山核桃属(Carya Nutt.)隶属于胡桃科(Juglandaceae),大约有18 个种,主要分布在北美东部及亚洲东南部[1]。在我国,山核桃属植物主要有5 个种,分别为山核桃(Carya cathayensis)、云南山核桃(Carya tonkinensis)、贵州山核桃(Carya kweichowensis)、湖南山核桃(Carya hunanensis)和大别山山核桃(Carya dabieshanesis)。其中,山核桃主产于浙江临安、淳安、桐庐和安吉等地区[2]。山核桃坚果风味醇厚,含有各种维生素,具有极高的营养价值,是国内外著名的坚果之一[3],此外,山核桃还具有重要的工业及医药价值[4]。近年来,随着生活水平的提高,人们对山核桃的需求呈逐年上升的趋势[5]。然而,山核桃多生长于山坡沟坎,且树体高大,采摘难度极大,因管理和采摘山核桃而造成的伤亡事故时有发生[6]。因此,开展山核桃矮化和半矮化的种质资源创新与培育,对我国山核桃产业的可持续发展具有重要的现实意义。
赤霉素(gibberellins,GAs)作为调控植物生长发育的五大激素之一,在植物[7]、细菌[8]、真菌[9]中广泛存在。研究表明,GAs 影响高等植物生物生活的各个阶段,如种子的萌发[10-11]、茎的伸长[12-13]、花器官的诱导及发育[14-15]、果实的形成[16-17]、木质素的积累[18]、叶绿素的表达[19-20]等。GAs 属于双萜类化合物,由4 个异戊二烯单位组成[21]。随着植物功能基因组学和蛋白质组学的发展,GAs 研究取得重大进展,GAs 的生物合成代谢途径及其关键酶在模式植物拟南芥中已比较清楚[22]。GAs生物合成过程中的关键酶主要包括古巴焦磷酸合酶(ent-copalyl diphosphate synthase,CPS)、内根-贝壳杉烯合酶(entkaurene synthase,KS)、内根-贝壳杉烯氧化酶(entkaurene oxidase,KO)、内根-贝壳杉烯酸氧化酶(entkaurenoic acid oxidase,KAO)、GA20 氧化酶(GA20-oxydase,GA20ox)、GA3β-羟化酶(GA3β-hydroxidase,GA3ox)及 GA2 氧化酶(GA2- oxydase,GA2ox)[23]。在GAs的合成代谢过程中,这些关键酶主要通过三个阶段发挥作用,第一阶段发生在质体中,CPS和KS将GAs合成前体牻牛儿基牻牛儿基焦磷酸(geranylgeranyl diphosphate,GGDP)转化为内根-贝壳杉烯(ent-kaurene);第二阶段主要发生在内质网,内根-贝壳杉烯被细胞色素P450单加氧酶KO和KAO 氧化,形成GA12-醛,它是GA 的最初产物,进一步转化成GA12,GA12作为GAs生物合成中重要的中间物质,在GA13 氧化酶(GA13-oxydase,GA13ox)的作用下还可转变为GA53;第三阶段发生在细胞质,GA12/GA53 被GA20ox 氧化酶催化为GA9/GA10,该产物又进一步被GA3ox 氧化酶转化为有生物活性的GA1/GA4,而GA2ox氧化酶则将有生物活性的GA1和GA4转化为无生物活性的GA8/GA34[24](图1)。
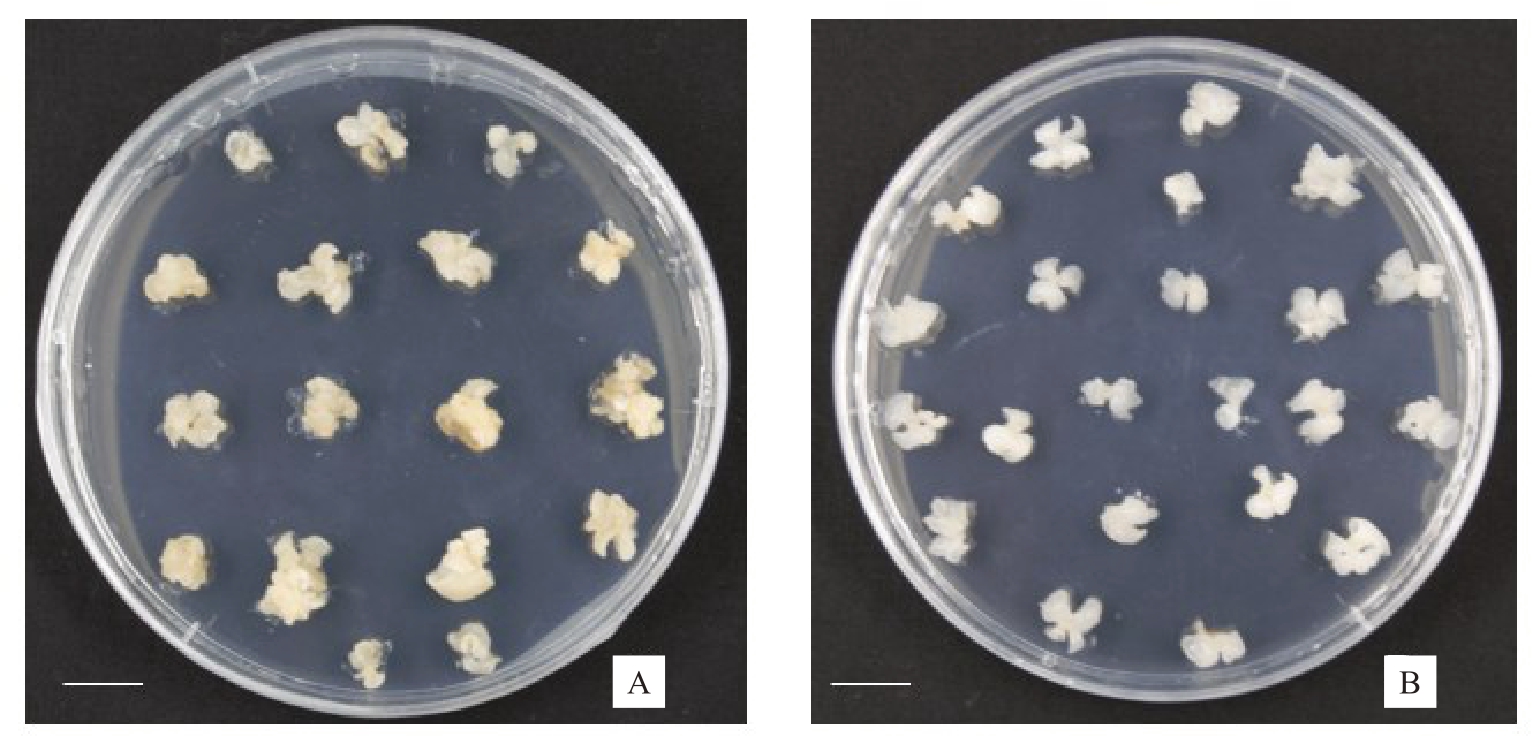
图1 山核桃和核桃体细胞胚
Fig.1 Somatic embryos of hickory and walnut
A.山核桃体细胞胚;B.核桃体细胞胚。标尺=100 mm。
A.Somatic embryos of hickory;B.Somatic embryos of walnut.Bars=100 mm.
GA3ox(GA3oxidase)是重要的GAs生物合成调控酶之一,属于2-酮戊二酸依赖性双加氧酶(2-oxoglutarate-dependent dioxygenase,2-ODDs)。该家族由多个基因家族编码,是植物基因组中第二大类酶家族,参与各种氧合/羟化反应,在植物发育、转录调节、核酸修饰及修复、次级代谢合成途径中发挥重要作用[25]。GA3ox调控植物的生长发育是一个复杂的过程,涉及到信号转导、合成代谢、内源激素水平以及周围的环境信号[26-27],如水分[28]、光照[29]、温度[30]、时空表达[31]等因素。目前,GA3ox 基因已经在拟南芥(Arabidopsis thaliana)[32]、烟草(Nicotiana taba-cum)[33]、南瓜(Cucurbita moschata)[34]、小麦(Triticum aestivum)[35]、杨树(Populus)[36]、苹果(Malus domestica)[37]和葡萄(Vitis vinifera)[38]等多种植物中得到分离克隆。同时随着突变体和转基因技术等相关研究的不断深入,人们对GA3ox的生物学功能有了一定的了解。资料显示,GA3ox 的表达量可以影响植物的株高[39]。从矮杆小麦‘宁98-210’(含有矮杆基因Rht12)扩增得到GA3ox1,通过实时荧光定量PCR(qRT-PCR)分析,结果显示,与正常株高的‘中国春’相比,TaGA3ox1 基因主要在矮杆小麦材料的倒一节结中的表达量较高,说明TaGA3ox1在缩短矮化品种小麦的节结部位起着关键调控作用[40];水稻矮化小穗突变体d18 的新等位基因3β-羟化酶(GA3ox2),在突变体中表达量显著降低,茎部细胞长度显著变短,植株表现出矮化表型[41];玉米中典型的GAs缺陷型突变体d1编码GA3ox氧化酶,阻断催化GAs 合成的最后一步,突变体植株矮小[42];土豆StGA3ox2 基因突变,可造成节间变短、植株变矮[43]。此外,有研究表明,GA3ox还可在一定程度影响植物体内叶绿体的合成[44],如水稻GA3ox 缺陷型突变体植株中,叶片颜色变深,叶绿素增加,说明GA3ox 参与调控叶绿素的表达[45];GA3ox 还能通过调节纤维发育影响茎秆生长情况,从而调节植物的株高[46-47]。
目前,关于GA3ox基因在果树中的研究较少,笔者以山核桃为材料,首次克隆山核桃CcGA3ox 基因,利用生物信息学的方法对其进行分析,进一步开展遗传转化并验证其生物学功能,为培育半矮化的山核桃创新种质奠定基础。
1 材料和方法
1.1 植物材料
植物材料均来自于省部共建亚热带森林培育国家重点实验室,包括山核桃体细胞胚和核桃(Juglans regia L.)体细胞胚(简称“体胚”)。体胚培养在(25±2)℃的暗箱中,再生植株培养在(25±2)℃、光照周期为16 h/8 h(光/暗)、光照强度为40~50 μmol·m-2·s-1的培养室中[48]。总RNA 的提取选用山核桃体胚,遗传转化材料选用生长良好的核桃体细胞胚。GFP 荧光验证和PCR 验证选用转化后生长至E3代的稳定遗传的核桃体胚和核桃再生植株。
1.2 方法
1.2.1 山核桃CcGA3ox 基因克隆与载体构建 过表达载体由TaKaRa 公司的pCAMBIA1300(简称pC1300)改造而来,即在多克隆位点前后分别连接花椰菜花叶病毒(CaMV)的35S 强启动子和绿色荧光蛋白基因GFP;大肠杆菌E. coli DH5α 感受态细胞购自于杭州有康生物技术有限公司;农杆菌GV3101 感受态细胞购自于上海唯地生物科技有限公司。
选用北京天根生化科技有限公司的多糖多酚植物总RNA 提取试剂盒提取山核桃体胚的RNA,采用杭州昊枫生物科技有限公司Plant DNAzol植物基因组DNA 快速提取试剂提取DNA,基因克隆及后续的验证过程采用TaKaRa 公司的cDNA 反转录试剂盒、Primer STAR 高保真酶、DNA Marker、rTaqDNA聚合酶、连接酶和各种限制性内切酶进行操作,凝胶回收及质粒提取采用上海生物工程有限公司的SanPrep柱式取试剂盒。
根据山核桃CcGA3ox基因的全长CDS序列,按照喏唯赞CloneExpress II One Step Cloning Kit 试剂盒的操作要求,基于In-Fusion 的载体构建技术[49]进行构建载体,利用Premier 5.0软件设计克隆引物,其序列见表1。根据Saito等[50]的PCR扩增体系进行扩增,并调整PCR 反应程序为:94 ℃预变性2 min;98 ℃变性10 s;55 ℃退火30 s;68 ℃延伸2 min;共32 个循环;68 ℃延伸7 min。将PCR 反应产物进行1.2%琼脂糖凝胶电泳,同时,将pC1300 质粒进行Bam HⅠ和SalⅠ双酶切,分别胶回收目的条带和双酶切电泳产物,将目的条带和酶切后的质粒载体进行连接,将连接产物35S::CcGA3ox::GFP转入DH5α大肠杆菌感受态细胞[51],取阳性单克隆菌株进行PCR鉴定并送至杭州有康生物技术有限公司测序鉴定,测序结果比对正确的质粒转化农杆菌[52],PCR验证正确的菌株用于保菌及后续实验。将克隆获得的序列拟命名为CcGA3ox,并将构建的过表达载体拟命名为35S::CcGA3ox::GFP。
表1 引物序列
Table 1 Primers sequence

注:GGATCC、GTCGAC 分别为Bam HⅠ、SalⅠ的酶切位点。
Note:GGATCC and GTCGAC are Bam HⅠand SalⅠrestriction sites,respectively.
?
1.2.2 山核桃CcGA3ox 基因的生物信息学分析利用美国国立生物技术信息中心(NCBI)网站中的BLAST 对氨基酸序列进行序列相似性分析(https://blast.ncbi.nlm.nih.gov/);利用软件PROSITE Scan 对基因进行保守区域预测分析;利用ORF Finder(https://ncbiinsights.ncbi.nlm.nih.gov/tag/orffinder/)分析开放阅读框;利用ExPASy(https://web.expasy.org/cgi-bin/protparam/protparam)对氨基酸的理化性质进行分析;利用PSORT(https://www.genscript.com/tools/psort)在线分析软件进行亚细胞定位预测,利用TMHMM Server v.2.0 软件进行蛋白序列跨膜区分析;使用MEGA 7.0 软件对氨基酸序列进行多序列比对分析,建立多物种系统发育树。
1.2.3 山核桃CcGA3ox 基因在核桃中的遗传转化 将构建好的载体35S::CcGA3ox::GFP 转入农杆菌GV3101 感受态细胞中并在含有利福平(50 mg·mL-1)和卡那霉素(50 mg·mL-1)的LB 液体培养基悬浮培养至OD600值为0.8~1.0,5 000 r·min-1离心5 min 富集菌株,倒掉上清液,再加入含有乙酰丁香酮(40 mg·L-1)的液体DKW培养基进行悬浮[53]。选取生长良好的核桃体胚,在悬浮液中浸染10~15 min后,放置于加有乙酰丁香酮(40 mg·L-1)的DKW 固体培养基中共培养3 d,再转入含有潮霉素(80 mg·L-1)和羧苄青霉素(300 mg·L-1)抗生素的DKW 固体培养基中进行阳性体胚筛选,培养3~4 周后进行鉴定。具体步骤参照Zhang等[54]的方法。
利用体式荧光显微镜(Carl Zeiss Stereo D13covery V12,Axio Cam MRc system)在蓝色激发光(488 nm)下,观察山核桃35S::CcGA3ox::GFP 转化体胚的荧光激发情况[55],并对具有绿色荧光激发体细胞胚进行PCR鉴定(引物序列见表1)。经PCR鉴定的阳性体胚长至子叶胚阶段后,将体胚脱水干化3 d后,置于萌发培养基中进行植株再生,之后对再生植株进行PCR阳性鉴定。
1.2.4 山核桃CcGA3ox 基因功能的初步验证 从鉴定的阳性植株顶芽开始向下截取1.5 cm,培养15 d观察植株表型,并选取植株顶芽、叶片、茎段混样提取RNA,使用The iQ5 Real-Time PCR Detection System 仪器进行qRT-PCR,测定转基因植株中Cc-GA3ox 的相对表达量。反应程序为[56]:95 ℃10 min,95 ℃10 s,60 ℃31 s,40 个循环;95 ℃15 s,60 ℃1 min,95 ℃30 s,60 ℃15 s。采用2-△△CT[57]方法计算定量结果。植物组织叶绿素的提取采用乙醇提取法[58],采用紫外分光光度计测定波长665 nm和649 nm的吸光度值,采用公式[59]计算结果,数据处理及显著性差异分析使用Excle和SPSS 2.0软件。
2 结果与分析
2.1 山核桃CcGA3ox基因的克隆和序列分析
根据设计的引物,采用PCR技术克隆扩增获得山核桃CcGA3ox 基因编码区全长,琼脂糖凝胶电泳显示约1 000 bp 大小条带(图2-A),与目的基因(1 116 bp)大小一致。为了进一步构建山核桃CcGA3ox 基因过量表达载体,将目的条带胶回收后的产物与双酶切后的pC1300 质粒连接,转化DH5α大肠杆菌感受态细胞,挑选单克隆进行筛选培养,对得到的菌液进行PCR鉴定,电泳显示条带在1 000 bp左右(图2-B),测序比对正确的序列命名为Cc-GA3ox。构建的CcGA3ox 基因过表达载体命名为35S::CcGA3ox::GFP。
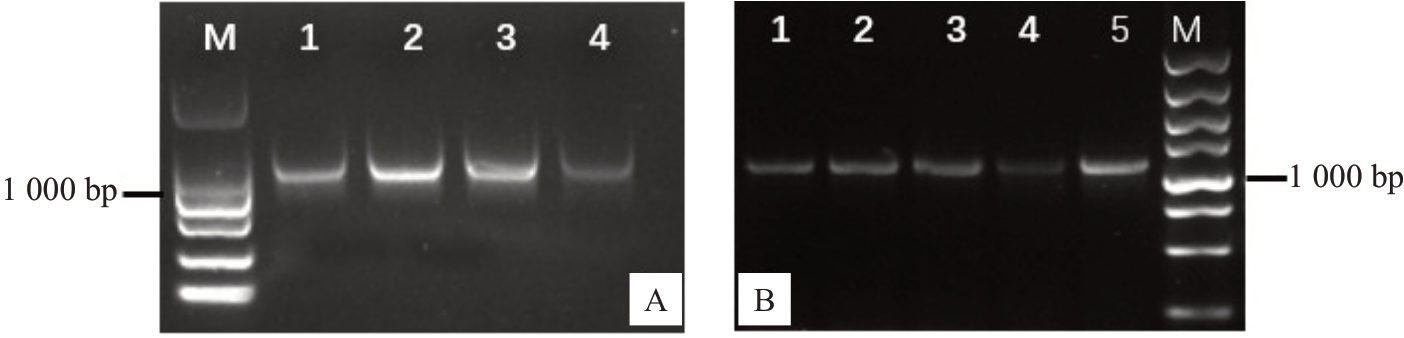
图2 山核桃CcGA3ox 基因克隆及载体构建电泳分析
Fig.2 Electrophoretogram of GA3ox gene cloning and vector construction in hickory
A.山核桃CcGA3ox 基因扩增电泳图。M.Maker DL2000,泳道1~4 为目的条带;B.山核桃CcGA3ox 基因大肠杆菌菌检电泳图。M.Maker DL5000,泳道1~5 为目的条带。
A.Amplification electrophoresis map of CcGA3ox gene.M.Maker DL2000,Lane 1-4 is the target;B.Electrophoretogram of CcGA3ox Escherichia coli.M.Maker is DL 5000.Lane 1-5 is the target.
2.2 山核桃CcGA3ox基因的生物信息学分析
ORF Finder 软件分析结果表明,山核桃Cc-GA3ox开放阅读框的长度为1 116 bp,编码371个氨基酸;用ExPASy 在线软件预测该基因所编码蛋白的相对分子质量为40.69 kDa,等电点(pI)为6.67,小于7.0,为酸性蛋白质,分子式为C1 824H2 836N498O537S11,总疏水性系数为-0.209,为亲水性蛋白;用PSORT在线软件分析预测CcGA3ox 蛋白质在细胞内的分布,其中有56.5%位于细胞质中,21.7%位于细胞核,13%位于线粒体,极少量分布在细胞膜和溶酶体中;应用TMHMM Server v.2.0 对CcGA3ox 蛋白的跨膜区域进行分析,结果显示,该蛋白的跨膜区域值为零,在跨膜区域中氨基酸序列的期望值为0.028 33,当该结果大于18时才有跨膜区域,因此该蛋白没有跨膜区域(图3);利用PROSITE Scan软件分析预测蛋白结构域和功能位点,发现该蛋白含有保守的2OG-FeⅡ-Oxy(288-297)结构域和3 个Fe2+结合位点(230,232,287),以及数目不等的基元,包括6 个酪蛋白激酶Ⅱ磷酸化位点(25~28、43~46、45~48、145~148、190~193、331~334)、5 个N-糖基化位点(85~88、242~245、243~246、279~282、292 295)、2 个蛋白激酶C 磷酸化位点(97~99、349~351)、2 个酰基化位点(205~210、280~285),这些保守基元在信号识别以及遗传转录中具有重要作用(图4)。
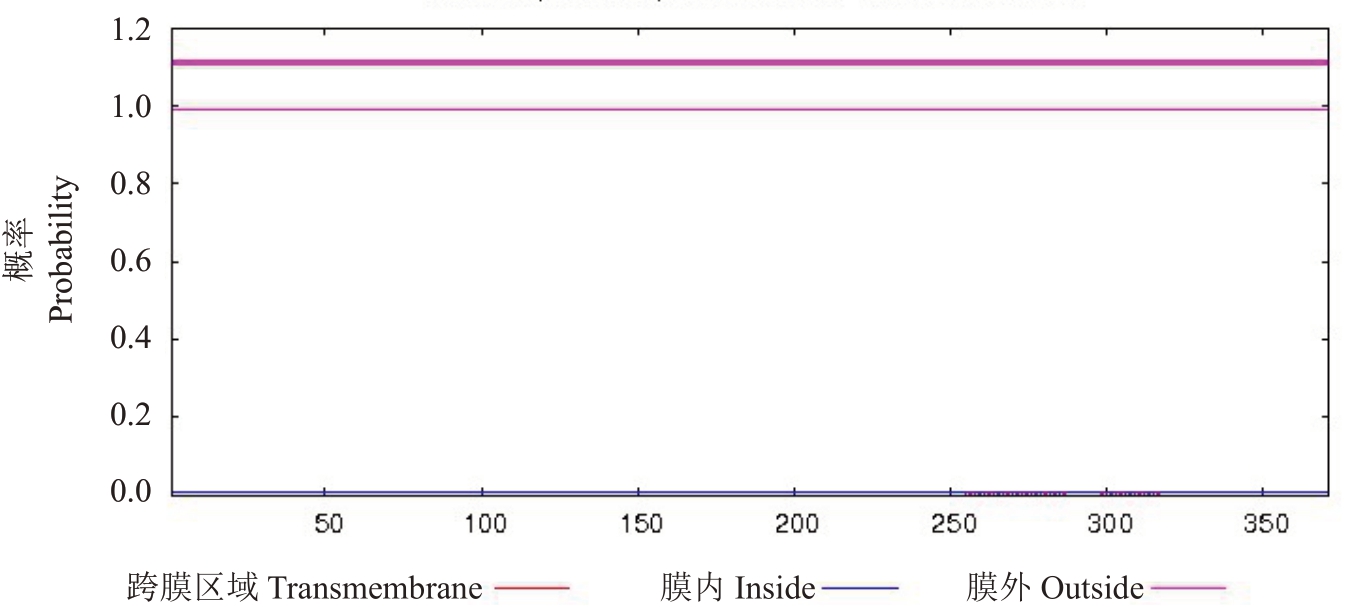
图3 山核桃CcGA3ox 跨膜区域的预测分析
Fig.3 Prediction of Cc transmembrane region of hickory

图4 山核桃CcGA3ox 基因保守结构域及功能结合位点
Fig.4 Conserved domain and functional site of CcGA3ox gene in hickory
运用NCBI 在线软件中的BLAST 将CcGA3ox氨基酸序列与NCBI 中Nr(RefSeq non-redundant proteins)数据库中选取的39 个物种的GA3ox 氨基酸序列进行比对,经过Clustal W比对并利用MEGA 7.0 中的NJ(Neighbor joining)法构建进化树,利用iTOL在线软件对进化树进行修饰,最后得到不同物种的GA3ox 氨基酸序列进化树。图5 显示,山核桃CcGA3ox 氨基酸序列与薄壳山核桃GA3ox(L1184S0079)的相似度最高,达到98.6%,同属一个小分支,亲缘关系最近;其次是与核桃JrGA3ox达到97.84%的同源率,亲缘关系较近;与板栗CmGA3ox的同源率为84.64%,与阔叶栎QlGA3ox的同源率为83.56%,与巴西橡胶树HbGA3ox 同源率为80.7%,与麻风树JcGA3ox 同源率为79.23%。发现在同一支内,各物种之间的差异较小,但随着分支的不断扩大,亲缘关系值逐渐减小。
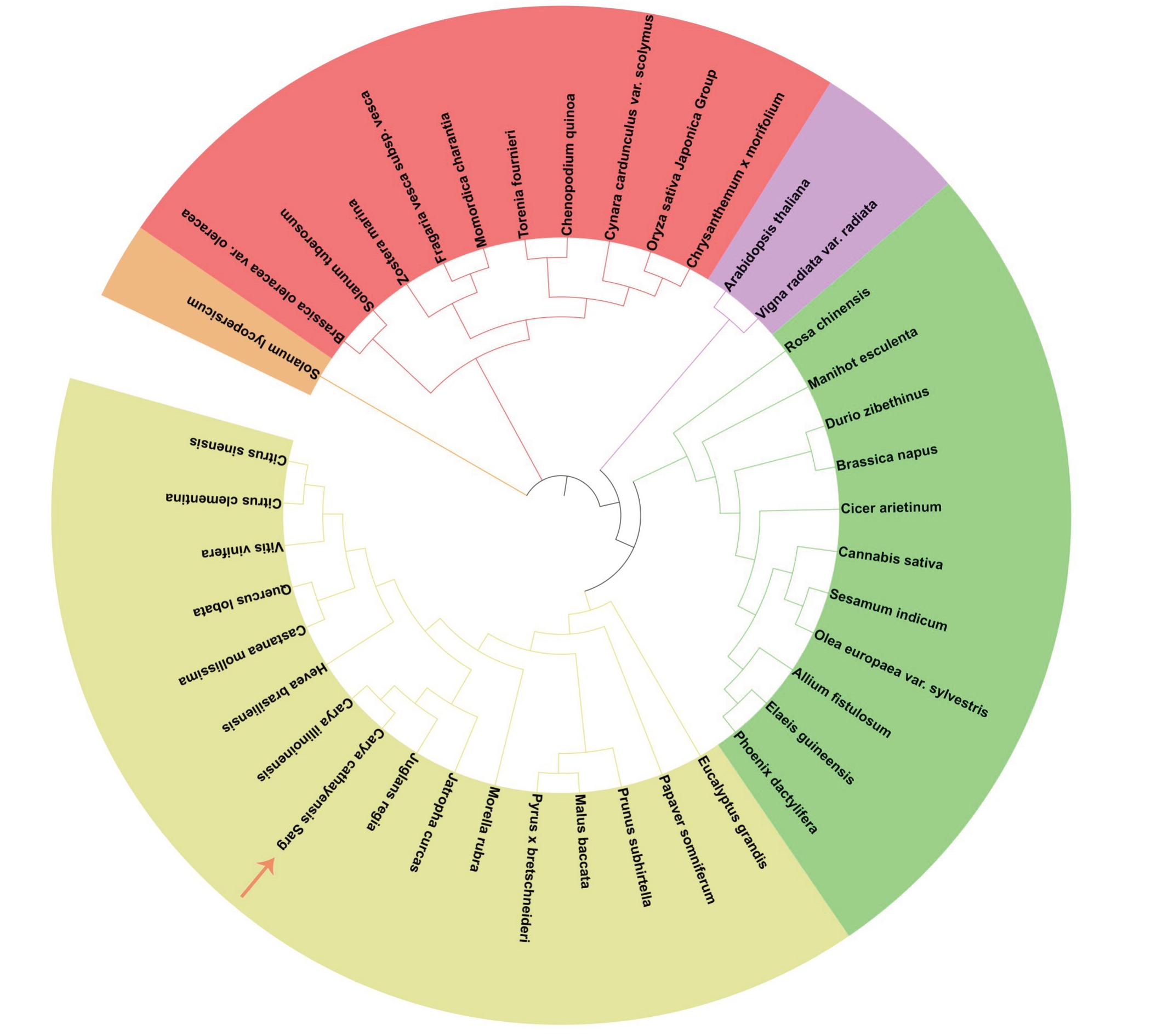
图5 不同物种的GA3ox 氨基酸序列的系统进化树
Fig.5 Phylogenetic tree of GA3ox amino acid sequences in different species
山核桃Carya cathayensis. CcGA3ox (CCA0475S0001); 薄壳山核桃Carya illinoinensis. CiGA3ox (L1184S0079); 核桃Juglans regia. Jr-GA3ox (XP_018827425.1); 板栗Castanea mollissima. CmGA3ox (AEW67998.1); 阔叶栎Quercus lobata. QlGA3ox (XP_030968798.1); 巴西橡胶树Hevea brasiliensis.HbGA3ox(XP_021660459.1);麻风树Jatropha curcas.JcGA3ox(XP_012073283.1);葡萄Vitis vinifera.VvGA3ox(XP_002284981.1); 橙子Citrus sinensis. CsGA3ox (XP_006465424.1); 柚子Citrus clementina. CcGA3ox (XP_006427125.2); 杨梅Morella rubra.MrGA3ox(KAB1218614.1);白梨Pyrus bretschneideri.PbGA3ox(XP_009339604.1);山荆子Malus baccata.MbGA3ox(TQE04418.1);罂粟Papaver somniferum.PsGA3ox(XP_026404546.1);巨桉Eucalyptus grandis.EgGA3ox(XP_010061961.1);大叶早樱Cerasus subhirtella.CsGA3ox(BAD91162.1);海枣.Phoenix dactylifera.PdGA3ox(XP_008785607.1);油棕Elaeis guineensi.EgGA3ox(XP_010925284.1);大葱Allium fistulosum.AfGA3ox(BAG32267.1);橄榄树Olea europaea.OeGA3ox(XP_022884986.1);芝麻Sesamum indicum.SiGA3ox(XP_011072274.1);大麻Cannabis sativa. CsGA3ox (XP_030488464.1); 鹰嘴豆Cicer arietinum. CaGA3ox (XP_004498636.1); 油菜Brassica napus. BnGA3ox (XP_013710717.1);榴莲Durio zibethinus.DzGA3ox(XP_022777151.1);木薯Manihot esculenta.MeGA3ox(XP_021611256.1);月季花Rosa chinensis. RcGA3ox (XP_024168805.1); 案头菊 Chrysanthemum morifolium. CmGA3ox (BAG48320.1); 水稻 Oryza sativa. OsGA3ox (XP_015634638.1);刺棘蓟Cynara cardunculus.CcGA3ox(XP_024980281.1);藜麦Chenopodium quinoa.CqGA3ox(XP_021731922.1);蓝猪耳Torenia fournieri. TfGA3ox (BAJ65442.1); 苦瓜Momordica charantia. McGA3ox (XP_022145559.1); 草莓Fragaria vescasubsp. Fv GA3ox (XP_004292966.1);大叶藻Zostera marina.ZmGA3ox(KMZ69735.1);马铃薯Solanum tuberosum.StGA3ox(XP_006341659.1);卷心菜Brassica oleracea. BoGA3ox (XP_013619987.1); 绿豆Vigna radiata.VrGA3ox (XP_014516137.1); 拟南芥Arabidopsis thaliana.AtGA3ox (NP_193900.1);番茄Solanum lycopersicum.SlGA3ox(XP_004253252.1).
根据与山核桃的亲缘关系的远近,可将GA3ox分为5 个簇族。其中山核桃、薄壳山核桃、核桃、板栗、阔叶栎、巴西橡胶树、麻风树、葡萄、橙子、柚子、杨梅、白梨、山荆子、罂粟、巨桉、大叶早樱为第一簇族,与山核桃遗传距离关系较近,亲缘关系值为61.29%~98.60%;海枣、油棕、大葱、橄榄树、芝麻、大麻、鹰嘴豆、油菜、榴莲、木薯、月季花为第二簇族,与山核桃遗传距离关系相对较近,亲缘关系值为43.12%~76.20%;案头菊、水稻、刺棘蓟、藜麦、蓝猪耳、苦瓜、草莓、大叶藻、马铃薯、卷心菜为第三簇族,亲缘关系值为36.91%~67.92%;豆、拟南芥为第四簇族,亲缘关系值为43.39%~63.90%;番茄单独为一簇族。
2.3 山核桃CcGA3ox 基因在核桃中的遗传转化及阳性检测
目前,由于山核桃体细胞胚遗传转化体系还不够成熟,笔者选取同属于胡桃科生长健壮的核桃体细胞胚作为遗传转化的初始材料(图6-A~B),用制备好的35S::CcGA3ox::GFP过表达载体的农杆菌工程菌进行侵染,将农杆菌侵染的体胚记为E0 代,所选用的E0代体胚均由一个体细胞胚分化而来,再将E0代其表面增殖的胚状体记为E1,以此类推。培养至E3 代,体式荧光显微镜下观察发现,转化体细胞胚生长健壮,可正常发育和增殖,与对照体胚相似,其发育过程经历了4 个时期,分别为球形胚、心形胚、鱼雷形胚以及子叶形胚阶段。在白光视野下,对照体胚和转化体胚形态相似,均为白色透明状;在蓝色激发光(488 nm)条件下,35S::CcGA3ox::GFP 阳性转化核桃体胚呈现明亮的绿色荧光(该类体胚定为GFP 阳性体胚),而对照体胚呈现较弱的绿色荧光(图6)。数据统计结果表明,E1 代体胚GFP 阳性率为32%;E2 代体胚GFP 阳性率为56%;E3 代体胚GFP 阳性率为77%,说明转化的体细胞胚能够稳定遗传(表2)。
表2 核桃体胚培养代数对阳性率的影响
Table 2 The effect of generation on the rate of positive somatic embryos in walnut
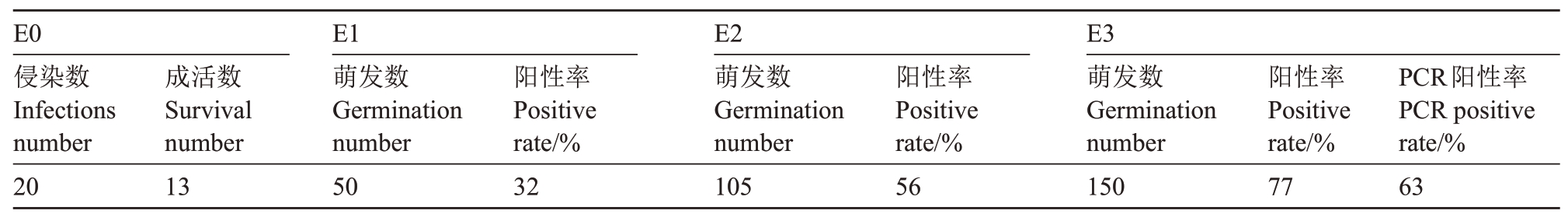
?
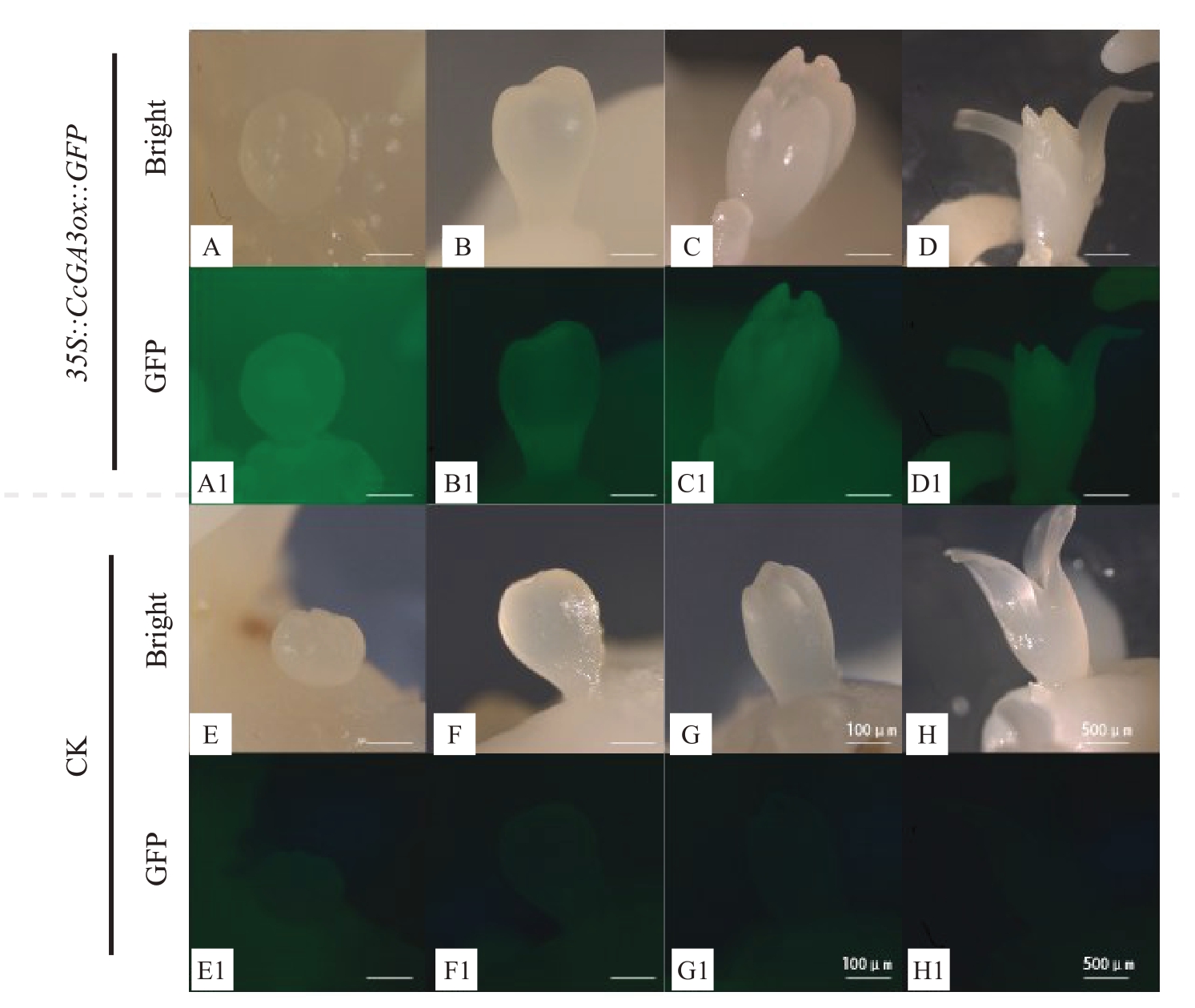
图6 核桃35S::CcGA3ox::GFP 转化体胚荧光表达
Fig.6 Fluorescent expression of walnut 35S::CcGA3ox::GFP transformed somatic embryos
A~D.白光下的转化核桃体胚;A1~D1.蓝光激发下的转化核桃体胚;E~H.白光下的对照核桃体胚;E1~H1. 蓝光激发下的对照核桃体胚;A、E 为球形胚;B、F 为心形胚;C、G 为鱼雷形胚;D、H 为子叶形胚。标尺=100 μm。
A-D.Transgenic somatic embryo of walnut under natural light;A1-D1.Transgenic somatic embryo of walnut stimulated by blue light;E-H.Control somatic embryo of walnut under natural light;E1-H1.Control somatic embryo of walnut stimulated by blue light;A and E:spherical embryos;B and F:heart-shaped embryos;C and G:torpedo embryos;D and H:cotyledon embryos.Bar=100 μm.
为进一步验证体胚的阳性,将GFP 阳性体胚培养至E3代提取DNA,进行PCR检测,为排除假阳性的可能性,用外源GFP基因(729 bp)进行验证,发现凝胶电泳显示条带大小为750 bp左右,与GFP基因大小相符合;同时用目的基因CcGA3ox(1 116 bp)进行再次验证,目的基因条带的PCR 检验结果显示(图7),得到大小为1 000 bp 左右的电泳条带,与目的基因大小相符(图7-A、C)。因此说明获得阳性验证体胚,且体胚PCR验证阳性率为63%。将PCR验证的阳性体胚进行脱水干化处理3~5 d,置于添加BA和IBA的改良DKW固体培养基中促其萌发,生长成3~5 cm再生植株,提取DNA,进行GFP和目的基因的双重PCR 验证,检测到大小约为750 bp 和1 000 bp 的电泳条带,说明得到阳性转化35S::Cc-GA3ox::GFP核桃再生植株(图7-B、D)。
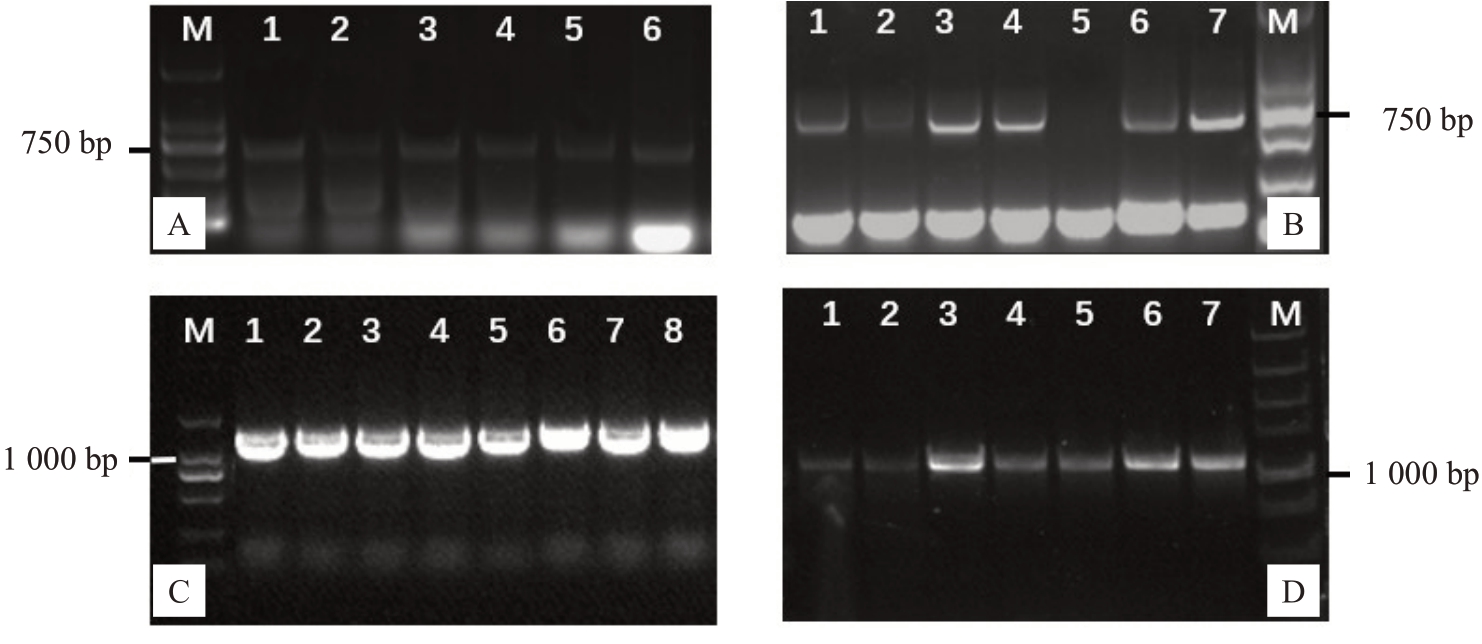
图7 35S::CcGA3ox::GFP 转核桃体胚及再生植株PCR 鉴定
Fig.7 PCR identification of transformed somatic embryos and regenerated plants of 35S::CcGA3ox::GFP in walnut
A.阳性体胚GFP 基因PCR 鉴定。M.Maker DL2000,泳道1~6 为阳性体胚。B.阳性再生植株GFP 基因PCR 鉴定,M.Maker DL2000,泳道1~7 为阳性再生植株。C.阳性体胚目的基因PCR 鉴定。M.Maker DL5000,泳道1~8 为阳性体胚。D.阳性再生植株目的基因PCR 鉴定。M.Maker DL5000,泳道1~7 为阳性植株。
A.Identification of GFP gene from positive somatic embryos by PCR.M.Maker DL2000,lane 1-6 as positive somatic embryos.B.Identification of GFP gene in positive regenerated plants by PCR.M.Maker DL2000,lane 1-7 as positive regenerated plants.C.Identification of target gene from positive somatic embryos by PCR. M. Maker DL5000,lane 1-8 as positive somatic embryos. D. Identification of target gene in positive regenerated plants by PCR.M.Maker DL5000,lane 1-7 as positive regenerated plants.
2.4 山核桃CcGA3ox基因的功能验证
2.4.1 核桃35S::CcGA3ox::GFP再生植株的表型分析 为了研究山核桃CcGA3ox 基因过量表达与株高的相关性,笔者选取了3 组阳性再生植株进行培养观察,3个株系分别命名为35S::CcGA3ox::GFP-1、35S::CcGA3ox::GFP-2、35S::CcGA3ox::GFP-3,所选植株截取的长度为从顶尖开始向下1.5 cm,带有2~4枚复叶。结果显示,在生长15 d 后,核桃35S::Cc-GA3ox::GFP阳性核桃植株与对照植株相比,再生植株生长旺盛,株高较高,节间长度和叶片长度更长(图8-A~C)。株高和节间长度统计结果显示,对照植株平均高度为20.3 mm,节间平均长度为3.8 mm。再生植株株系35S::CcGA3ox::GFP-1 平均株高为25.2 mm,比对照增加了24%,节间平均长为4.8 mm,比对照增加了26%;35S::CcGA3ox::GFP-2平均株高为25.1 mm,比对照增加了24%,节间平均长度为4.8 mm,比对照增加了24% ;35S::Cc-GA3ox::GFP-3 平均株高为25.8 mm,比对照增加了27%,节间平均长度为4.3 mm,比对照增加了27%(图9)。

图8 核桃35S::CcGA3ox::GFP 阳性再生植株的表型分析
Fig.8 Phenotypic analysis of regenerated plants of walnut 35S::CcGA3ox::GFP
A.对照植株和阳性再生植株在0 d 的表型;B.对照植株和再生阳性植株在15 d 的表型;C.对照植株及阳性再生植株株茎及叶片表型对比。标尺=10 mm。
A.The phenotypes of a control and regenerated positive plants at 0 day;B.The phenotypes of a control and regenerated positive plants at 15 days;C.The phenotypes of a control and regenerated positive plants at stem and leaves;Bar=10 mm.
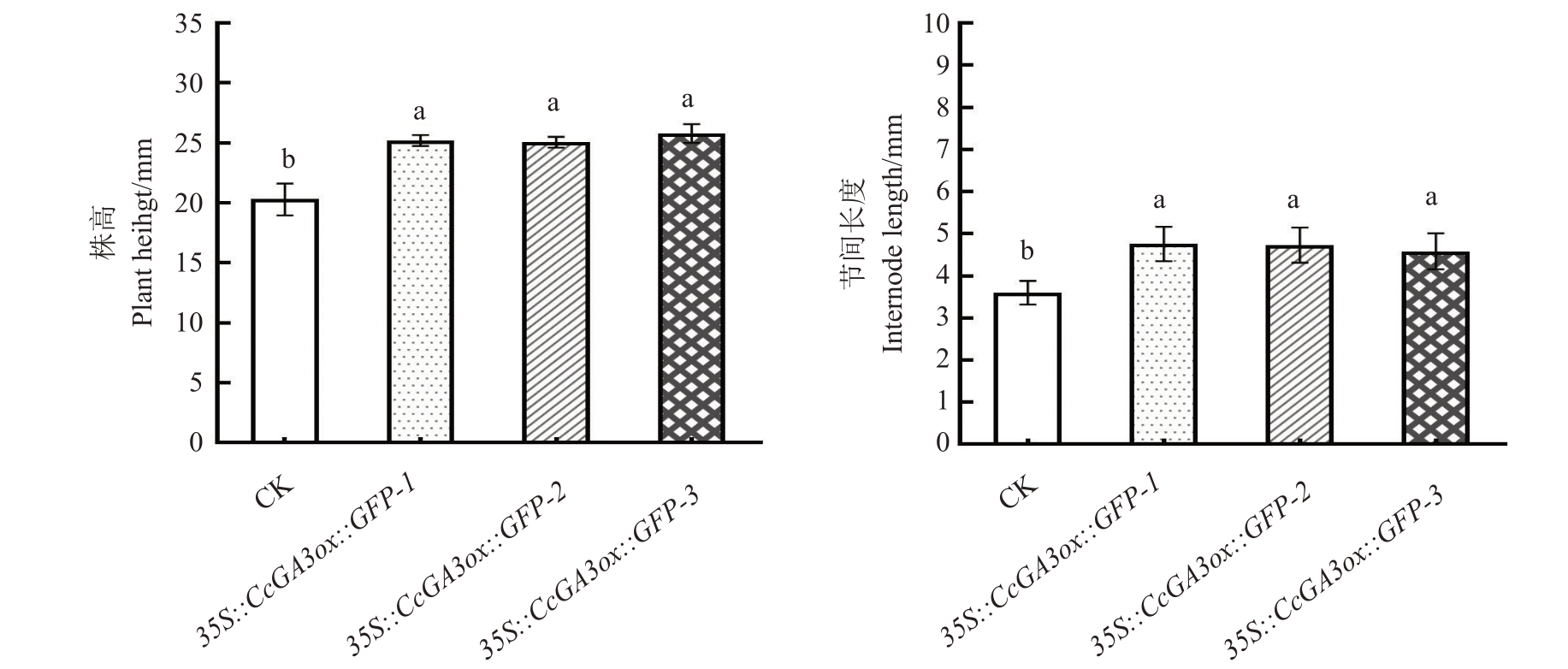
图9 核桃35S::CcGA3ox::GFP 再生植株株高及节间长度分析
Fig.9 Analysis of plant height and internode length of walnut 35S::CcGA3ox::GFP regenerated plants
单因素方差分析显著性,不同小写字母表示差异显著(p <0.05)。下同。
Significance of one-way ANOVA,different small letters indicated significant difference(p <0.05).The same below.
为探究山核桃CcGA3ox 基因在阳性转化植株和未转化植株中表达量的差异性,提取35S::Cc-GA3ox::GFP 再生植株的RNA,通过qRT-PCR 检测CcGA3ox基因在核桃再生植株中的相对表达量。结果显示,35S::CcGA3ox::GFP 株系阳性植株Cc-GA3ox 基因的表达量均显著高于对照植株,平均相对表达量是对照植株的137 倍。其中,35S::Cc-GA3ox::GFP-1株系的相对表达量是对照植株的130倍;35S::CcGA3ox::GF-2 株系的相对表达量是对照植株的141 倍;35S::CcGA3ox::GF-3 株系的相对表达量是对照植株的140倍(图10)。
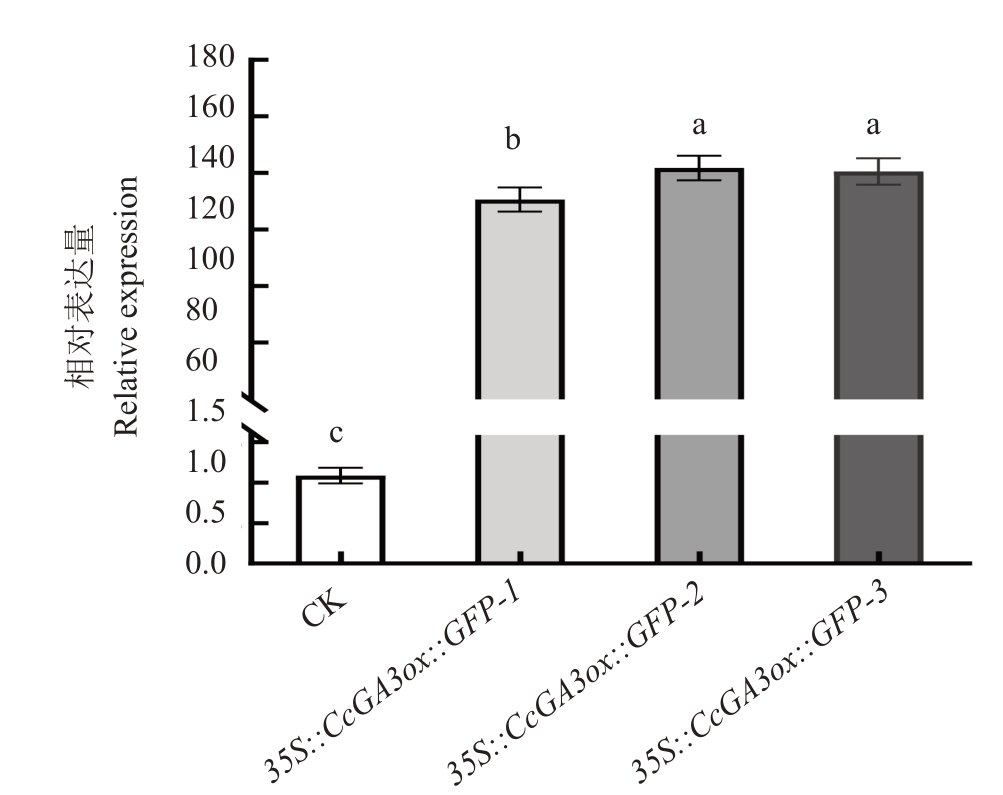
图10 核桃35S::CcGA3ox::GFP 再生植株相对表达量分析
Fig.10 Relative expression analysis of regenerated plants of walnut 35S::CcGA3ox::GFP
2.4.2 山核桃CcGA3ox 植株中叶绿素含量分析通过表型观察,山核桃35S::CcGA3ox::GFP 再生植株阳性株系呈现肉眼所见的叶色变浅。选取3个株系的阳性再生植株,进行叶绿素含量的测定,结果显示,与对照植株相比,阳性再生植株的叶绿素含量显著降低。35S::CcGA3ox::GFP-1、35S::CcGA3ox::GFP-2 和35S::CcGA3ox::GFP-3 阳性再生植株的叶绿素a 含量(w,后同)分别为0.272 5、0.385 0 和0.255 7 mg·g-1,显著低于对照(0.520 5 mg·g-1);3 个阳性再生植株的叶绿素b含量分别为0.363 8、0.403 6和0.346 7 mg·g-1,显著低于对照(0.508 7 mg·g-1);同样,3个阳性再生植株总叶绿素含量分别为0.636 3、0.788 7、0.602 4 mg·g-1,显著低于对照(1.029 1 mg·g-1)。与对照植株相比,阳性再生植株平均叶绿素a 含量减少41.5%,平均叶绿素b 含量减少29.3%,总叶绿素含量下降34.4%(图11)。
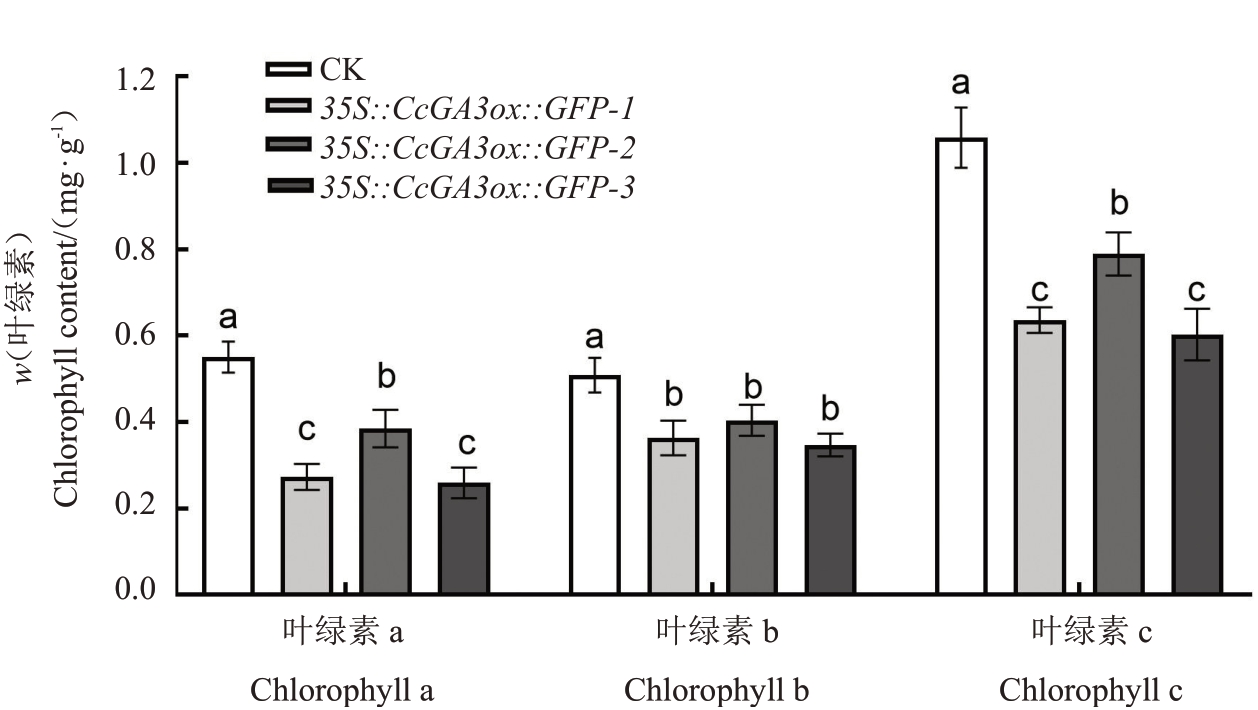
图11 核桃35S::CcGA3ox::GFP 再生植株叶绿素含量分析
Fig.11 Analysis of chlorophyll content of regenerated plants in walnut 35S::CcGA3ox::GFP
3 讨 论
GAs 参与植物生长发育的整个过程,是植物的重要调控激素之一,GAs 的缺乏会导致植物生长缓慢,出现矮化表型、雄性不育等现象[60]。GA3ox作为GAs 合成过程中关键限速酶之一,参与调控GAs 最后合成代谢阶段[61]。目前,有关GA3ox 基因的研究大多集中在模式植物和农作物方面,如水稻、拟南芥、小麦、玉米等[31]。在果树中,有关GA3ox 基因生物学功能验证方面报道较少。本研究中,笔者基于Huang 等[62]山核桃的转录组数据,通过基因克隆获得编码山核桃GA3ox 基因的完整开放阅读框序列,通过对该基因的生物信息学分析,发现山核桃Cc-GA3ox 基因序列长度为1 116 bp,编码371 个氨基酸,相对分子质量为40.69 kDa,等电点为6.67,分子式为C1 824H2 836N498O537S11。不同物种的同源序列比对结果表明,山核桃CcGA3ox基因属于2-酮戊二酸依赖性双加氧酶(2-ODDs)[63],这与葡萄、小麦GA3ox中的比对结果一致[38,40]。PSORT 在线软件分析表明,山核桃CcGA3ox 蛋白主要定位于细胞质,不存在信号肽和跨膜结构域,这与铁皮石斛GA3ox 蛋白[64]类似。进一步将山核桃CcGA3ox基因编码的氨基酸序列与其他物种的氨基酸序列在NCBI中进行比对,发现其与薄壳山核桃亲缘CiGA3ox 关系最近,同源率高达98.6%;此外,其与核桃、板栗、阔叶栎GA3ox 亲缘关系较近,同源率分别为97.84%、84.64%、83.56%,与小麦、油菜、拟南芥、水稻的同源率分别为61.03%、59.7%、44.33%、38.46%,说明在进化过程中,亲缘关系越近,同源率越高。
研究表明,果树树体的矮化机制与以赤霉素为中心的激素代谢密切相关[65]。GAs相关合成代谢酶的基因表达量可改变植株高度[66]。GA20ox 作为GAs 合成途径中的关键酶,在GAs 合成最后步骤中起到限速酶的作用从而调控GAs 含量。果树中GA20ox 基因的遗传转化研究表明,GA20ox 表达量的变化明显改变转化植株体内的GAs含量,从而影响植物株高[61]。在柑橘中过表达GA20ox基因,过表达植株中的GA1含量是对照植株的1.8~2.8倍,过表达植株的高度与对照相比明显增高,节间增长,而GA20ox 基因沉默表达植株的GA1 含量仅为对照植株的46%~62%,表现出矮化表型[67]。在梨中,同样发现GA20ox 基因在不同品种的表达量与植株高度呈正相关,不同发育阶段新梢叶片中GA20ox基因的表达强度为‘中矮1 号’<‘锦香’<‘早酥’,与植株高矮表现一致[68]。与GA20ox相似,GA3ox基因表达量的改变也可影响植株高度。杂交获得的短枝型苹果‘苏帅’与其亲本相比,枝条粗壮节间较短,植株较矮,通过对‘苏帅’和其亲本的内源激素测定分析,发现‘苏帅’中GA3ox4 同源基因表达量显著低于亲本[69]。紫花苜蓿MsDWF1 基因编码GA3ox,该基因自然突变体植株呈现矮化表型,且这种矮化表型可被GA3 恢复,但不能被野生型砧木恢复[70]。以上研究结果表明,GA3ox 基因正向调控植株高度的变化。笔者在本研究中通过过量表达山核桃GA3ox基因,发现核桃再生植株高度显著高于对照,表明过量表达该基因可促进植株长高,该结论与前人研究结果一致。
研究显示,GAs 调控植株高度主要通过节间伸长或缩短实现,一般来说,节的数量不变[67]。Dennis等[71]构建了转PsGA3ox 基因的过表达豌豆株系,发现转化植株平均节间长度比对照植株增加22%,节间明显伸长,但节的数量不变,进一步检测结果表明,转化植株PsGA3ox 在节间组织中的相对表达量为对照植株的5 倍。土豆StGA3ox2 基因受到RNA干扰后,再生植株呈现矮化性状,与对照相比,节间变短[43]。在本研究中,在CcGA3ox 的过表达核桃植株中,茎秆的伸长主要是通过节间伸长实现的,而非节间数目的增多。
此外,GAs 还在植物的生长过程中间接调控植物体内叶绿素的合成[45]。研究表明,GAs 主要通过DELLA 蛋白介导调控叶绿素的合成[19],同时,GAs通过影响叶绿素合成代谢关键酶的作用,维持叶绿素含量,减缓植物衰老[20],CND41 作为一个负调控叶绿体转录水平的结合蛋白,CND41反义突变转基因烟草植株矮化,叶片深绿,开花延迟,GAs 含量降低,并推测GA20ox氧化酶转录水平降低[72]。与野生型水稻相比,水稻GAs 生物合成基因3b-羟基化酶(GA3ox)功能丧失的突变体叶片面积减少,每单位叶面积中叶绿素含量增加,具有更强的光合作用,叶片颜色更深[45]。玉米GAs 缺乏矮化突变体d1,编码GA3ox 氧化酶并阻断GAs 形成,出现节间缩短表型,叶片宽且呈深绿色[42]。在麻风树中过量表达Jc-GA2ox6基因,通过调控植株内源GA1和GA4水平,从而产生具有更高叶绿素含量的深绿色叶片及矮小的表型[73]。本研究中过量表达CcGA3ox 可使核桃转化株系的叶绿素含量显著降低,与前人研究结果一致。
GA3ox 基因在调控植物生长发育[31]、遗传代谢[26]、非生物胁迫[30]等方面起着重要作用,随着研究的进一步深入,GA3ox 基因在调控果树生长发育中的机制将会被逐渐揭示,本研究也为分子水平利用基因敲除、定向突变等手段深入研究GA3ox 的生物学功能奠定了基础。
4 结 论
从山核桃中分离获得CcGA3ox基因,并对该基因序列进行生物信息学分析。功能鉴定结果初步表明,山核桃CcGA3ox基因过表达可促进核桃植株高度增加,降低叶绿素含量,为进一步明确该基因的功能特征及其参与调控植株生长的分子机制提供了良好的理论依据。
[1] 郑万钧.中国树木志[M].北京:中国林业出版社,1985:23-76.ZHENG Wanjun. Chinese tree chronicles[M]. Beijing: China Forestry Press,1985:23-76.
[2] 吕芳德,黄菁.山核桃属植物研究进展[J].经济林研究,2005,23(2):72-75.LÜ Fangde,HUANG Jing. Advances of Carya Nutt[J]. Nonwood Forest Research,2005,23(2):72-75.
[3] 杨颖.临安市山核桃生态化经营模式的研究[D].临安:浙江农林大学,2017.YANG Ying. Research on ecology management model of Carya cathayensis in Lin’an county[D]. Lin’an: Zhejiang Agricultural and Forestry University,2017.
[4] 陈咪佳.山核桃主要营养成分比较及其加工影响的研究[D].临安:浙江农林大学,2017.CHEN Mijia. The research on comparison of main nutritional components of hickory and influence of processing effect[D].Lin’an:Zhejiang Agricultural and Forestry University,2017.
[5] 郑燕.安徽省山核桃产业发展现状及对策研究[D].合肥:安徽农业大学,2016.ZHENG Yan. Research on the present situation and development of Carya cathayensiss Sarg. industry in Anhui province[D].Hefei:Anhui Agricultural University,2016.
[6] 许小峰,张蔚,徐九华,杨林,邓世维.便携式山核桃动力采摘设备的研究[J].木材加工机械,2012,23(5):51-54.XU Xiaofeng,ZHANG Wei,XU Jiuhua,YANG Lin,DENG Shiwei. Research on picking equipment of portable hickory[J].Wood Processing Machinery,2012,23(5):51-54.
[7] WAILER E W.The biochemistry and physiology of gibberellins,volsⅠandⅡ[J]. Plant,Cell and Environment,2010,7(8): 631-632.
[8] HEDDEN P,PHILLIPS A L. Gibberellin metabolism: New insights revealed by the genes[J].Trends in Plant Science,2001,5(12):523-530.
[9] HEDDEN P. Gibberellin metabolism and its regulation[J]. Journal of Plant Growth Regulation,2002,20(4):317-318.
[10] JONES R L,JACOBSEN J V.Regulation of synthesis and transport of secreted proteins in cereal aleurone[J]. International Review of Cytology,1991,126:49-88.
[11] BIEMELT S,TSCHIERSCH H,SONNEWALD U.Impact of altered gibberellin metabolism on biomass accumulation,lignin biosynthesis,and photosynthesis in transgenic tobacco plants[J].Plant Physiology,2004,135(1):254-265.
[12] ACHARD P,GENSCHIK P. Releasing the brakes of plant growth: how GAs shutdown DELLA proteins[J]. Journal of Experimental Botany,2009,60(4):1085-1092.
[13] KING K E,MORITZ T,HARBERD N P. Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA[J].Genetics,2001,159(2):767-776.
[14] DONG B,DENG Y,WANG H B,GAO R,STEPHEN G K,CHEN S M,CHEN F D,JIANG J F. Gibberellic acid signaling is required to induce flowering of chrysanthemums grown under both short and long days[J]. International Journal of Molecular Sciences,2017,18(6):1259
[15] YAMGUCHI N,WINTER C M,WU M F,KANNO Y,YAMAGUCHI A,SEO M,WAGNER D. Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis[J].Science,2014,344(6184):638-641.
[16] CHORY J,LI J. Gibberellins,brassinosteroids and light-regulated development[J]. Plant Cell and Environment,1997,20(6):801-806.
[17] GHOSH A,CHIKARA J,CHAUDHARY D R,PRAKASH A R,BORICHA G,ZALA A. Paclobutrazol arrests vegetative growth and unveils unexpressed yield potential of Jatropha curcas[J].Journal of Plant Growth Regulation,2010,29(3):307-315.
[18] 李哲馨,钮世辉,高琼,李伟.赤霉素调控木质部发育的细胞学研究[J].北京林业大学学报,2014,36(2):68-73.LI Zhexin,NIU Shihui,GAO Qiong,LI Wei. Cytological study of gibberellin regulated xylem development[J]. Journal of Beijing Forestry University,2014,36(2):68-73.
[19] MA Z X,HU X P,CAI W J,HUANG W H,ZHOU X,LUO Q,YANG H Q,WANG J W,HUANG J R. Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin- regulated chlorophyll biosynthesis under light conditions[J].Plos Genetics,2014,10(8):e1004519.
[20] LI J R,YU K,WEI J R,MA Q,WANG B Q,YU D. Gibberellin retards chlorophyll degradation during senescence of Paris polyphylla[J].Biologia Plantarum,2010,54(2):395-399.
[21] 谈心,马欣荣.赤霉素生物合成途径及其相关研究进展[J].应用与环境生物学报,2008,14(4):571-577.TAN Xin,MA Xinrong.Advance in research of gibberellin biosynthesis pathway and related progress[J]. Chinese Journal of Applied and Environmental Biology,2008,14(4):571-577
[22] OLSZEWSKI N,SUN T P,GUBLER F. Gibberellin signaling:biosynthesis,catabolism,and response pathways[J]. The Plant Cell,2002,14(Suppl.):61-80.
[23] 周明兵,汤定钦.高等植物赤霉素生物合成及其关键酶的研究进展[J].浙江林学院学报,2004,21(3):344-348.ZHOU Mingbing,TANG Dingqin. Research progress of gibberellin biosynthesis and its key enzymes in higher plants[J]. Journal of Zhejiang Forestry College,2004,21(3):112-116.
[24] 王金祥,李玲,潘瑞炽.高等植物中赤霉素的生物合成及其调控[J].植物生理学通讯,2002,38(1):1-8.WANG Jinxiang,LI Ling,PAN Ruizhi. Gibberellin biosynthesis and its regulation in higher plants[J]. Plant Physiology Communications,2002,38(1):1-8.
[25] HAN F M,ZHU B G. Evolutionary analysis of three gibberellin oxidase genesin rice,Arabidopsis,and soybean[J]. Gene,2011,473(1):23-35.
[26] ACHARD P,GONG F,CHEMINANT S,ALIOUA M,HEDDEN P,GENSCHIK P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism[J].The Plant Cell,2008,20(8):2117-2129.
[27] OZGA J A,YU J,REINECKE D M.Pollination-,development-,and auxin- specific regulation of gibberellin 3β- hydroxylase gene expression in pea fruit and seeds[J]. Plant Physiology,2003,131(3):1137-1146.
[28] TOYOMASU T,KAWAIDE H,MITSUHASHI W,INOIE Y.KAMIYA Y.Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds[J]. Plant Physiology,1998,118(4):1517-1523.
[29] 牛亚利,赵芊,张肖晗,艾秋实,宋水山.霉素信号在非生物胁迫中的作用及其调控机制研究进展[J].生物技术通报,2015,31(10):31-37.NIU Yali,ZHAO Qian,ZHANG Xiaohan,AI Qiushi,SONG Shuishan. Research progress on the role and regulation mechanism of gibberellin signal in response to abiotic stress[J]. Biotechnology Bulletin,2015,31(10):31-37.
[30] YANG L J,YANG D B,YAN X J,CUI L,WANG Z Y,YUAN H Z.The role of gibberellins in improving the resistance of tebuconazole-coated maize seeds to chilling stress by microencapsulation[J].Scientific Reports,2016,6:35447.
[31] 杨意宏,徐浩,陈段芬,高志民.高等植物赤霉素3-β-双氧化酶基因研究进展[J].生物技术通报,2018,34(3):18-22.YANG Yihong,XU Hao,CHEN Duanfen,GAO Zhimin. Research advance on the gene for gibberellin 3-β-dioxygenase gene in higher plants[J]. Biotechnology Bulletin,2018,34(3):18-22.
[32] CHIANG H H,HWANG I,GOODMAN H M. Isolation of the Arabidopsis GA4 locus[J].Plant Cell,1995,7(2):195-201.
[33] ITOH H,TANAKA-UEGUCHI M,KAWAIDE H,CHEN X B,KAMIYA Y,MATSUOKA M. The gene encoding tobacco gibberellin 3β-ydroxylase is expressed at the site of GA action during stem elongation and flower organ development[J].The Plant Journal,1999,20(1):15-24.
[34] LANGE T,KAPPLER J,FISCHER A,FRISSE A,PADEFFKE T,SCHMIDKE S,LANGE M J P. Gibberellin biosynthesis in developing pumpkin seedlings[J]. Plant Physiology,2005,139(1):213-223.
[35] APPLEFORD N E J,EVANS D J,LENTON J R,GASKIN P,CROKER S J,DEVOS K M,PHILLIPS A L.HEDDEN P.Function and transcript analysis of gibberellin-biosynthetic enzymes in wheat[J].Planta,2006,223(3):568-582.
[36] ISRAEISSON M,MELLEROWICZ E,CHONO M,GULLBERG J,MORITZ T.Cloning and overproduction of gibberellin 3-oxidase in hybrid aspen trees effects on gibberellin homeostasis and development[J]. Plant Physiology,2004,135(1): 221-230.
[37] 赵慧君,余海忠,王海燕,朱文权.苹果赤霉素3-氧化酶1(Md-GA3ox1)在烟草中的表达研究[J].湖北文理学院学报,2015,36(2):17-20.ZHAO Huijun,YU Haizhong,WANG Haiyan,ZHU Wenquan.Research on apple GA3-oxidase 1(MdGA3ox1)expression in tobacco[J].Journal of Hubei University of Arts and Science,2015,36(2):17-20.
[38] 刘炳臣,王茜,王跃进,张朝红.葡萄活性赤霉素合成关键基因VvGA3ox 家族的克隆和表达分析[J]. 园艺学报,2016,43(12):2293-2303.LIU Bingchen,WANG Qian,WANG Yuejin,ZHANG Chaohong. Cloning and expression analysis of the key gene family Vvga3ox in active gibberellin synthesis in grapevine[J]. Acta Horticulturae Scinica,2016,43(12):2293-2303.
[39] 乔枫,赵开军.植物中赤霉素代谢酶与株高的关系[J].生物技术通报,2011,27(3):1-6.QIAO Feng,ZHAO Kaijun. Relationship between plant height and gibberellin metabolic enzymes[J]. Biotechnology Bulletin,2011,27(3):1-6.
[40] 刘颖,阳文龙,郭小丽,翟晓,刘冬成,孙家柱,夏石头,张爱民.Rht12 矮秆小麦GA3ox 基因的克隆与表达分析[J].分子植物育种,2015,13(2):241-253.LIU Ying,YANG Wenlong,GUO Xiaoli,ZHAI Xiao,LIU Dongcheng,SUN Jiazhu,XIA Shitou,ZHANG Aimin,Cloning and expression analysis of GA3ox gene from Rht12 dwarf wheat[J].Molecular Plant Breeding,2015,13(2):241-253.
[41] 徐江民,方云霞,曾龙军,徐娜,焦然,胡娟,马路,肖飒清,黄玲,胡江,饶玉春,王跃星.水稻矮化小穗突变d18 新等位基因的发现及生理功能分析[J].中国科学:生命科学,2018,48(6):692-704.XU Jiangmin,FANG Yunxia,ZENG Longjun,XU Na,JIAO Ran,HU Juan,MA Lu,XIAO Saqing,HUANG Ling,HU Jiang,RAO Yuchun,WANG Yuexing. Identification and physiological function analysis of a new dwarf spikelet Dwarf18-allele mutant in rice[J].Scientia Sinica Vitae,2018,48(6):692-704.
[42] CHEN Y,HOU M M,LIU L J,WU S,SHEN Y,ISHIYAMA K,KOBAYASHI M,MCCARTY D R,TAN B C. The maize DWARF1 encodes a gibberellin 3-oxidase and is dual localized to the nucleus and cytosol[J]. Plant Physiology,2014,166(4):2028-2039.
[43] ROUMELIOTIS E,KLOOSTERMAN B,OORTWIJN M,LANGE T,VISSER R G F,BACHEM C W B.Down regulation of StGA3ox genes in potato results in altered GA content and affect plant and tuber growth characteristics[J]. Journal of Plant Physiology,2013,170(14):1228-1234.
[44] WOLF F T,HABER A H. Chlorophyll content of gibberellintreated wheat seedlings[J].Nature,1960,186(4720):217-218.
[45] JIANG X S,LI H Y,WANG T,PENG C L,WANG H Y,WU H,WANG X J. Gibberellin indirectly promotes chloroplast biogenesis as a means to maintain the chloroplast population of expanded cells[J].Plant Journal,2012,72(5):768-780.
[46] 王富廷,杨炜茹,张启翔. 垂枝梅花赤霉素GA20ox、GA3ox、GID1 基因克隆与表达分析[C]//张启翔.中国观赏园艺研究进展,北京:中国林业出版社,2014.WANG Futing,YANG Weiru,ZHANG Qixiang.Molecular cloning and expression analysis of GA20ox,GA3ox and GID1 of Prunus mume[C]// ZHANG Qixiang. Advances in Omamental Horticulture of China,Beijing:China Forestry Press,2014.
[47] 王增光,柴国华,王芝瑶,唐贤丰,孙长江,周功克,马三梅.拟南芥AtGA3ox1 和AtGA3ox2 基因影响茎秆次生细胞壁增厚的分子机理[J].遗传,2013,35(5):655-665.WANG Zengguang,CHAI Guohua,WANG Zhiyao,TANG Xianfeng,SUN Changjiang,ZHOU Gongke,MA Sanmei. Molecular mechanism of AtGA3ox1 and Atga3ox2 genes affecting secondary wall thickening in stems in Arabidopsis[J].Hereditas,2013,35(5):655-665.
[48] 胡恒康,江香梅,张启香,陈贝,黄坚钦.碳源对山核桃体细胞胚发生和植株再生的影响[J]. 浙江农林大学学报,2011,28(6):911-917.HU Hengkang,JIANG Xiangmei,ZHANG Qixiang,CHEN Bei,HUANG Jianqin. Somatic embryogenesis and plant regeneration from Carya cathayensis embryos using different carbon source[J]. Journal of Zhejiang Agricultural and Forestry University,2011,28(6):911-917.
[49] TUO D C,SHEN W T,YAN P,LI X Y,ZHOU P. Rapid construction of stable infectious full-length cDNA clone of papaya leaf distortion mosaic virus using in-fusion cloning[J]. Viruses,2015,7(12):6241-6250.
[50] SAITO T,BAI S L,ITO A,SAKAMOTO D,SAITO T,UBI B E,TMAI T,MARIGUCHI T. Expression and genomic structure of the dormancy-associated MADS box genes MADS13 in Japanese pears(Pyrus pyrifolia Nakai)that differ in their chilling requirement for endodormancy release[J].Tree Physiology,2013,33(6):654-667.
[51] SAMBROOK J,FRITSCH F J,MANIATIS T. Molecular cloning: a laboratory manual[J]. Biochemical Education,1983,11(2):182-183.
[52] HOOD E E,GELVIN S B,MELCHERS L S,HOEKEMA A.New Agrobacterium helper plasmids for gene transfer to plants[J].Transgenic Research,1993,2(4):208-218.
[53] 刘会君.核桃JrAMT 基因的克隆与功能分析[D].杭州:浙江农林大学,2019.LIU Huijun. Cloning and functional analysis of walnut JrAMT gene[D].Hangzhou:Zhejiang Agricultural and Forestry University,2019.
[54] ZHANG Q X,WALAWAGE S L,TRICOLI D M,DANDEKAR A M,LESLIE C A.A red fluorescent protein(DsRED)from Discosoma sp. as a reporter for gene expression in walnut somatic embryos[J].Plant Cell Reports,2015,34(5):861-869.
[55] LESLIE C A,HACKETT W P,MCGRANAHAN G H. Improved rooting methods for walnut(Juglans)microshoots[J].Acta Horticulturae,2010,861:365-372.
[56] GUO Y P,LI W,SUN H Y,WANG N,YU H S. CHEN H G.Detection and quantification of Rhizoctonia cerealis in soil using real-time PCR[J].Journal of General Plant Pathology,2012,78(4):247-254.
[57] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2- Δ Δ Ct method[J].Methods,2011,25:402-408.
[58] 张志良,翟伟菁,李小芳.植物生理学实验指导[M].北京:高等教育出版社,2009.ZHANG Zhiliang,ZHAI Weijing,LI Xiaofang. Experimental guidance of plant physiology[M]. Beijing: Higher Education Press,2009.
[59] 邱振鲁,李筱涵,朱蕾,张稳轩,刘文薪,于洪泉.赤霉素、6-糠氨基嘌呤与萘乙酸对风信子花期及叶绿素含量的影响[J].绿色科技,2018(16):253-255.QIU Zhenlu,LI Xiaohan,ZHU Lei,ZHANG Wenxuan,LIU Wenxin,YU Hongquan. Effects of gibberellin,6-furylaminopurine and NAA on flowering period and chlorophyll content of hyacinth[J]. Journal of Green Science and Technology,2018(16):253-255.
[60] 陈晶晶,胡玉林,胡会刚,段雅婕,庞振才,谢江辉.植物矮化相关基因的研究进展[J]. 广东农业科学,2014,41(15):126-132.CHEN Jingjing,HU Yulin,HU Huigang,DUAN Yajie,PANG Zhencai,XIE Jianghui. Research advances on dwarfing gene in plants[J]. Guangdong Agricultural Science,2014,41(15): 126-132.
[61] 岳川,曾建明,曹红利,王新超,章志芳.高等植物赤霉素代谢及其信号转导通路[J].植物生理学报,2012,48(2):118-128.YUE Chuan,ZENG Jianming,CAO Hongli,WANG Xinchao,ZHANG Zhifang.Gibberellin metabolism and signal pathway in higher plants[J]. Plant Physiology Journal,2012,48(2): 118-128.
[62] HUANG Y J,XIAO L H,ZHANG Z R,……,HUANG J Q.The genomes of pecan and Chinese hickory provide insights into Carya evolution and nut nutrition[J]Gigaence,2019,8(5):1-17.
[63] YAMAGUCHI S,KAMIYA Y. Gibberellin biosynthesis: Its regulation by endogenous and environmental signals[J]. Plant and Cell Physiology,2000,41(3):251-257.
[64] 刘思思,张岗,陈晓梅,李淑超,陈娟,郭顺星.铁皮石斛赤霉素3-氧化酶基因的克隆及表达分析[J]. 中草药,2016,47(6):990-996.LIU Sisi,ZHANG Gang,CHEN Xiaomei,LI Shuchao,CHEN Juan,GUO Shunxing. Cloning and expression analysis of gibberellin 3-oxidase gene of Dendrobium candidum[J]. Chinese Traditional and Herbal Drugs,2016,47(6):990-996.
[65] FAUST M.决定树体大小的理论机制[J].吴帮良译国外农学(果树),1992(1):13-14.FAUST M. The theoretical mechanism of determining tree size[J]. WU Bangliang Trans Foreign Agriculture (fruit trees),1992(1):13-14.
[66] 王弋,董晨,魏永赞,郑雪文,李伟才.GA 信号途径及其调控果树生长发育的研究进展[J].果树学报,2018,35(4):110-121.WANG Yi,DONG Chen,WEI Yongzan,ZHENG Xuewen,LI Weicai. Research progress on GA signaling pathway and its function in regulating fruit tree growth and development[J].Journal of Fruit Science,2018,35(4):110-121.
[67] FAGOAGA C,TADEO F R,IGLESIAS D J,HUERTA L,LISO I. VIDAL A M,TALON M T,NAVARRO L,CARCÍAMATÍNEZ J L,PEÑA L.Engineering of gibberellin levels in citrus by sense and antisense overexpression of a GA20-oxidase gene modifies plant architecture[J].Journal of Experimental Botany,2007,58(6):1407-1420.
[68] 宣利利,欧春青,王斐,王志刚,程飞飞,李振茹,姜淑苓.梨矮化砧木中矮1 号GA20-氧化酶基因克隆与表达分析[J].果树学报,2011,28(5):883-887.XUAN Lili,OU Chunqing,WANG Fei,WANG Zhigang,CHENG Feifei,LI Zhenru,JIANG Shuling. Isolation and expression analysis of gibberellin 20- oxidase gene from pear dwarfing Zhongai 1[J]. Journal of Fruit Science,2011,28(5):883-887.
[69] 辛璐.短枝型苹果‘苏帅’赤霉素相关差异基因的筛选与表达分析[D].南京:南京农业大学,2015.XIN Lu.Screening and expression of the difference for gibberellin related genes of spur-type apple‘Sushuai’[D]. Nanjing:Nanjing Agricultural University,2015.
[70] DALMADI Á,KALÓ P,JAKAB J,SASKŐI A,PETROVICS T,DEÁK G,KISS G B. Dwarf plants of diploid Medicago sativa carry a mutation in the gibberellin 3-β-hydroxylase gene[J].Plant Cell Reports,2008,27(8):1271-1279.
[71] REINECKE D M,WICKRAMARATHNA A D,OZGA J A,KUREPIN L V,JIN A L,GOOD A G,PHARIS R P.Gibberellin 3-oxidase gene expression patterns influence gibberellin biosynthesis,growth,and development in pea[J]. Plant Physiology,2013,163(2):929-945.
[72] NAKANO T,NAGATA N,KIMURA T,SEKIMOTO M,KAWAIDE H,MURAKAMI S,KANEKO Y,MATSUSHIMA H,KAMIYA Y,SATO F,YOSHIDA S. CND41,a chloroplast nucleoid protein that regulates plastid development,causes reduced gibberellin content and dwarfism in tobacco[J]. Physiologia Plantarum,2003,117(1):130-136.
[73] HU Y X,TAO Y B,XU Z F.Overexpression of Jatropha Gibberellin 2-oxidase 6 (JcGA2ox6) induces dwarfism and smaller leaves,flowers and fruits in Arabidopsis and Jatropha[J]. Frontiers in Plant Science,2017,8:2103.