碳氮代谢[10]及糖代谢[11]进行调节,促进植物生长发育,增加产量,延迟叶片衰老,影响果实成熟和贮藏[12],并能提高植物的抗逆性[13]。高文华[14]研究发现,100~150µmol·L-1褪黑素处理能显著促进西瓜果实可溶性总糖、可溶性固形物、维生素C 以及番茄红素的积累,而高浓度(大于150µmol·L-1)褪黑素处理的西瓜则品质欠佳。在果树的研究上,褪黑素能够促进梨果实发育,增加梨果实单果质量,并通过调节蔗糖相关合成分解酶编码基因的表达进一步调控转化酶、蔗糖合酶、蔗糖磷酸合酶活性,使梨果实中蔗糖和山梨醇含量增加,同时增加可溶性固形物含量,从而改善梨果实品质[15-16]。褪黑素(100 和200 µmol·L-1)通过增加苹果幼树的主干直径、树高、侧枝长度、侧枝数量和叶绿素含量促进其生长[17]。褪黑素在桃的研究上主要集中在桃果实的采后处理方面,即褪黑素能够缓解贮藏期间的低温冷害,延长贮藏期[18],有关褪黑素对桃生长及果实品质的影响尚未见报道。鉴于此,笔者以早熟桃为试验材料,在桃果实成熟前1 个月对其叶片进行不同浓度褪黑素的喷施处理,研究褪黑素对桃生长和果实品质的影响,以期筛选能促进桃生长和提高其果实品质的最佳褪黑素浓度,为褪黑素在果树上的应用提供参考。
1 材料和方法
1.1 试验地概况
试验地位于四川省成都市温江区(30°36′N,103°41′E),属亚热带湿润气候区,四季分明,日照偏少,年均气温16.0 ℃,平均降雨量865.9 mm。土壤为潮土,其基本理化性质为:pH值7.62,有机质含量(w,后同)12.38 mg·g-1,全氮含量0.750 mg·g-1,全磷含量11.88 mg·g-1,全钾含量15.38 mg·g-1,碱解氮含量57.29 mg·kg-1,速效磷含量35.28 mg·kg-1,速效钾含量21.96 mg·kg-1,水溶性钙含量15.36 mg·kg-1,水溶性镁含量3.128 mg·kg-1,水溶性钾含量2.056 mg·kg-1,水溶性钠含量3.219 mg·kg-1。
供试材料为5 a(年)生早熟桃‘早蜜’,属水蜜桃(Prunus persica)类,砧木为毛桃。桃树种植方式为高垄栽培,每行种植桃树20株,株行距3.5 m×3.5 m,每5 株作开沟处理,桃树株高2 m 左右,冠幅3 m 左右。树形为开心形,每年冬季和夏季各修剪1次。
1.2 试验设计
2019 年5 月初,果实进入快速膨大期,选取20株树势健壮基本一致无病虫害的‘早蜜’桃树,挂牌标记,并用双层遮光袋为所有果实套袋。对桃树整株进行叶面喷施褪黑素(0、50、100、150 和200 µmol·L-1),喷施程度以叶片正背面均匀布满雾状水滴为度[19]。每株为1 个重复,每个处理4 次重复,完全随机排列。之后每隔7 d 喷施1 次,共喷4 次。试验植株按常规栽培措施管理。2019 年6 月中旬,当桃果实达到商业成熟度80%时进行枝条和果实的采收。2019 年5 月1 日—6 月15 日的日均气温22.8 ℃,平均高温25.5 ℃,平均低温17.6 ℃,极端高温30 ℃,极端低温12 ℃,降雨量102.3 mm。
1.3 测定项目与方法
从每株树的东南西北方向对相似位置长势相对一致的当年生新梢从基部进行取样,每株取8~10枝,并以8~10 片叶长度标准将新梢分为基部、中部和顶部3部分,用游标卡尺分别量取3部分茎长和底部茎粗。取功能叶片测定光合色素(叶绿素a、叶绿素b、叶绿素总量和类胡萝卜素)含量、抗氧化氢酶(CAT)活性,超氧化物歧化酶(SOD)活性、可溶性蛋白含量及丙二醛(MDA)含量[20]。在每株树的东西南北方向(含内膛和枝条上部)随机采集果实20个,用电子秤称取果实质量(单果质量),用HP-230型硬度仪测定果实硬度,用数显卡尺测定果实纵横径,果形指数=果实横径/果实纵径。果实可溶性固形物含量用手持式折光仪取果汁测定,维生素C 含量采用2,6-二氯靛酚蓝比色法测定[21],可滴定酸含量采用NaOH 滴定法测定[21],并测定果实苯丙氨酸解氨酶(PAL)[21]、多酚氧化酶(PPO)[20]、抗坏血酸过氧化物酶(APX)[20]和脂氧合酶(LOX)活性[22]。取果实样品切碎置于烘箱中105 ℃杀青,75 ℃烘干至恒重,粉碎过0.149 mm筛,用于测定果实可溶性总糖及糖组分含量(蔗糖、果糖、葡萄糖、山梨醇)[23]。
1.4 数据处理
采用Microsoft Excel 及SPSS 20.0 进行数据统计与分析处理。
2 结果与分析
2.1 褪黑素对桃树枝条生长的影响
随褪黑素浓度的增加,桃树枝条基部茎长呈先增后减趋势(表1),其中褪黑素浓度为50~150µmol·L-1时均显著高于对照;中部和顶部茎长的变化趋势与基部相似,但均在褪黑素浓度为100µmol·L-1时最大,较对照分别增加了19.36%(p <0.05)和28.72%(p <0.05)。经50~150µmol·L-1褪黑素处理后,桃树枝条基部茎粗较对照均显著增加,褪黑素浓度高于150µmol·L-1时,与对照无显著差异;随褪黑素浓度的增加,桃树枝条中部茎粗呈先增后减趋势,在褪黑素浓度为150µmol·L-1时最大,较对照增加了10.74%(p <0.05)。桃树枝条顶部茎粗随褪黑素浓度的增加呈无规则波状变化趋势,在褪黑素浓度为50 µmol·L-1时最大,较对照增加了21.38%(p <0.05)。
表1 褪黑素对桃树枝条生长的影响
Table 1 Effects of melatonin on branch growth of peach
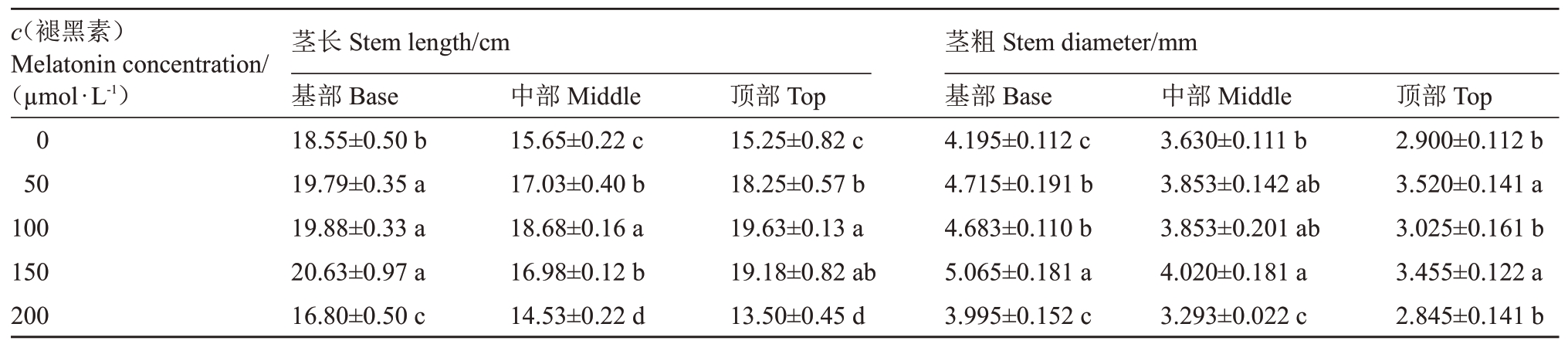
注:小写字母不同表示处理间差异达到0.05 显著水平,下同。
Note:Different lowercase letters indicated significant differences among treatments at 0.05 levels(p <0.05).The same below.
?
2.2 褪黑素对桃树叶片光合色素含量的影响
经褪黑素处理后,桃树叶片光合色素含量较对照有增加也有减少(图1)。褪黑素浓度为150µmol·L-1时,叶绿素a 含量与对照有显著差异,较对照减少了4.29%(p <0.05),褪黑素浓度为100µmol·L-1时,叶绿素a 含量较对照减少了0.46%(p >0.05),其余2 个处理的叶绿素a 含量与对照无显著差异。褪黑素浓度为100~150µmol·L-1时,叶绿素b含量较对照均显著减少,但低于100µmol·L-1时与对照无显著差异,高于150µmol·L-1时较对照显著增加。仅在褪黑素浓度为150µmol·L-1时,总叶绿素含量较对照有所减少,其余处理与对照均无显著差异。褪黑素处理后,叶绿素a/b值较对照有增加也有减少,其类胡萝卜素含量除褪黑素浓度为50 和200µmol·L-1时与对照无显著差异外,其余处理均低于对照。
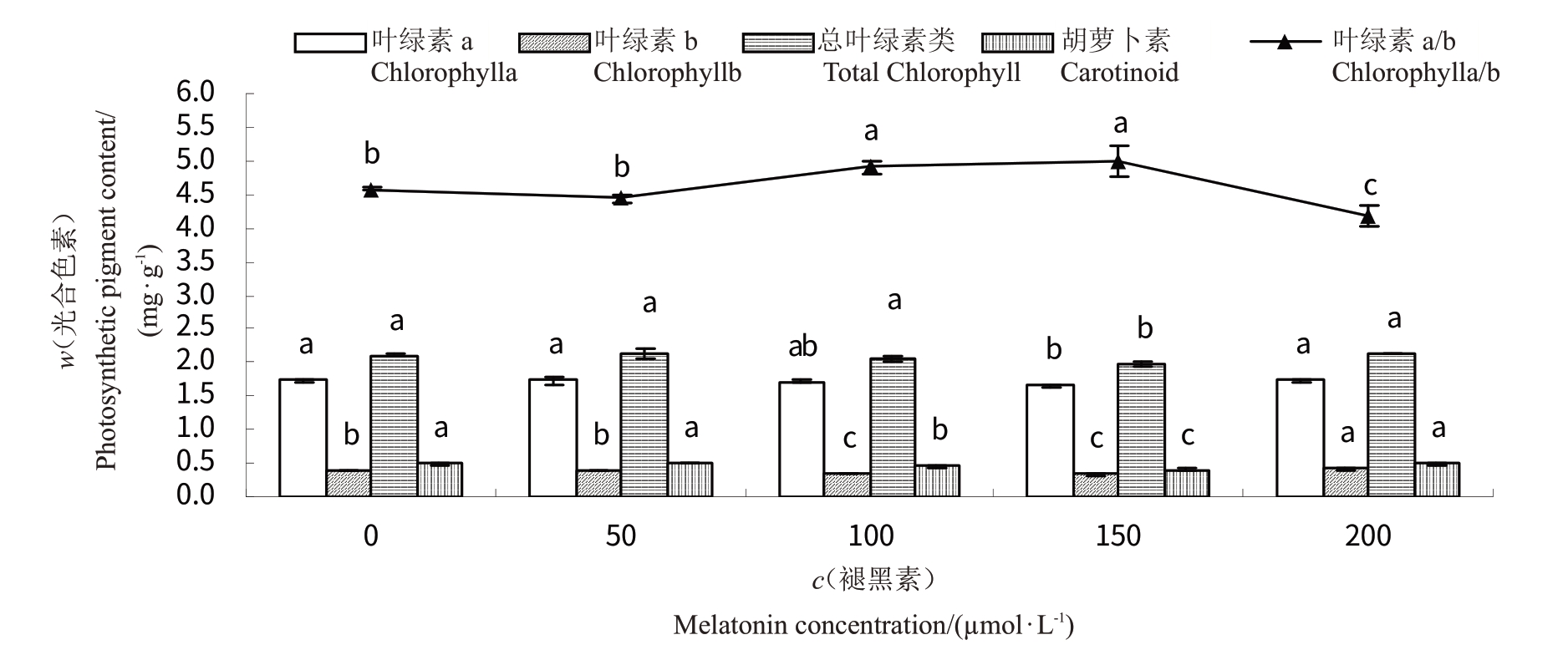
图1 褪黑素对桃树叶片光合色素含量的影响
Fig.1 Effects of melatonin on photosynthetic pigment content in peach leaves
2.3 褪黑素对桃树叶片抗氧化酶活性与渗透调节物质含量的影响
经褪黑素处理后,除了褪黑素浓度为150µmol·L-1时,桃树叶片SOD 活性与对照无显著差异,其余处理均显著低于对照,在褪黑素浓度为50µmol·L-1时最小。褪黑素浓度为50 和100µmol·L-1,桃树叶片CAT活性较对照提高了10.33%(p <0.05)和12.65%(p <0.05);褪黑素浓度为150 和200µmol·L-1,CAT活性较对照降低了23.51%(p <0.05)和12.68%(p <0.05)。在褪黑素浓度为50 µmol·L-1时,桃树叶片MDA 含量较对照有所增加;而褪黑素浓度高于50µmol·L-1后,桃树叶片MDA 含量较对照均有所减少。褪黑素浓度为50µmol·L-1时,桃树叶片可溶性蛋白含量与对照无显著差异;褪黑素浓度为100、150和200µmol·L-1时,桃树叶片可溶性蛋白含量均低于对照,较对照分别减少了8.86%(p <0.05)、8.32%(p <0.05)和9.81%(p <0.05)(表2)。
表2 褪黑素对桃树叶片抗氧化酶活性与渗透调节物质含量的影响
Table 2 Effects of melatonin on antioxidant enzyme activity and osmotic substance content of peach leaves
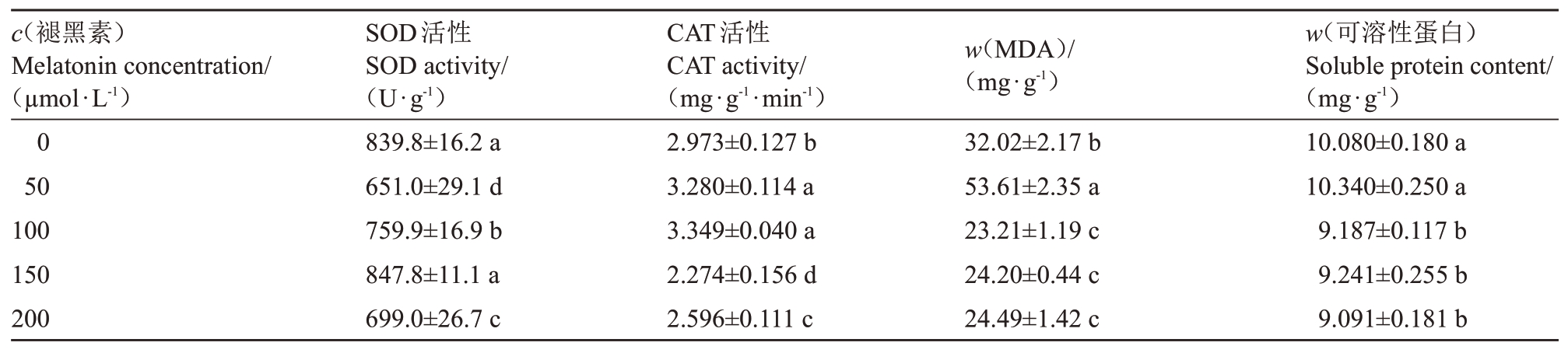
?
2.4 褪黑素对桃果实相关代谢酶活性的影响
褪黑素显著提高了桃果实PAL 活性,在褪黑素浓度为200µmol·L-1时最大,较对照提高了42.00%(p <0.05,表3)。桃果实APX 活性的大小顺序为:150 µmol·L- 1>100 µmol·L- 1>50 µmol·L- 1>200µmol·L-1>0 µmol·L-1。褪黑素处理的桃果实PPO活性均显著低于对照,在褪黑素浓度为50µmol·L-1时最小,较对照降低了29.13%(p <0.05)。褪黑素处理后,桃果实LOX 活性均显著高于对照,在褪黑素浓度为150 µmol·L- 1 时最大,较对照提高了44.71%(p <0.05)。
表3 褪黑素对桃果实相关代谢酶活性的影响
Table 3 Effects of melatonin on related metabolic enzyme activity of peach fruit
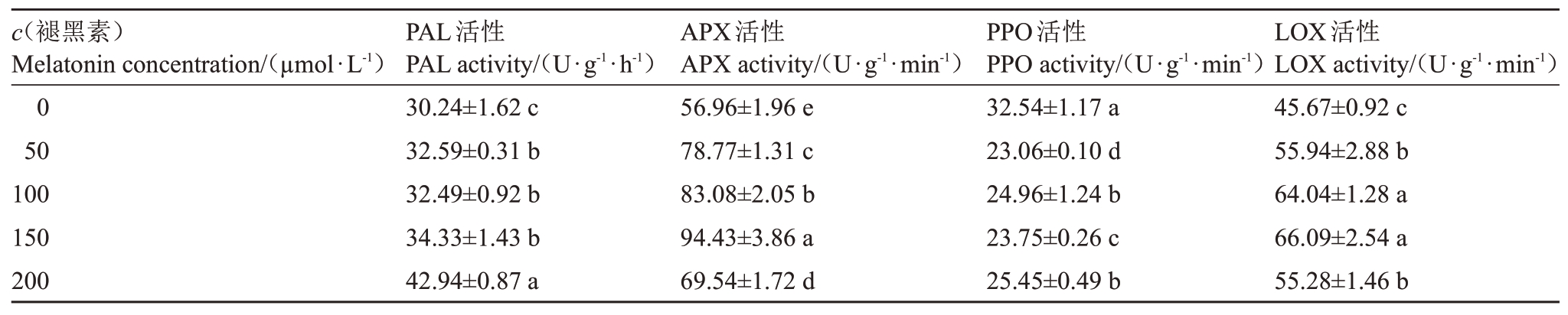
?
2.5 褪黑素对桃果实外观品质的影响
褪黑素浓度为100和150µmol·L-1时,桃果实单果质量较对照分别增加了13.57%(p <0.05)和14.62%(p <0.05),其余各处理均显著低于对照(表4)。桃果实纵径和横径的变化趋势与单果质量相似,均在褪黑素浓度为100µmol·L-1时最大,较对照分别增加了6.28%(p <0.05)和4.81%(p <0.05),在褪黑素浓度为200µmol·L-1时最小,较对照分别减少了7.1%(p <0.05)和11.59%(p <0.05)。桃果实硬度随褪黑素浓度的增加呈先增后减再增趋势,在褪黑素浓度为150µmol·L-1时最小,较对照减少了20.32%(p <0.05)。各处理的桃果形指数之间无显著差异。
表4 褪黑素对桃果实外观品质的影响
Table 4 Effects of melatonin on appearance quality of peach fruit
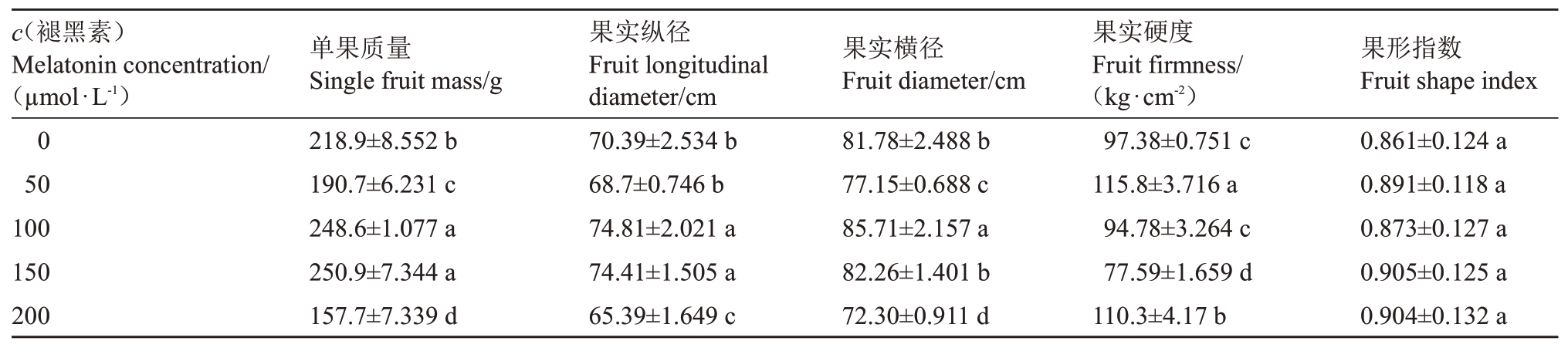
?
2.6 褪黑素对桃果实内含物含量的影响
桃果实维生素C含量随褪黑素浓度的增加呈先增后减趋势(表5),在褪黑素浓度为100µmol·L-1时最大,较对照增加了31.40%(p <0.05),在褪黑素浓度为200 µmol·L-1时最小,较对照减少了38%(p <0.05)。褪黑素浓度为100 和150 µmol·L-1时,桃果实的可溶性固形物含量显著高于对照,较对照分别增加了18.83%(p <0.05)和21.37%(p <0.05),其余处理与对照无显著差异。褪黑素浓度为50 和200µmol·L-1时,桃果实的可滴定酸含量显著低于对照,较对照分别减少了25.28%(p <0.05)和19.06%(p <0.05),其余处理与对照无显著差异。
表5 褪黑素对桃果实内含物含量的影响
Table 5 Effects of melatonin on internal solution content in peach fruit

?
2.7 褪黑素对桃果实不同糖组分含量的影响
除褪黑素浓度为150 µmol·L-1外,其余各处理的桃果实可溶性总糖含量较对照均显著减少(图2)。褪黑素浓度为100和150µmol·L-1时,桃果实蔗糖含量均高于对照,较对照分别增加了10.11%(p <0.05)和17.04%(p <0.05),其余各处理均低于对照。桃果实果糖含量随褪黑素浓度的增加呈先增后减趋势,在褪黑素浓度为100µmol·L-1时最大,较对照增加了21.37%(p <0.05)。褪黑素处理后桃果实山梨醇含量变化趋势与果糖含量相似,但均显著高于对照,也在褪黑素浓度为100µmol·L-1时最大,较对照增加了24.11%(p <0.05)。褪黑素浓度为100和150µmol·L-1时,桃果实葡萄糖含量均低于对照,较对照分别减少了37.25%(p <0.05)和26.16%(p <0.05),其余各处理与对照无显著差异。

图2 褪黑素对桃果实不同糖组分含量的影响
Fig.2 Effects of melatonin on different sugar component content in peach fruit
3 讨 论
褪黑素对植物生长具有调控作用[24]。王震[17]研究发现,在浇灌200µmol·L-1褪黑素处理后,苹果幼树生长量(主干直径、树高、侧枝长度和侧枝数量)和叶片质量均有所提高,而400µmol·L-1则显示出抑制效果。本试验也得到相似结果,50~150µmol·L-1褪黑素对桃树新梢(基部、中部和顶部)茎粗和茎长均表现为促进作用,而200µmol·L-1褪黑素则表现出抑制作用。这主要是由于褪黑素与吲哚乙酸作用效果相似,对植物生长促进与抑制作用存在一定的规律性,低浓度褪黑素能够促进植物生长,高浓度褪黑素则会抑制植物生长[9]。同时,褪黑素与植物中多种激素有着不同的互作模式[25],如褪黑素与吲哚乙酸间存在信号对话,能增强吲哚乙酸的生物合成[25-26]。因此,高浓度褪黑素对桃树枝条生长的抑制作用也可能是褪黑素诱导植物体内吲哚乙酸等生长素浓度的升高而导致的[25-27]。
从代谢角度看,褪黑素的抗氧化能力能够维持植物光合作用的过程,防止叶绿素降解,提高光系统Ⅱ的效率[28]。在本试验中,50 和200 µmol·L-1褪黑素提高了桃叶片光合色素含量,且100~150µmol·L-1褪黑素显著提高了桃叶片的叶绿素a/b值,提高了对光能的利用率[29],表明褪黑素能够改善桃叶片吸收的光能分配,使更多的光能用于碳固定,通过影响光合色素含量来调控桃叶的光合作用[28,30]。在正常生理条件下,植物体内产生的O2·-、H2O2和OH-等ROS会被植物自身的抗氧化酶系统清除:SOD 将O2·-转化为H2O2,H2O2再被CAT 转化为H2O,从而使植物体内ROS的产生和清除处于动态平衡状态[31-32]。在逆境胁迫下,褪黑素可通过提高植物抗氧化酶活性和降低MDA含量[33],降低植物叶片中O2·-产生的速率和H2O2含量,增加有机渗透调节物质可溶性蛋白含量,维持细胞内离子平衡,达到保护细胞的目的[34]。本试验中,50~100µmol·L-1褪黑素处理的桃叶片具有较低的SOD活性和较高的CAT活性,既防止了H2O2的积累,又避免了O2·-和H2O2反应生成毒性更强的羟基自由基[35]。100~200µmol·L-1褪黑素显著降低了桃叶片MDA 含量和可溶性蛋白含量,表明此时桃叶片细胞内的生理反应保持一种动态平衡。这些结果说明,褪黑素刺激抗氧化酶,在细胞水平上防止氧化应激引起氧化损伤,从而减轻了ROS对桃叶的伤害[36]。APX、PAL、LOX和PPO均是果树果实中代谢的关键性酶,而果树果实的抗氧化能力、抗病性与酚类物质代谢有关[37]。本试验研究结果表明,褪黑素处理提高了桃果实抗氧化酶APX活性和抗病相关酶PAL 活性,并降低了PPO 活性,这可能增强了桃果实的抗氧化能力,并抑制了桃果实酶促褐变的发生[38]。同时,褪黑素诱导了桃果实PAL 和LOX活性水平的升高,这就可能缓解了不可逆硬化的发生,增强了果实芳香物质代谢和色素物质转化[39]的能力,有利于桃果实后期正常的软熟和转色。
研究表明,褪黑素可有效提高园艺植物的产量和改善果实品质[40-43],在本试验中,100~150µmol·L-1褪黑素提高了桃果实的单果质量、纵横径。在跃变型果实上,褪黑素处理增加了番茄果实内乙烯含量,加速跃变峰的出现,进而促进番茄果实着色和软化[44],张婷婷等[45]在杏果实上的研究也有此发现。在非跃变型果实上,褪黑素改变乙烯合成和信号转导基因的表达,促进葡萄果实成熟[46]。其他研究也表明,100~150µmol·L-1褪黑素处理可能促进了跃变型果实的成熟,使得果实硬度有所降低,但不同浓度的褪黑素在处理不同物种、不同熟度的果实时可能会得到截然相反的结果[26],如Gao 等[47]研究表明,100µmol·L-1褪黑素处理完全成熟的桃,能有效延缓采收后桃果实的衰老。本试验结果表明,浓度为50和200µmol·L-1褪黑素处理降低了桃果实单果质量和纵横径,提高了桃果实硬度,这与前人的研究一致[46-47]。维生素C、可溶性固形物、糖、酸是桃果实内在品质形成的重要基础,也是影响消费者满意度的主要因素[48],高品质的果品不仅能够满足消费者的需求,而且在市场上竞争力强,经济效益高[49]。外源褪黑素能提高番茄果实品质,表现为可溶性固形物、有机酸、抗坏血酸和番茄红素含量的提高[42],以及可溶性糖和β-胡萝卜素含量的提高[50]。本试验中,100~150µmol·L-1褪黑素提高了桃果实的内在品质,显著增加了桃果实的维生素C 和可溶性固形物含量,但对可滴定酸含量影响不显著,其余褪黑素浓度处理与对照差异不显著或显著降低。这些结果与前人的研究一致[42,48,50],说明褪黑素能够用于提高桃果实的内在品质。
甜味强度取决于总糖量以及特定的糖分布(每种糖的相对含量),因果糖、葡萄糖和山梨醇的相对甜度大约是蔗糖的1.75、0.75 和0.6 倍[51]。糖也会影响口感属性和香气感知,其中果糖甜度值较高,葡萄糖风味最好[52],蔗糖和山梨醇与整体味道和香气高度相关[53]。本试验研究表明,桃果实不同糖组分含量大小为:蔗糖>葡萄糖>果糖>山梨醇,这与前人的研究一致[23]。蔗糖是桃果实中糖分的主要积累形式,在果实发育过程中呈现加速积累的趋势,在果实开始成熟前达到最大含量[23]。葡萄糖和果糖含量在桃果实发育的早期较高,随后逐渐下降直至成熟[54]。山梨醇是桃主要的光合产物,随着桃果实发育,山梨醇成为含量最低的糖类,直到果实成熟[53]。本试验结果表明,褪黑素处理后,桃果实的可溶性总糖含量较对照有所减少或无显著差异,但有效改变了桃果实的4 种糖含量,其果糖和山梨醇含量均高于对照。100~150µmol·L-1褪黑素降低了桃果实葡萄糖含量并提高了桃果实的蔗糖含量;50 和200µmol·L-1褪黑素则显著提高了桃果实山梨醇含量,降低了桃果实的蔗糖含量。这些结果说明,褪黑素通过调控桃果实的糖组分分配和含量进而改变桃果实的甜度,同时通过调控蔗糖代谢在一定程度上影响了果实的发育和成熟[11]。
[1] 纪萍.中国桃产业国际竞争力及出口影响因素研究[D].杨凌:西北农林科技大学,2011.JI Ping. Study on the international competitiveness of Chinese peach industry and the influencing factors of export[D]. Yangling:Northwest Agriculture and Forestry University,2011.
[2] 金睦皓,毛双,刘鹏凌.我国桃产业出口贸易的现状分析及应对策略[J].江苏农业科学,2019,47(12):334-338.JIN Muhao,MAO Shuang,LIU Pengling.An analysis of the current situation of China's peach industry export trade and countermeasures[J]. Jiangsu Agricultural Sciences,2019,47(12): 334-338.
[3] 张凤敏,宫美英.我国桃生产中存在的问题与对策[J].河北果树,2006(6):1-3.ZHANG Fengmin,GONG Meiying. Problems and countermeasures in peach production in China[J].Hebei Fruits,2006(6):1-3.
[4] 和岳,王明力.桃果实贮藏保鲜技术的发展现状[J].贵州农业科学,2011,39(11):181-183.HE Yue,WANG Mingli. Developing status of peach fresh-keeping technology in China[J]. Guizhou Agricultural Sciences,2011,39(11):181-183.
[5] 李秋利,高登涛,魏志峰,王志强,司鹏,郭景南.茉莉酸酯类对春美桃果实品质及贮藏性的影响[J].河南农业科学,2015,44(12):93-98.LI Qiuli,GAO Dengtao,WEI Zhifeng,WANG Zhiqiang,SI Peng,GUO Jingnan.Effect of jasmonic acid esters on fruit quality and storage quality of Chunmei peach[J]. Journal of Henan Agricultural Sciences,2015,44(12):93-98.
[6] 林竹,苏淑钗,马超,白倩,杨少燕.油菜素内酯对肥城桃光合作用和果实品质的影响[J].经济林研究,2016,34(2):73-78.LIN Zhu,SU Shuchai,MA Chao,BAI Qian,YANG Shaoyan.Effects of EBR on photosynthesis and fruit quality of Feicheng peach[J].Nonwood Forest Research,2016,34(2):73-78.
[7] SUN Z,LI Y,ZHOU J,ZHU S H. Effects of exogenous nitric oxide on contents of soluble sugars and related enzyme activities in‘Feicheng’peach fruit[J].Journal of the Science of Food Agriculture,2011,91(10):1795-1800.
[8] ARNAO M B. Phytomelatonin: discovery,content,and role in plants[J].Advances in Botany,2014,2014:1-11.
[9] 徐芳,周海鹏,郭早霞,于宏安,袁玉川,巩智刚,王玉华.植物褪黑素及其抗逆性研究[J].基因组学与应用生物学,2013,32(2):260-266.XU Fang,ZHOU Haipeng,GUO Zaoxia,YU Hong’an,YUAN Yuchuan,GONG Zhigang,WANG Yuhua. The melatonin and its resistance to stress in plants[J].Genomics and Applied Biology,2013,32(2):260-266.
[10] ERDAL S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms[J].Plant Cell Reports,2019,38(1):1001-1012.
[11] ZHAN H,SU T,HUO L,WEI L,JIANG Y,MA F. Unveiling the mechanism of melatonin impacts on maize seedling growth:sugar metabolism as a case[J].Journal of Pineal Research,2015,59(2):255-266.
[12] ARNAO M B,HERNÁNDEZ-RUIZ J. Functions of melatonin in plants: a review[J]. Journal of Pineal Research,2015,59(2):133-150.
[13] WEEDA S,ZHANG N,ZHAO X L,NDIP G,GUO Y D,BUCK G A,FU CG,REN S X.Arabidopsis transcrip to me analysis reveals key roles of melatonin in plant defense systems[J].PLoS One,2014,9(3):e93462.
[14] 高文华. 外源褪黑素处理对西瓜生长及产量和品质的影响[D].杨凌:西北农林科技大学,2019.GAO Wenhua. Effects of exogenous melatonin on growth,yield and quality of watermelon[D]. Yangling: Northwest Agriculture and Forestry University,2019.
[15] LIU J,YUE R,SI M,WU M,CONG L,ZHAI R,YANG C Q,WANG Z G,MA F W,XU L F. Effects of exogenous application of melatonin on quality and sugar metabolism in‘Zaosu’pear fruit[J]. Journal of Plant Growth Regulation,2019,38(3):1161-1169.
[16] 刘建龙.外源褪黑素对梨果实发育、采后品质和抗轮纹病的影响及其调控机制研究[D].杨凌:西北农林科技大学,2019.LIU Jianlong. Effects of exogenous melatonin on fruit development,postharvest quality and resistance to ring rot and its regulation mechanism[D].Yangling: Northwest Agriculture and Forestry University,2019.
[17] 王震.多巴胺和褪黑素对苹果幼树生长的影响[D].杨凌:西北农林科技大学,2014.WANG Zhen. Effects of dopamine and melatonin on the growth of apple young trees[D]. Yangling: Northwest Agriculture and Forestry University,2014.
[18] GAO H,LU Z M,YANG Y,WANG D N,YANG T,CAO M M,CAO W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism[J].Food Chemistry,2017,245:659-666.
[19] 于会丽,司鹏,邵微,杨晓静,乔宪生,杜新慧.不同铁肥在‘春蜜’桃上的应用效果[J].中国果树,2018(5):30-32.YU Huili,SI Peng,SHAO Wei,YANG Xiaojing,QIAO Xiansheng,DU Xinhui.The application effect of different iron fertilizer on‘Chunmi’peach[J].China Fruits,2018(5):30-32.
[20] 熊庆娥.植物生理学实验教程[M].成都:四川科学技术出版社,2003.XIONG Qing’e.Plant physiology experiment report[M].Chengdu:Sichuan Science and Technology Press,2003.
[21] 李小方,张志良.植物生理学实验指导[M].5 版北京:高等教育出版社,2016.LI Xiaofang,ZHANG Zhiliang. Experimental guidance of plant physiology[M].5 ed.Beijing:Higher Education Press,2016.
[22] 陈昆松,徐昌杰,许文平,吴敏,张上隆.猕猴桃和桃果实脂氧合酶活性侧定方法的建立[J].果树学报,2003,20(6):436-438.CHEN Kunsong,XU Changjie,XU Wenping,WU Min,ZHANG Shanglong. Improved method for detecting lipoxygenase activity from kiwifruit and peach fruit[J]. Journal of Fruit Science,2003,20(6):436-438.
[23] 黄丽萍,张倩茹,尹蓉,杨萍,杜海燕,张静.不同品种桃果实糖、酸、Vc 含量分析[J].农学学报,2017,7(10):56-60.HUANG Liping,ZHANG Qianru,YIN Rong,YANG Ping,DU Haiyan,ZHANG Jing.Content analysis of sugar,acid and Vc in fruits of different peach varieties[J]. Journal of Agriculture,2017,7(10):56-60.
[24] WEI W,LI Q T,CHU Y N,REITER R J,YU X M,ZHU D H,ZHANG W K,MA B,LIN Q,ZHANG J S,CHEN S Y.Melatonin enhances plant growth and abiotic stress tolerance in soybean plants[J]. Journal of Experimental Botany,2015,66(3):695-707.
[25] 张贵友,刘伟华,戴尧仁.植物中的褪黑素及其功能[J].中草药,2003,34(1):87-89.ZHANG Guiyou,LIU Weihua,DAI Yaoren.Presence and possible function of melatonin in plants[J]. Chinese Traditional and Herbal Drugs,2003,34(1):87-89.
[26] 巩彪,史庆华. 园艺作物褪黑素的研究进展[J]. 中国农业科学,2017,50(12):2326-2337.GONG Biao,SHI Qinghua.Review of melatonin in horticultural crops[J].Scientia Agricultura Sinica,2017,50(12):2326-2337.
[27] 杨力.褪黑素和生长素诱导的差异转录组及拟南芥和二穗短柄草中14-3-3 基因家族功能分析[D].北京:中国科学院大学,2017.YANG Li. Differential transcriptome induced by melatonin and auxin and functional analysis of 14-3-3 gene family in Arabidopsis and Dichondra[D]. Beijing: University of Chinese Academy of Sciences,2017.
[28] ERLAND L A,MURCH S J,REITER R J,SAXENA P K. A new balancing act: The many roles of melatonin and serotonin in plant growth and development [J]. Plant Signaling & Behavior,2015,10(11):e1096469.
[29] 陈晓强.不同桃品种光合特性研究[D].南京:南京农业大学,2006.CHEN Xiaoqiang. Photosynthetic characteristics of different peach varieties[D]. Nanjing: Nanjing Agricultural University,2006.
[30] 耿庆伟,邢浩,郝桂梅,孙永江,翟衡,杜远鹏.黑素对臭氧胁迫下‘赤霞珠’葡萄叶片光合作用的影响[J].园艺学报,2016,43(8):1463-1472.GENG Qingwei,XING Hao,HAO Guimei,SUN Yongjiang,ZHAI Heng,DU Yuanpeng. Effect of exogenous melatonin on photosynthesis of‘Cabernet Sauvigon’grape leaves under ozone stress[J]. Acta Horticulturae Sinica,2016,43(8): 1463-1472.
[31] 张梦如,杨玉梅,成蕴秀,周滔,段晓艳,龚明,邹竹荣.植物活性氧的产生及其作用和危害[J].西北植物学报,2014,34(9):1916-1926.ZHANG Mengru,YANG Yumei,CHENG Yunxiu,ZHOU Tao,DUAN Xiaoyan,GONG Ming,ZOU Zhurong.Generation of reactive oxygen species and their functions and deleterious effects in plants[J].Acta Botanica Boreali-Occidentalia Sinica,2014,34(9):1916-1926.
[32] 董亮,何永志,王远亮,董志扬.超氧化物歧化酶(SOD)的应用研究进展[J].中国农业科技导报,2013,15(5):53-58.DONG Liang,HE Yongzhi,WANG Yuanliang,DONG Zhiyang.Research progress on application of superoxide dismutase(SOD)[J]. Journal of Agricultural Science and Technology,2013,15(5):53-58.
[33] 包宇. 外源褪黑素对低温胁迫下番茄幼苗生理指标的影响[D].重庆:西南大学,2014.BAO Yu.Effects of exogenous melatonin on physiological index of tomato seedling under low temperature stress[D].Chongqing:Southwestern University,2014.
[34] 付晴晴,谭雅中,翟衡,杜远鹏.葡萄中褪黑素对NaHCO3胁迫的响应及外源褪黑素缓解NaHCO3 胁迫的作用机制[J].植物生理学报,2017,53(12):2114-2124.FU Qingqing,TAN Yazhong,ZHAI Heng,DU Yuanpeng. The response of melatonin to NaHCO3 stress and the mechanism of exogenous melatonin treatment in alleviating NaHCO3 stress in grapevine[J]. Plant Physiology Communications,2017,53(12):2114-2124.
[35] 逯明辉,宋慧,李晓明,陈劲枫. 冷害过程中黄瓜叶片SOD、CAT 和POD 活性的变化[J]. 西北植物学报,2005,25(8):1570-1573.YUN Minghui,SONG Hui,LI Xiaoming,CHEN Jinfeng.Changes of SOD,CAT and POD activities in cucumber leaves during cold damage[J].Acta Botanica Boreali-Occidentalia Sinica,2005,25(8):1570-1573.
[36] BONNEFONGT- POUSSELOT D,COLLIN F,JORE D,GARDÈS-ALBERT M. Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro[J]. Journal of Pineal Research,2011,50(3):328-335.
[37] 宋晓雪,胡文忠,毕阳,姜爱丽.鲜切果蔬酶促褐变关键酶的研究进展[J].食品工业科技,2013,34(15):354-357.SONG Xiaoxue,HU Wenzhong,BI Yang,JIANG Aili.Research progress in key enzymes for enzymatic browning of fresh-cut fruits and vegetables[J].Science and Technology of Food Industry,2013,34(15):390-393.
[38] 陈艺晖,林河通,林艺芬,张居念,赵云峰.拟茎点霉侵染对龙眼果实采后果皮褐变和活性氧代谢的影响[J].中国农业科学,2011,44(23):4858-4866.CHEN Yihui,LIN Hetong,LIN Yifen,ZHANG Junian,ZHAO Yunfeng. Effects of Phomopsis ionganae Chi infection on browning and active oxygen metabolism in pericarp of harvested longan fruits[J]. Scientia Agricultura Sinica,2011,44 (23):4858-4866.
[39] 李超.大久保桃果实采后贮藏保鲜及其品质控制的研究[D].杨凌:西北农林科技大学,2008.LI Chao. Postharvest storage and quality control of Okubo peach fruit[D]. Yangling: Northwest Agriculture and Forestry University,2008.
[40] 梁博文.多巴胺和褪黑素对干旱和养分胁迫下苹果矿质养分吸收的调控研究[D].杨凌:西北农林科技大学,2018.LIANG Bowen. Regulation of dopamine and melatonin on mineral nutrient uptake of apple under drought and nutrient stress[D]. Yangling: Northwest Agriculture and Forestry University,2018.
[41] LIU J L,YUE R R,SI M,WU M,CONG L,ZHAI R,YANG C Q,WANG Z G,MA F W,XU L F.Effects of exogenous application of melatonin on quality and sugar metabolism in‘Zaosu’pear fruit[J]. Journal of Plant Growth Regulation,2019,38(3):1161-1169.
[42] LIU J,ZHANG R,SUN Y,LIU Z,JIN W,SUN Y. The beneficial effects of exogenous melatonin on tomato fruit properties[J].Scientia Horticulturae,2016,207:14-20.
[43] VERDE A,MIGUEZ J M,GALLARDO M.Melatonin and related bioactive compounds in commercialized date palm fruits(Phoenix dactylifera L.): Correlation with some antioxidant parameters[J]. European Food Research and Technology,2019,245(1):51-59.
[44] SUN Q,ZHANG N,WANG J,ZHANG H J,LI D B,SHI J,LI R,WEEDA S,ZHAO B,REN S X,GUO Y D. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life[J]. Journal of Experimental Botany,2015,66(3):657-668.
[45] 张婷婷,李永才,毕阳,张彦东,胡培芳,王调兰,张苗.采后褪黑素处理对杏果实成熟的影响[J].中国果树,2019(1):27-31.ZHANG Tingting,LI Yongcai,BI Yang,ZHANG Yandong,HU Peifang,WANG Tiaolan,ZHANG Miao. Effect of postharvest melatonin treatment on ripening of apricot fruit[J].China Fruits,2019(1):27-31.
[46] 许丽丽,岳倩宇,卞凤娥,翟衡,姚玉新.褪黑素对葡萄果实成熟及乙烯和ABA 含量的影响[J]. 植物生理学报,2017,53(12):2181-2188.XU Lili,YUE Qianyu,BIAN Feng’e,ZHAI Heng,YAO Yuxin.Effects of melatonin treatment on grape berry ripening and contents of ethylene and ABA[J]. Plant Physiology Communications,2017,53(12):2181-2188.
[47] GAO H,ZHANG Z K,CHAI H K,CHENG N,YANG Y,WANG D N,YANG T,CAO W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit[J].Postharvest Biology and Technology,2016,118:103-110.
[48] REIG G,IGLESIAS I,GATIUS F,ALEGRE S.Antioxidant capacity,quality,and anthocyanin and nutrient contents of several peach cultivars [Prunus persica (L.) Batsch] grown in Spain[J].Journal of Agricultural and Food Chemistry,2013,61(26):6344-6357.
[49] LIVERANI A,BRANDI F,QUACQUARELLI I,SIRRI S,GIOVANNINI D. Superior taste and keeping quality are steady goals of the peach breeding activity at CRA-FRF,Italy[J].Acta Horticulturae,2015,(1084):179-186.
[50] 杜天浩,周小婷,朱兰英,张静,邹志荣.褪黑素处理对盐胁迫下番茄果实品质及挥发性物质的影响[J].食品科学,2016,37(15):69-76.DU Tianhao,ZHOU Xiaoting,ZHU Lanying,ZHANG Jing,ZOU Zhirong. Effect of melatonin treatment on tomato fruit quality and volatile compounds under salt stress[J]. Food Science,2016,37(15):69-76.
[51] 赵剑波,姜全,郭继英,李绍华.桃不同种质资源成熟果实葡萄糖、果糖含量比例研究[J].中国农业大学学报,2008,13(2):30-34.ZHAO Jianbo,JIANG Quan,GUO Jiying,LI Shaohua. Study on glucose/fructose in fruit of peach germplasm resources[J].Journal of China Agricultural University,2008,13(2):30-34.
[52] 李梦鸽,邓群仙,吕秀兰,刘建,程雪丽.‘美人指’葡萄果实糖积累和蔗糖代谢相关酶活性的研究[J].西北农林科技大学学报(自然科学版),2016,44(8):185-190.LI Mengge,DENG Qunxian,LÜ Xiulan,LIU Jian,CHENG Xueli. Sugar accumulation and sucrose-metabolizing enzymes activities of‘Manicure Finger’grape[J]. Journal of Northwest Agriculture and Forestry University (Natural Science Edition),2016,44(8):185-190.
[53] CIRILLI M,BASSI D,CIACCIULLI A. Sugars in peach fruit:A breeding perspective[J]. Horticulture Research,2016,3(1): 1-12.
[54] DESNOUES E,GIBON Y,BALDAZZI V,SIGNORET V,GÉNARD M,QUILOT-TURION B.Profiling sugar metabolism during fruit development in a peach progeny with different fructose-to-glucose ratios[J].BMC Plant Biology,2014,14(1):336.